Abstract
This review article explores the critical role of additives in enhancing the performance and durability of thermochemical energy storage (TCES) materials, particularly in limestone-based systems. It evaluates various strategies, including hydration and the use of fine particles, along with additives like Al2O3 and ZrO2, to address challenges like performance degradation and sintering over multiple cycles. Additionally, the review examines how multicyclic stability and material activity toward CO2 are related. It emphasizes the importance of selecting support materials that optimize both stability and reactivity. Furthermore, it highlights the need for systematic investigation into the selection, synthesis methods, and additive percentages to identify optimal formulations for improved multicyclic stability. Finally, it underscores the importance of understanding the mechanisms of interaction between additives and CaO/CaCO3 matrices to guide the design of effective additive-integrated systems. This comprehensive analysis provides valuable insights into current methodologies, emerging trends, and future directions for advancing sustainable energy storage technologies.
1. Introduction
In our ongoing efforts to have a cleaner and longer-lasting energy future, the need for better ways to store energy is a crucial topic in scientific discussions [1]. The increasing use of renewable energy sources like solar and wind power is a positive step in fighting climate change and reducing our dependence on limited fossil fuels [2,3,4]. However, there’s a big challenge—these eco-friendly sources do not generate energy all the time. This on-and-off pattern, called intermittency, poses a significant hurdle to relying solely on renewables [5].
Renewable energy generation, influenced by variable weather conditions, requires a seamless mechanism to address the fluctuations between surplus and deficit [6]. Energy storage emerges as an integral component in the pursuit of a resilient and dependable energy grid [7,8,9]. Its primary function involves the accumulation of excess energy during peak production and its subsequent release during periods of low generation, thereby stabilizing the intermittent nature of renewable energy availability [10]. This operational feature not only alleviates issues related to intermittency but also establishes a critical foundation for the widespread acceptance and integration of sustainable energy sources [11].
The collaboration between energy storage and grid resilience becomes evident when we consider incorporating renewable energy into our current power systems [12]. The adaptable characteristics of energy storage systems play a crucial role in maintaining a balance between the ever-changing dynamics of energy production and consumption. This proves instrumental in providing a defense against disruptions and preventing blackouts [13]. As energy storage alleviates the burden on traditional power plants, it becomes a pivotal element in strengthening the fundamental structure of our energy infrastructure [14].
Essentially, energy storage is not just a temporary solution; it is the essential element driving the shift towards a sustainable energy environment [15]. With nations worldwide pledging to achieve ambitious renewable energy goals, the scalability and effectiveness of energy storage technologies take center stage [16]. It serves as the channel through which intermittent renewables smoothly blend into the energy mix, propelling us towards a future marked by low carbon emissions [17]. The necessity for energy storage becomes the guiding principle leading us through the intricate process of deploying renewable energy sources [18].
In summary, the requirement for energy storage in the scientific discourse goes beyond being a mere technological necessity; it represents the central point for the sustainable energy transition. From addressing the intermittency of renewable energy sources to strengthening grid resilience and expediting the shift towards a cleaner energy framework, energy storage emerges as a game changer in this defining era. The ongoing dedication to advancing energy storage technologies is not solely a scientific pursuit; it is a clear call for the harmonious coexistence of environmental responsibility and global energy security [19].
2. Types of Energy Storage
As mentioned in the previous section, energy storage is crucial for maintaining a stable and reliable energy supply, especially as we integrate more renewable energy sources with intermittent generation patterns [20]. Energy storage technologies can be categorized into five types based on the process through which energy is stored: mechanical [21,22], electrochemical (or batteries) [23,24], thermal [25,26], electrical [26,27], and hydrogen storage technologies [28,29,30]. Figure 1 summarizes these types of energy storage technologies along with their sub-categories.
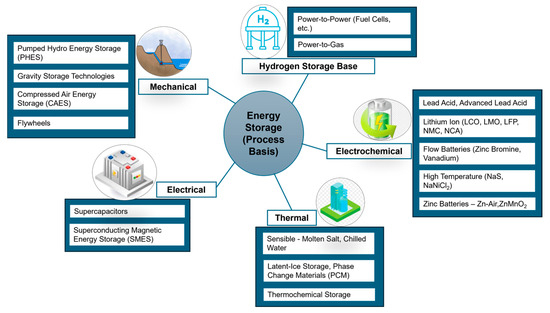
Figure 1.
Types of energy storage-process basis.
Technologies used for mechanical energy storage, store energy in the form of gravitational potential energy, kinetic energy (associated with motion), or potential energy resulting from compression. These technologies can be further categorized as Pumped Hydro Storage (PHS) [31], Gravity Energy Storage [32], Compressed Air Energy Storage (CAES) [33], and Flywheel Storage [34] technologies. In PHS, surplus electricity is used to pump water from a lower reservoir to a higher one during periods of low demand. When electricity demand is high, the stored water is released and used to generate electricity through turbines. CAES stores energy by compressing air and storing it in underground caverns. During peak demand, the compressed air is released and expanded through turbines to generate electricity. Whereas, in Flywheel Energy Storage, energy is stored in the form of kinetic energy by spinning a rotor (flywheel) at high speeds. When energy is needed, the kinetic energy is converted back to electricity [21].
In an electrical energy storage system, electricity is stored by using an electric charge/electric field, or magnetic field. It includes supercapacitors [35] and superconducting magnetic energy storage [36]. A supercapacitor, also known as an ultracapacitor, is a powerful type of capacitor. It has much higher capacitance than regular capacitors but operates at a lower voltage. It acts as a bridge between electrolytic capacitors and rechargeable batteries. Superconducting Magnetic Energy Storage (SMES) systems store energy by utilizing the magnetic field generated through the flow of direct current in a superconducting coil. This coil is cooled to a temperature below its superconducting critical temperature using cryogenic methods [37].
Hydrogen energy storage is a form of chemical energy storage in which energy is used to produce hydrogen [38]. Hydrogen storage technologies operate on the fundamental idea of using electricity (from renewable sources) to carry out water electrolysis, generating hydrogen and oxygen [39]. The produced hydrogen is stored [40] and can be utilized either for direct combustion in applications like heating or for electricity generation through polymer electrolyte fuel cells [41].
Electrochemical energy storage includes a range of secondary batteries that convert the chemical energy within their active materials into electrical energy through a reverse electrochemical oxidation-reduction reaction [24,42]. Secondary batteries are typically classified into distinct groups based on the electrochemical system they employ. These include standard batteries such as lead-acid [43] and Ni-Cd [44], as well as modern batteries like Ni-MH [45], Li-ion [46], and Li-polymer [47]. Special batteries, such as Ag-Zn and Ni-H2, represent another category, while technologies like flow batteries (e.g., Br2-Zn, vanadium redox) and high-temperature batteries (e.g., Na-S, Na-metal chloride) constitute additional segments [48]. This diverse range of commercially available secondary batteries caters to various applications and technological requirements.
Thermal Energy Storage (TES) allows the storage of heat energy in the form of temperature, phase change, or a reversible chemical reaction [49]. Multiple case studies have been performed for better conversion of solar to heat energy for TES, such as optimized solar air collectors, PCM nanoadditive solar stills, and improved energy storage in permanent magnets and Mg2+-doped CaCO3/PEG composites [50,51,52,53]. TES are categorized into sensible heat storage (SHS) [54], latent heat storage (LHS) [55], and thermochemical energy storage (TCES) [56]. SHS involves storing heat in a material by changing its temperature, like water or oil, that can later be released when needed. LHS involves the storage and release of heat during the phase transition of materials called phase change materials (PCMs) (e.g., salts, alkali nitrates, hydroxides, or chlorides) [57]. TCES stores energy through reversible chemical reactions. During charging, the chemical reaction absorbs heat, and during discharging, the reverse reaction releases heat, for example, calcination (endothermic) and carbonation (exothermic) of calcium carbonate [58]. TCES not only possesses the highest energy densities but also offers other benefits over SHS and LHS technologies. The characteristics comparison of all three types of TES is summarized in Table 1.

Table 1.
Characteristics of TES Technologies [59].
Each type of energy storage has its advantages and disadvantages, and the choice depends on factors such as the application, duration of energy storage, scale, and specific requirements of the energy storage system. As technology continues to advance, there’s ongoing research to improve the efficiency, cost-effectiveness, and sustainability of these energy storage methods.
Energy storage technologies are also categorized based on storage duration: short-duration energy storage (SDES) and long-duration energy storage (LDES) [60]. SDES systems store energy for up to a few hours, while LDES systems can store energy from hours to days, or in some cases, from days to months [61]. Both of these energy storage systems are essential to balance the grid on hourly, daily, weekly, and even seasonal timescales. The requirement for longer energy storage increases as the percentage share of renewable energy increases in the grid [62]. Figure 2 illustrates the relationship between the fraction of annual energy sourced from variable renewable generators (specifically wind and solar) at a regional or local level and the maximum duration of electricity storage necessary to ensure continuous demand fulfillment, presented on a logarithmic scale. Arrows are employed to denote differing levels of stringency regarding curtailment, transmission, and grid flexibility assumptions, with a leftward arrow indicating more restrictive conditions and a rightward arrow indicating more lenient conditions. For instance, in scenarios where curtailment is minimized (arrow pointing left), a longer storage duration is required compared with scenarios where greater curtailment is permitted (arrow pointing right) [63]. Beyond a 60% threshold for variable renewables, there is a notable escalation in the demand for both daily and weekly storage [63]. The necessity for seasonal storage (LDES for months) becomes particularly pronounced beyond an 80% penetration of variable renewables [63]. Consequently, the imperative role of energy storage in facilitating system flexibility and energy management emerges as crucial for the effective integration of substantial proportions of wind and solar energy sources.
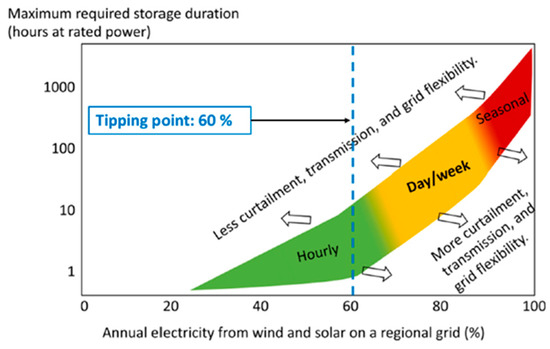
Figure 2.
Maximum duration of electricity storage needed versus fraction of annual energy from variable renewable generators (wind and solar) [63].
Figure 3 presents the storage duration of some energy storage technologies against the size of energy storage along with their function on top [59]. All kinds of electrochemical batteries (lead acid, li-ion batteries) and capacitors lie under the category of SDES [64]. CAES, PHS, and thermal energy storage (molten salts) are considered LDES and scalable to GWs of energy storage, can be used for bulk power management [65]. TCES, which is a sub-category of TES, is not present in Figure 3, but it can be placed alongside the molten salts (LHS). Because TCES possesses the highest energy densities among the other TECs (8–10 times higher than SHS and two times higher than LHS) [26], TCES will be the focus of this review.
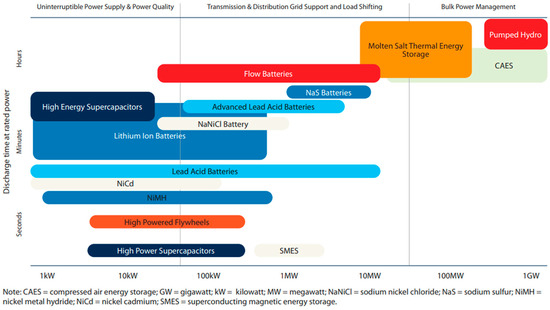
Figure 3.
Storage duration of some energy storage technologies against the size of energy storage technologies [59].
3. Introduction to Thermochemical Energy Storage
TCES is a promising technology that involves the use of chemical reactions to store and release energy. TCES systems utilize reversible chemical reactions to store thermal energy, which can be later retrieved for various applications. These systems typically involve the conversion of a material between two chemical states, one absorbing energy during an endothermic reaction (charging) and the other releasing energy during an exothermic reaction (discharging).
An ideal TCES system should possess several key attributes. Firstly, it should exhibit high energy storage density to minimize the system’s weight and volume requirements, with target values set at 300 kWh m−3 or >1000 kJ kg−1 [66]. Secondly, high working temperatures are essential for maximizing power generation efficiency, with the charging step falling between 700 °C and 1500 °C [66]. Additionally, materials must demonstrate high cycling and chemical/thermal stability to continue prolonged operation, with a focus on maintaining chemical reaction reversibility and multicyclic stability [66]. Strong mechanical properties are crucial for ensuring long-term integrity during repeated cycles, as solid materials are prone to morphological degradation at high temperatures. Moreover, storage materials should be inexpensive, abundant, and low in toxicity (targeted cost of $4.2 MJ−1, DOE 2020) [66]. Fast and stable kinetics of both forward and reverse reactions are necessary to facilitate energy charging and discharging steps, while favorable reaction thermodynamics play a decisive role in selecting suitable reactions based on working temperatures and reaction enthalpy.
Finally, high solar absorptivity and thermal conductivity are vital for maximizing heat storage efficiency, which is crucial for driving endothermic reactions in TCES systems. Therefore, improving these properties represents a significant challenge in the context of TCES [67].
Table 2 summarizes the properties of popular TCES systems, along with their reversible chemical reactions, phase-phase interaction types, and material types. Three types of phase-phase interaction are mentioned in Table 2: solid–gas, liquid–gas, and gas–gas. Among the three, solid–gas systems (carbonates, hydroxides, hydrides, and metal oxides) are considered the most promising TCES because the primary products of these reactions are solids that can be conveniently stored [68].

Table 2.
Properties of popular TCES systems.
Carbonates offer low material costs and abundance, making them suitable for large-scale use, although they face challenges with limited reversibility and cyclic stability [91]. Hydroxides, also economically viable and abundant, demonstrate good reversibility but are susceptible to agglomeration and side reactions [92]. Metal hydrides display high volumetric energy density but are hindered by hydrogen embrittlement and high material costs [93]. Metal oxides exhibit good reversibility yet suffer from toxicity and high costs [94]. Ammonia synthesis, while easy to control, encounters issues with low operating temperatures and storage costs [89]. Methane reforming, despite high operating temperatures, lacks volumetric energy density and poses toxicity concerns [90]. The SO3/O2/SO2 system operates at high temperatures but has a low energy density and requires a catalyst [90].
4. Limestone-Based Thermochemical Energy Storage
One of the most promising classes of gas–solid systems for TCES consists of those relying on the reversible decomposition of limestone, also known as calcium carbonate. Limestone is decomposed into CaO and CO2 by absorbing heat (calcination), while during the reverse reaction the heat is released (carbonation).
This approach offers numerous advantages, including low material cost (approximately $10 per tonne for CaCO3/CaO), widespread availability, nontoxicity, exceptionally high energy density (reaching up to 4 GJ m−3) and reaction enthalpy (ΔH860°C = 165.9 kJ) [95], operation at very high temperatures (exceeding 800 °C), catalyst-free operation, byproduct-free reactions, and extensive experimental validation from Carbon Capture and Storage (CCS) applications [58].
Despite the advantageous characteristics of limestone-based TCES, it is afflicted by significant drawbacks, particularly concerning the multicyclic stability of CaCO3/CaO. Specifically, the reduction in CaO reactivity necessitates the use of oversized equipment due to the substantial presence of unreactive solids within the system. As CaO deactivation progresses, the volume of inert solid material requiring transportation, preheating, cooling, and processing throughout the plant escalates, resulting in reduced overall efficiency.
The multicycle stability and performance degradation of the CaCO3/CaO system have been extensively reported and studied for CO2 storage and capture (CCS) [96,97,98]. The multicycle performance of limestone strongly depends on the operating conditions of the process. The operating conditions for CCS are different from those for TCES (Table 3).

Table 3.
Comparison of CCS and TCES operation conditions [99,100,101].
It has been widely reported that the calcination reaction goes to completion under appropriate conditions, while the carbonation reaction often does not. The carbonation reaction is divided into two stages. Initially, carbonation occurs rapidly as CO2 molecules are adsorbed onto the exposed surface of CaO particles. This early stage is not only influenced by the intrinsic reaction kinetics but also by factors such as mass and heat transfer efficiency to the particle surface, which can significantly affect the carbonation rate. As the reaction progresses, a thin layer of CaCO3 forms on the CaO surface, leading to a transition to a diffusion-controlled stage. Here, the rate of carbonation becomes limited by the diffusion of CO2 molecules through the formed CaCO3 layer before reaching the underlying CaO surface for further reaction. This shift highlights the importance of considering both reaction kinetics and diffusion phenomena in understanding the overall carbonation process. This incomplete carbonation affects the overall efficiency of the energy storage system.
The observed decline in carbonation activity of CaO over multiple cycles is primarily attributed to CaO deactivation mechanisms, including sintering, pore plugging, and agglomeration. Sintering refers to the process where CaO particles coalesce due to high temperatures, reducing the available surface area and slowing carbonation reactions. Pore plugging occurs when pores within the CaO material become filled with CaCO3, hindering the diffusion of CO2 into the material. Agglomeration involves the formation of larger CaO particles, which reduces the overall active surface area of the material. This issue has been extensively studied in the context of CO2 capture applications and, more recently, in thermochemical energy storage (TCS) applications, where it directly impacts the energy density of the system.
5. Strategies to Improve Energy Storage in Limestone-Based Systems
Some strategies have been devised to address the issue of multicycle performance degradation of limestone, including hydration [102], Ca-based sorbents/additives, and the use of fine CaO/CaCO3 particles.
Hydration involves the exposure of CaO to water vapor, leading to the formation of calcium hydroxide (Ca(OH)2). This process can help mitigate CaO deactivation by forming a protective layer of Ca(OH)2 on the CaO surface, which can inhibit sintering and enhance the regeneration ability of CaO during carbonation. Specifically, upon hydration, CaO undergoes conversion into Ca(OH)2, resulting in an expansion of molar volume (CaO = 16.9 cm3 mol−1; Ca(OH)2 = 33.7 cm3 mol−1) and a reduction in density (CaO = 3.32 g cm−3; Ca(OH)2 = 2.20 g cm−3) [103]. This hydration process leads to swelling of the CaO, followed by the decomposition of the formed hydrate, inducing the development of fractures within the material’s core. Consequently, the material’s porosity and surface area are increased, facilitating enhanced diffusion of CO2 during carbonation reactions and mitigating pore blockage phenomena [103]. Research studies have demonstrated the effectiveness of hydration in improving the cyclic stability and reactivity of CaO-based sorbents for CO2 capture and thermochemical energy storage [103,104,105].
The size of particles plays a significant role in the multicyclic performances of limestone [106,107], alongside factors like crystallinity [108], morphology [109], and the inclusion of additives [110]. Large or highly crystalline particles exhibit slower reaction kinetics and suffer from plugging during carbonation. Conversely, smaller particles enable faster carbonation kinetics at lower temperatures, leading to improved multicyclic stability by minimizing sintering and pore-plugging issues [111,112].
Research indicates that reducing limestone particle size below 45 μm can notably increase residual CaO multicyclic conversion [58]. This effect is attributed to the relative thickness of the CaCO3 product layer compared with pore size within the CaO skeleton, where coarse particles are prone to severe pore-plugging and subsequent sintering, decreasing the available CaO surface area. Fine particles (<45 μm) mitigate pore-plugging phenomena due to their higher surface-to-volume ratio, enhancing CO2 accessibility [113].
Despite benefits in multicyclic stability, fine particle usage in large-scale reactors poses challenges such as agglomeration and poor gas/solid contact [114,115]. However, studies employing high-intensity acoustic waves demonstrate enhanced carbonation performances of fine limestone particles in fluidized bed reactors, mitigating sintering-induced CaO deactivation [116].
The vulnerability of limestone-based materials to sintering over multiple cycles, where particles fuse together, is due to the low Tammann temperature (Tt) of CaCO3. The Tammann temperature is the temperature at which sintering begins [117]. To overcome this challenge and maintain the reactivity of CaCO3/CaO over multiple cycles, a promising solution is the doping or mixing of an additive with heat-resistant properties such as Al2O3, SiO2, TiO2, ZrO2, Y2O3, MgO, etc., which have high Tt values [105,118,119,120,121]. These additive materials help with sintering resistance, ensuring better stability over repeated carbonation and calcination cycles. These additives can be classified into two groups based on their interaction with CaO: reactive additives and non-reactive additives. Reactive additives include Al2O3, SiO2, ZrO2, etc.; they react with CaO and form ternary oxides (Table 4). Non-reactive additives do not react with CaO; examples of such additives are Y2O3, MgO, CeO2, etc. (Table 5) [117].
Al2O3 emerges as a prominent choice among materials for creating ternary oxides with CaO, owing to its impressive attributes such as remarkable thermal stability (notably demonstrated by a Tt of 900 °C), cost-efficiency, and strong mechanical resilience [117]. Recent advancements in the multicyclic performances of CaO-based sorbents supported on Al2O3 are outlined in Table 4. Incorporation of Al2O3 into CaO-based sorbents leads to the formation of diverse Ca-Al ternary oxides, such as Ca3Al2O6 [122], Ca5Al6O14 [123], Ca9Al6O18 [124], and Ca12Al14O33 [125]. Consequently, these mixed oxides contribute to the stabilization of CaO particles, enhancing their resistance to sintering during repeated operational cycles [126].
It is crucial to emphasize that the multicyclic performance achievable by Al2O3-stabilized sorbents is heavily influenced by the synthesis methodology employed, as it dictates the distribution of CaO, Al2O3, and Ca-Al ternary oxides within the final material [117]. In this context, an array of synthesis techniques has been proposed in the scientific literature for the fabrication of Al2O3-supported CaO-based sorbents, including wet mixing, coprecipitation, sol-gel, chemical vapor deposition, atomic layer deposition, and others. These methods offer distinct advantages in terms of control over material morphology, porosity, and chemical composition, thus influencing the sorbents’ performance characteristics [127].
ZrO2-supported CaO-based sorbents have been extensively investigated using various synthesis techniques, primarily due to the high thermal stability of ZrO2 (as high as 1221 °C). In contrast to Al2O3 and SiO2 supports, the interaction between CaO and ZrO2 is relatively straightforward, resulting mainly in the formation of CaZrO3 [126,128]. CaZrO3 is a perovskite with a high melting point of 2345 °C, a small thermal expansion coefficient, high strength, and chemical and thermal stability at elevated temperatures [129,130]. A thorough examination of Table 4 reveals that ZrO2-supported CaO-based sorbents exhibit notable multicyclic stability, particularly when a high mass ratio of ZrO2 is utilized. For instance, research conducted by Koirala et al. found that a ZrO2-stabilized sorbent containing 76 wt.% of CaZrO3 maintained its CO2 uptake (0.11 gCO2/gsorbent) over 1200 cycles without any significant decline [131]. However, it is worth noting that while these sorbents demonstrate impressive multicyclic stability, the higher amount of ZrO2 will reduce the amount of CaO by reacting with it to form CaZrO3, which may lead to reduced CaO reactivity, thereby limiting the overall CO2 uptake capacity [128].
SiO2 stands out as another widely accessible and cost-effective support material extensively utilized in the production of stabilized CaO-based sorbents [130]. Nanostructured silica plays a crucial role in enhancing the dispersion of CaO agglomerates and mitigating sintering phenomena [132]. It has been demonstrated that this beneficial effect stems from the interaction between SiO2 and CaO, leading to the formation of calcium silicates (Ca2SiO4), which impart greater thermal stability to the CaO framework. Consequently, this results in a higher effective conversion over multiple cycles compared with raw limestone [133]. Notably, Ca2SiO4 exhibits higher thermal stability (characterized by a Tt of 929 °C) compared with SiO2 (which has a Tt of 664 °C) [134]. During the multicycle process, Ca2SiO4 undergoes a phase transformation, causing a volume expansion of the sorbent, which counteracts sintering phenomena [134].
The incorporation of MgO in CaCO3/CaO has been extensively explored [135,136]. Huang et al. homogeneously dispersed and mixed MgO nanoparticles (ranging from 50 to 100 nm) with CaO grains on the particle surface, resulting in improved sintering resistance and enhanced multicyclic stability [137]. The cyclic stability of MgO-stabilized sorbents is notably influenced by the MgO amount, as reflected in the MgO-to-CaO weight ratio [105]. It has been established that an adequate amount of MgO is essential for achieving effective and stable reactive performance. Regardless of the MgO/CaO ratio, MgO-stabilized sorbents typically exhibit a higher specific surface area and pore volume compared with the original material [138]. However, an increase in MgO content leads to a reduction in specific surface area. There exists an optimum MgO/CaO ratio that maximizes multicyclic CO2 uptake; beyond a certain MgO amount, sintering resistance diminishes [139].
Apart from MgO, Y2O3 (with a Tt of 1083 °C) has been identified as an effective stabilizer for CaO-based materials, mitigating sintering tendencies [117]. Y2O3 nanoparticles were evenly distributed on CaO particles by Zhang et al., resulting in enhanced reaction kinetics and a linear correlation between the maximum carbonation rate and specific pore volume (less than 220 nm) [140]. Different synthesis methods, such as thermal decomposition and wet impregnation, have been employed to synthesize Y2O3-stabilized CaO-based materials, with no significant difference observed in multicyclic performances, indicating minimal impact of the synthesis procedure [141].

Table 4.
The performance of reactive additives and their operating conditions.
Table 4.
The performance of reactive additives and their operating conditions.
| Additive/Support | Synthesis Method | Ternary Metal Oxide | Temperature Carb./Calci. | CO2 vol % Carb./Calci | Testing Conditions | CO2 Uptake (gCO2/gsorb) Last Cycle | Ref. |
|---|---|---|---|---|---|---|---|
| Al2O3 | |||||||
| wt.% Al2O3 | |||||||
| 9 | wet mixing | Ca12Al14O33/Ca3Al2O6 | 650/850 °C | 20/0 | TGA—100 cycles | 0.34 | [142] |
| 9 | carbon gel templating | Ca12Al14O33 | 750/750 °C | 55/0 | TGA—30 cycles | 0.55 | [143] |
| 18 | flame spray pyrolysis | Ca12Al14O33 | 850/950 °C | 100/30 | TGA—100 cycles | 0.25 | [144] |
| 5 | dry mixing | Ca3Al2O6 | 650/900 °C | 15/70 | TGA—20 cycles | 0.14 | [119] |
| 15 | atomic layer deposition | Ca12Al14O33/Ca3Al2O6 | 650/900 °C | 20/100 | TGA—10 cycles | 0.41 | [145] |
| 9 | chemical vapor deposition | Ca3Al2O6 | 650/950 °C | 20/100 | TGA—20 cycles | 0.41 | [122] |
| 10 | sol–gel | Ca5A6O14 | 650/800 °C | 15/0 | TGA—50 cycles | 0.45 | [123] |
| 34 | sol–gel | Ca3Al2O6 | 650/850 °C | 15/0 | TGA—100 cycles | 0.41 | [146] |
| 20 | Ball milling | Ca5Al6O14/Ca9Al6O18 | 900/900 °C | 100/0 | Sieverts—50 cycles | 0.49 | [126] |
| ZrO2 | |||||||
| wt.% CaZrO3 | |||||||
| 34 | sol–gel | - | 650/850 °C | 15/0 | TGA—50 cycles | 0.46 | [120] |
| 34 | sol–gel | - | 650/920 °C | 10/80 | Fluidized bed—20 cycles | 0.31 | [120] |
| 26 | sol–gel | - | 900/900 °C | 80/0 | TGA—20 cycles | 0.65 | [147] |
| 29 | sol–gel | - | 650/800 °C | 50/0 | TGA—90 cycles | 0.34 | [148] |
| 76 | flame spry pyrolysis | - | 700/700 °C | 50/50 | TGA—1200 cycles | 0.11 | [131] |
| 58 | flame spry pyrolysis | - | 700/700 °C | 30/0 | TGA—100 cycles | 0.21 | [149] |
| 10 | citrate sol–gel | - | 650/780 °C | 100/0 | TGA—10 cycles | 0.69 | [150] |
| 29 | sol–gel | - | 650/900 °C | 15/0 | TGA—30 cycles | 0.45 | [151] |
| 29 | spray drying | - | 650/950 °C | 90/90 | TGA—100 cycles | 0.45 | [152] |
| 7 | Ball milling | - | 850/1000 °C | 100/100 | TGA—11 cycles | 0.22 | [153] |
| 20 | Wet precipitation | - | 884/884 °C | 100/0 | TGA—40 cycles | 0.7 | [128] |
| SiO2 | |||||||
| wt.% SiO2 | |||||||
| 70 | wet impregnation | Ca2SiO4 | 650/850 °C | 15/0 | TGA—80 cycles | 0.07 | [154] |
| 9 | one-pot synthesis route | - | 650/950 °C | 100/0 | TGA—50 cycles | 0.26 | [132] |
| 20 | dry mixing | Ca2SiO4/CaSiO3 | 650/850 °C | 15/0 | TGA—100 cycles | 0.18 | [155] |
| 33 | wet mixing | - | 700/910 °C | 100/0 | TGA—40 cycles | 0.42 | [156] |
| 10 | freeze-drying | Ca2SiO4 | 700/920 °C | 100/100 | TGA—30 cycles | 0.21 | [157] |
| 2 | Ball milling | - | 900/900 °C | 100/0 | TGA—20 cycles | 0.18 | [158] |

Table 5.
The performance of non-reactive additives and their operating conditions.
Table 5.
The performance of non-reactive additives and their operating conditions.
| Additive/Support | Synthesis Method | Temperature Carb./Calci. | CO2 vol % Carb./Calci | Testing Conditions | CO2 Uptake (gCO2/gsorb) Last Cycle | Ref. |
|---|---|---|---|---|---|---|
| MgO | ||||||
| wt.% MgO | ||||||
| 16 | carbon gel templating | 650/900 °C | TGA—10 cycles | 0.55 | [159] | |
| 8 | one-pot recrystallization | 650/900 °C | 20/100 | TGA—10 cycles | 0.47 | [160] |
| 15 | wet mechanochemical activation | 650/900 °C | 20/100 | TGA—30 cycles | 0.30 | [161] |
| 25 | wet mixing | 650/900 °C | 15/0 | TGA—24 cycles | 0.56 | [162] |
| 25 | wet mixing | 650/850 °C | 20/0 | TGA—50 cycles | 0.27 | [163] |
| 41 | wet mixing | 650/900 °C | 100/0 | dual fixed bed—10 cycles | 0.28 | [164] |
| 34 | sol–gel | 650/850 °C | 15/0 | TGA—100 cycles | 0.32 | [165] |
| 20 | wet mixing | 600/900 °C | 15/0 | fixed bed—10 cycles | 0.25 | [166] |
| 26 | dry mixing | 758/850 °C | 100/0 | dual fixed bed—50 cycles | 0.53 | [167] |
| 6 | sol–gel | 675/950 °C | 15/80 | TGA—50 cycles | 0.58 | [168] |
| 26 | wet mixing | 650/850 °C | 15/0 | TGA—50 cycles | 0.40 | [169] |
| Y2O3 and CeO | ||||||
| 20 wt.% Y2O3 | calcination | 650/850 °C | 20/0 | TGA—10 cycles | 0.57 | [140] |
| 20 wt.% Y2O3 | calcination | 650/950 °C | 25/100 | TGA—10 cycles | 0.49 | [140] |
| 15Ca/Ce ratio | sol–gel combustion | 600/700 °C | 50/0 | TGA—18 cycles | 0.59 | [170] |
Additionally, CeO2 (with a Tt of 1064 °C) has been successfully integrated as a stabilizer in CaO-based materials, attributed to its high thermal stability and ability to facilitate oxygen mobility and vacancy generation, thereby enhancing CO2 diffusion and O2− mobility [171,172]. Various Ca/Ce molar ratios have been explored in CeO2-supported CaO-based material synthesis, revealing a loose-shell-connected cross-linking structure with CeO2 acting as a physical barrier to prevent CaO crystallite growth and sintering [170].
Moreover, the simultaneous incorporation of multiple inert materials has been investigated to exploit synergistic effects on multicyclic stability. For instance, TiO2 and Al2O3 were simultaneously used to fabricate hierarchical core–shell microarchitectures. CaO-based materials are enriched in Ca, supported by Al2O3, and stabilized by TiO2 [173]. Similarly, a Y2O3/MgO-stabilized CaO-based material was synthesized via a sol–gel method to enhance cyclic stability and CO2 uptake capacity [174]. Table 6 summarizes the performance of multiple additives used in pairs.

Table 6.
The performance of multiple additives used in pairs and their operating conditions.
In summary, the selection of support material for stabilizing CaO-based materials must consider both multicyclic stability and material activity towards CO2. While metal oxide-stabilized materials typically exhibit better multicyclic stability due to the stable framework formed, the presence of metal oxides may reduce CaO activity due to the formation of ternary oxides, necessitating a balance between mitigating CO2 uptake (carbonation) decline and minimizing CaO activity reduction. Moreover, the utilization of novel sintering-resistant materials necessitates consideration of higher material costs and environmental impacts associated with synthesis processes. Furthermore, the majority of synthetic materials in the literature are produced as fine powders, posing handling and processing challenges.
The selection and characterization of additives, including their synthesis methods and doping ratios, require systematic investigation to identify optimal formulations for enhanced multicyclic stability. Additionally, the mechanisms underlying the interaction between additives and CaO/CaCO3 matrices need to be elucidated to guide the design of tailored additive-integrated systems.
6. Challenges and Limitations
The exploration of additives in limestone-based thermochemical energy storage (TCES) systems opens up avenues for further research and development in several critical areas. Understanding the interaction between additives and the primary material, such as calcium carbonate (CaCO3) or calcium oxide (CaO), is essential for optimizing the performance and durability of TCES systems.
Future research should focus on systematically evaluating a broader range of additives beyond those already investigated, such as Al2O3, SiO2, MgO, Y2O3, CeO2, and TiO2. This exploration could involve novel materials with potentially superior properties in terms of sintering resistance, thermal stability, and catalytic effects. Additionally, detailed characterization studies, such as in-situ synchrotron experiments like powder X-ray diffraction (PXRD) or Small/Wide Angle X-ray Scattering (SAXS/WAXS), are necessary to clarify the mechanisms underlying the interaction between additives and the primary material, including surface morphology, chemical bonding, and phase transformations.
The effectiveness of additives in enhancing the multicyclic stability of TCES systems is highly dependent on their concentration and uniform dispersion within the material matrix. Further research is needed to optimize these parameters to achieve the maximum benefit while minimizing costs and processing complexity. Advanced synthesis techniques, such as atomic layer deposition, sol-gel methods, and mechanochemical activation, offer precise control over additive distribution and morphology and warrant investigation for their applicability in large-scale TCES systems. On the other hand, the environmental impact of such synthesis methods should be carefully evaluated, and perhaps greener alternative synthesis methods should be considered.
In-depth mechanistic studies are required to unravel the specific roles played by additives in mitigating sintering, pore plugging, and agglomeration phenomena in TCES materials. Computational modeling and simulation techniques can provide valuable insights into the thermodynamic and kinetic aspects of additive interactions, for example, how additives can enhance ion mobility at high temperatures, facilitate the carbonation reaction, and aid in the rational design of optimized materials with tailored properties for enhanced TCES performance.
As TCES technologies transition from laboratory-scale research to practical implementation, considerations of scalability, cost-effectiveness, and sustainability become paramount. Future research activities should address the scalability of additive synthesis and integration processes, as well as the economic feasibility of large-scale production and deployment. Life cycle assessments and techno-economic analyses can help identify the most promising additive formulations and processing routes for commercialization, taking into account factors such as raw material availability, energy consumption, and environmental impact.
The successful deployment of TCES systems hinges on their seamless integration with renewable energy sources, such as solar and wind power, to enable grid stabilization and energy arbitrage. Research efforts should focus on developing synergistic approaches that optimize the coupling between TCES and renewable energy technologies, leveraging advances in control strategies, predictive modeling, and system optimization algorithms. Collaborative research initiatives involving academia, industry, and government stakeholders can facilitate the rapid translation of fundamental research findings into practical solutions for real-world energy challenges.
By addressing these research implications, the field of limestone-based TCES can advance towards the realization of cost-effective, high-performance energy storage solutions that play a pivotal role in enabling the widespread adoption of renewable energy and achieving global sustainability goals.
7. Conclusions
In conclusion, this review has underscored the critical role of additives in enhancing the performance and durability of limestone-based thermochemical energy storage (TCES) systems. By evaluating various strategies such as hydration, fine particle utilization, and the incorporation of additives like Al2O3 and ZrO2, this paper has addressed challenges such as performance degradation and sintering over multiple cycles. It is evident from the discussion that the selection of support materials must carefully balance multicyclic stability with material activity towards CO2. While metal oxide-stabilized materials offer better multicyclic stability, their potential to reduce CaO activity necessitates a nuanced approach to optimize performance.
Future research should explore a wider range of additives, prioritize detailed characterization studies, and employ advanced synthesis techniques to optimize additive distribution and morphology while minimizing costs. Mechanistic studies and computational modeling offer avenues to better understand the specific roles of additives in TCES materials. As TCES technologies advance towards practical use, scalability, cost-effectiveness, and sustainability considerations must be addressed. By embracing these implications and fostering collaboration, the field can move towards cost-effective, high-performance energy storage solutions.
Funding
M.V.S. acknowledges the financial support from the UCD Ad Astra Fellowship Program and the Technology Transfer Strengthening Initiative Program from Enterprise Ireland (reference grant number 68001). R.A. acknowledges the financial support from the UCD Ad Astra Studentship Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Quaschning, V.V. Renewable Energy and Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Edenhofer, O.; Pichs-Madruga, R.; Sokona, Y.; Seyboth, K.; Kadner, S.; Zwickel, T.; Eickemeier, P.; Hansen, G.; Schlömer, S.; von Stechow, C. Renewable Energy Sources and Climate Change Mitigation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Karduri, R.K. Integrating Renewable Energy into Existing Power Systems: Challenges and Opportunities. Int. J. Adv. Res. Manag. Archit. Technol. Eng. (IJARMATE) 2018, 2. [Google Scholar] [CrossRef]
- Jones, L.E. Renewable Energy Integration: Practical Management of Variability, Uncertainty, and Flexibility in Power Grids; Academic press: Cambridge, MA, USA, 2017. [Google Scholar]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Sullivan, G.; Griffiths, C.; Jewell, E.; Searle, J.; Elvins, J. Cycling Stability of Calcium-Impregnated Vermiculite in Open Reactor Used as a Thermochemical Storage Material. Energies 2023, 16, 7225. [Google Scholar] [CrossRef]
- Shchegolkov, A.; Shchegolkov, A.; Demidova, A. The use of nanomodified heat storage materials for thermal stabilization in the engineering and aerospace industry as a solution for economy. In Proceedings of the International Conference on Modern Trends in Manufacturing Technologies and Equipment (ICMTMTE 2018), Sevastopol, Russian, 10–14 September 2018. [Google Scholar]
- Stecca, M.; Elizondo, L.R.; Soeiro, T.B.; Bauer, P.; Palensky, P. A Comprehensive Review of the Integration of Battery Energy Storage Systems Into Distribution Networks. IEEE Open J. Ind. Electron. Soc. 2020, 1, 46–65. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Jasiūnas, J.; Lund, P.D.; Mikkola, J. Energy system resilience–A review. Renew. Sustain. Energy Rev. 2021, 150, 111476. [Google Scholar] [CrossRef]
- Nkwanyana, T.B.; Siti, M.W.; Wang, Z.H.; Toudjeu, I.; Mbungu, N.T.; Mulumba, W. An assessment of hybrid-energy storage systems in the renewable environments. J. Energy Storage 2023, 72, 108307. [Google Scholar] [CrossRef]
- Nadeem, F.; Hussain, S.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative review of energy storage systems, their roles, and impacts on future power systems. IEEE Access 2018, 7, 4555–4585. [Google Scholar] [CrossRef]
- Semadeni, M. Energy Storage as an Essential Part of Sustainable Energy Systems: A Review on Applied Energy Storage Technologies; CEPE Working Paper No. 24; CEPE: Zurich, Switzerland, 2003. [Google Scholar]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Teske, S. Achieving the Paris Climate Agreement Goals: Global and Regional 100% Renewable Energy Scenarios with Non-Energy GHG Pathways for +1.5 C and +2 C; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Dell, R.M.; Rand, D.A.J. Energy storage—A key technology for global energy sustainability. J. Power Sources 2001, 100, 2–17. [Google Scholar] [CrossRef]
- Azzuni, A.; Breyer, C. Energy security and energy storage technologies. Energy Procedia 2018, 155, 237–258. [Google Scholar] [CrossRef]
- Zsiborács, H.; Baranyai, N.H.; Vincze, A.; Zentkó, L.; Birkner, Z.; Máté, K.; Pintér, G. Intermittent renewable energy sources: The role of energy storage in the European power system of 2040. Electronics 2019, 8, 729. [Google Scholar] [CrossRef]
- Alami, A.H. Mechanical Energy Storage for Renewable and Sustainable Energy Resources; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Mahmoud, M.; Ramadan, M.; Olabi, A.G.; Pullen, K.; Naher, S. A review of mechanical energy storage systems combined with wind and solar applications. Energy Convers. Manag. 2020, 210, 112670. [Google Scholar] [CrossRef]
- Moseley, P.T. Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Newnes: Oxford, UK, 2014. [Google Scholar]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Dincer, I.; Rosen, M.A. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Alva, G.; Lin, Y.X.; Fang, G.Y. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Ferreira, H.L.; Garde, R.; Fulli, G.; Kling, W.; Lopes, J.P. Characterisation of electrical energy storage technologies. Energy 2013, 53, 288–298. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Becherif, M.; Ramadan, H.S.; Cabaret, K.; Picard, F.; Simoncini, N.; Bethoux, O. Hydrogen Energy Storage: New Techno-Economic Emergence Solution Analysis. Energy Procedia 2015, 74, 371–380. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Zemtsova, N.V.; Stanishevskiy, Y.M.; Vetcher, A.A. Recent Advantages on Waste Management in Hydrogen Industry. Polymers 2022, 14, 4992. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Al-Hadhrami, L.M.; Alam, M.M. Pumped hydro energy storage system: A technological review. Renew. Sustain. Energy Rev. 2015, 44, 586–598. [Google Scholar] [CrossRef]
- Berrada, A.; Loudiyi, K. Gravity Energy Storage; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Budt, M.; Wolf, D.; Span, R.; Yan, J.Y. A review on compressed air energy storage: Basic principles, past milestones and recent developments. Appl. Energy 2016, 170, 250–268. [Google Scholar] [CrossRef]
- Amiryar, M.E.; Pullen, K.R. A Review of Flywheel Energy Storage System Technologies and Their Applications. Appl. Sci. 2017, 7, 286. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Vulusala, G.V.S.; Madichetty, S. Application of superconducting magnetic energy storage in electrical power and energy systems: A review. Int. J. Energy Res. 2018, 42, 358–368. [Google Scholar] [CrossRef]
- Holla, R.V. Energy storage methods-Superconducting magnetic energy storage—A Review. J. Undergrad. Res. 2015, 5, 49–54. [Google Scholar] [CrossRef]
- Panchenko, V.A.; Kovalev, A.A.; Litti, Y.V.; Daus, Y.V. Prospects for the production of green hydrogen: Review of countries with high potential*. Int. J. Hydrogen Energy 2023, 48, 4551–4571. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.M. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Zuttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Buckley, D.N.; O’Dwyer, C.; Quill, N.; Lynch, R.P. Electrochemical energy storage. Energy Storage Options Their Environ. Impact 2018, 46, 115. [Google Scholar]
- Zhang, Y.; Zhou, C.-G.; Yang, J.; Xue, S.-C.; Gao, H.-L.; Yan, X.-H.; Huo, Q.-Y.; Wang, S.-W.; Cao, Y.; Yan, J. Advances and challenges in improvement of the electrochemical performance for lead-acid batteries: A comprehensive review. J. Power Sources 2022, 520, 230800. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Jain, A.; Khurana, P.; Kumar, D.; Thatai, S. Ni-Cd Batteries. In Rechargeable Batteries: History, Progress, and Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 177–194. [Google Scholar]
- Feng, F.; Geng, M.; Northwood, D.O. Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: A review. Int. J. Hydrogen Energy 2001, 26, 725–734. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Long, L.Z.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Folorunso, O.; Olukanmi, P.O.; Shongwe, T. Progress towards sustainable energy storage: A concise review. Eng. Rep. 2023, 5, e12731. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Zayed, M.E.; Kabeel, A.E.; Shboul, B.; Ashraf, W.M.; Ghazy, M.; Irshad, K.; Rehman, S.; Zayed, A.A.A. Performance augmentation and machine learning-based modeling of wavy corrugated solar air collector embedded with thermal energy storage: Support vector machine combined with Monte Carlo simulation. J. Energy Storage 2023, 74, 109533. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Enweremadu, C.C.; Kareem, M.W.; Arogundade, A.I.; Irshad, K.; Islam, S.; Oladosu, K.O.; Elfaghi, A.M.; Didane, D.H. Experimental investigation of double slope solar still integrated with PCM nanoadditives microencapsulated thermal energy storage. Desalination 2023, 553, 116477. [Google Scholar] [CrossRef]
- Khan, I.U.; Tirth, V.; Algahtani, A.; Khan, R.; Sohail, M.; Ali, A.; Islam, S.; Irshad, K. Optimization of Single alpha-Phase for Promoting Ferromagnetic Properties of 44Fe-28Cr-22Co-3Mo-1Ti-2V Permanent Magnet with Varying Co Concentration for Energy Storage. Materials 2022, 15, 2344. [Google Scholar] [CrossRef] [PubMed]
- Zahir, M.H.; Rahman, M.M.; Basamad, S.K.S.; Mohaisen, K.O.; Irshad, K.; Rahman, M.M.; Aziz, M.A.; Ali, A.; Hossain, M.M. Preparation of a Sustainable Shape-Stabilized Phase Change Material for Thermal Energy Storage Based on Mg2+-Doped CaCO3/PEG Composites. Nanomaterials 2021, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Sensible heat thermal storage energy and exergy performance evaluations. Renew. Sustain. Energy Rev. 2016, 53, 897–923. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- Rostami, S.; Afrand, M.; Shahsavar, A.; Sheikholeslami, M.; Kalbasi, R.; Aghakhani, S.; Shadloo, M.S.; Oztop, H.F. A review of melting and freezing processes of PCM/nano-PCM and their application in energy storage. Energy 2020, 211, 118698. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A.; Giménez, P. The Calcium-Looping (CaCO3/CaO) process for thermochemical energy storage in Concentrating Solar Power plants. Renew. Sustain. Energy Rev. 2019, 113, 109252. [Google Scholar] [CrossRef]
- Khan, M.I.; Asfand, F.; Al-Ghamdi, S.G. Progress in research and technological advancements of thermal energy storage systems for concentrated solar power. J. Energy Storage 2022, 55, 105860. [Google Scholar] [CrossRef]
- Schoenung, S.M.; Hassenzahl, W.V. Long-vs. Short-Term Energy Storage Technologies Analysis: A Life-Cycle Cost Study: A Study for the DOE Energy Storage Systems Program; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2003. [Google Scholar]
- Aspitarte, L.; Woodside, C.R. A techno-economic survey of energy storage media for long-duration energy storage applications. Cell Rep. Sustain. 2024, 1, 100007. [Google Scholar] [CrossRef]
- Cárdenas, B.; Swinfen-Styles, L.; Rouse, J.; Hoskin, A.; Xu, W.; Garvey, S.D. Energy storage capacity vs. renewable penetration: A study for the UK. Renew. Energy 2021, 171, 849–867. [Google Scholar] [CrossRef]
- The European Association for Storage of Energy. Energy Storage Targets 2030 and 2050; The European Association for Storage of Energy: Brussels, Belgium, 2024. [Google Scholar]
- Zou, C.F.; Zhang, L.; Hu, X.S.; Wang, Z.P.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef]
- Hameer, S.; van Niekerk, J.L. A review of large-scale electrical energy storage. Int. J. Energy Res. 2015, 39, 1179–1195. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Gonzalez-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Raganati, F.; Ammendola, P. Review of Carbonate-Based Systems for Thermochemical Energy Storage for Concentrating Solar Power Applications: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 1777–1808. [Google Scholar] [CrossRef]
- Khosa, A.A.; Xu, T.X.; Xia, B.Q.; Yan, J.; Zhao, C.Y. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system—A review. Sol. Energy 2019, 193, 618–636. [Google Scholar] [CrossRef]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D.; Palumbo, R. Design and experimental investigation of a horizontal rotary reactor for the solar thermal production of lime. Energy 2004, 29, 811–821. [Google Scholar] [CrossRef]
- Bagherisereshki, E.; Tran, J.; Lei, F.Q.; AuYeung, N. Investigation into SrO/SrCO3 for high temperature thermochemical energy storage. Sol. Energy 2018, 160, 85–93. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z.Z. Metal Hydrides for High-Temperature Power Generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- Ward, P.A.; Teprovich, J.A.; Liu, Y.F.; He, J.; Zidan, R. High temperature thermal energy storage in the CaAl2 system. J. Alloys Compd. 2018, 735, 2611–2615. [Google Scholar] [CrossRef]
- Wierse, M.; Werner, R.; Groll, M. Magnesium Hydride for Thermal-Energy Storage in a Small-Scale Solar Thermal Power-Station. J. Less-Common Met. 1991, 172, 1111–1121. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanovic, B. High Temperature Metal Hydrides as Heat Storage Materials for Solar and Related Applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 2002, 7, 2254–2256. [Google Scholar] [CrossRef]
- Fernandes, S.M.; Landzberg, M.J. Pregnancy in patients with tetralogy of fallot: Invited commentary. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 175–176. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Cobalt Oxide-Based Structured Thermochemical Reactors/Heat Exchangers for Solar Thermal Energy Storage in Concentrated Solar Power Plants. In Proceedings of the ASME 2014 8th International Conference on Energy Sustainability collocated with the ASME 2014 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 4: Screening of oxides for use in cascaded thermochemical storage concepts. Sol. Energy 2016, 139, 695–710. [Google Scholar] [CrossRef]
- Miguel, S.Á.D.; Gonzalez-Aguilar, J.; Romero, M. 100-Wh Multi-purpose Particle Reactor for Thermochemical Heat Storage in Concentrating Solar Power Plants. Energy Procedia 2014, 49, 676–683. [Google Scholar] [CrossRef]
- Fahim, M.A.; Ford, J.D. Energy-Storage Using the Bao2-Bao Reaction Cycle. Chem. Eng. J. Biochem. Eng. J. 1983, 27, 21–28. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. Thermodynamic potential of molten copper oxide for high temperature solar energy storage and oxygen production. Appl. Energy 2017, 201, 69–83. [Google Scholar] [CrossRef]
- Dunn, R.; Lovegrove, K.; Burgess, G. A Review of Ammonia-Based Thermochemical Energy Storage for Concentrating Solar Power. Proc. IEEE 2012, 100, 391–400. [Google Scholar] [CrossRef]
- Pelay, U.; Lu, L.A.; Fan, Y.L.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Airò Farulla, G.; Cellura, M.; Guarino, F.; Ferraro, M. A Review of Thermochemical Energy Storage Systems for Power Grid Support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Wang, K.; Yan, T.; Li, R.; Pan, W. A review for Ca(OH)2/CaO thermochemical energy storage systems. J. Energy Storage 2022, 50, 104612. [Google Scholar] [CrossRef]
- Dubey, S.K.; Kumar, K.R.; Tiwari, V.; Srivastva, U. Impacts, Barriers, and Future Prospective of Metal Hydride-Based Thermochemical Energy Storage System for High-Temperature Applications: A Comprehensive Review. Energy Technol. 2024, 12, 2300768. [Google Scholar] [CrossRef]
- Han, X.Y.; Wang, L.; Ling, H.S.; Ge, Z.W.; Lin, X.P.; Dai, X.J.; Chen, H.S. Critical review of thermochemical energy storage systems based on cobalt, manganese, and copper oxides. Renew. Sustain. Energy Rev. 2022, 158, 112076. [Google Scholar] [CrossRef]
- Anwar, R.; Vijayaraghavan, R.K.; McNally, P.J.; Dardavila, M.M.; Voutsas, E.; Sofianos, M.V. Investigating the activity of Ca2Fe2O5 additives on the thermochemical energy storage performance of limestone waste. RSC Adv. 2023, 13, 32523–32531. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Pérez-Maqueda, L.A.; Gimenez-Gavarrell, P. Scaling-up the Calcium-Looping Process for CO2 Capture and Energy Storage. KONA Powder Part. J. 2021, 38, 189–208. [Google Scholar] [CrossRef]
- Valverde, J.M.; Raganati, F.; Quintanilla, M.A.S.; Ebri, J.M.P.; Ammendola, P.; Chirone, R. Enhancement of CO2 capture at Ca-looping conditions by high-intensity acoustic fields. Appl. Energy 2013, 111, 538–549. [Google Scholar] [CrossRef]
- Tregambi, C.; Salatino, P.; Solimene, R.; Montagnaro, F. An experimental characterization of Calcium Looping integrated with concentrated solar power. Chem. Eng. J. 2018, 331, 794–802. [Google Scholar] [CrossRef]
- Alovisio, A.; Chacartegui, R.; Ortiz, C.; Valverde, J.M.; Verda, V. Optimizing the CSP-Calcium Looping integration for Thermochemical Energy Storage. Energy Convers. Manag. 2017, 136, 85–98. [Google Scholar] [CrossRef]
- Chacartegui, R.; Alovisio, A.; Ortiz, C.; Valverde, J.M.; Verda, V.; Becerra, J.A. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle. Appl. Energy 2016, 173, 589–605. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Calcium-looping for thermochemical energy storage in concentrating solar power applications: Evaluation of the effect of acoustic perturbation on the fluidized bed carbonation. Chem. Eng. J. 2020, 392, 123658. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Limestone calcination under calcium-looping conditions for CO(2) capture and thermochemical energy storage in the presence of H(2)O: An in situ XRD analysis. Phys. Chem. Chem. Phys. 2017, 19, 7587–7596. [Google Scholar] [CrossRef] [PubMed]
- Zeman, F. Effect of steam hydration on performance of lime sorbent for CO2 capture. Int. J. Greenh. Gas Control 2008, 2, 203–209. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Suzuki, Y. Limestone Calcination with CO2 Capture (II): Decomposition in CO2/Steam and CO2/N2 Atmospheres. Energy Fuels 2008, 22, 2326–2331. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Acharya, B.; Dutta, A. CaO-based CO2 sorbents: A review on screening, enhancement, cyclic stability, regeneration and kinetics modelling. J. CO2 Util. 2018, 23, 179–199. [Google Scholar] [CrossRef]
- Khinast, J.; Krammer, G.F.; Brunner, C.; Staudinger, G. Decomposition of limestone: The influence of CO2 and particle size on the reaction rate. Chem. Eng. Sci. 1996, 51, 623–634. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- Biasin, A.; Segre, C.U.; Strumendo, M. CaCO3 Crystallite Evolution during CaO Carbonation: Critical Crystallite Size and Rate Constant Measurement by In-Situ Synchrotron Radiation X-ray Powder Diffraction. Cryst. Growth Des. 2015, 15, 5188–5201. [Google Scholar] [CrossRef]
- Ma, X.T.; Li, Y.J.; Yan, X.Y.; Zhang, W.; Zhao, J.L.; Wang, Z.Y. Preparation of a morph-genetic CaO-based sorbent using paper fibre as a biotemplate for enhanced CO2 capture. Chem. Eng. J. 2019, 361, 235–244. [Google Scholar] [CrossRef]
- Khosa, A.A.; Zhao, C.Y. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. Carbonation Kinetics of Fine CaO Particles in a Sound-Assisted Fluidized Bed for Thermochemical Energy Storage. Kona Powder Part. J. 2022, 39, 240–250. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Relevant influence of limestone crystallinity on CO(2) capture in the Ca-looping technology at realistic calcination conditions. Environ. Sci. Technol. 2014, 48, 9882–9889. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Guerrero, M.; Sarrion, B.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Valverde, J.M. Large-scale high-temperature solar energy storage using natural minerals. Sol. Energy Mater. Sol. Cells 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Gas-solid fluidization of cohesive powders. Chem. Eng. Res. Des. 2018, 133, 347–387. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. Role of Acoustic Fields in Promoting the Gas-Solid Contact in a Fluidized Bed of Fine Particles. Kona Powder Part. J. 2015, 32, 23–40. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P.; Chirone, R. CO2 adsorption on fine activated carbon in a sound assisted fluidized bed: Effect of sound intensity and frequency, CO2 partial pressure and fluidization velocity. Appl. Energy 2014, 113, 1269–1282. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.B.; Sun, Z.K. Review on the Development of Sorbents for Calcium Looping. Energy Fuels 2020, 34, 7806–7836. [Google Scholar] [CrossRef]
- Sarrión, B.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Valverde, J.M. Role of calcium looping conditions on the performance of natural and synthetic Ca-based materials for energy storage. J. CO2 Util. 2018, 28, 374–384. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Antzara, A.N.; Arregi, A.; Heracleous, E.; Lemonidou, A.A. In-depth evaluation of a ZrO2 promoted CaO-based CO2 sorbent in fluidized bed reactor tests. Chem. Eng. J. 2018, 333, 697–711. [Google Scholar] [CrossRef]
- Sánchez Jiménez, P.E.; Perejón, A.; Benítez Guerrero, M.; Valverde, J.M.; Ortiz, C.; Pérez Maqueda, L.A. High-performance and low-cost macroporous calcium oxide based materials for thermochemical energy storage in concentrated solar power plants. Appl. Energy 2019, 235, 543–552. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.H.; Wei, S.Y.; Su, Y.L.; Qin, Y.K. Development of highly effective CaO@Al2O3 with hierarchical architecture CO2 sorbents via a scalable limited-space chemical vapor deposition technique. J. Mater. Chem. A 2018, 6, 3462–3470. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Z.M.; Zhao, C.J.; Cheng, Z.M. Catalytic performance of Ni/CaO-Ca5Al6O14 bifunctional catalyst extrudate in sorption-enhanced steam methane reforming. Catal. Today 2016, 259, 347–353. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, L.; Zhou, Z.M.; Cheng, Z.M. Core-shell structured CaO-Ca9Al6O18@Ca5Al6O14/Ni bifunctional material for sorption-enhanced steam methane reforming. Chem. Eng. Sci. 2017, 163, 114–122. [Google Scholar] [CrossRef]
- Ma, X.T.; Li, Y.J.; Qian, Y.; Wang, Z.Y. A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture. Energies 2019, 12, 2617. [Google Scholar] [CrossRef]
- Møller, K.T.; Ibrahim, A.; Buckley, C.E.; Paskevicius, M. Inexpensive thermochemical energy storage utilising additive enhanced limestone. J. Mater. Chem. A 2020, 8, 9646–9653. [Google Scholar] [CrossRef]
- Wang, N.N.; Feng, Y.C.; Liu, L.; Guo, X. Effects of preparation methods on the structure and property of Al-stabilized CaO-based sorbents for CO2 capture. Fuel Process. Technol. 2018, 173, 276–284. [Google Scholar] [CrossRef]
- Anwar, R.; Navrátil, J.; Vijayaraghavan, R.K.; McNally, P.J.; Otyepka, M.; Blonski, P.; Sofianos, M.V. Upcycling natural Limestone waste for thermochemical energy storage by utilising tailored CaZrO3 nanoadditives. Mater. Adv. 2023, 4, 1905–1915. [Google Scholar] [CrossRef]
- Alay, E.A.S.M.; Nazir, S.; Cottenier, S.; Shaukat, A. Evaluation of thermodynamics, formation energetics and electronic properties of vacancy defects in CaZrO(3). Sci. Rep. 2017, 7, 8439. [Google Scholar] [CrossRef] [PubMed]
- Soria-Hoyo, C.; Valverde, J.M.; van Ommen, J.R.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Sayagués, M.J. Synthesis of a nanosilica supported CO2 sorbent in a fluidized bed reactor. Appl. Surf. Sci. 2015, 328, 548–553. [Google Scholar] [CrossRef]
- Koirala, R.; Gunugunuri, K.R.; Pratsinis, S.E.; Smirniotis, P.G. Effect of Zirconia Doping on the Structure and Stability of CaO-Based Sorbents for CO2 Capture during Extended Operating Cycles. J. Phys. Chem. C 2011, 115, 24804–24812. [Google Scholar] [CrossRef]
- Li, C.C.; Wu, U.T.; Lin, H.P. Cyclic performance of CaCO3@mSiO2 for CO2 capture in a calcium looping cycle. J. Mater. Chem. A 2014, 2, 8252–8257. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. [Google Scholar] [CrossRef]
- Zhao, M.; Shi, J.; Zhong, X.; Tian, S.C.; Blamey, J.; Jiang, J.G.; Fennell, P.S. A novel calcium looping absorbent incorporated with polymorphic spacers for hydrogen production and CO2 capture. Energy Environ. Sci. 2014, 7, 3291–3295. [Google Scholar] [CrossRef]
- Silaban, A.; Narcida, M.; Harrison, D.P. Characteristics of the reversible reaction between CO2(g) and calcined dolomite. Chem. Eng. Commun. 1996, 146, 149–162. [Google Scholar] [CrossRef]
- Albrecht, K.O.; Wagenbach, K.S.; Satrio, J.A.; Shanks, B.H.; Wheelock, T.D. Development of a CaO-Based CO2 Sorbent with Improved Cyclic Stability. Ind. Eng. Chem. Res. 2008, 47, 7841–7848. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, Q.W.; Louis, B.; Wang, Q. A facile Solvent/Nonsolvent Preparation of Sintering-Resistant MgO/CaO Composites for High-Temperature CO2 Capture. Energy Technol. 2018, 6, 2469–2478. [Google Scholar] [CrossRef]
- Daud, F.D.M.; Vignesh, K.; Sreekantan, S.; Mohamed, A.R. Improved CO2 adsorption capacity and cyclic stability of CaO sorbents incorporated with MgO. New J. Chem. 2016, 40, 231–237. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, L.; Yang, Z.Q.; Tang, Q. Removal of CO2 by CaO/MgO and CaO/Ca9Al6O18 in the Presence of SO. Energy Fuels 2011, 25, 5514–5520. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Z.G.; Peng, Y.; Su, W.K.; Sun, X.X.; Li, J.H. Investigation on a novel CaO-Y2O3 sorbent for efficient CO2 mitigation. Chem. Eng. J. 2014, 243, 297–304. [Google Scholar] [CrossRef]
- Derevschikov, V.S.; Lysikov, A.I.; Okunev, A.G. High Temperature CaO/Y2O3 Carbon Dioxide Absorbent with Enhanced Stability for Sorption-Enhanced Reforming Applications. Ind. Eng. Chem. Res. 2011, 50, 12741–12749. [Google Scholar] [CrossRef]
- Zhang, M.M.; Peng, Y.X.; Sun, Y.Z.; Li, P.; Yu, J.G. Preparation of CaO-Al2O3 sorbent and CO2 capture performance at high temperature. Fuel 2013, 111, 636–642. [Google Scholar] [CrossRef]
- Broda, M.; Muller, C.R. Synthesis of highly efficient, Ca-based, Al(2)O(3)-stabilized, carbon gel-templated CO(2) sorbents. Adv. Mater 2012, 24, 3059–3064. [Google Scholar] [CrossRef]
- Koirala, R.; Reddy, G.K.; Smirniotis, P.G. Single Nozzle Flame-Made Highly Durable Metal Doped Ca-Based Sorbents for CO2 Capture at High Temperature. Energy Fuels 2012, 26, 3103–3109. [Google Scholar] [CrossRef]
- Kurlov, A.; Armutlulu, A.; Donat, F.; Studart, A.R.; Müller, C.R. CaO-Based CO2 Sorbents with a Hierarchical Porous Structure Made via Microfluidic Droplet Templating. Ind. Eng. Chem. Res. 2020, 59, 7182–7188. [Google Scholar] [CrossRef]
- Angeli, S.D.; Martavaltzi, C.S.; Lemonidou, A.A. Development of a novel-synthesized Ca-based CO2 sorbent for multicycle operation: Parametric study of sorption. Fuel 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Sultana, K.S.; Tran, D.T.; Walmsley, J.C.; Ronning, M.; Chen, D. CaO Nanoparticles Coated by ZrO2 Layers for Enhanced CO2 Capture Stability. Ind. Eng. Chem. Res. 2015, 54, 8929–8939. [Google Scholar] [CrossRef]
- Broda, M.; Müller, C.R. Sol-gel-derived, CaO-based, ZrO2-stabilized CO2 sorbents. Fuel 2014, 127, 94–100. [Google Scholar] [CrossRef]
- Lu, H.; Khan, A.; Pratsinis, S.E.; Smirniotis, P.G. Flame-Made Durable Doped-CaO Nanosorbents for CO2 Capture. Energy Fuels 2009, 23, 1093–1100. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, K.B. Introduction of chemically bonded zirconium oxide in CaO-based high-temperature CO2 sorbents for enhanced cyclic sorption. Chem. Eng. J. 2019, 355, 850–857. [Google Scholar] [CrossRef]
- Zhao, C.J.; Zhou, Z.M.; Cheng, Z.M. Sol-gel-Derived Synthetic CaO-Based CO2 Sorbents Incorporated with Different Inert Materials. Ind. Eng. Chem. Res. 2014, 53, 14065–14074. [Google Scholar] [CrossRef]
- Zhao, M.; He, X.; Ji, G.Z.; Song, Y.Q.; Zhao, X. Zirconia incorporated calcium looping absorbents with superior sintering resistance for carbon dioxide capture from or processes. Sustain. Energy Fuels 2018, 2, 2733–2741. [Google Scholar] [CrossRef]
- Sarrion, B.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A.; Valverde, J.M. Pressure Effect on the Multicycle Activity of Natural Carbonates and a Ca/Zr Composite for Energy Storage of Concentrated Solar Power. ACS Sustain. Chem. Eng. 2018, 6, 7849–7858. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Valverde, J.M. Nanosilica supported CaO: A regenerable and mechanically hard CO2 sorbent at Ca-looping conditions. Appl. Energy 2014, 118, 92–99. [Google Scholar] [CrossRef]
- Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.A. Enhancement of fast CO2 capture by a nano-SiO2/CaO composite at Ca-looping conditions. Environ. Sci. Technol. 2012, 46, 6401–6408. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Yu, C.T.; Chiang, P.C.; Wang, C.F. Development of high-temperature CO2 sorbents made of CaO-based mesoporous silica. Chem. Eng. J. 2010, 161, 129–135. [Google Scholar] [CrossRef]
- Zhao, M.; Song, Y.Q.; Ji, G.Z.; Zhao, X. Demonstration of Polymorphic Spacing Strategy against Sintering: Synthesis of Stabilized Calcium Looping Absorbents for High-Temperature CO2 Sorption. Energy Fuels 2018, 32, 5443–5452. [Google Scholar] [CrossRef]
- Chen, X.Y.; Jin, X.G.; Liu, Z.M.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Naeem, M.A.; Armutlulu, A.; Kierzkowska, A.; Müller, C.R. Development of high-performance CaO-based CO2 sorbents stabilized with Al2O3 or MgO. Energy Procedia 2017, 114, 158–166. [Google Scholar] [CrossRef]
- Broda, M.; Kierzkowska, A.M.; Müller, C.R. Development of Highly Effective CaO-based, MgO-stabilized CO2 Sorbents via a Scalable “One-Pot” Recrystallization Technique. Adv. Funct. Mater. 2014, 24, 5753–5761. [Google Scholar] [CrossRef]
- Kurlov, A.; Broda, M.; Hosseini, D.; Mitchell, S.J.; Pérez-Ramírez, J.; Müller, C.R. Mechanochemically Activated, Calcium Oxide-Based, Magnesium Oxide-Stabilized Carbon Dioxide Sorbents. ChemSusChem 2016, 9, 2380–2390. [Google Scholar] [CrossRef]
- Liu, W.; Feng, B.; Wu, Y.; Wang, G.; Barry, J.; da Costa, J.C. Synthesis of sintering-resistant sorbents for CO2 capture. Environ. Sci. Technol. 2010, 44, 3093–3097. [Google Scholar] [CrossRef]
- Lan, P.Q.; Wu, S.F. Synthesis of a Porous Nano-CaO/MgO-Based CO2 Adsorbent. Chem. Eng. Technol. 2014, 37, 580–586. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C. Development of a CaO-based sorbent with improved cyclic stability for CO2 capture in pressurized carbonation. Chem. Eng. J. 2011, 171, 197–205. [Google Scholar] [CrossRef]
- Antzara, A.; Heracleous, E.; Lemonidou, A.A. Development of CaO-based Mixed Oxides as Stable Sorbents for Post-Combustion CO2 capture via Carbonate Looping. Energy Procedia 2014, 63, 2160–2169. [Google Scholar] [CrossRef]
- Phromprasit, J.; Powell, J.; Assabumrungrat, S. Metals (Mg, Sr and Al) modified CaO based sorbent for CO2 sorption/desorption stability in fixed bed reactor for high temperature application. Chem. Eng. J. 2016, 284, 1212–1223. [Google Scholar] [CrossRef]
- Li, L.Y.; King, D.L.; Nie, Z.M.; Howard, C. Magnesia-Stabilized Calcium Oxide Absorbents with Improved Durability for High Temperature CO2 Capture. Ind. Eng. Chem. Res. 2009, 48, 10604–10613. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhang, P.P.; Duan, Y.F.; Zhao, C.S. Reactivity enhancement of calcium based sorbents by doped with metal oxides through the sol-gel process. Appl. Energy 2016, 162, 390–400. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Xu, Y.Q.; Ding, N.; Shen, Q.W.; Zheng, C.G. Wet mixing combustion synthesis of CaO-based sorbents for high temperature cyclic CO2 capture. Chem. Eng. J. 2015, 267, 111–116. [Google Scholar] [CrossRef]
- Wang, S.; Fan, S.; Fan, L.; Zhao, Y.; Ma, X. Effect of cerium oxide doping on the performance of CaO-based sorbents during calcium looping cycles. Environ. Sci. Technol. 2015, 49, 5021–5027. [Google Scholar] [CrossRef] [PubMed]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B Environ. 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Guo, H.X.; Feng, J.Q.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Effect of micro-structure and oxygen vacancy on the stability of (Zr-Ce)-additive CaO-based sorbent in CO2 adsorption. J. CO2 Util. 2017, 19, 165–176. [Google Scholar] [CrossRef]
- Peng, W.; Xu, Z.; Luo, C.; Zhao, H. Tailor-Made Core-Shell CaO/TiO2-Al2O3 Architecture as a High-Capacity and Long-Life CO2 Sorbent. Environ. Sci. Technol. 2015, 49, 8237–8245. [Google Scholar] [CrossRef]
- He, D.L.; Qin, C.L.; Zhang, Z.H.; Pi, S.; Ran, J.Y.; Pu, G. Investigation of Y2O3/MxOy-Incorporated Ca-Based Sorbents for Efficient and Stable CO2 Capture at High Temperature. Ind. Eng. Chem. Res. 2018, 57, 11625–11635. [Google Scholar] [CrossRef]
- Hlaing, N.N.; Sreekantan, S.; Othman, R.; Hinode, H.; Kurniawan, W.; Thant, A.A.; Mohamed, A.R.; Salim, C. A novel (Zr-Ce) incorporated Ca(OH)2 nanostructure as a durable adsorbent for CO2 capture. Mater. Lett. 2014, 133, 204–207. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.J.; Yan, X.Y.; Zhao, J.L.; Wang, Z.Y. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure. Appl. Energy 2020, 263, 114650. [Google Scholar] [CrossRef]
- Kim, S.M.; Kierzkowska, A.M.; Broda, M.; Müller, C.R. Sol-gel synthesis of MgAl2O4-stabilized CaO for CO2 capture. Energy Procedia 2017, 114, 220–229. [Google Scholar] [CrossRef]
- Li, L.Y.; King, D.L.; Nie, Z.M.; Li, X.S.; Howard, C. MgAl2O4 Spinel-Stabilized Calcium Oxide Absorbents with Improved Durability for High-Temperature CO2 Capture. Energy Fuels 2010, 24, 3698–3703. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.D.; Guo, Y.F.; Zhao, C.W.; Zhang, J.B.; Liu, W.Q.; Lu, P. Stabilized Performance of Al-Decorated and Al/Mg Co-Decorated Spray-Dried CaO-Based CO2 Sorbents. Chem. Eng. Technol. 2019, 42, 1283–1292. [Google Scholar] [CrossRef]
- Sedghkerdar, M.H.; Mahinpey, N.; Soleimanisalim, A.H.; Sun, Z.K.; Chen, Z.W.; Lim, J.; Kaliaguine, S. Core-shell structured CaO-based pellets protected by mesoporous ceramics shells for high-temperature CO2 capture. Can. J. Chem. Eng. 2016, 94, 2038–2044. [Google Scholar] [CrossRef]
- Naeem, M.A.; Armutlulu, A.; Imtiaz, Q.; Muller, C.R. CaO-Based CO(2) Sorbents Effectively Stabilized by Metal Oxides. Chemphyschem 2017, 18, 3280–3285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).