Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation
Abstract
1. Introduction
2. Biological Hydrogen Production
2.1. Microorganisms Participating in Dark Fermentation
2.2. Artificial Microbial Consortium
3. Hydrogen Production from Biomass
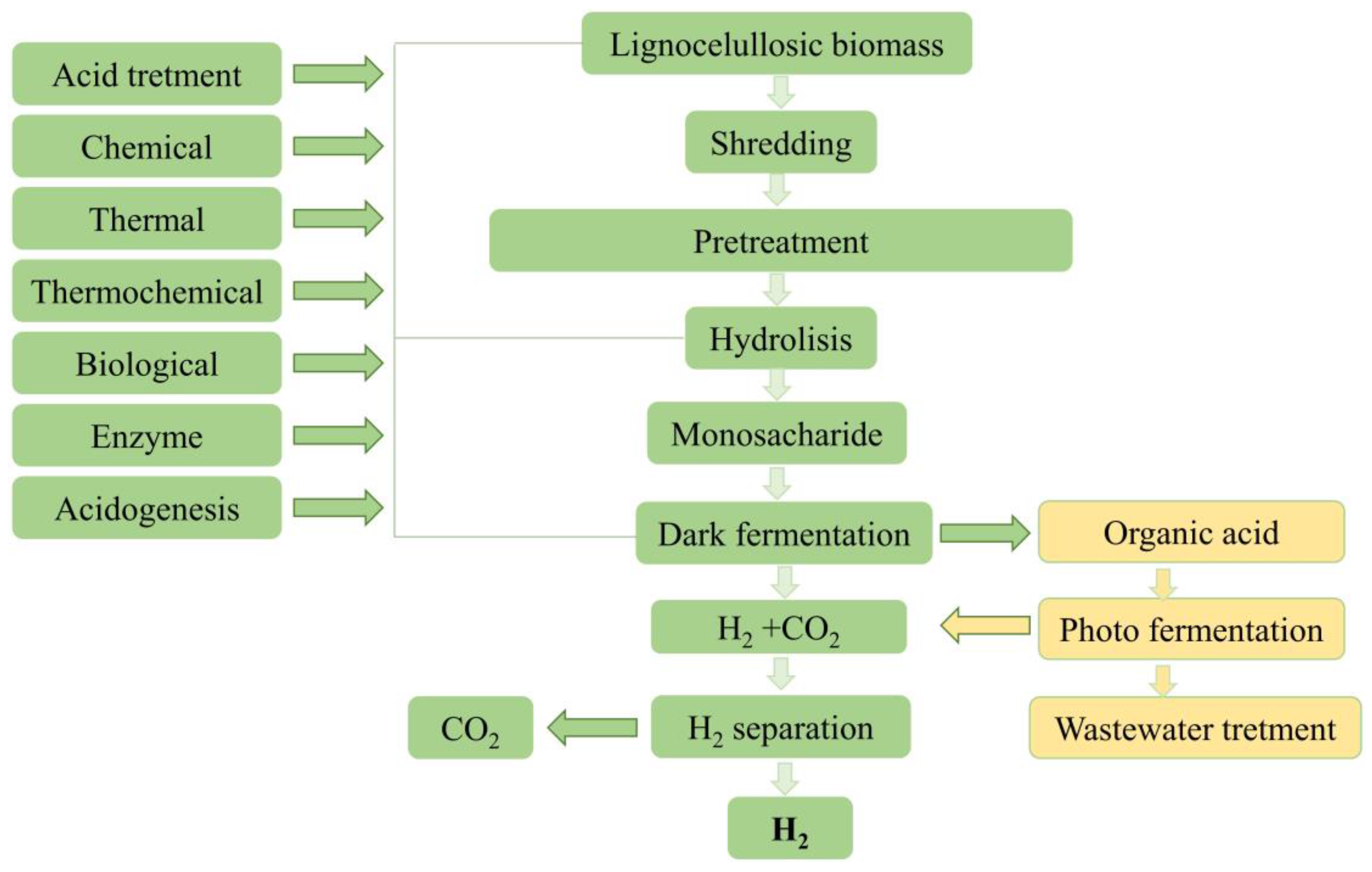
3.1. Lignocellulosic Biomass and Crop Residues Containing Sugars
3.2. Wastewaters
4. Hydrogen Management
4.1. Hydrogen Storage
4.1.1. Natural Caves and Salt Mine Caves
4.1.2. Underground Hydrogen Storage in a Gas Mixture
4.1.3. Special Cases of Hydrogen Storage in Subsurface Reactors for the Methanation Process
4.2. Hydrogen Transport
4.3. Hydrogen Economics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghasemian, S.; Faridzad, A.; Abbaszadeh, P.; Taklif, A.; Ghasemi, A.; Hafezi, R. An overview of global energy scenarios by 2040: Identifying the driving forces using cross-impact analysis method. Int. J. Environ. Sci. Technol. 2020, 1–24. [Google Scholar] [CrossRef]
- Ahmadov, A.K.; van der Borg, C. Do natural resources impede renewable energy production in the EU? A mixed-methods analysis. Energy Policy 2019, 126, 361–369. [Google Scholar] [CrossRef]
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, M. Social, Economic, and Environmental Impacts of Renewable Energy Resources. In Wind Solar Hybrid Renewable Energy System; Okedu, K.E., Tahour, A., Aissaou, A.G., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-591-4. [Google Scholar]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Seetharaman; Moorthy, K.; Patwa, N.; Saravanan; Gupta, Y. Breaking barriers in deployment of renewable energy. Heliyon 2019, 5, e01166. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Hydrogen Strategy for a Climate EN Neutral Europe; European Commission: Brussels, Belgium, 2020.
- IRENA—International Renewable Energy Agency Hydrogen. Available online: https://www.irena.org/Energy-Transition/Technology/Hydrogen (accessed on 8 February 2023).
- Chen, R.; Wang, Y.Z.; Liao, Q.; Zhu, X.; Xu, T.F. Hydrolysates of lignocellulosic materials for biohydrogen production. BMB Rep. 2013, 46, 244–251. [Google Scholar] [CrossRef]
- Panić, I.; Cuculić, A.; Ćelić, J. Color-Coded Hydrogen: Production and Storage in Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1995. [Google Scholar] [CrossRef]
- Sen, S.; Bansal, M.; Razavi, S.; Khan, A.S. The Color Palette of the Colorless Hydrogen. Available online: https://jpt.spe.org/twa/the-color-palette-of-the-colorless-hydrogen (accessed on 8 February 2023).
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen Production from Lignocellulosic Biomass: Technology and Sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Weger, L.B.; Leitão, J.; Lawrence, M.G. Expected impacts on greenhouse gas and air pollutant emissions due to a possible transition towards a hydrogen economy in German road transport. Int. J. Hydrogen Energy 2021, 46, 5875–5890. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Talapko, D. Influence of Distributed Electrical Energy Production on the Availability Enhancement in Telecommunication and Datacenter Facility; University of Zagreb: Zagreb, Croatia, 2020. [Google Scholar]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Salimi, M.; Hosseinpour, M.; Borhani, T.N. The Role of Clean Hydrogen Value Chain in a Successful Energy Transition of Japan. Energies 2022, 15, 6064. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Talapko, J.; Talapko, D.; Matić, A.; Škrlec, I. Microorganisms as New Sources of Energy. Energies 2022, 15, 6365. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The economics and the environmental benignity of different colors of hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- Ramamurthy, P.C.; Singh, S.; Kapoor, D.; Parihar, P.; Samuel, J.; Prasad, R.; Kumar, A.; Singh, J. Microbial biotechnological approaches: Renewable bioprocessing for the future energy systems. Microb. Cell Fact. 2021, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Mohsen, A.; Elwakeel, K.Z. Biomass-to-sustainable biohydrogen: Insights into the production routes, and technical challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrogen Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Kanwal, F.; Torriero, A.A.J. Biohydrogen-A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-hydrogen production through dark fermentation: An overview. Biomass Convers. Biorefin. 2022, 1–26. [Google Scholar] [CrossRef]
- Jose, V. Photobiological hydrogen as a renewable fuel. Holist. Approach Environ. 2021, 11, 67–71. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 529. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefin. 2020, 1–19. [Google Scholar] [CrossRef]
- Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156. [Google Scholar] [CrossRef]
- Sittijunda, S.; Baka, S.; Jariyaboon, R.; Reungsang, A.; Imai, T.; Kongjan, P. Integration of Dark Fermentation with Microbial Electrolysis Cells for Biohydrogen and Methane Production from Distillery Wastewater and Glycerol Waste Co-Digestion. Fermentation 2022, 8, 537. [Google Scholar] [CrossRef]
- El Bari, H.; Lahboubi, N.; Habchi, S.; Rachidi, S.; Bayssi, O.; Nabil, N.; Mortezaei, Y.; Villa, R. Biohydrogen production from fermentation of organic waste, storage and applications. Clean. Waste Syst. 2022, 3, 100043. [Google Scholar] [CrossRef]
- AntonopouLou, G.; Ntaikou, I.; Stamatelatou, K.; Lyberatos, G. Biological and fermentative production of hydrogen. In Handbook of Biofuels Production. Processes and Technologies; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 205–347. ISBN 978-1-84569-679-5. [Google Scholar]
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 1095. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Gokuladoss, V. Fusion of Vermicompost and Sewage Sludge as Dark Fermentative Biocatalyst for Biohydrogen Production: A Kinetic Study. Energies 2022, 15, 6917. [Google Scholar] [CrossRef]
- Camacho, C.I.; Estévez, S.; Conde, J.J.; Feijoo, G.; Moreira, M.T. Dark fermentation as an environmentally sustainable WIN-WIN solution for bioenergy production. J. Clean. Prod. 2022, 374, 134026. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.J.; Lee, K.S.; Lin, C.Y.; Chen, C.Y.; Chang, J.S. Biomass based hydrogen production by dark fermentation-recent trends and opportunities for greener processes. Curr. Opin. Biotechnol. 2018, 50, 136–145. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Wiktorowska-Sowa, E.; Piotrowski, J.; Salamon, A.; Kaźmierczak, W.; Błaszczyk, M.K.; Barberan, A.; Chen, Y.; et al. Dynamics and Complexity of Dark Fermentation Microbial Communities Producing Hydrogen From Sugar Beet Molasses in Continuously Operating Packed Bed Reactors. Front. Microbiol. 2021, 11, 612344. [Google Scholar] [CrossRef]
- Vasmara, C.; Pindo, M.; Micheletti, D.; Marchetti, R. Initial pH influences microbial communities composition in dark fermentation of scotta permeate. Int. J. Hydrogen Energy 2018, 43, 8707–8717. [Google Scholar] [CrossRef]
- Tang, T.; Chen, Y.; Liu, M.; Du, Y.; Tan, Y. Effect of pH on the performance of hydrogen production by dark fermentation coupled denitrification. Environ. Res. 2022, 208, 112663. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Beyond the Theoretical Yields of Dark-Fermentative Biohydrogen. Indian J. Microbiol. 2018, 58, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Keskin, T.; Abubackar, H.N.; Yazgin, O.; Gunay, B.; Azbar, N. Effect of percolation frequency on biohydrogen production from fruit and vegetable wastes by dry fermentation. Int. J. Hydrogen Energy 2019, 44, 18767–18775. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies 2022, 15, 4876. [Google Scholar] [CrossRef]
- Bonk, F.; Chaturvedi, T.; Torres, A.I.; Schmidt, J.E.; Thomsen, M.H.; Stephanopoulos, G. Exploring Opportunities for the Production of Chemicals from Municipal Solid Wastes within the Framework of a Biorefinery. Comput. Aided Chem. Eng. 2015, 37, 2123–2128. [Google Scholar] [CrossRef]
- Androga, D.D.; Özgür, E.; Eroglu, I.; Gündüz, U.; Yücel, M. Photofermentative Hydrogen Production in Outdoor Conditions. In Hydrogen Energy—Challenges and Perspectives; Minic, D., Ed.; IntechOpen: London, UK, 2012; pp. 77–120. ISBN 978-953-51-0812-2. [Google Scholar]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface Microbial Hydrogen Cycling: Natural Occurrence and Implications for Industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Kutty, P.; Pachapur, P.; Brar, S.K.; Le Bihan, Y.; Galvez-Cloutier, R.; Buelna, G. Seed Pretreatment for Increased Hydrogen Production Using Mixed-Culture Systems with Advantages over Pure-Culture Systems. Energies 2019, 12, 530. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Vasmara, C.; Galletti, S.; Cianchetta, S.; Ceotto, E. Advancements in Giant Reed (Arundo donax L.) Biomass Pre-Treatments for Biogas Production: A Review. Energies 2023, 16, 949. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.; Wang, M.; Zhang, Z.; Feng, Y. Enhancement of magnetic field on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2021, 341, 125764. [Google Scholar] [CrossRef]
- Oswald, F.; Stoll, I.K.; Zwick, M.; Herbig, S.; Sauer, J.; Boukis, N.; Neumann, A. Formic Acid Formation by Clostridium ljungdahlii at Elevated Pressures of Carbon Dioxide and Hydrogen. Front. Bioeng. Biotechnol. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Dzulkarnain, E.L.N.; Audu, J.O.; Wan Dagang, W.R.Z.; Abdul-Wahab, M.F. Microbiomes of biohydrogen production from dark fermentation of industrial wastes: Current trends, advanced tools and future outlook. Bioresour. Bioprocess. 2022, 9, 16. [Google Scholar] [CrossRef]
- Masset, J.; Calusinska, M.; Hamilton, C.; Hiligsmann, S.; Joris, B.; Wilmotte, A.; Thonart, P. Fermentative hydrogen production from glucose and starch using pure strains and artificial co-cultures of Clostridium spp. Biotechnol. Biofuels 2012, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Morra, S. Fantastic [FeFe]-Hydrogenases and Where to Find Them. Front. Microbiol. 2022, 13, 853626. [Google Scholar] [CrossRef]
- Schuchmann, K.; Chowdhury, N.P.; Müller, V. Complex Multimeric [FeFe] Hydrogenases: Biochemistry, Physiology and New Opportunities for the Hydrogen Economy. Front. Microbiol. 2018, 9, 2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Spengler, K.; Terberger, K.; Boehm, M.; Appel, J.; Barske, T.; Timm, S.; Battchikova, N.; Hagemann, M.; et al. Pyruvate:ferredoxin oxidoreductase and low abundant ferredoxins support aerobic photomixotrophic growth in cyanobacteria. eLife 2022, 11, e71339. [Google Scholar] [CrossRef]
- Germane, K.L.; Liu, S.; Gerlach, E.S.; Savage, A.M.; Renberg, R.L.; Zu, T.N.K.; Dong, H.; Walck, S.D.; Servinsky, M.D.; Sund, C.J. Hydrogen-Cycling during Solventogenesis in Clostridium acetobutylicum American Type Culture Collection (ATCC) 824 Requires the [NiFe]-Hydrogenase for Energy Conservation. Fermentation 2018, 4, 55. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Technol. 2019, 49, 825–865. [Google Scholar] [CrossRef]
- Buckel, W. Energy Conservation in Fermentations of Anaerobic Bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of Cellulose and Hemicellulose by Ruminal Microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Zheng, Y.; Kahnt, J.; Kwon, I.H.; Mackie, R.I.; Thauer, R.K. Hydrogen formation and its regulation in Ruminococcus albus: Involvement of an electron-bifurcating [FeFe]-hydrogenase, of a non-electron-bifurcating [FeFe]-hydrogenase, and of a putative hydrogen-sensing [FeFe]-hydrogenase. J. Bacteriol. 2014, 196, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Ergal, İ.; Zech, E.; Hanišáková, N.; Kushkevych, I.; Fuchs, W.; Vítěz, T.; Vítězová, M.; Bochmann, G.; Rittmann, S.K.-M.R. Scale-Up of Dark Fermentative Biohydrogen Production by Artificial Microbial Co-Cultures. Appl. Microbiol. 2022, 2, 215–226. [Google Scholar] [CrossRef]
- Vivijs, B.; Haberbeck, L.U.; Mfortaw Mbong, V.B.; Bernaerts, K.; Geeraerd, A.H.; Aertsen, A.; Michiels, C.W. Formate hydrogen lyase mediates stationary-phase deacidification and increases survival during sugar fermentation in acetoin-producing enterobacteria. Front. Microbiol. 2015, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, A.; Zhuang, G. Construction of Environmental Synthetic Microbial Consortia: Based on Engineering and Ecological Principles. Front. Microbiol. 2022, 13, 829717. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhou, J.J.; Quan, C.S.; Xiu, Z.L. Advances in industrial microbiome based on microbial consortium for biorefinery. Bioresour. Bioprocess. 2017, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Ergal, İ.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the Thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443. [Google Scholar] [CrossRef]
- Ergal, İ.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M. Design and engineering of artificial microbial consortia for biohydrogen production. Curr. Opin. Biotechnol. 2022, 73, 74–80. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.-S. Biohydrogen Production From Renewable Biomass Resources. In Biomass, Biofuels, Biochemicals, Biohydrogen; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar] [CrossRef]
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third generation biohydrogen production by Clostridium butyricum and adapted mixed cultures from Scenedesmus obliquus microalga biomass. Fuel 2015, 153, 128–134. [Google Scholar] [CrossRef]
- Kabir, F.; Gulfraz, M.; Raja, G.; Inam-ul-Haq, M.; Batool, I.; Awais, M.; Habiba, U.; Gul, H. Comparative study on the usability of lignocellulosic and algal biomass for production of alcoholic fuels. BioResources 2019, 14, 8135–8154. [Google Scholar] [CrossRef]
- Allouache, A.; Majda, A.; Toudert, A.Z.; Amrane, A.; Ballesteros, M. Cellulosic bioethanol production from Ulva lactuca macroalgae. Cellul. Chem. Technol. 2021, 55, 629–635. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Rahimian Koloor, S.S.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass: Challenges and future directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013. [Google Scholar] [CrossRef] [PubMed]
- Björkmalm, J.; Byrne, E.; Van Niel, E.W.J.; Willquist, K. A non-linear model of hydrogen production by Caldicellulosiruptor saccharolyticus for diauxic-like consumption of lignocellulosic sugar mixtures. Biotechnol. Biofuels 2018, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Luque, R.; Dutta, N.; Usman, M.; Luo, G.; Zhang, S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustain. Chem. 2022, 3, 35–55. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic biomass for bioethanol: Recent advances, technology trends, and barriers to industrial development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Kucharska, K.; Gębicki, J. Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management. Molecules 2022, 27, 7658. [Google Scholar] [CrossRef]
- Dauptain, K.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Carrere, H. Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour. Technol. 2020, 313, 123665. [Google Scholar] [CrossRef]
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-treatment technologies for dark fermentative hydrogen production: Current advances and future directions. Waste Manag. 2018, 71, 734–748. [Google Scholar] [CrossRef]
- Bielen, A.A.M.; Verhaart, M.R.A.; van der Oost, J.; Kengen, S.W.M. Biohydrogen Production by the Thermophilic Bacterium Caldicellulosiruptor saccharolyticus: Current Status and Perspectives. Life 2013, 3, 52–85. [Google Scholar] [CrossRef] [PubMed]
- Pecorini, I.; Baldi, F.; Iannelli, R. Biochemical Hydrogen Potential Tests Using Different Inocula. Sustainability 2019, 11, 622. [Google Scholar] [CrossRef]
- Van De Werken, H.J.G.; Verhaart, M.R.A.; VanFossen, A.L.; Willquist, K.; Lewis, D.L.; Nichols, J.D.; Goorissen, H.P.; Mongodin, E.F.; Nelson, K.E.; Van Niel, E.W.J.; et al. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2008, 74, 6720–6729. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Progress in Ameliorating Beneficial Characteristics of Microbial Cellulases by Genetic Engineering Approaches for Cellulose Saccharification. Front. Microbiol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Improvements in fermentative biological hydrogen production through metabolic engineering. J. Environ. Manag. 2012, 95, S360–S364. [Google Scholar] [CrossRef]
- Bhatia, R.K.; Sakhuja, D.; Mundhe, S.; Walia, A. Renewable Energy Products through Bioremediation of Wastewater. Sustainability 2020, 12, 7501. [Google Scholar] [CrossRef]
- Preethi; Usman, T.M.M.; Rajesh Banu, J.; Gunasekaran, M.; Kumar, G. Biohydrogen production from industrial wastewater: An overview. Bioresour. Technol. Rep. 2019, 7, 100287. [Google Scholar] [CrossRef]
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Vasmara, C.; Marchetti, R. Initial pH influences in-batch hydrogen production from scotta permeate. Int. J. Hydrogen Energy 2017, 42, 14400–14408. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Ismail, S.; Ni, S.Q.; Ihsanullah, I.; Ahmad, R.; Khan, A.; Tawfik, A.; Nizami, A.S.; Lee, M. Harvesting biohydrogen from industrial wastewater: Production potential, pilot-scale bioreactors, commercialization status, techno-economics, and policy analysis. J. Clean. Prod. 2022, 340, 130809. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Adeniran, J.A.; Lototskyy, M.; Asadi, A. Simultaneous brewery wastewater treatment and hydrogen generation via hydrolysis using Mg waste scraps. J. Clean. Prod. 2020, 276, 123198. [Google Scholar] [CrossRef]
- Pitchaimuthu, S.; Sridharan, K.; Nagarajan, S.; Ananthraj, S.; Robertson, P.; Kuehnel, M.F.; Irabien, Á.; Maroto-Valer, M. Solar Hydrogen Fuel Generation from Wastewater—Beyond Photoelectrochemical Water Splitting: A Perspective. Energies 2022, 15, 7399. [Google Scholar] [CrossRef]
- Zhang, J.; Kan, X.; Shen, Y.; Loh, K.C.; Wang, C.H.; Dai, Y.; Tong, Y.W. A hybrid biological and thermal waste-to-energy system with heat energy recovery and utilization for solid organic waste treatment. Energy 2018, 152, 214–222. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: A review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Pires, M.; Meloni, E.; Skov, I.R.; Vilardi, G.; Zuorro, A.; Belikov, J.; Perea-Moreno, A.-J.; Toczyłowska-Mamí Nska, R.; Mamí Nski, M.Ł. Wastewater as a Renewable Energy Source—Utilisation of Microbial Fuel Cell Technology. Energies 2022, 15, 6928. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of Hydrogen as an Alternative Fuel for Next-Generation Industrial Applications; Challenges and Expected Opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Wollin, K.M.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Mangerich, A.; Gundert-Remy, U.; Partosch, F.; Röhl, C.; Schupp, T.; et al. Critical evaluation of human health risks due to hydraulic fracturing in natural gas and petroleum production. Arch. Toxicol. 2020, 94, 967–1016. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Rep. 2022, 8, 461–499. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Yang, C.; Li, Y.; Wang, T.; Ma, H.; Shi, H.; Li, J.; Liu, J. Repair of irregularly shaped salt cavern gas storage by re-leaching under gas blanket. J. Nat. Gas Sci. Eng. 2017, 45, 848–859. [Google Scholar] [CrossRef]
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038. [Google Scholar] [CrossRef]
- Sampim, T.; Kokkaew, N.; Parnphumeesup, P. Risk Management in Biomass Power Plants Using Fuel Switching Flexibility. Energy Procedia 2017, 138, 1099–1104. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Biswas, S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Review on Synthesis of Methane as a Pathway for Renewable Energy Storage With a Focus on Solid Oxide Electrolytic Cell-Based Processes. Front. Energy Res. 2020, 8, 229. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Dominguez-Gonzalez, G.; Muñoz-Hernandez, J.I.; Bunn, D.; Garcia-Checa, C.J. Integration of Hydrogen and Synthetic Natural Gas within Legacy Power Generation Facilities. Energies 2022, 15, 4485. [Google Scholar] [CrossRef]
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Hanišáková, N.; Onderka, V.; Urbanová, I. Underground gas storage as a promising natural methane bioreactor and reservoir? J. Energy Storage 2022, 47, 103631. [Google Scholar] [CrossRef]
- Balsalobre-Lorente, D.; Radulescu, M.; Shahzad, U.; Rehman, A.; Neacsa, A.; Eparu, C.N.; Stoica, D.B. Hydrogen—Natural Gas Blending in Distribution Systems—An Energy, Economic, and Environmental Assessment. Energies 2022, 15, 6143. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C. Membranes 2021, 11, 282. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Aziz, M.; TriWijayanta, A.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Rizi, H.A.Y.; Shin, D.; Hossein Ali, Y.R.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energies 2022, 15, 8246. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Bethoux, O. Hydrogen Fuel Cell Road Vehicles and Their Infrastructure: An Option towards an Environmentally Friendly Energy Transition. Energies 2020, 13, 6132. [Google Scholar] [CrossRef]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production: Processes, Systems and Technologies; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83. [Google Scholar] [CrossRef]
- Vostakola, F.; Salamatinia, M.; Horri, A.; Fallah Vostakola, M.; Salamatinia, B.; Horri, B.A. A Review on Recent Progress in the Integrated Green Hydrogen Production Processes. Energies 2022, 15, 1209. [Google Scholar] [CrossRef]
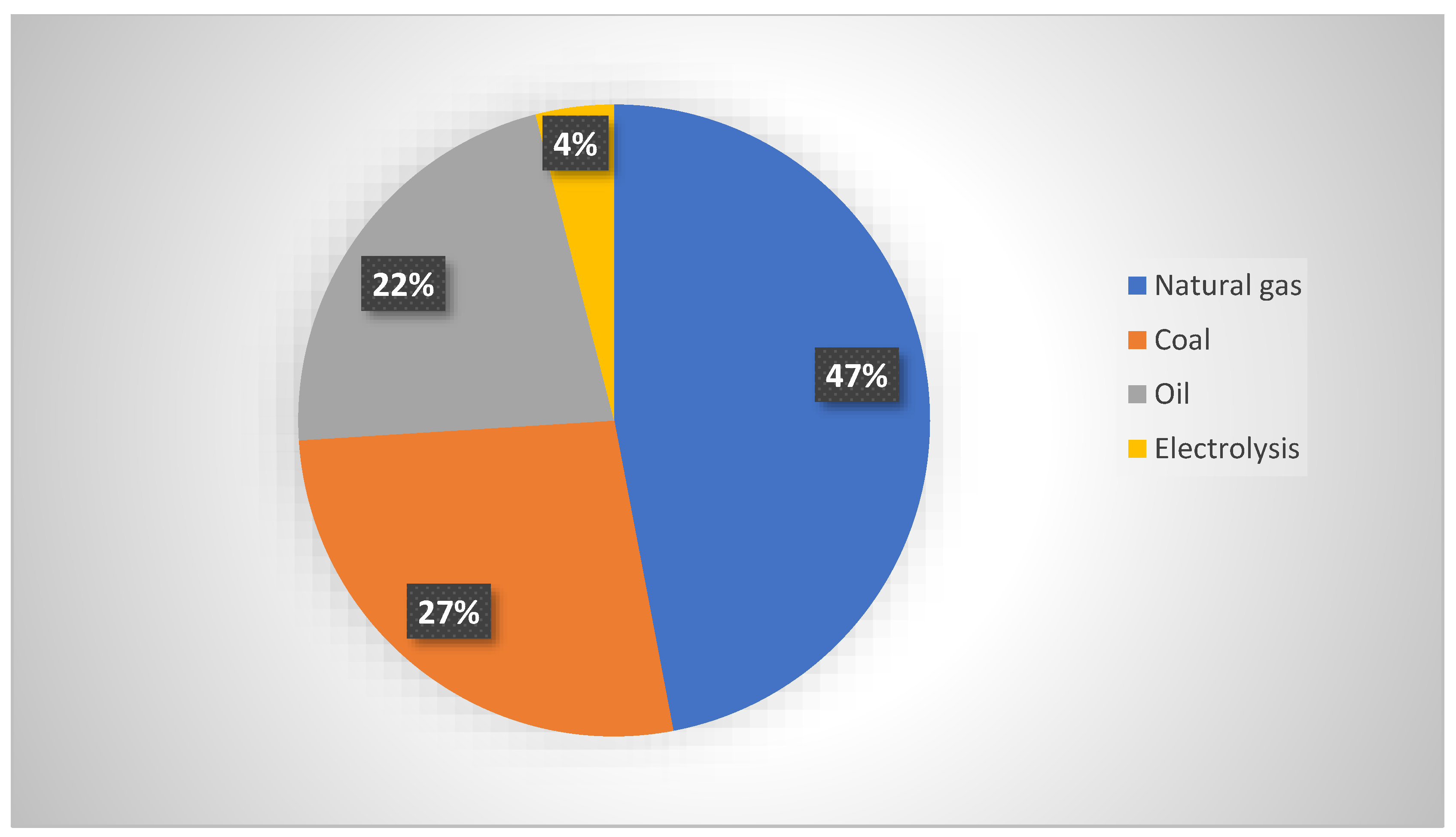

| Hydrogen Type (Designated Color) | Hydrogen Derived from |
|---|---|
| White | Natural source |
| Black | Coal |
| Gray | Methane |
| Brown | Lignite |
| Blue | Methane with carbon capture |
| Turquoise | Methane pyrolysis |
| Green | Renewable energy |
| Pink | Nuclear |
| Advantages of Dark Fermentation Compared to Photo-Fermentation and Bio-Photolysis | Ref. | |
|---|---|---|
| 1. | The rate of dark fermentation is significantly higher compared to photo-fermentation and bio-photolysis processes | [36] |
| 2. | Dark fermentation has the potential to utilize a wide range of potential substrates, including renewable biomass and organic waste materials, resulting in relatively lower costs. | [37] |
| 3. | Dark fermentation does not require direct input of light energy. Therefore, it is capable of continuously producing hydrogen day and night. | [38] |
| 4. | Its simple reactor design gives it efficiency and economic feasibility | [39] |
| 5. | The estimated cost of hydrogen production by dark fermentation is 340 times lower than that of photosynthetic processes. | [40] |
| Substrate | Organism | pH | Temp | Hydrogen Yield |
|---|---|---|---|---|
| Cornstalk 20 g/dm3 | Thermoanaerobacterium thermosaccharolyticum | 7.5 | 55 °C | 6.38 mmol/g substrate |
| Rice straw 1% w/v | Thermotoga neapolitana | 7.5 | 75 °C | 2.27 mmol/g straw |
| Wheat straw 5 g/dm3 | Thermoanaerobacterium thermosaccharolyticum | 7.0 | 60 °C | 3.53 mmol/g substrate |
| Cornstalk 15 g/dm3 | Clostridium sartagoforme | 6.5 | 35 °C | 87.2 cm3/g substrate |
| Sugarcane bagasse 1% | Caldicellulosiruptor saccharolyticus | - | 70 °C | 2.3 mol/mol glucose |
| Corn leaves 0.9% | Caldicellulosiruptor saccharolyticus | - | 70 °C | 1.80 mol/mol glucose |
| Soybean straw | Mixed cultures | 7.0 | 35 °C | 5.46 cm3/g substrate |
| Pine tree wood | Mixed cultures from sewage sludge digester | 7.0 | 35 °C | 0.99 mol/mol substrate |
| Cattle wastewater | Sewage sludge | 5.5 | 45 °C | 12.41 mmol/g substrate |
| Wastewater from the brewery plant | Mixed cultures (from activated sludge) | 5.5 | 35 °C | 2 mol/mol hexose |
| Genus | Species | Hydrogen Yields | Ref. |
|---|---|---|---|
| Clostridium | C. acetobutylicum | 2.0 mol/mol glucose | |
| C. beijerinckii | 2.31 mol/mol xylose | ||
| C. butiricum | 2.78 mol/mol saccharose | ||
| C. thermolacticum | 3.0 mol/mol lactose | ||
| Ruminococcus | R. albus | 2.01 mol/mol glucose | |
| R. albus | 0.59 mol/mol cellulose | [27] | |
| Thermotoga | T. maritima | 2.2 mol/mol glucose | |
| Thermoanaerobacterium | T. thermosaccharolyticum | 2.4 mol/mol glucose |
| Genus | Species | Hydrogen Yields | Ref. |
|---|---|---|---|
| Citrobacter | C. amalonaticus | 1.24 mol/mol glucose | [27] |
| C. freundii | 0.83 mol/mol glucose | ||
| C. intermedius | 1.1 mol/mol glucose | ||
| Klebsiellae | K. pneumoniae | 2.07 mol/mol glucose | [27] |
| Enterobacter | Enterobacter aerogenes | 2.16 mol/mol glucose | [69] |
| Enterobacter cloacae | 3.9 mol/mol glucose | [27] | |
| Escherichia | Escherichia coli | 2 mol/mol glucose | [27] |
| Microorganisms Consortium | Hydrogen Yield |
|---|---|
| E. aerogenes–C. acetobutylicum | 5.58 mol/mol glucose |
| Ruminococcus albus–Wolinella succinogenes | 3.91 mol/mol glucose |
| Caldicellulosiruptor saccharolyticus–C. kristjanssonii | 3.7 mol/mol glucose |
| Citrobacter freundii–C. butyricum | 2.12 mol/mol glucose |
| C. saccharolyticus–C. owensensi | 4.42 mol/mol glucose |
| Thermatoga neapolitana–C. saccharolyticus | 2.8 mol/mol glucose |
| Enterobacter cloacae–Bacillus cereus | 3.0 mol/mol glucose |
| Escherichia coli–C. butyricum | 1.65 mol/mol glucose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talapko, D.; Talapko, J.; Erić, I.; Škrlec, I. Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation. Energies 2023, 16, 3321. https://doi.org/10.3390/en16083321
Talapko D, Talapko J, Erić I, Škrlec I. Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation. Energies. 2023; 16(8):3321. https://doi.org/10.3390/en16083321
Chicago/Turabian StyleTalapko, Domagoj, Jasminka Talapko, Ivan Erić, and Ivana Škrlec. 2023. "Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation" Energies 16, no. 8: 3321. https://doi.org/10.3390/en16083321
APA StyleTalapko, D., Talapko, J., Erić, I., & Škrlec, I. (2023). Biological Hydrogen Production from Biowaste Using Dark Fermentation, Storage and Transportation. Energies, 16(8), 3321. https://doi.org/10.3390/en16083321






