Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production
Abstract
1. Introduction
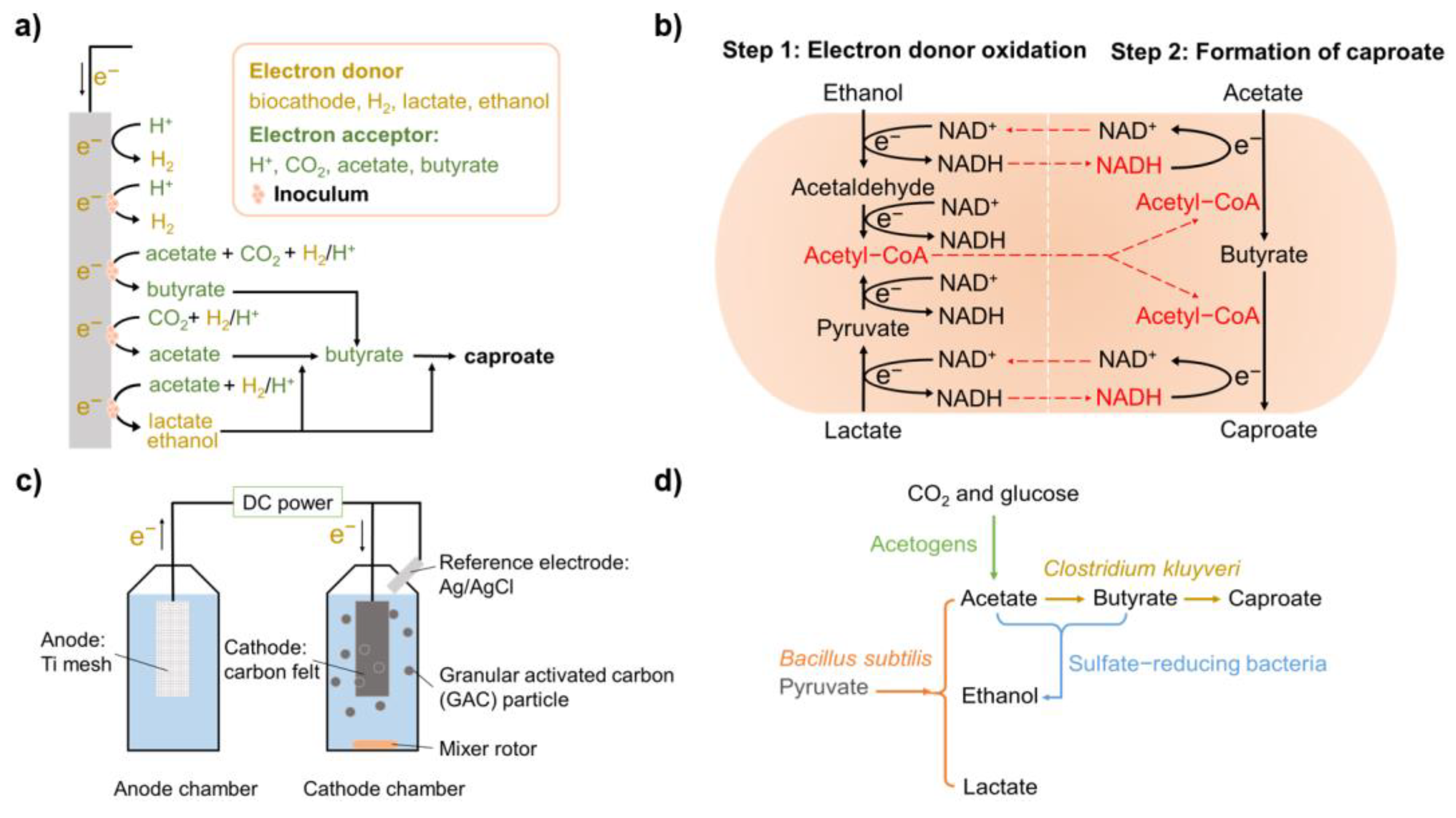
2. Working Principle of Electricity-Driven Medium Chain Carboxylic Acids Fermentation
3. Functional Microbes and Metabolic Pathway of Electricity-Driven Medium Chain Carboxylic Acids Production from Organic Waste
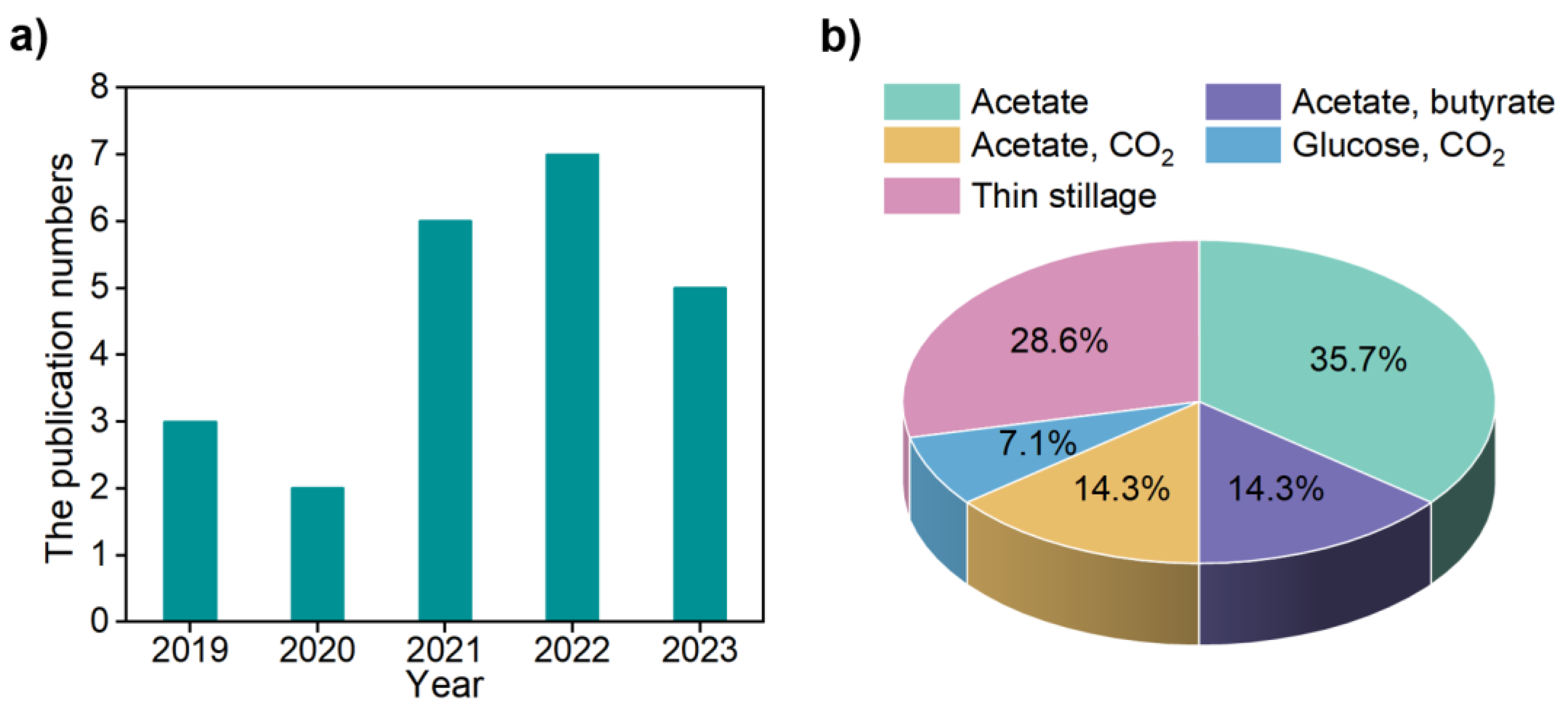
3.1. Functional Microorganisms
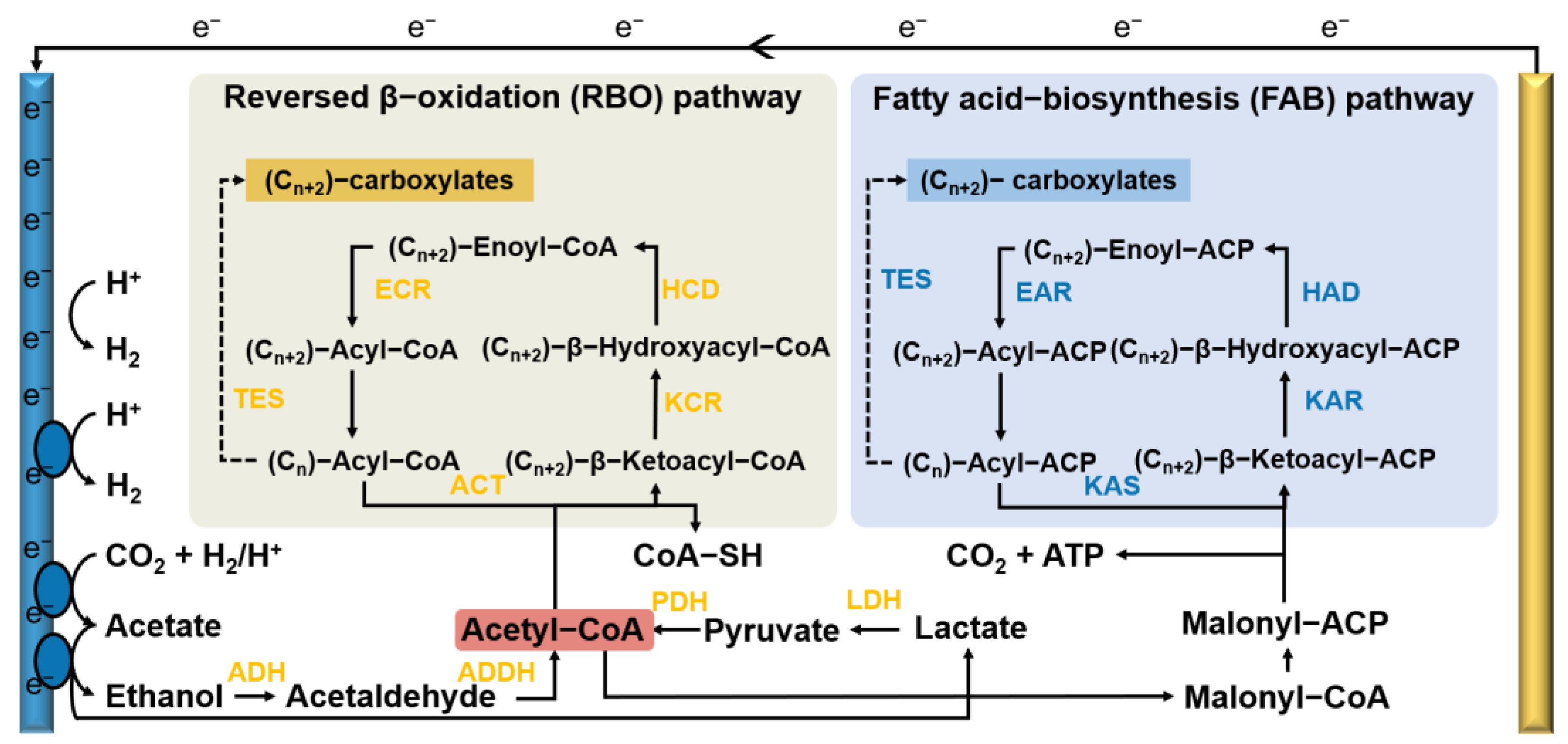
3.2. Metabolic Pathway
4. Enhanced Strategies for Electricity-Steering Medium Chain Carboxylic Acids Generation from Organic Waste
4.1. Substrate Modulation
4.2. Tuning Applied Voltage/Current
4.3. Electrode Acclimation and Cathode Material Optimation
4.4. Microbial Cooperation and Stimulation
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, R.; Deng, C.; Zhang, W.; Hollmann, F.; Murphy, J.D. Production of bio-alkanes from biomass and CO2. Trends Biotechnol. 2021, 39, 370–380. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Mohan, S.V. Understanding acidogenesis towards green hydrogen and volatile fatty acid production–critical analysis and circular economy perspective. Chem. Eng. J. 2023, 141550. [Google Scholar] [CrossRef]
- Satchwell, A.J.; Scown, C.D.; Smith, S.J.; Amirebrahimi, J.; Jin, L.; Kirchstetter, T.W.; Brown, N.J.; Preble, C.V. Accelerating the deployment of anaerobic digestion to meet zero waste goals. Environ. Sci. Technol. 2018, 52, 13663–13669. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Duan, X.; Chen, Y. Integrated metagenomic and metaproteomic analyses unravel ammonia toxicity to active methanogens and syntrophs, enzyme synthesis, and key enzymes in anaerobic digestion. Environ. Sci. Technol. 2021, 55, 14817–14827. [Google Scholar] [CrossRef]
- Kiyasudeen, K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Prospects of Organic Waste Management and the Significance of Earthworms; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Chen, C.; Zhang, X.; Liu, C.; Wu, Y.; Zheng, G.; Chen, Y. Advances in downstream processes and applications of biological carboxylic acids derived from organic wastes. Bioresour. Technol. 2022, 346, 126609. [Google Scholar] [CrossRef]
- Fugol, M.; Prask, H.; Szlachta, J.; Dyjakon, A.; Pasławska, M.; Szufa, S. Improving the energetic efficiency of biogas plants using enzymatic additives to anaerobic digestion. Energies 2023, 16, 1845. [Google Scholar] [CrossRef]
- Lelicińska-Serafin, K.; Manczarski, P.; Rolewicz-Kalińska, A. An Insight into post-consumer food waste characteristics as the key to an organic recycling method selection in a circular economy. Energies 2023, 16, 1735. [Google Scholar] [CrossRef]
- Yesil, H.; Calli, B.; Tugtas, A.E. A hybrid dry-fermentation and membrane contactor system: Enhanced volatile fatty acid (VFA) production and recovery from organic solid wastes. Water Res. 2021, 192, 116831. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Huang, H.; Duan, X.; Dong, L. Improved anaerobic digestion under ammonia stress by regulating microbiome and enzyme to enhance VFAs bioconversion: The new role of glutathione. Chem. Eng. J. 2022, 433, 134562. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Biological production of medium-chain carboxylates through chain elongation: An overview. Biotechnol. Adv. 2022, 55, 107882. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcon, J.; Trably, E.; Bernet, N. Electrofermentation: How to drive fermentation using electrochemical systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yong, Y.-c.; Fang, Z.; Yan, H.; Li, J.; Meng, J. Highly selective butanol production by manipulating electron flow via cathodic electro-fermentation. Bioresour. Technol. 2023, 374, 128770. [Google Scholar] [CrossRef]
- Tharak, A.; Katakojwala, R.; Kajla, S.; Mohan, S.V. Chemolithoautotrophic reduction of CO2 to acetic acid in gas and gas-electro fermentation systems: Enrichment, microbial dynamics, and sustainability assessment. Chem. Eng. J. 2023, 454, 140200. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Q.; Zhang, P.; Zhang, Q.; Wu, Y.; Li, F.; Tao, X.; Wang, S.; Nabi, M.; Zhou, Y. Effect of acid/ethanol ratio on medium chain carboxylate production with different VFAs as the electron acceptor: Insight into carbon balance and microbial community. Energies 2019, 12, 3720. [Google Scholar] [CrossRef]
- Shrestha, S.; Xue, S.; Kitt, D.; Song, H.; Truyers, C.; Muermans, M.; Smets, I.; Raskin, L. Anaerobic dynamic membrane bioreactor development to facilitate organic waste conversion to medium-chain carboxylic acids and their downstream recovery. ACS EST Eng. 2021, 2, 169–180. [Google Scholar] [CrossRef]
- Xu, J.; Guzman, J.J.; Angenent, L.T. Direct medium-chain carboxylic acid oil separation from a bioreactor by an electrodialysis/phase separation cell. Environ. Sci. Technol. 2020, 55, 634–644. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, Z.; Wei, W.; Chen, X.; Mannina, G.; Ni, B.-J. A novel strategy for efficiently transforming waste activated sludge into medium-chain fatty acid using free nitrous acid. Sci. Total Environ. 2023, 862, 160826. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Zuo, J. Performance and mechanisms of medium-chain fatty acid production by anaerobic fermentation of food waste without external electron donors. Bioresour. Technol. 2023, 374, 128735. [Google Scholar] [CrossRef]
- Contreras-Davila, C.A.; Carrion, V.J.; Vonk, V.R.; Buisman, C.N.J.; Strik, D. Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Res. 2020, 169, 115215. [Google Scholar] [CrossRef]
- Andersen, S.J.; Candry, P.; Basadre, T.; Khor, W.C.; Roume, H.; Hernandez-Sanabria, E.; Coma, M.; Rabaey, K. Electrolytic extraction drives volatile fatty acid chain elongation through lactic acid and replaces chemical pH control in thin stillage fermentation. Biotechnol. Biofuels 2015, 8, 221. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, H.; Wu, W.; Ali Bacar, M.; Wang, Q.; Gao, M.; Wu, C.; Xia, C.; Qian, D.; Chong, W.W.F.; et al. Product spectrum analysis and microbial insights of medium-chain fatty acids production from waste biomass during liquor fermentation process: Effects of substrate concentrations and fermentation modes. Bioresour. Technol. 2023, 368, 128375. [Google Scholar] [CrossRef]
- Ma, H.; Wu, W.; Yu, Z.; Zhao, J.; Fu, P.; Xia, C.; Lam, S.S.; Wang, Q.; Gao, M. Medium-chain fatty acid production from Chinese liquor brewing yellow water by electrofermentation: Division of fermentation process and segmented electrical stimulation. Bioresour. Technol. 2022, 360, 127510. [Google Scholar] [CrossRef]
- Khor, W.C.; Andersen, S.; Vervaeren, H.; Rabaey, K. Electricity-assisted production of caproic acid from grass. Biotechnol. Biofuels 2017, 10, 180. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-phased conversion of acid whey waste into medium-chain carboxylic acids via lactic acid: No external e-donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef]
- Wu, S.L.; Sun, J.; Chen, X.; Wei, W.; Song, L.; Dai, X.; Ni, B.J. Unveiling the mechanisms of medium-chain fatty acid production from waste activated sludge alkaline fermentation liquor through physiological, thermodynamic and metagenomic investigations. Water Res. 2020, 169, 115218. [Google Scholar] [CrossRef]
- Sarria, S.; Kruyer, N.S.; Peralta-Yahya, P. Microbial synthesis of medium-chain chemicals from renewables. Nat. Biotechnol. 2017, 35, 1158–1166. [Google Scholar] [CrossRef]
- Candry, P.; Ganigue, R. Chain elongators, friends, and foes. Curr. Opin. Biotechnol. 2021, 67, 99–110. [Google Scholar] [CrossRef]
- de Leeuw, K.D.; Buisman, C.J.N.; Strik, D. Branched medium chain fatty acids: Iso-caproate formation from iso-butyrate broadens the product spectrum for microbial chain elongation. Environ. Sci. Technol. 2019, 53, 7704–7713. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.; Plugge, C.M.; Strik, D.P.; Grootscholten, T.I.; Buisman, C.J.; Hamelers, H.V. Chain elongation with reactor microbiomes: Open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, Y.; Chen, Y.; Liu, M.; Bao, X.; Guo, W. Opportunities and challenges in microbial medium chain fatty acids production from waste biomass. Bioresour. Technol. 2021, 340, 125633. [Google Scholar] [CrossRef]
- Lü, F.; Wang, Z.; Zhang, H.; Shao, L.; He, P. Anaerobic digestion of organic waste: Recovery of value-added and inhibitory compounds from liquid fraction of digestate. Bioresour. Technol. 2021, 333, 125196. [Google Scholar] [CrossRef]
- Shi, X.; Wu, L.; Wei, W.; Ni, B.-J. Insights into the microbiomes for medium-chain carboxylic acids production from biowastes through chain elongation. Crit. Rev. Env. Sci. Tec. 2022, 52, 3787–3812. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.H.; Pandey, A. Dark fermentation: Production and utilization of volatile fatty acid from different wastes-A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef]
- Xu, J.; Guzman, J.J.; Andersen, S.J.; Rabaey, K.; Angenent, L.T. In-line and selective phase separation of medium-chain carboxylic acids using membrane electrolysis. Chem. Commun. 2015, 51, 6847–6850. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Grootscholten, T.I.M.; Steinbusch, K.J.J.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Bioelectrochemical production of caproate and caprylate from acetate by mixed cultures. ACS Sustain. Chem. Eng. 2013, 1, 513–518. [Google Scholar] [CrossRef]
- Wu, P.; Liu, H.; Li, J.; Ding, P.; Zhang, C.; Zhang, J.; Jiang, Q.; Zhang, Y.; Cu, M.-h.; Xu, J.-j. Acetate-to-bioproducts by chain elongation microbiome catalysis under applied voltage regulation. Energy Convers. Manag. 2021, 248, 114804. [Google Scholar] [CrossRef]
- Jiang, Y.; Chu, N.; Zhang, W.; Zhang, L.; Jianxiong Zeng, R. Electrofermentation regulates mixed culture chain elongation with fresh and acclimated cathode. Energy Convers. Manag. 2020, 204, 112285. [Google Scholar] [CrossRef]
- Virdis, B.; Hoelzle, R.D.; Marchetti, A.; Boto, S.T.; Rosenbaum, M.A.; Blasco-Gomez, R.; Puig, S.; Freguia, S.; Villano, M. Electrofermentation: Sustainable bioproductions steered by electricity. Biotechnol. Adv. 2022, 59, 107950. [Google Scholar] [CrossRef]
- Raes, S.M.T.; Jourdin, L.; Buisman, C.J.N.; Strik, D.P. Bioelectrochemical chain elongation of short-chain fatty acids creates steering opportunities for selective formation of n-butyrate, n-valerate or n-caproate. ChemistrySelect 2020, 5, 9127–9133. [Google Scholar] [CrossRef]
- Carvajal-Arroyo, J.M.; Andersen, S.J.; Ganigué, R.; Rozendal, R.A.; Angenent, L.T.; Rabaey, K. Production and extraction of medium chain carboxylic acids at a semi-pilot scale. Chem. Eng. J. 2021, 416, 127886. [Google Scholar] [CrossRef]
- Raes, S.M.T.; Jourdin, L.; Buisman, C.J.N.; Strik, D.P. Continuous long-term bioelectrochemical chain elongation to butyrate. ChemElectroChem 2017, 4, 386–395. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Q.; Chu, N.; Zeng, R.J.; Jiang, Y. Deciphering mixotrophic microbial electrosynthesis with shifting product spectrum by genome-centric metagenomics. Chem. Eng. J. 2023, 451, 139010. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Wu, P.; Li, J.; Zhang, J. Clostridium kluyveri enhances caproate production by synergistically cooperating with acetogens in mixed microbial community of electrofermentation system. Bioresour. Technol. 2023, 369, 128436. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wu, P.; Zhang, C.; Zhang, J. Quorum sensing signals stimulate biofilm formation and its electroactivity for chain elongation: System performance and underlying mechanisms. Sci. Total Environ. 2023, 859, 160192. [Google Scholar] [CrossRef]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A comprehensive understanding of electro-fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.-H.; Song, Y.-C.; Kim, S.-H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2021, 323, 124598. [Google Scholar] [CrossRef]
- Izadi, P.; Fontmorin, J.-M.; Virdis, B.; Head, I.M.; Eileen, H.Y. The effect of the polarised cathode, formate and ethanol on chain elongation of acetate in microbial electrosynthesis. Appl. Energy 2021, 283, 116310. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Li, L.; Shi, Z.; Ke, S.; He, Q. Granular activated carbon stimulated caproate production through chain elongation in fluidized cathode electrofermentation systems. J. Clean. Prod. 2022, 365, 132757. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, X.; Cai, W.; Cui, K.; Patil, S.A.; Guo, K. Recent progress on microbial electrosynthesis reactor designs and strategies to enhance the reactor performance. Biochem. Eng. J. 2023, 190, 108745. [Google Scholar] [CrossRef]
- Roy, S.; Schievano, A.; Pant, D. Electro-stimulated microbial factory for value added product synthesis. Bioresour. Technol. 2016, 213, 129–139. [Google Scholar] [CrossRef]
- Li, Z.; Cai, J.; Gao, Y.; Zhang, L.; Liang, Q.; Hao, W.; Jiang, Y.; Zeng, R.J. Efficient production of medium chain fatty acids in microbial electrosynthesis with simultaneous bio-utilization of carbon dioxide and ethanol. Bioresour. Technol. 2022, 352, 127101. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, X.; Li, M.; Han, L.; Liu, X.; Zhang, F.; Hou, X. A three chamber bioelectrochemical system appropriate for in-situ remediation of nitrate-contaminated groundwater and its reaction mechanisms. Water Res. 2019, 158, 401–410. [Google Scholar] [CrossRef]
- Vasudevan, D.; Richter, H.; Angenent, L.T. Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes. Bioresour. Technol. 2014, 151, 378–382. [Google Scholar] [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Hoelzle, R.D.; Virdis, B.; Batstone, D.J. Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol. Bioeng. 2014, 111, 2139–2154. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.; Angenent, L.T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, Z.; Varrone, C.; Zhou, A.; He, Z.; Li, H.; Zhang, J.; Liu, W.; Yue, X. Elucidating the microbial ecological mechanisms on the electrofermentation of caproate production from acetate via ethanol-driven chain elongation. Environ. Res. 2022, 203, 111875. [Google Scholar] [CrossRef]
- Dessi, P.; Sanchez, C.; Mills, S.; Cocco, F.G.; Isipato, M.; Ijaz, U.Z.; Collins, G.; Lens, P.N.L. Carboxylic acids production and electrosynthetic microbial community evolution under different CO2 feeding regimens. Bioelectrochemistry 2021, 137, 107686. [Google Scholar] [CrossRef]
- Sharma, M.; Aryal, N.; Sarma, P.M.; Vanbroekhoven, K.; Lal, B.; Benetton, X.D.; Pant, D. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer using a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem. Commun. 2013, 49, 6495–6497. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Schiel-Bengelsdorf, B.; Durre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.; Sang, B.I. Metabolic cascade of complex organic waste to medium-chain carboxylic acids: A review on the state-of-the-art multi-omics analysis for anaerobic chain elongation pathways. Bioresour. Technol. 2022, 344, 126211. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Zuo, J.; Hu, J. Production of n-caproate using food waste through thermophilic fermentation without addition of external electron donors. Bioresour. Technol. 2022, 343, 126144. [Google Scholar] [CrossRef]
- Chan, D.I.; Vogel, H.J. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem. J. 2010, 430, 1–19. [Google Scholar] [CrossRef]
- Han, W.; He, P.; Shao, L.; Lu, F. Metabolic interactions of a chain elongation microbiome. Appl. Environ. Microbiol. 2018, 84, e01614–e01618. [Google Scholar] [CrossRef]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Candry, P.; Radic, L.; Favere, J.; Carvajal-Arroyo, J.M.; Rabaey, K.; Ganigue, R. Mildly acidic pH selects for chain elongation to caproic acid over alternative pathways during lactic acid fermentation. Water Res. 2020, 186, 116396. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Chen, C.; Yin, Y.; Zhao, G.; Chen, Y. Physiological responses of Methanosarcina barkeri under ammonia stress at the molecular level: The unignorable lipid reprogramming. Environ. Sci. Technol. 2023, 57, 3917–3929. [Google Scholar] [CrossRef]
- Mukherjee, T.; Venkata Mohan, S. Metabolic flux of Bacillus subtilis under poised potential in electrofermentation system: Gene expression vs product formation. Bioresour. Technol. 2021, 342, 125854. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by nitrogen and phosphorus supplementation. Bioresour. Technol. 2021, 340, 125622. [Google Scholar] [CrossRef]
- Yu, D.; Cheng, S.; Cao, F.; Varrone, C.; He, Z.; Liu, W.; Yue, X.; Zhou, A. Unveiling the bioelectrocatalyzing behaviors and microbial ecological mechanisms behind caproate production without exogenous electron donor. Environ. Res. 2022, 215, 114077. [Google Scholar] [CrossRef]
- Liu, P.; Liang, P.; Jiang, Y.; Hao, W.; Miao, B.; Wang, D.; Huang, X. Stimulated electron transfer inside electroactive biofilm by magnetite for increased performance microbial fuel cell. Appl. Energy 2018, 216, 382–388. [Google Scholar] [CrossRef]
- Schievano, A.; Sciarria, T.P.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-fermentation–merging electrochemistry with fermentation in industrial applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Chen, Y.; Zheng, G.; Chen, Y. Source separation, transportation, pretreatment, and valorization of municipal solid waste: A critical review. Environ. Dev. Sustain. 2022, 24, 11471–11513. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, D.; Zhu, T.; Zhang, Y.; Horn, H.; Liu, Y. Ferrate pretreatment-anaerobic fermentation enhances medium-chain fatty acids production from waste activated sludge: Performance and mechanisms. Water Res. 2023, 229, 119457. [Google Scholar] [CrossRef]
- Ma, H.; Lin, Y.; Jin, Y.; Gao, M.; Li, H.; Wang, Q.; Ge, S.; Cai, L.; Huang, Z.; Van Le, Q.; et al. Effect of ultrasonic pretreatment on chain elongation of saccharified residue from food waste by anaerobic fermentation. Environ. Pollut. 2021, 268, 115936. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Huang, H.; Qin, Z.; Liu, C.; Chen, Y. Amino acid configuration affects volatile fatty acid production during proteinaceous waste valorization: Chemotaxis, quorum sensing, and metabolism. Environ. Sci. Technol. 2022, 56, 8702–8711. [Google Scholar] [CrossRef]
- Urban, C.; Xu, J.; Sträuber, H.; dos Santos Dantas, T.R.; Mühlenberg, J.; Härtig, C.; Angenent, L.T.; Harnisch, F. Production of drop-in fuels from biomass at high selectivity by combined microbial and electrochemical conversion. Energy Environ. Sci. 2017, 10, 2231–2244. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Kerkhoven, E.J.; Nielsen, J. Barriers and opportunities in bio-based production of hydrocarbons. Nat. Energy 2018, 3, 925–935. [Google Scholar] [CrossRef]

| Feedstock | Electron Donor | Inoculum Source | Dominant Microbes | Maximum MCCAs Concentration/Productivity | Electrochemical Conditions | pH | Temperature (°C) | Reference |
|---|---|---|---|---|---|---|---|---|
| Acetic acid | Cathode; H2 and ethanol generated in situ | Microbes from a continuously operating anaerobic fixed film reactor for C4–C8 fatty acids production | Clostridium kluyveri | 0.19 g/(L·d) | −0.9 V | 6 | 30 | [37] |
| Acetate, butyrate | Cathode; H2 and ethanol generated in situ | Acclimated microbes from CE reactors | Clostridia, Oscillibacter, and Caproiciproducens | 7.8 ± 0.4 mM | −0.95 V | 5 | 30 | [50] |
| Acetic acid, butyric acid | Cathode; H2 and ethanol generated in situ | A mixed culture of sulfate reducers | Curvibacter, Deltaproteobacteria, Desulfovibrionales, Desulfobacteraceae, and Syntrophobacteraceae | 0.02 g/L | 160–210 Am2 | 7.4 | 18–22 | [61] |
| Acetate, ethanol | Cathode; H2 and ethanol generated in situ | Enriched caproate-producing microbial consortium | Clostridium_sensu_stricto, Acetabacterium | 7454 mg COD/L | −0.8 V | 6.0 | 22 ± 2 | [59] |
| Acetate, ethanol | Cathode; ethanol; H2 and ethanol generated in situ | Enriched microbes from CE reactor | Clostridium_sensu_stricto_12, Dysgonomonas, and Acetoanaerobium | 3.40 g/(L·d) | −1.1 V | 7.2 (Initial pH) | 30 | [39] |
| Acetate, ethanol | Cathode; ethanol; H2 and ethanol generated in situ | Acclimated sludge from CE reactors | Clostridia, Actinobacteria, and Negativicutes | 4.3 g/L | −0.7 V | 7.0 (Initial pH) | 36 ± 1 | [46] |
| Acetate, ethanol | Cathode; ethanol; H2 and ethanol generated in situ | Acclimated CE mixed culture | Clostridium_sensu_stricto_12, Desulfovibrio, Clostridium_sensu_stricto_13 | 2.45 g/L | −0.7 V | / | / | [45] |
| Acetate, ethanol | Cathode; ethanol; H2 and ethanol generated in situ | Acclimated CE mixed culture + Clostridium kluyveri | Clostridium_sensu_stricto_12, Desulfovibrio, Clostridium_sensu_stricto_13 | 4.68 g/L | −0.7 V | / | / | [45] |
| Thin stillage | Cathode; ethanol; lactate; H2 and ethanol generated in situ | Thin stillage | Megasphaera elsdenii, Lactobacillus spp. | 1.144 ± 0.275 g COD/(L·d) | 100 mA | 5.4–5.7 | 35 | [22] |
| Liquor wastewater | Cathode; ethanol; lactate; H2 and ethanol generated in situ | Domesticated pit mud | Rummeliibacillus, Clostridium_sensu_stricto 12, and Caproiciproducens | 4.04 g/L | −1.0 V | 6.5 (Initial pH) | 35 ± 2 | [23] |
| Yellow water from a winery | Cathode; ethanol; lactate; H2 and ethanol generated in situ | Domesticated shallow pit mud | Clostridium and Caproiciproducens | 2.14 g/L | −0.8 V | 6.5 | 35 | [24] |
| Substrate | Substrate Concentration | Electron Donor | Applied Voltage/Current | Voltage Supply Mode | Cathode Type | MCCAs Concentration or Productivity | Carbon/Electron Conversion or Recovery Rate (%) | MCFAs Selectivity (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Substrate modulation—composition regulation of the electron acceptor | |||||||||
| Acetic acid | 100 mM acetic acid | Cathode; H2 and ethanol generated in situ | −0.9 V | Continuous | Graphite felt | 739 mg/L caproate, 36 mg/L caprylate | Carbon recovery: 31%, electron recovery: 45% | 26% | [37] |
| Acetic acid, n-butyrate | 30 mM acetic acid, 30 mM n-butyrate | Cathode; H2 and ethanol generated in situ | 29 mA | Continuous | Graphite felt | 0.3 g/L caproate | / | 83.4% | [41] |
| Acetate, CO2 | 10 mM acetic acid, 1 L/h CO2 gas | Cathode; H2, ethanol, and lactate generated in situ | 15 mA | Continuous | Graphite | 0.07 ± 0.04 g/(L·d) | Electron recovery: 58.9 ± 9.8% | / | [43] |
| Substrate modulation—tuning the ratio of electron donor to electron acceptor | |||||||||
| Acetate, ethanol | 25 mM acetate | Cathode; H2 and ethanol generated in situ | −0.8 V | Continuous | Carbon cloth coated with Pt/C catalyst | 501 mg COD/L caproate | Carbon recovery: 51.4% | About 23% | [59] |
| 25 mM acetate, 37.5 mM ethanol | 3016 mg COD/L caproate | Carbon recovery: about 92% | About 76% | ||||||
| 25 mM acetate, 75 mM ethanol | 7454 mg COD/L caproate | Carbon recovery: 98.6% | About 88% | ||||||
| Substrate modulation—altering substrate concentration | |||||||||
| Thin stillage | 0.14 gC/(L·d) acetate, 0.1 gC/(L·d) ethanol | Cathode; ethanol; lactate; H2 and ethanol generated in situ | 100 mA | Continuous | 0.057 g COD/(L·d) caproate | Carbon conversion: 60 ± 2% | 3% | [22] | |
| 0.28 gC/(L·d) acetate, 0.1 gC/(L·d) ethanol | Cathode; ethanol; lactate; H2 and ethanol generated in situ | 100 mA | Continuous | 1.144 g COD/(L·d) caproate, 0.104 g COD/(L·d) heptanoate | Carbon conversion: 60 ± 11% | 12% | |||
| Glucose, CO2 | 0 g/L glucose, 4.2 g/L NaHCO3 | Cathode; H2 and ethanol generated in situ | −1.0 V | Continuous | Pre-enriched acetogens on the cathode carbon felt. | / | Electron recovery: 27.2% | 0% | [44] |
| 0.1 g/L glucose, 4.2 g/L NaHCO3 | / | Electron recovery: 23.5% | 0% | ||||||
| 0.2 g/L glucose, 4.2 g/L NaHCO3 | 0.37 ± 0.07 g/L caproate | Electron recovery: 23.1% | 12.8 ± 1.5% | ||||||
| Tuning applied voltage/current | |||||||||
| Acetate | 8.34 g/L acetate | Cathode; H2 and ethanol generated in situ | 0 | Continuous | Carbon felts | Not detected | / | / | [38] |
| −0.6 V | 0.12 g/(L·d) caproate | Electron recovery: 84% | / | ||||||
| −1.2 V | Not detected | / | / | ||||||
| −1.8 V | Not detected | Electron recovery: 22% | / | ||||||
| −2.5 V | Not detected | Electron recovery: 20% | / | ||||||
| Ethanol, CO2,acetate | 100 mM ethanol, 25 mM acetate, 4.2 g/L NaHCO3 | Cathode; ethanol; H2 and ethanol generated in situ | −0.8 V | Continuous | Acclimated cathode | 2.4 g/L caproate approximately | / | About 41% | [39] |
| 200 mM ethanol, 50 mM acetate, 4.2 g/L NaHCO3 | −0.8 V | Continuous | 7.3 g/L caproate approximately | / | About 73% | ||||
| 100 mM ethanol, 25 mM acetate, 4.2 g/L NaHCO3 | −1.1 V | Continuous | 1.3 g/L caproate approximately | / | About 20% | ||||
| 200 mM ethanol, 50 mM acetate, 4.2 g/L NaHCO3 | −1.1 V | Continuous | 4.2 g/L caproate approximately | / | About 45% | ||||
| Acetate, CO2 | 10 mM acetic acid, 1 L/h CO2 gas | Cathode; H2, ethanol and lactate generated in situ | 5 mA | Continuous | Fresh cathode: graphite | 0 | Electron recovery: 73.7 ± 12.6% | / | [43] |
| 15 mA | 0.07 ± 0.04 g/(L·d) | Electron recovery: 58.9 ± 9.8% | / | ||||||
| Chinese liquor wastewater | 11.90 ± 0.14 g/L ethanol, 13.21 ± 0.11 g/L lactate, 8 g/L acetate, 0.08 ± 0.03 g/L propionate, 0.74 ± 0.13 g/L butyrate, 0.98 ± 0.04 g/L caproate | Cathode; ethanol; lactate; H2 and ethanol generated in situ | −0.8 V | Electrical stimulation in first half (0–6 d) | / | 1.47 g/L caproate | / | / | [24] |
| Electrical stimulation in first half (6–12 d) | 2.14 g/L caproate | / | / | ||||||
| Continuous electrical stimulation (0–12 d) | 1.93 g/L caproate | / | / | ||||||
| Without electricity | 0.55 g/L caproate | / | / | ||||||
| Electrode acclimation and cathode material optimation | |||||||||
| Ethanol, CO2, acetate | 100 mM ethanol, 50 mM acetate, 4.2 g/L NaHCO3 | Cathode; ethanol; H2 and ethanol generated in situ | −0.8 V | Continuous | Fresh cathode: carbon felt | 2.0 g/L caproate approximately | Electron recovery: 101.28% ± 7.88% | 36.16 ± 1.67% | [39] |
| 100 mM ethanol, 50 mM acetate, 4.2 g/L NaHCO3 | −1.1 V | Continuous | 1.8 g/L caproate approximately | Electron recovery: 93.45% ± 0.11% | 36.22 ± 1.89% | ||||
| 100 mM ethanol, 25 mM acetate, 4.2 g/L NaHCO3 | −0.8 V | Continuous | Acclimated cathode | 2.4 g/L caproate approximately | / | About 41% | |||
| 200 mM ethanol, 50 mM acetate, 4.2 g/L NaHCO3 | −0.8 V | Continuous | 7.3 g/L caproate approximately | / | About 74% | ||||
| 300 mM ethanol, 75 mM acetate, 4.2 g/L NaHCO3 | −1.1 V | Continuous | 6.0 g/L caproate approximately | / | About 38% | ||||
| Acetate, butyrate | 5.0 g/L acetate, 50 mmol/L butyrate | Cathode; H2 and ethanol generated in situ | −0.95 V | Continuous | Fresh fluidized cathode: carbon felt | 3.4 ± 0.2 mM caproate | Carbon recovery: about 39%; electron recovery: about 35% | / | [50] |
| Fresh fluidized cathode: carbon felt with 8% filling ratio granular activated carbon | 7.8 ± 0.4 mM caproate | Carbon recovery: 69.3 ± 0.5%; electron recovery: 61.1% ± 1.9% | / | ||||||
| Glucose, CO2 | 0.2 g/L glucose, 4.2 g/L NaHCO3 | Cathode; H2 and ethanol generated in situ | −1.0 V | Continuous | Fresh cathode without acetogens pre-enrichment | Not detected | Electron recovery: 70.3% | / | [44] |
| Pre-enrichment of acetogens on the cathode carbon felt | 0.37 ± 0.07 g/L caproate | Electron recovery: 23.1% | 12.8 ± 1.5% | ||||||
| Microbial cooperation and stimulation | |||||||||
| Ethanol, acetate | 6 g/L ethanol, 2.0 g/L sodium acetate | Cathode; ethanol; H2 and ethanol generated in situ | −0.7 V | Continuous | Acclimated cathode carbon felts | 2.45 g/L caproate | Carbon conversion: 83.37% | / | [45] |
| 4.68 g/L caproate(bioaugmentation by C. kluyveri) | Carbon conversion: 92.07% | / | |||||||
| Ethanol, acetate | 6 g/L ethanol, 2.73 g/L sodium acetate | Cathode; ethanol; H2 and ethanol generated in situ | −0.7 V | Continuous | Acclimated cathode carbon felts | 3.77 g/L caproate (with quorum sensing signals 10 μM C6-HSL) | / | / | [46] |
| 4.30 g/L caproate (10 μM C8-HSL) | / | / | |||||||
| 3.96 g/L caproate (10 μM 3OC10-HSL) | / | / | |||||||
| Glucose, CO2 | 0.2 g/L glucose, 4.2 g/L NaHCO3 | Cathode; ethanol and H2 generate in situ | −1.0 V | Continuous | Fresh cathode without acetogens pre-enrichment | Not detected | Electron recovery: 70.3% | / | [44] |
| Pre-enriched acetogens on the cathode carbon felt | 0.37 ± 0.07 g/L caproate | Electron recovery: 23.1% | 12.8 ± 1.5% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yin, Y.; Chen, C.; Zhang, X.; Zhou, J.; Zhang, Q.; Chen, Y. Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production. Energies 2023, 16, 2571. https://doi.org/10.3390/en16062571
Liu C, Yin Y, Chen C, Zhang X, Zhou J, Zhang Q, Chen Y. Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production. Energies. 2023; 16(6):2571. https://doi.org/10.3390/en16062571
Chicago/Turabian StyleLiu, Chao, Yue Yin, Chuang Chen, Xuemeng Zhang, Jing Zhou, Qingran Zhang, and Yinguang Chen. 2023. "Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production" Energies 16, no. 6: 2571. https://doi.org/10.3390/en16062571
APA StyleLiu, C., Yin, Y., Chen, C., Zhang, X., Zhou, J., Zhang, Q., & Chen, Y. (2023). Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production. Energies, 16(6), 2571. https://doi.org/10.3390/en16062571







