Abstract
Molten salt is an excellent medium for heat transfer and storage. The unique microstructure of carbon nanomaterials leads to good mechanical stability, low density, high thermal conductivity, and high strength, etc. The addition of carbon nanomaterials to molten salt to form molten salt nanofluid can remarkably enhance the specific heat capacity and thermal conductivity of molten salt and reduce the molten salt viscosity, which is of great importance to increase the heat storage density and reduce the heat storage cost. Nevertheless, some challenges remain in the study of such nanofluids. The main challenge is the dispersion stability of carbon nanomaterials. Therefore, to improve research on carbon nanofluids, this paper summarizes the progress of carbon-based molten salt nanofluid research worldwide including the preparation methods of molten salt nanofluids, the improvement of heat transfer performance, and the improvement of heat storage performance. The effects of carbon nanoparticle concentration, size, and type on the heat transfer and storage performance of molten salt are derived, and the effects of nanoparticle shape on the heat transfer performance of molten salt are analyzed while more promising preparation methods for carbon-based molten salt nanofluids are proposed. In addition, the future problems that need to be solved for high-temperature molten salt-based carbon nanofluids are briefly discussed.
1. Introduction
The direct conversion of traditional fossil energy sources into electricity and heat has become a major source of energy worldwide due to its low cost and stable output. However, traditional fossil energy reserves are limited, and their application has caused serious damage to the ecological environment. Therefore, the development of clean energy research has become the focus of global energy development and trends. The main sources of clean energy are solar, wind, and tidal energy, among which solar energy is considered the most ideal alternative to fossil energy because of its excellent properties. Solar power generation has two main types: photovoltaic and solar thermal. Photovoltaic power generation is a technology that uses solar panels to convert light energy directly into electricity but is not equipped with an energy storage system, generates unstable power, and largely affects the power grid [1,2,3,4,5,6,7]. Solar thermal power generation technology [8,9,10,11,12,13,14] refers to gathering solar energy and converting it into thermal energy through a thermal storage medium, and then transmitting the thermal energy to the power generation system to generate electricity, which is a green, clean renewable energy generation technology and is one of the most ideal ways to solve the energy problem in the future [15,16].
Heat transfer and storage technology is an important part of solar thermal power generation, where the key issue to be addressed is the heat transfer and storage medium. Common heat transfer storage media include water, water vapor, thermal oil, liquid metal, etc., but these media have low upper limit temperature, high pressure, short service life, and high price, which seriously affect the efficiency and cost of solar thermal power generation systems [17,18]. By contrast, molten salts with low melting point, low cost, large heat transfer temperature difference, good thermal stability, and other advantages such as solar thermal power generation, industrial chemical processing, waste heat utilization, and other aspects of the medium and high-temperature heat transfer storage medium has been widely used in some fields, and has been highly valued by engineering fields and scientific researchers [19]. At present, the commonly used molten salts in industry are mainly nitrates [20,21,22,23,24], chloride salts [25,26], carbonates [27,28], and fluoride salts [29,30], etc. However, carbonates are prone to decomposition at high temperatures, chloride salts are very corrosive and greatly increase pipeline maintenance costs, and although nitrates have the advantages of low price, low vapor pressure, and wide temperature range, their thermal conductivity is low. These shortcomings limit the development of molten salt heat storage and need to be further improved.
Molten salt nanofluid is a heat transfer storage fluid formed by adding nanoparticles to molten salt, which can substantially enhance the specific heat capacity (SHC) and thermal conductivity of molten salt, and reduce the molten salt viscosity, for increasing the heat storage density and reducing the heat storage cost. Nanomaterials have different properties compared with macroscopic materials, such as surface effects and quantum tunneling effects [31,32]. Since these effects occur at the nano level, nanomaterials have better thermophysical properties than conventional materials. Therefore, nanomaterials have a better effect of enhancing the thermal properties of molten salts compared with conventional materials. Nanoparticles can be broadly classified into different types: metallic, metal oxide, and non-metallic. Metal nanoparticles have nano surface effects and thermal properties similar to those of other nanomaterials, providing them thermophysical properties that conventional metallic materials do not possess [33,34,35,36]. However, metal nanoparticles are mostly precious metal materials, which are more expensive and corrosive, which is not conducive to the reduction of thermal storage costs. Regarding metal oxide nanoparticles [37,38,39], although the price of metal nanoparticles is relatively lower than that of metal oxide nanoparticles, the thermal conductivity is relatively low. Non-metallic nanoparticles are mainly divided into two categories: carbon nanomaterials and inorganic non-metallic materials. Although inorganic non-metallic materials have the advantage of low cost, their specific surface area and thermal conductivity are small, which is not conducive to strengthening the thermal storage performance of molten salts.
Compared with the nanoparticles above, carbon nanomaterials have a larger surface area, minimal erosion and corrosion, and a smaller density. In addition, graphene and carbon nanotubes (CNTs) have black surfaces and the ability to absorb sunlight [40]. The unique microstructure of carbon nanomaterials results in good mechanical stability, low density, high thermal conductivity, and high strength [41]. Table 1 shows taking advantage of their excellent thermal properties and compounding them with molten salts can substantially improve the specific heat or thermal conductivity of conventional materials to obtain molten salt nanofluids with high thermal storage or thermal conductivity [42,43,44,45]. Figure 1 summarizes the progress of research on carbon-based molten salt nanofluids worldwide in recent years including the preparation methods of molten salt nanofluids, the improvement of heat transfer properties, and the enhancement of heat storage properties, and proposes future problems to be solved for high-temperature molten salt-based carbon nanofluids.

Table 1.
Improvement of heat storage and heat transfer properties of nano carbon materials.
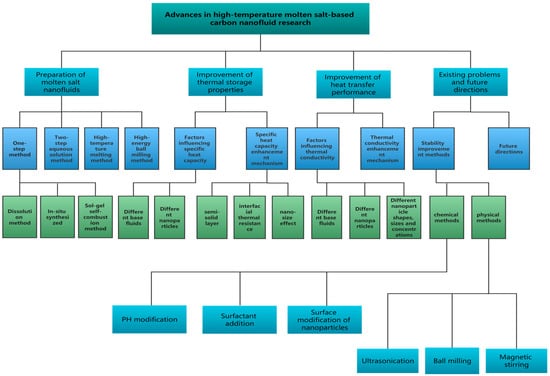
Figure 1.
Schematic of research progress in high temperature molten salt-based carbon nanofluids.
2. Preparation of Molten Salt Nanofluids
The physical properties of molten salt nanofluids differ considerably from those of suspensions composed of conventional-sized particles due to the microscale effect between the nanoparticles and the molten salt. Owing to the microscale effect, nanoparticles are poorly dispersed in molten salt and are prone to agglomeration, forming several larger agglomerates that affect the thermophysical properties of molten salt nanofluids. Therefore, how to improve the dispersion of nanoparticles in molten salts and enhance the stability of nanofluids has become the focus of research on the thermophysical properties and enhanced heat transfer of molten salt nanofluids. Currently, four main methods are used for the preparation of molten salt nanofluids: one-step method, two-step water-soluble method, high-temperature melting method, and high-energy ball milling method [57].
2.1. One-Step Method
The one-step method is a general term for a class of methods for preparing nanofluids that synchronizes nanoparticle preparation with nanoparticle dispersion in molten salts, which can mitigate the agglomeration effect of nanoparticles to some extent. Common one-step methods include dissolution, in situ synthesis, and sol–gel self-combustion [58]. Figure 2 shows the one-step preparation process.
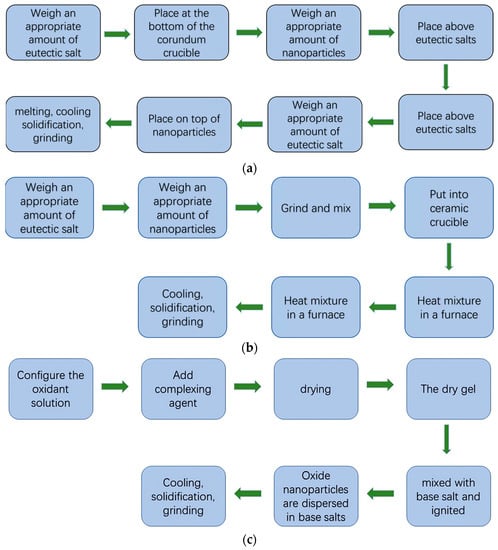
Figure 2.
One-step preparation of molten salt nanofluidic flow. (a) Dissolution method; (b) In situ synthesized; (c) Sol–gel self-combustion method.
In the dissolution method for the preparation of molten salt nanofluids, nanoparticles are dissolved or dispersed directly in a molten salt base solution by using a suitable molten salt as a solvent and adding the nanoparticles directly into the molten salt.
The dissolution method is a method in which a suitable molten salt is mixed directly with the nanoparticles so that the nanoparticles are directly dissolved and dispersed in the molten salt. The nanofluid is prepared by placing the eutectic salt at the bottom of a corundum crucible, laying the nanoparticles flat on top of the eutectic salt, and performing high-temperature melting under an argon atmosphere to avoid water absorption by the nanoparticles. Nanofluids prepared by the dissolution method have good stability [58], but this method has substantial limitations and requires attention to the matching of the melting point and decomposition temperature of the high-temperature molten salt with the nanoparticles; otherwise, it can easily cause agglomeration of nanoparticles and make the nanofluids less stable [57].
The in-situ synthesis method uses chemical reactions among different elements or compounds under certain conditions to generate nanoparticles and disperse them directly in the molten salt to prepare molten salt nanofluids [59]. The method uses nanoparticles as precursors and adjusts the temperature above the nanoparticle decomposition temperature and below the molten salt decomposition temperature so that the nanoparticles are decomposed and mixed directly in the molten salt base solution, and finally separated by centrifugation. The sample is obtained by washing. The advantages of this method are that the surface of the molten salt is free of contamination, the compatibility between nanoparticles and molten salt is good, and the pretreatment of the molten salt is eliminated, but the particle size distribution of the nanoparticles generated by this method is not uniform, which may make the specific heat distribution of the molten salt nanofluid uneven [58].
The sol–gel self-combustion method is a new preparation method that combines the traditional sol–gel method with the self-combustion method. The method starts with the selection of a suitable molten salt as the oxidant solution, the addition of a complexing agent to form a sol–gel solution, and drying to obtain a dry sol–gel, which is then mixed with the base salt and ignited. This method has high requirements for the selection of the complexing agent and oxidant [60], as the complexing agent acts as a fuel during the reaction, is the source of spontaneous combustion by the auto ignition method, and has many uncontrollable variables in the operation, which is very demanding for the operator and not conducive to industrial production.
These three methods are categorized as one-step preparation of nanofluids, which share the common feature of simultaneous nanoparticle preparation and dispersion, and equally have their own characteristics. In situ synthesis has the advantage that the nanoparticles are generated directly in the molten salt base solution, but the resulting nanoparticles have an uneven size distribution. The accuracy of the experimental results is not guaranteed.
In summary, nanofluid prepared by the one-step method has better dispersion and higher stability, but the one-step preparation is more complex, and the preparation involves many redox reactions, which are more demanding on the materials, and the carbon nanomaterials are prone to combustion and difficult to undergo redox reactions. Thus, preparing high-temperature molten salt-based carbon nanofluid by the one-step method is difficult.
2.2. Two-Step Aqueous Solution Method
In the two-step aqueous solution method, nanofluids are prepared by dispersing nanoparticles into deionized water by certain dispersion means (e.g., ultrasonic shock) and then evaporating the aqueous solution with an over heater. The preparation process of the two-part aqueous solution method is shown in Figure 3.
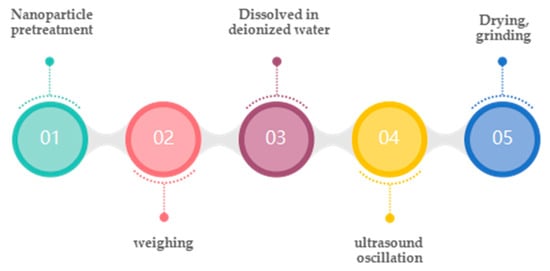
Figure 3.
Two-step preparation of molten salt nanofluidic flow [57].
Jo et al. [61,62,63] prepared molten salt nanofluids by adding multi-walled carbon nanotube (MWCNTs) nanoparticles of 10–30 nm in diameter and gum Arabic (GA) to binary carbonates (K2CO3-Li2CO3) by a two-step water solubility method to investigate the effect of the dispersant on the SHC (specific heat) of MWCNTs-enhanced molten salts and study the thermal stability of nanofluids. The dispersant itself did not improve the specific heat of molten salt, and the molten salt with only MWCNTs underwent evident agglomeration and insignificant specific heat enhancement. By contrast, the sample with dispersant had a maximum specific heat enhancement of 22%, which was apparent, and the CNTs were well dispersed. The dispersion of nanoparticles can considerably affect the increase of SHC of molten salt nanofluid.
2.3. High-Temperature Melting Method
The high-temperature melting method [64] is divided into two types: one-step and two-step. The one-step method directly mixes nanoparticles and inorganic salts at a specific ratio, places them in a muffle furnace, heats the mixed molten salts to the molten state, keeps them at a high temperature for a period, and then cools, grinds, and crushes them to obtain the sample, finally. The two-step method is based on the one-step method. It places the samples into the muffle furnace again, heats them to the molten state, keeps them at a high temperature for a period, and then cools, grinds, and crushes them. The sample is obtained, or the inorganic salt is heated to the molten state first. Then, the nanoparticles are mixed into the molten salt by mechanical stirring, and then cooled, ground, and crushed. Finally, the sample is obtained. The particles are fully dispersed. The particles are fully dispersed. The preparation process of high temperature melting method is shown in Figure 4.
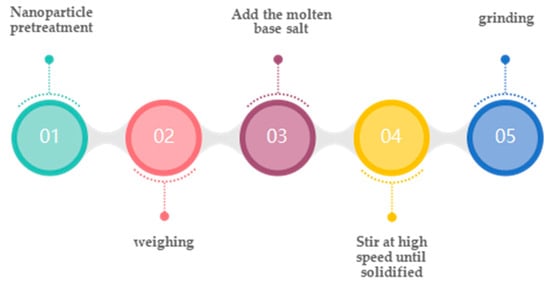
Figure 4.
High-temperature melting preparation of molten salt nanofluidic flow [57].
Wu et al. [49] successfully prepared molten salt nanofluids by mixing solar salt (NaNO3-KNO3) and MWCNT nanoparticles with 12–15 nm particle size using the high-temperature melting method. The composite molten salt nanomaterials were subjected to thermal stability cycling experiments at 300 °C, 400 °C, and 500 °C. After seven days of thermal stability cycling, the weight losses of the samples were 0.6%, 1%, and 2%, which were within 2%.
2.4. High-Energy Ball Milling Method
The basic principle of the mechanochemical method is to prepare new materials by using mechanical energy to induce chemical reactions or induce changes in the material organization, structure, and properties, which is an important way to prepare ultrafine materials. The high-energy ball milling method uses ball milling equipment to mix the materials, specifically, molten salt and CNT (carbon nanotubes), in several grinding jars in a turn-table, transported by grinding balls, and rotating the jars around a rotating axis to mix the raw materials thoroughly, resulting in a molten salt composite. Figure 5 shows the High-energy ball milling preparation process.

Figure 5.
High-energy ball milling method preparation of molten salt nanofluidic flow.
Yuan et al. [51] prepared molten salt nanocomposites with different concentrations by adding MWCNTs nanoparticles to binary carbonates (K2CO3-Li2CO3) through a high-energy ball milling method, investigated the variation of thermal conductivity of the composites at different concentrations, and introduced a new theoretical calculation method of thermal conductivity. When the mass fraction of CNT was between 0.1% and 1.75%, the effective thermal conductivity of the composite molten salts increased with the addition of CNT, and the maximum enhancement reached 50.72%. When the mass fraction of CNT exceeded 1.75%, the effective thermal conductivity seriously decreased, and a serious agglomeration occurred when it reached 2.5%.
In summary, the two-step aqueous solution method, the high-temperature melt method, and the high-energy ball milling method can all be used to prepare high-temperature molten salt-based carbon nanofluids. However, although the molten salt nanofluids prepared by the two-step water-solution method are uniformly dispersed, they are less stable and must be formed into stable suspensions with the help of corresponding dispersants. However, the dispersants are mostly organic materials, which have difficulty withstanding high temperatures above 200 °C. They can only be tried for the development of low-temperature molten salt nanofluids, and the two-step preparation requires immense amounts of deionized water and high-power consumption of ultrasonic equipment, which restricts their industrial applications. The high-energy ball milling method can obtain nano powders of pure elemental composites, but problems such as low purity of the prepared composites and uneven particle size distribution are observed. The nanofluid prepared by the high-temperature melting method has better thermal stability compared with the two-step method and better purity and particle size distribution than the high-energy ball milling method, which is currently a good method for preparing nanofluids.
3. Improvement of Thermal Storage Properties
The higher the thermal storage capacity is, the greater the amount of solar energy that can be absorbed, thus increasing the efficiency of power generation. Table 2 shows the addition of carbon nanomaterials can remarkably increase the thermal storage performance of molten salts, which plays a key role in enhancing the thermal storage capacity and heat transfer efficiency of the system, improving the stability of the heat exchange system and reducing the cost of heat transfer and storage.

Table 2.
Improvement of heat storage performance of carbon nanomaterials.
3.1. Specific Heat Capacity of Molten Salt-Based Carbon Nanomaterials
The SHC enhancement of molten salt nanofluids has been an important research area of interest to scholars, but no uniform conclusion has been made on the factors affecting the SHC enhancement of molten salt nanofluids. Yan et al. [46] prepared different concentrations of molten salt nanofluids by physically blending BNNSs (hexagonal boron nitride is also known as white graphene) with different mass fractions into solar salts as the base salt, and investigated the changes of SHC and thermal stability of nanofluids at different concentrations. The introduction of BNNSs into the nitrate eutectic led to an enhanced SHC, with a maximum increase in SHC intensity of 29.8% for a white graphene nanoparticle (GNP) mass fraction of 1.5% in the nanofluid compared with pure eutectic salt but a decrease in SHC of 7.2% when the nanoparticle mass fraction was 2.0%, a phenomenon attributed to the agglomeration of the nanoparticles, after 20 cycles of hot and cold shock. The SHC of the nanofluid did not decrease considerably and showed good thermal stability. Hamdy et al. [71] synthesized molten salt nanocomposites by adding different weight fractions of MWCNTs to solar salt. The experimental results showed the addition of 0.1, 0.5, and 1.0 wt% concentrations of MWCNTs increased the SHC of molten salt by 36.11%, 33.30%, and 10.11%, respectively. Xiao et al. [47] prepared molten salt composites by dispersing expanded graphite into solar salt via the ultrasonic aqueous solution method. When the nanoparticle doping amount was 2%, the SHC of the molten salt was enhanced by 110% and the nanoparticles were well dispersed. The above experimental results show different nanoparticles have dissimilar degrees of SHC enhancement for molten salts. Different scholars added the same nanoparticles to the same base salt, but the SHC enhancement was also different. Wu et al. [67] prepared MWCNTs nanocomposites with different concentrations using chloride salt (KCl-MgCl2) as the base salt. The increase in SHC was greatest when the nanoparticle concentration was 1%, and the specific heat of the molten salt increased by nearly 100% in the high-temperature working section of 370–420 °C. Moreover, the specific heat increased with the increase in nanofluid temperature. Wang et al. [68] prepared nanocomposites by adding 1 wt% of MWCNTs to chlorinated salt (KCl-MgCl2). The enhancement of the specific heat of the chloride salt by CNT was small, with a maximum specific heat enhancement of 9.2%. This result may be due to differences in the materials and manipulation practices used in the authors’ experiments.
As carbon nanomaterials are poorly dispersed, improving the dispersion of nanoparticles has an important influence on the SHC of nanoparticle-enhanced molten salts. The addition of dispersants is an important method to enhance the dispersibility of nanoparticles. Kim et al. [69] prepared graphite composites using binary carbonates (Li2CO3-K2CO3) as the base salt and investigated the SHC of the nanocomposites under different concentrations of nanoparticles and different concentrations of GA (Gum Arabic). GA would decompose between 250 °C and 350 °C with minimal or no effect or even a negative effect on the specific heat of the molten salt. Figure 6 (Data from Ref. [69]) shows when the GA addition was 1wt%, the SHC of the base salt gradually increases with the increase of nanoparticle concentration, but the enhancement is limited, and the maximum enhancement does not exceed 30%; when the GA addition was 5wt%, the SHC of the base salt is almost twice that of the pure molten salt; and when the nanoparticle concentration exceeded 0.1 wt%, the SHC almost no longer changed. Thus, the dispersion uniformity of nanoparticles is beneficial to the enhancement of the SHC of the molten salt. However, with the addition of too many nanoparticles, the increase in specific heat of the molten salt is very insignificant, but it will instead cause agglomeration of nanoparticles. Shin et al. [70] used a two-step aqueous solution method to prepare high-temperature nanofluids by dispersing MWCNTs nanoparticles with mass concentrations of 0.05%, 0.1%, and 1.0% into Li2CO3 and K2CO3 (62:38 molar ratio) eutectic salts. To reduce nanoparticle agglomeration, the experiments used sodium dodecyl sulfate as surfactant and a syringe pump device to evaporate water and dispense the mixed solution dropwise into the beaker, which greatly reduced nanoparticle agglomeration and improved nanoparticle dispersion compared with boiling the nanofluid at high temperature. The specific heat of the nanofluids all increased compared with pure carbonates, with a maximum increase of 17% when the MWCNTs concentration was 1%, and no optimum concentration was found, which is expected to be more than 1%. Dispersants can improve the dispersion of carbon nanomaterials and therefore the degree to which carbon nanoparticles enhance the SHC of the molten salt. The following are methods to improve the dispersibility of nanomaterials.
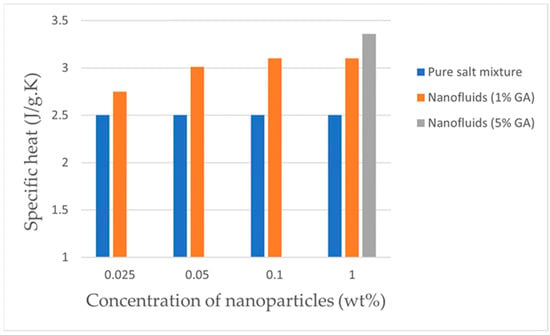
Figure 6.
Specific heat of nanofluids in liquid phases with respect nanoparticle concentration [69].
In addition to adding dispersants, electrifying nanoparticles and using hybridized nanoparticles are effective methods for improving their dispersibility. Yuan et al. [72] used the double electric layer model and molecular dynamic (MD) simulations to focus on the effect of charged single-walled carbon nanotubes (SWCNTs) on the heat capacity of complex carbonate salts (Li2CO3-K2CO3). The Coulomb pairing energy was much larger than the van der Waals pairing energy in all cases where SWCNTs (single-walled carbon nanotubes) were charged, the electrostatic force was the dominant force, and an increase in SWCNTs charge hardly increased the van der Waals pairing energy, with a maximum increase in Coulomb energy of 21.28%, as a larger SWCNTs surface charge led to local enrichment of positive and negative charges. As the SWCNTs charge increased, the nanofluid Cp (specific heat capacity) increased monotonically with a maximum specific heat increase of 19.2%. This result suggests that the enrichment of positive and negative charges leads to a greater internal energy, which in turn leads to a greater SHC. Vaka et al. [55,56] prepared nanofluids by dispersing graphene–TiO2 hybrid nanoparticles into a five-membered mixed molten salt (NaNO3-KNO3-LiNO3-CaNO3-CsNO3) by ultrasonic dispersion. The maximum specific heat enhancement was 19.6% when the heterogeneous nanoparticles were added at 0.05%. After seven days of thermal cycling, the specific heat of the eutectic salt decreased by 28.94%, whereas the specific heat of the nanofluid decreased by only 2.88%. The nanofluid showed good thermal stability within the applicable temperature range, and the heterogeneous nanoparticles did not decompose. However, the authors did not explain the greater enhancement of the specific heat of the molten salt by the hybrid nanoparticles compared with that by the single nanoparticles, and the potential behavior of graphene hybridization in terms of energy storage needs to be explored further.
Derbenev et al. [73,74] used the Debye–Huckel equation to solve the electrostatic problem for two colloidal particles, providing a unified approach to understanding pairwise electrostatic interactions among spherical colloidal particles and a link to the complex arrangement of charged particles in colloidal systems and in the environment. Theory aims to solve the major problem of electrostatic interactions among spheres of charged media. The switching behavior between charge repulsion and attraction de-pends on the ratio of the long and short axes of the spheres. When the long and short axes are equal, theory yields a solution equivalent to that of a spherical particle. Nanoparticles come in many shapes other than spheres, and studying the interaction of spherical particles alone is not sufficient; studying the interaction of arbitrarily shaped particles is essential. Siryk et al. [75] considered the interaction of two arbitrarily shaped dielectric particles immersed in a solvent containing a dissociated salt and assumed the linearized Poisson–Boltzmann equation holds. This work extends the recent results for dielectric spheres to arbitrarily shaped particles and establishes for the first time a rigorous (to the Debye–Huckel level of accuracy) analytical theory of the electrostatic interaction of these particles at arbitrary distances. Numerical tests have confirmed the theory proposed by the authors has wide application prospects. Obolensky et al. [76] applied the exact Debye–Huckel level theory to quantify the errors inherent in DLVO and other errors associated with replacing multiparticle interactions with sums of pairwise interactions (even though the latter are calculated exactly). Asymmetric dielectric shielding, i.e., the enhanced repulsion among charged dielectrics immersed in a high-dielectric-constant medium, is demonstrated in the presence of free ions in the medium. This setup allows us to derive the electrostatic forces among particles more precisely and go about the equilibrium more accurately. Large-scale modeling has been a difficult task, and Denton et al. [77] presented an accurate, promising statistical mechanic theory for calculating effective electrostatic interactions in colloid–nanoparticle mixtures that is useful for large-scale modeling.
In addition to the two factors above, the difference in base salt is an important factor affecting the SHC enhancement of molten salt nanofluids. Wang [68] prepared nanocomposites by adding 1 wt% of MWCNTs to chloride salt (KCl-MgCl2) and ternary carbonate (Li2CO3-Na2CO3-K2CO3). The enhancement of specific heat for chloride salts by CNTs was small, with a maximum specific heat enhancement of 9.2%, in contrast to a larger enhancement for carbonates, with a maximum specific heat enhancement of 46.6% for the base salt in the temperature range of 520–560 °C and an average specific heat enhancement of 36.89% in the working temperature range of the nanofluid. However, this paper did not explain the principle of the different enhancement of the two salts by nanoparticles or the mechanism of the interaction between nanoparticles and molten salts. Xie et al. [65,66] prepared nanofluids by mixing GNPs and molten salt by ultrasonic dispersion using solar salt and Hitec salt as the base salt. The specific heat of the nanofluid was not directly proportional to the number of nanoparticles added to the solar salt. Figure 7 (Data from Ref. [65]) shows the specific heat enhancement rate was largest at 16.7% when the nanoparticles were doped at 1 wt%, whereas the specific heat value decreased instead at 0.1 wt% doping, and the reason for the change was not clear. The increase in specific heat was only 7.5% at 1 wt% nanoparticle doping in the Hitec molten salt, the increase in specific heat was not proportional to the amount of nanoparticle doping, and the conventional heat balance model was not applicable to explain this change in specific heat. Xiao et al. [78] prepared nanofluids by adding GNPs to mixed molten salts by a two-step aqueous solution method using solar and Hetic salts as base salts, and investigated the rheological behavior of the nanoparticles on the molten salts. The addition of graphene to Hetic and solar salts resulted in a remarkable increase in the viscosity of the nanofluids, which could be attributed to the larger size of the graphene. The increase in viscosity had a substantial effect on the enhancement of the thermal properties of the molten salt. Therefore, further systematic experiments need to be established to investigate the flow and heat transfer properties of graphene–molten salt nanofluid. Lee et al. [79] configured graphite–molten salt nanofluids with different concentrations using solar salt and binary carbonate (Li2CO3-K2CO3) as the base salt by a two-step aqueous solution method, and investigated the solvent composition and the effects of solvent composition and nanoparticle concentration on the specific heat of the molten salt. The carbonate-based graphite nanofluid, with increasing nanoparticle concentration, gradually increased the specific heat of the carbonate, with the maximum degree of increase reaching 23.5% when the graphite nanoparticle mass fraction was 1%. The specific heat of nitrate-based graphite nanofluids exhibited a behavior completely different from that of carbonate-based graphite nanofluids. The specific heat of the molten salt decreased when the graphite nanoparticle mass fraction was 0.1%, and as the graphite nanoparticle concentration increased, the decrease effect was gradually counteracted, even above the specific heat of the base salt. The authors speculated that the reduction in specific heat of nitrate-based graphite nanofluids was related to the content of NaNO3, but no relevant experiments have been set up to demonstrate it.
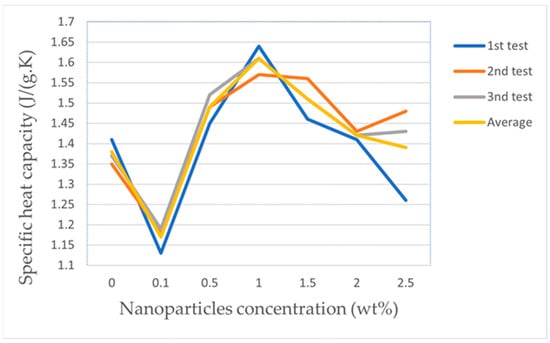
Figure 7.
SHC (Specific heat capacity) for molten salt and nanofluids with different GNP concentrations. [65].
In summary, the type of nanoparticle, the means of nanoparticle dispersion, and the type of base salt are all important factors in the enhancement of the SHC of nanofluids. However, these three factors are not the only ones that affect the SHC of nanofluids, as the experimental techniques and the environment of the test bench may lead to changes in the SHC of molten salt nanofluids.
3.2. Mechanism of Molten Salt Nanostructured Specific Heat Capacity Enhancement
Several scholars have proposed different explanations for the study of the mechanism of enhanced specific heat capacity of nanofluids.
There are possibilities for the development of nanodendritic structures as a mechanism for heat capacity enhancement. These nanostructures have enhanced heat capacity due to their increased surface area, but no definitive explanation has been given for the formation of such structures. These structures may be caused by microscopic segregation in the eutectic salt due to the varied levels of attraction among the different types of salt and nanoparticles, but this does not explain how these segregated salts develop into dendritic structures. Rizvi et al. [80] used binary mixed molten salts as a study object and proposed forming deviated nuclei on the surface of nanoparticles through heterogeneous nucleation and forming dendritic crystals through 1D grain growth. Nanoparticles generally have impurity groups carrying negative charges, whereas in binary mixed molten salts, each component is attracted to nanoparticles to different degrees. One salt is attracted and grows on the surface of nanoparticles, and then the salt forms a local separation and starts to repel other salts, lines into bias-shaped nuclei, and grows to form dendritic crystals. The dendritic crystals can be dissolved in water, proving that they are molten salts and not nanoparticles.
The semi-solid layer also becomes the compression layer. Semi-solid layer theory is one of the main mechanisms for the enhancement of the SHC of nanofluids. Tao et al. [81] prepared nanofluids by adding SWCNTs, MWCNTs, graphene, and fullerene to binary carbonates. For composites with the addition of SWCNTs and MWCNTs nanoparticles, the molten salt would form a crystalline layer on the surface of MWCNTs, which is known as a semi-solid layer, and it is the main reason for the enhancement of Cp. The formation of a mesh structure facilitated the formation of thermally conductive connections, thus substantially increasing the thermal conductivity. For graphene nanocomposites, it formed a thickness of 100–500 nm reticular structure, which had a large specific surface area, but did not contribute much to the effective thermal conductivity connection in its thickness direction. For fullerene nanocomposites, because of the spherical structure of its particles, which formed thicker nanolayers and larger particles on its surface, the reinforcing effect on the SHC was almost negligible.
Yuan et al. [82] investigated the thermal effects of charged SWCNTs with molten carbonates through MD simulations. The surface charge of SWCNTs affected the compressed ion layer around the SWCNTs, which in turn influenced the specific heat (Cp) enhancement of the nanofluid. Ions within the compressed ion layer were redistributed. In addition, changing the negative surface charge substantially affected the uptake of ions within the compressed ion layer. The surface charge of SWCNTs stimulated the enrichment of positive and negative charges, which in turn generated greater pairwise energy. Thus, enrichment of positive and negative charges should be the main cause of Cp enhancement. The results show that negatively charged SWCNTs have the best enhancement of Cp for carbonate salts, with an enhancement rate of up to 19.16%.
Jo et al. [83] investigated the mechanism of SHC enhancement by MD (molecular dynamics) simulations. The thickness and specific surface area of the compressed liquid layer on the nanoparticle surface had a considerable effect on the enhancement of SHC of molten salts, the compressed liquid layer changed the solvent distribution, and graphite nanoparticles were more attractive to K+, which also contributed to the increase in specific heat of molten salts. However, the authors have not explored which mechanisms lead to the enhancement of the solid phase SHC of nanomaterials, and further studies are needed.
The mechanisms proposed for the increase in SHC of molten salt nanofluids include the nanosize effect, semi-solid layer, and interfacial thermal resistance, but no consistent conclusion has been reached. Therefore, further in-depth studies are needed to reveal the influencing factors and mechanisms for the increase of SHC of molten salt nanofluids.
3.3. Microstructure of Nanofluids
The microstructure of nanofluids is a hot topic worldwide. By looking at the microstructure of nanofluids, the mechanisms by which nanoparticles enhance the properties of the base fluid can be investigated, whether specific nanostructures are generated around nanoparticles can be observed, the size of individual nanoparticles and clusters entering the molten salt can be measured and observed, and the dispersion of nanoparticles in the molten salt regime can be assessed.
In the academic world, many scholars believe the anomalous enhancement of the thermophysical properties of nanofluids is due to the creation of special nanostructures around nanoparticles. Therefore, scholars have observed the microstructure of nanofluids by scanning electron microscope (SEM). Tariq et al. [84] prepared nanofluids by dispersing CNTs in ternary eutectic salts (LiNO3-KNO3-NaNO3) by a two-step aqueous solution method. The SEM image shows the CNTs formed a mesh structure in the eutectic salt, this mesh structure became more compact as the concentration of CNTs increased, and the SHC of the nanofluid gradually decreased, so this mesh nanostructure was an important factor in the anomalous change in the SHC of the nanofluid. Liu et al. [85] prepared molten salt nanofluids by dispersing MWCNTs in solar salts using a two-step aqueous solution method. The SHC of the molten salt increased abnormally with increasing concentration of MWCNTs. The SEM images show many stacked structures were formed on the surface of the nanoparticles, and the presence of these structures led to enhanced thermal resistance at the molten salt interface, resulting in enhanced SHC of the molten salt. Hu et al. [86] investigated the addition of Al2O3 nanoparticles to binary carbonates (Li2CO3-K2CO3) to obtain high-temperature nanofluids. The SEM images show that at different concentrations of nano salts, the surface of the base salt was smooth with no special structures, the surface of the nanoparticle-doped composite had some dotted special structures, and as the concentration of nanoparticles increased, these special structures also increased. These special structures greatly increased the surface area of the molten salt, thus improving its thermophysical properties.
In summary, the presence of nanostructures in molten salts increases the surface area and interfacial thermal resistance of molten salts, allowing them to absorb and store more heat, which is of great importance for reducing the cost of molten salt heat storage.
4. Improvement of Heat Transfer Performance
Thermal conductivity is the main parameter reflecting the ability of a medium to transfer heat, and the measurement of thermal conductivity is a major part of the study of the heat transfer properties of molten salt nanofluids. Methods to improve the effective thermal conductivity of nanofluids include Brownian motion, aggregation of nanoparticles, size effects, and fluid delamination at the solid–liquid interface. The improvement of thermal conductivity depends mainly on the size, shape, concentration, fluid temperature, dispersion, and surface area of the solid–liquid interface of the nanoparticles [87,88,89].
Carbon materials have good thermal conductivity; with the development of nanotechnology, the preparation cost of carbon nanomaterials has gradually decreased, and many researchers have used carbon nanomaterials as nanoparticles for nanofluids; numerous experimental studies found carbon nanoparticles have high enhancement to nanofluidic base fluids [90,91,92,93,94,95]. Molten salts are an excellent medium for heat transfer and storage because of their low melting point, low cost, large heat transfer temperature difference, and good thermal stability, but the thermal conductivity of molten salts is generally low and does not meet industrial needs well. In recent years, some scholars have started to focus on adding carbon material nanoparticles to molten salts to improve their thermal conductivity, as shown in Table 3.

Table 3.
Improvement of heat transfer performance of nano carbon materials.
4.1. Thermal Conductivity of Molten Salt-Based Carbon Nanotube Nanofluids
CNTs are one-dimensional nanomaterials with excellent thermal properties, and recent experimental studies have shown that CNTs can improve the thermal conductivity of molten salts. Zhang [96] preferably selected GA as a dispersant and prepared nanofluids containing different mass fractions of MWCNTs using a two-step aqueous solution method. The average thermal conductivity of the molten salts increased the most when the addition of nanoparticles was at 0.5%, reaching 49.1%, whereas the addition of 1% resulted in no change in thermal conductivity, and the optimum addition of nanoparticles should be below 1%. In this paper, although the authors propose a model of nanoparticle-enhanced thermal conductivity of molten salts and four factors influencing the thermal conductivity of nanofluids, micro convection due to Brownian motion, and the effect of the liquid film layer between the nanoparticles and the base fluid, the small size effect, and the agglomeration effect, they do not investigate the mechanism of thermal conductivity enhancement, and the GA dispersant chosen by the authors decomposes at high temperatures, which adversely affects the stability of molten salt nanofluids.
To improve the stability of nanofluid and the dispersion of nanoparticles and avoid the adverse effects of dispersant decomposition on molten salt nanofluid, Wu et al. [49] prepared molten salt–MWCNT composites using the high-temperature melting method. The molten salt thermal conductivity was greatly improved, with a maximum thermal conductivity of 293% when MWCNTs were added at 0.3wt%; the thermal conductivity of the molten salt showed a decrease when the addition of MWCNTs was 0.1 wt%, but the authors did not explain the reasons for the increase and decrease of the thermal conductivity of the molten salt. The extremely agglomerative nature of CNTs makes the dispersion of CNTs by high-temperature melting methods more limited, and the nanoparticle dispersion is small, which is detrimental to the thermal conductivity of the nanofluid. Surface modification of nanoparticles is a very promising method to improve their dispersibility. Gorji et al. [97] conducted stability and thermal conductivity tests on doped functionalized SWCNTs and plain SWCNTs nanofluids. The nanofluids doped with functionalized nanoparticles exhibited better thermal conductivity and thermal storage capacity and improved thermal stability after three months of thermal stability tests. Omrani et al. [98] dispersed six different length-to-diameter ratios of MWCNTs-COOH in 0.05 vol% deionized water, adjusted the nanofluid pH value to neutral, and investigated the effect of MWCNT aspect ratio on the dispersion energy, thermal conductivity, and rheological properties of the nanofluids. The neutral pH value and the addition of functional groups to the surface of CNTs could effectively improve the dispersion of CNTs and reduce the agglomeration of nanoparticles. The thermal conductivity of the samples increased linearly with increasing nanofluid temperature and CNT length-to-diameter ratio, with a maximum thermal conductivity improvement reaching 36% and a decrease in fluid viscosity. CNTs are tubular nanoparticles, and their curl and length-to-diameter ratio, among others, have an important effect on their thermal conductivity. In this paper, the authors only study the enhancement of the thermal conductivity of molten salts made by CNTs and not the enhancement of the thermal conductivity of nanofluids by the change of curl of CNTs. Moreover, some scholars have objected to the linear increase of the thermal conductivity of nanofluids with the length-to-diameter ratio of CNTs proposed by the authors.
To calculate the effective thermal conductivity of carbonate doped CNTs, Yuan et al. [51] proposed a theoretical calculation method based on a 3D random curl structure model. To validate the method, CNT composite molten salts were prepared using a high-energy ball milling method. The curl degree of the CNTs was determined using an image recognition technique. The effective thermal conductivity of the composite molten salt was measured to validate the calculated model. The error of the theoretical calculation model was less than 8.5% for the well-mixed CNT composite molten salt, indicating that the calculation model can be used to determine the effective thermal conductivity of the CNT composite molten salt. In addition, the influence of key factors such as the curl degree and length-to-diameter ratio of CNTs on the effective thermal conductivity of the composite molten salt was revealed. First, the effective thermal conductivity λeff of the composite molten salt decreased with the increase of the curl degree of the CNTs. Second, increasing the CNT L/D ratio to 175 can increase the λeff of the composite molten salt, and the thermal conductivity almost ceased to increase when the CNT L/D ratio exceeded 175. Third, the increase in thermal conductivity of CNTs was a key factor in the increase in λeff of the composite molten salt. Therefore, CNTs with a length-to-diameter ratio of 175 and a small curl have the greatest improvement in the thermal conductivity of molten salt nanofluids.
4.2. Thermal Conductivity of Molten Salt-Based Graphene and Other Carbon Nanofluids
Graphene has a thermal conductivity range of approximately 4000–6600 at room temperature [99] and is considered a very promising material for enhancing the thermal conductivity of molten salts.
Saranprabhu et al. [48]. prepared graphene molten salt composites by mixing solar salt with experimentally produced graphene oxide by using the ball milling method. The thermal conductivity of the composites was enhanced during the concentration of graphene oxide from 0.125% to 0.5%, with a maximum thermal conductivity enhancement of 17%. However, the authors neither explained the mechanism of the thermal conductivity enhancement nor further increased the nanoparticle concentration to investigate its effect on the thermal conductivity. To investigate the enhancement of thermal conductivity of molten salt by graphene further, Hamdy et al. [50] prepared molten salt nanocomposites with different concentrations by adding reduced graphene oxide (RGO) to solar salt. RGO had a good effect on the enhanced thermal conductivity of the molten salt, and the maximum strength of the thermal conductivity was enhanced by 52.1% at an RGO mass fraction of 1.5%. The authors speculated two main reasons for the enhanced thermal conductivity of the composites: (i) RGO was well dispersed and formed a special layered structure in the molten salt, which acted as a thermal conductivity network, thus enhancing the heat flow. (ii) Graphene had a special sheet-like structure, and this structure provided a higher heat flow to the composite, which led to an increased thermal conductivity. However, the authors have not tested either of these hypotheses through experiments or simulations and are only at the stage of theoretical reasoning. For a further understanding of the mechanism of thermal conductivity enhancement of GNPs on molten salts, Lyu et al. [54] prepared graphene nanosheet (GNS)–molten salt composites doped with different concentrations of GNS by a two-step aqueous solution method into NaNO3 and explored the mechanism of thermal conductivity enhancement by MD simulations. The thermal conductivity of the material gradually increased with the increase of GNS doping, and the maximum increase of thermal conductivity reached 245% when the GNS doping amount was 3%. Simulations showed the phonon band involved in energy transfer at low and medium frequencies was substantially wider for NaNO3 than for pure NaNO3. In addition, the phonon vibrations of GNS and NaNO3 were more harmonious in the low- and medium-frequency bands. The result was an improved thermal conductivity, so the authors speculate that phonon vibration matching is responsible for the enhanced thermal conductivity of the molten salt, which provides a new idea for the choice of nanoparticle doping amount. However, this is only a reasonable speculation based on simulations and does not prove that phonon energy transfer is the cause of the increased thermal conductivity of molten salt nanofluids, which needs to be verified by more rigorous experiments and simulations in the future.
In summary, CNTs and graphene have a positive effect on the improvement of the thermal conductivity of molten salts and are ideal materials for enhancing the thermal conductivity of molten salts. However, more types of carbon nanomaterials are available, other carbon nanomaterials also have very excellent thermal properties, and their enhancement of the thermal conductivity of molten salt nanofluids as nanoparticles is worthy of further exploration and exploration by scholars.
Xiao et al. [78] prepared molten salt composites by dispersing expanded graphite into solar salt via the ultrasonic aqueous solution method. When the nanoparticle doping amount was 2%, the thermal conductivity of the molten salt was greatly enhanced to 215%, and the expanded graphite was well dispersed in the molten salt as observed by SEM (scanning electron microscope) images, and the structure of the mixed molten salt itself was not changed. Ueki et al. [52] used a modified two-step aqueous solution method to disperse two different concentrations of silicon carbide (SiC) nanoparticles into Hitec salt to prepare molten salt nanofluids. The thermal conductivity of the nanofluids was higher than that of the base fluid, with an increase of about 1.46 times at 200 °C for a set of SiC additions of 0.6 wt%. At 250 °C, the thermal conductivity increased by a factor of approximately 1.50. However, the group with an addition of 1.2 wt% showed almost no increase in thermal conductivity, and substantial agglomeration of nanoparticles was observed. El-Sayed et al. [53] used ultrasonic dispersion and solid-state method of dispersion to disperse homogeneously cost-effective blast nanodiamond (ND) with an average particle size of 10 nm in binary nitrate molten salt (KNO3-NaNO3) to achieve good dispersion of ND. The mass loading of ND was varied and optimized, with a remarkable increase in thermal conductivity compared with the base binary molten salt and a maximum improvement in thermal conductivity of 93% at 3% nanoparticle addition. In addition, the thermal diffusivity of the developed ND-based molten salt was in-creased by 43% compared with the blank molten salt. The study speculated that the reason for the improved thermal conductivity of the composite was the excellent heat transfer effect of the ND particles [100,101,102], but no strengthening mechanism according to the body was provided.
In summary, the thermal conductivity of molten salt increases more than its SHC because carbon nanomaterials have a relatively high thermal conductivity and can form good heat transfer channels with the molten salt in molten salt nanofluid. Thus, heat can be transferred and transported well, and cannot be stored in large quantities within the nanofluid, but this is only a speculation at present, and more rigorous experimental verification is still required.
4.3. Effect of Nanoparticle Shape on the Thermal Conductivity of Nanofluids
Nanoparticles have many different shapes, typically spherical, cylindrical, and flaky. Owing to the different shapes of nanoparticles, many differences are noted in their heat transfer laws, which have attracted the attention of many researchers, and much theoretical research has been carried out. In this paper, two types of nanoparticles, spherical and cylindrical, are reviewed and summarized.
Murshed et al. [103] performed mathematical modeling to investigate the effect of the different shapes of nanoparticles on the effective thermal conductivity of nanofluids. In the modeling, the authors assumed that the nanofluids consisted of three components, particles, interfacial layers, and liquids; the nanoparticles were separated from one another; and the temperature field was continuous in each component. Using the above boundary conditions, the authors derived a model for calculating the different effective thermal conductivities of spherical and circular nanoparticles, and demonstrated the difference between spherical and cylindrical nanoparticles in enhancing the thermal conductivity of molten salts. Ghosh et al. [104,105] developed a multiscale heat transfer model using MD simulations to investigate the heat transfer properties of nanoparticles better. The heat transfer from nanoparticles colliding with a heat source is an important cause of heat transfer enhancement in nanofluids, and the authors compared the temperature change between spherical nanoparticles and cylindrical nanoparticles when colliding with a heat source. The results showed cylindrical nanoparticles can absorb heat more effectively, which indicates that cylindrical nanoparticles have better thermal conductivity enhancement for fluids.
The above simulation results show that cylindrical nanoparticles have better heat transfer enhancement compared with spherical nanoparticles, but the researchers speculate this phenomenon may be due to the cylindrical nanoparticles having a larger surface area [106]. To test these speculations, the researchers conducted an experimental study. Maheshwary et al. [107] configured TiO2-water nanofluids using nanoparticles with different shapes but otherwise consistent parameters and showed the nanofluids containing cylindrical nanoparticles were more thermally conductive than spherical nanoparticles, suggesting that the difference in nanoparticle shape led to a variance in the specific surface area of the nanoparticles, which led to their dissimilar thermal conductivities. Similarly, Murshedet et al. [108] configured TiO2-water nanofluids also containing cylindrical and spherical nanoparticles, using CTAB (ammonium bromide) as a surfactant to allow better dispersion of the nanoparticles and to reduce the interference caused by nanoparticle agglomeration. For a given particle concentration, higher thermal conductivity was observed for nanofluids containing cylindrical nanoparticles. Timofeeva et al. [106] measured the thermal conductivity of nanofluids using a transient hot wire method. The results showed that nanofluids containing cylindrical nanoparticles had the highest thermal conductivity. However, a different view has been proposed by others, who suggest that the greater thermal conductivity of cylindrical nanoparticles is related to their having a larger size than spherical nanoparticles. Kim et al. [109] configured nanofluids for nanoparticles by adding different shapes of Al2O3 to water. Thermal conductivity was related to suspension stability, which was influenced by the shape of the nanoparticles, whereas nanoparticle size had less influence on the stability of the suspension. Nanofluids containing brick-like nanoparticles had the highest stability, whereas nanofluids containing blade-like nanoparticles had the lowest stability.
In summary, the thermal conductivity of nanofluids is related to the variation in nanoparticle shape, and studies have shown that nanoparticle particle shape has a greater effect on thermal conductivity than particle size [110]. The above literature also shows particles with a larger specific surface area have higher thermal conductivity. Among carbon nanomaterials, graphite nanoparticles are spherical nanoparticles, and CNTs are cylindrical nanoparticles. Hence, CNTs have a greater advantage for the enhancement of thermal conductivity of heat transfer fluids. Research on the effect of carbon nanoparticle shape on the thermal conductivity of nanofluids in molten salt nanofluids is rare, and the effect of different shapes of carbon nanoparticles on the thermal conductivity of molten salts needs further experimental proof in the future.
4.4. Mechanism of Thermal Conductivity Enhancement of Molten Salts by Nanoparticles
Several scholars have proposed different explanations for the study of the thermal conductivity mechanism of nanofluids. In the current state of research, three inferences are considered possible reasons for the nanoparticles to enhance the thermal conductivity of base salt [57].
4.4.1. Brownian Motion Theory
Brownian motion is the never-ending irregular motion of particles suspended in a liquid or gas, and its effect on the thermal conductivity of nanofluids is mainly reflected in three aspects [111]: Collisions occur among nanoparticles due to Brownian motion, which completes the solid–solid heat exchange among nanoparticles, thus increasing the thermal conductivity of nanofluids. Owing to the extremely small size of nanoparticles, Brownian motion in the base fluid is very intense, thus causing the micro convection effect of the base fluid and increasing the thermal conductivity of the nanofluids. Collisions occur among molecules of the base fluid and nanoparticles, thus intensifying the heat exchange and increasing the thermal conductivity of the base fluid.
Many scholars have questioned this theory. William Evans et al. [112] used MD simulations to demonstrate that heat transport through a conduction mechanism quantified by thermal diffusivity is much faster than that quantified by particle diffusivity for nanoparticle motion, illustrating the negligible effect of micro convection caused by Brownian motion. By comparing the rate of thermal diffusion of the base fluid with the rate of energy transport due to Brownian motion, Keblinski et al. [102] concluded the Brownian diffusion rate is much smaller than the diffusion rate of hot carriers. Based on these calculations, the authors concluded the effect of Brownian motion on thermal conductivity is negligible. Cui et al. [113] carried out MD simulations based on the two said authors and optimized the model, again concluding the effect of micro convection caused by Brownian motion on the thermal conductivity of the nanofluid can be neglected.
4.4.2. Semi-Solid Layer Theory
Researchers have experimentally observed that a crystalline layer forms on the surface of nanoparticles, which is known as a nanolayer or semi-solid layer. The thermal properties of the semi-solid layer are speculated to be similar to those of molten salts near the melting point, and scholars have thought the nanolayer is one of the important mechanisms for the thermal conductivity enhancement of molten salt nanofluids [114,115,116]. Tao et al. [81] observed the nanolayer around nanoparticles through SEM images, and the thickness of the nanolayer around different nanoparticles varied: The nanolayer formed around SWCNTs and MWCNTs were relatively thin and could form effective thermal conductivity connections, so the thermal conductivity enhancement was greater. However, for graphene and fullerene, a thicker nanolayer appeared on their surfaces, which made forming effective thermal conductivity connections difficult, and the nanofluid thermal conductivity hardly increased or even decreased. The authors speculate that the nanosphere can only be enhanced by the presence of a nanosphere layer below 100 nm, which enhanced the effective thermal conductivity of the nanofluid. Teng et al. [117] investigated semi-solid layers. The results showed that from a microscopic point of view, the semi-solid layer possessed a stronger thermal conductivity than the base fluid, thus supporting the theory that the presence of a semi-solid layer strengthens the macroscopic thermal conductivity of nanofluids. However, no relevant literature proves whether the thermal conductivity within the semi-solid layer is dominated by solid or liquid thermal conductivity.
4.4.3. Nanoparticle Agglomeration Effect
Nanoparticles are generally small in diameter, have a large specific surface area, and have a large surface energy, which can easily attract anions and cations in molten salts and thus cause agglomeration. The effect of agglomeration on thermal conductivity has been studied extensively by many scholars. Combing the results of nanofluid research, the nanoparticle agglomeration effect reduces the improvement of thermal properties of molten salt nanofluids, and many scholars have used the addition of dispersants to molten salt nanofluids to weaken the agglomeration effect of nanoparticles to enhance the improvement of nanoparticle thermal conductivity of molten salts. Prasher et al. [118] argued agglomerated structures can enhance the thermal conductivity of molten salts; the agglomerates formed by nanoparticles, which provide a low-thermal-resistance thermal conductivity channel to the base fluid, can enhance the heat transfer. Zhang [96] suggested nanoparticle agglomeration and settling would reduce the thermal conductivity of the base fluid, but if the nanoparticles only agglomerated in small areas without settling, it would help improve the thermal conductivity. Therefore, the fact that the nanoparticle agglomeration effect on the enhancement of the thermal conductivity of the base fluid must be negative cannot be simply concluded, and whether a combination of other mechanisms exists should be investigated.
5. Existing Problems and Future Directions
5.1. Nanofluid Stability Issues and Future Directions for Enhancement
Although molten salt has been widely used in solar thermal power generation, its heat transfer and storage performance are poor. Using the high thermal conductivity of nanomaterials, introducing them into molten salt as additives to form a stable nanofluid suspension can substantially improve the heat transfer and storage performance of molten salt. Carbon nanomaterials have ultra-high thermal conductivity and are ideal materials for improving the heat transfer storage performance of molten salts, but carbon nanomaterials are highly prone to agglomeration due to their inherent properties, making forming stable suspensions difficult, which greatly limits the development and application of carbon-based nanofluids. Therefore, exploring an effective method for the dispersion of carbon materials is a problem that does not urgently need to be solved to study the SHC of carbon-based nanofluids. At present, the main methods to improve the dispersion of carbon nanoparticles are surface modification of nanoparticles, addition of surfactants, mixing nanoparticles, and pH control. [119]
5.1.1. Nanoparticle Surface Modification
The addition of functional groups to the nanoparticle surface and the charging of the nanoparticle surface can reduce the polarity of the carbon material and improve its dispersibility. Carbon nanomaterials are so prone to agglomeration mainly because they have a high polarity and react with the ions in the molten salt in a mutually exclusive manner, which makes dispersing carbon materials uniformly in the molten salt difficult. The existing surface modification methods for nanoparticles mainly include adding functional groups to the nanoparticle surface and making the nanoparticle surface electrically charged. Studies have shown that the addition of carboxylated functional groups to the surface of carbon nanomaterials can effectively improve the dispersion of nanoparticles. However, no corresponding study in the literature examines the mechanism of carboxylated functional groups to improve the dispersion of carbon nanomaterials, which can be explored in the future. According to Yuan Fan et al.’s simulations, the addition of appropriate surface charge can improve the attraction of carbon materials to the metal cations in the molten salt, enabling better binding of carbon nanoparticles to the molten salt. However, excessive surface charge will increase the repulsive force among nanoparticles, making them more prone to agglomeration.
5.1.2. Addition of Surfactants
The addition of surfactants increases the degree of dispersion of nanoparticles [120,121,122]. The active agent wets the nanoparticle surface, reduces the nanoparticle surface free energy, optimizes the spatial site resistance, increases the repulsive force among nanoparticles, inhibits particle agglomeration, and ensures the stability of the nanofluid [123]. The stability of nanofluid is not positively correlated with the amount of surfactant added, and adding too much surfactant makes the nanofluid less stable, so the optimal amount of addition should be determined [124,125]. Studies have shown the same surfactant has different effects on enhancing the stability of molten salt nanofluids with dissimilar carbon nanoparticles added, and the same molten salt-based carbon nanofluid has different effects on enhancing stability with dissimilar surfactant additions. A large body of literature shows for CNT–molten salt nanofluids, using GA as surfactant has the best improvement in nanofluid stability, and for graphene–molten salt nanofluids, sodium dodecyl benzoate sulfonate (SDBS) is the best surfactant, whereas the addition of surfactant alone does not increase the thermal physical properties of the eutectic salt.
Although surfactants can enhance the stability of nanofluids, surfactants decompose under high-temperature conditions and methods to improve the stability of nanofluids with surfactants lose their effectiveness. Therefore, the literature rarely discusses surfactants to improve the stability of high-temperature nanofluids, which could be explored in the future with focus. Therefore, surfactants to improve nanofluid stability are generally applied in the field of low-temperature nanofluids, and future research to improve the stability of high-temperature nanofluids needs to be explored further.
5.1.3. Hybrid Nanoparticles
In recent years, researchers have begun to focus on improving the thermal properties of molten salts by mixing nanoparticles, and this has also drawn attention to the effect of mixed nanoparticles on the stability of nanofluids [126]. Many methods are used for the preparation of hybrid nanoparticles, and different nanoparticles need to be mixed by different preparation methods. The main hybrid nanoparticle preparation methods are thermochemical synthesis technique [127], ball milling method [128], and in situ method [129]. By mixing nanoparticles, the thermal properties of different nanoparticles can be combined to provide a better reinforcement of the thermal properties of molten salts. According to the molecular forces among nanoparticles, mixing nanoparticles of different sizes and shapes can effectively reduce the forces among nanoparticles and molten salts, thus improving the stability of nanofluids. Vaka et al. [55,56] dispersed graphene–TiO2 hybrid nanoparticles by ultrasonic dispersion into five-membered mixed molten salts (NaNO3-KNO3-LiNO3-CaNO3-CsNO3) in nanofluid. The study showed the SHC of the mixed molten salts with the addition of heterogeneous nanoparticles was greatly enhanced, and the nanofluids showed good thermal stability after thermal stability tests.
In summary, surface modification of nanoparticles, addition of surfactants, and addition of hybrid nanoparticles can improve the stability of nanofluids, but surface modification will destroy the mechanical structure of nanoparticles and reduce the mechanical strength of nanoparticles, which is not conducive to the improvement of molten salt properties.
5.1.4. PH Control
The high surface activity of carbon nanomaterials can cause attraction and repulsion reactions with the anions and cations in the base fluid, which leads to agglomeration of nanoparticles and sedimentation, affecting the stability of the nanofluid. Adjusting the pH value of the base fluid can influence the concentration of free anions and cations in the medium and form a double electric layer around the nanoparticles, thus inhibiting the agglomeration of nanoparticles. The basic method of adjusting the pH of the molten salt is to add an acid-based solution to the base fluid to control the base fluid pH. The nanofluid pH is also influenced by the temperature and the different solution media, and the pH of the nanofluid decreases as the temperature increases. Placing the same nanoparticles into water and alkaline solutions reveals the nanoparticles have a better dispersion in water [119].
In summary, although PH control can improve the dispersion of nanoparticles and the stability of the colloidal system, the use of acid and alkaline solutions to adjust the PH value easily causes pollution to the base fluid and reduces the purity of the prepared composite. Moreover, the use of high-temperature molten salt nanofluid is higher, which makes the molten salt base fluid pH of strong acid environment of nanofluid PH value lower on the pipeline more corrosive [130], bringing additional economic loss. Therefore, the use of PH-controlled methods to improve the stability of molten salt nanofluids is less feasible.
5.2. Mechanisms and Future Directions for Improving the Heat Transfer and Storage Performance of Molten Salts
The addition of nanoparticles to molten salts to form stable nanofluids can substantially enhance the heat transfer and storage properties of molten salts. Therefore, the study of the mechanism of nanoparticles enhancing the heat transfer and storage performance of molten salts has attracted the attention of researchers. In a review of the current status of research on nanoparticle-enhanced heat transfer performance of molten salts, three inferences have been suggested as the reasons for nanoparticle-enhanced heat transfer performance of base fluids [57]: Brownian motion theory, semi-solid layer theory, and nanoparticle agglomeration effect. However, all three inferences are at the stage of theoretical derivation and have not been demonstrated by relevant experiments. The thermal storage performance of molten salts mainly depends on the SHC of molten salts. The proposed mechanisms for increasing the SHC of molten salt nanofluids include the nanosize effect, the semi-solid layer, and the interfacial thermal resistance, but these mechanisms are only at the stage of theoretical extrapolation, and no scholars have conducted rigorous experimental verification of them, and no unified conclusion has been formed.
The study of nanoparticles on the enhanced mechanism of heat transfer and storage in molten salts helps enhance the thermal conductivity of molten salts, reduce the cost of heat storage, improve the efficiency of heat transfer from molten salts, and lower the cost of solar thermal power generation. MD simulation is a method to simulate the physical trajectories and states of atoms and molecules based on the principles of Newtonian mechanics. For more complex molecular systems, the trajectories of the particles within the system are usually determined by numerically resolving the Newtonian equations of motion for the interacting particles, whereas the interparticle forces and potentials are determined using interparticle forces and potentials using molecularly based force fields. The molecular system of molten salt nanofluids is extremely complex and difficult to study experimentally at the molecular level. Microscopic models can be established by means of MD simulations to study the mechanism of intermolecular action of nanofluids, combined with the relevant phenomena observed experimentally, to explain the mechanism of nanoparticle enhancement of heat transfer in molten salts better. Computational fluid dynamics (CFD) is the science of approximating the integral and differential terms in the governing equations of fluid dynamics into discrete algebraic forms to obtain a system of algebraic equations, which can be solved to obtain numerical solutions at discrete time/space points. CFD can be used to establish the relevant algebraic equations to study the heat transfer laws and thermal energy storage mechanisms during the flow of nanofluids.
In summary, studying the strengthening mechanism of nanoparticles on the heat transfer and storage properties of molten salts is of great importance. MD simulations and CFD are very important aids to study the enhancement mechanism of nanoparticles on the heat transfer and storage performance of molten salts. In the future, the microscopic models of solid and liquid phases can be established with the help of these two methods, focusing on the mechanism of interaction between molten salts and nanoparticles and explaining the key factors of heat transfer and storage.
5.3. Viscosity and Corrosion
Viscosity is one of the very important thermophysical properties of molten salt nanofluids and has a major effect on the safety and economy of the system. Therefore, the study of nanofluid viscosity is of increasing interest to scientists. The concentration, shape, type, and size of the nanoparticles in molten salt nanofluids all have a considerable influence on the viscosity of the fluid.
Jo et al. [62] prepared nanofluids by adding different concentrations of CNTs to binary carbonates using a two-step aqueous solution method. The increases in nanofluid viscosity values were 1%, 93%, and 1130% at 550 °C for 1%, 2%, and 5% MWCNTs mass concentrations, respectively. Vaka et al. [56] prepared nanofluids by dispersing graphene–titanium dioxide hybrid nanoparticles into a five-membered mixed molten salt (NaNO3-KNO3-LiNO3-CaNO3). The effect of mixed nanoparticles on the viscosity of the molten salt was minimal, with viscosity changes of approximately −0.51% to 11.05% over the temperature range of 250–400 °C.
At present, the study of nanofluid viscosity is mostly limited to the field of low-temperature nanofluids, and high-temperature nanofluid viscosity is less studied because the measurement instruments are more expensive. Thus, further research and exploration are needed in the future.
Corrosion is a characteristic of all nanofluids, and the degree of corrosion is related to the maintenance costs of the equipment piping, which is of great importance in reducing the cost of power generation. Wang et al. [68] selected 304, 316L, 321, and 310S stainless steels for experimental studies on the corrosion characteristics of mixed molten salts. The results show the mixed molten salts mainly undergo intergranular corrosion on 316L and 304, and small pore corrosion on 321 and 310S, with 316L showing the best corrosion resistance characteristics. Vaka et al. [56] immersed SS316 in mixed molten salts and mixed nanofluids for costume comparison. The results indicated the surface of the sample immersed in the eutectic salt showed many pits, but the surface of the sample in the mixed nanofluid remained smooth. Hence, the addition of the mixed nanoparticles inhibited the corrosion of the sample by the molten salt.
In summary, the pipe material and the addition of nanoparticles have a substantial effect on the corrosion of molten salts. Therefore, selecting the right pipe material and adding the right nanoparticles are essential to reduce the maintenance costs of the pipes.
5.4. Costs
Although carbon nanomaterials have high thermal properties, they are relatively difficult to prepare and disperse, which contributes to the high cost of nanofluids. Further technical means are needed to improve and reduce costs in the future.
6. Conclusions
Carbon nanomaterials have excellent thermodynamic properties. Therefore, compounding carbon nanomaterials with traditional materials to obtain composite materials with high energy storage and high thermal conductivity has been a hot research topic worldwide in recent years. In this paper, the preparation methods, improvement of heat transfer properties, and improvement of thermal storage properties of carbon-based molten salt nanofluids by domestic and foreign scholars are summarized, and the following conclusions are drawn.
- Many methods are used for the preparation of molten salt nanofluids. The two-step aqueous solution method prepares nanofluids with poor thermal stability, and the high-energy ball milling method prepares composites with low purity and non-uniform particle size distribution. The one-step method involving redox should not be suitable for the preparation of carbon-based molten salt nanomaterials. Nanofluids prepared by the high-temperature melting method are thermally stable and can be used steadily at high temperatures for a long time with minimal change in SHC and mass. Therefore, nanofluids prepared by the high-temperature melting method are suitable for promotion as industrial production and can be focused on in the future.
- Composites prepared by adding carbon nanoparticles to high-temperature molten salt have greatly enhanced specific heat and thermal conductivity. They broaden the temperature applicability range of the base fluid, although the melting point and decomposition temperature do not change much. They are beneficial to increasing the energy storage density of the molten salt and reducing the cost of heat storage.
- The mechanism of specific heat and thermal conductivity of molten salts enhanced by nanomaterials is not yet unified and is still at the speculative stage. The heat transfer mechanism of carbon nanomaterials remains unclear, and the future research should focus on the heat transfer mechanism of carbon nanomaterials and the mechanism of interaction between nanomaterials and molten salts by means of MD simulation and CFD.
- Nanoparticle shape affects the thermal conductivity and stability of nanofluids. The thermal conductivity of nanofluids increases as the specific surface area of nanoparticles increases. Cylindrical nanoparticles have a larger specific surface area than other nanoparticles, such as spherical particles, and are therefore more conducive to the enhancement of fluid heat transfer properties.
- This paper summarizes a variety of methods to improve the dispersibility of carbon nanomaterials, among which adjusting the pH of the base fluid contaminates the nanofluid, the dispersant decomposes at high temperatures, and the cost of surface modification is high. Hybrid nanoparticles can combine the thermal properties of multiple nanoparticles while reducing the dispersion of nanoparticles. However, the preparation technology of hybrid nanoparticles is still immature, the complexity of selecting matching nanoparticles is much higher than that of ordinary nanoparticles, and further research on simpler preparation methods is needed in the future to reduce the cost of hybrid nanoparticles.
- Carbon nanomaterials have excellent thermodynamic properties, but only carbon materials such as CNTs and graphite are being studied. Research rarely examines the preparation of nanofluids by adding carbon nanofibers and ND to molten salts, which can be explored in the future.
Author Contributions
Conceptualization, X.C. and M.Z.; methodology, X.C. and M.Z.; formal analysis, M.Z.; investigation, M.Z.; resources, Y.W.; data curation, M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, X.C.; visualization, M.Z.; supervision, Y.W. and C.M.; project administration, Y.W. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We acknowledge the support of the National Key R&D Program of China (2022YFB2405202).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rubanenko, O.; Yanovych, V. Analysis of instability generation of Photovoltaic power station. In Proceedings of the 2020 IEEE 7th International Conference on Energy Smart Systems, Kyiv, Ukraine, 12–14 May 2020; Volume 7, pp. 128–133. [Google Scholar]
- Li, P.; Gao, X.; Li, Z.; Zhou, X. Effect of the temperature difference between land and lake on photovoltaic power generation. Renew. Energy 2022, 185, 86–95. [Google Scholar] [CrossRef]
- Eftekharnejad, S.; Vittal, V.; Heydt, G.T.; Keel, B.; Loehr, J. Impact of Increased Penetration of Photovoltaic Generation on Power Systems. IEEE Trans. Power Syst. 2013, 28, 893–901. [Google Scholar] [CrossRef]
- Jain, A.; Tripathyt, S.C.; Balasubramanian, R. Reliability and economic analysis of a power generation system including a photovoltaic system. Energy Conv. Manag. 1995, 36, 183–189. [Google Scholar] [CrossRef]
- Eftekharnejad, S.; Heydt, G.T.; Vittal, V. Optimal Generation Dispatch with High Penetration of Photovoltaic Generation. IEEE Trans. Sustain. Energy 2015, 6, 1013–1020. [Google Scholar] [CrossRef]
- Tafti, H.D.; Maswood, A.I.; Konstantinou, G.; Pou, J.; Blaabjerg, F. A General Constant Power Generation Algorithm for Photovoltaic Systems. IEEE Trans. Power Electr. 2018, 33, 4088–4101. [Google Scholar] [CrossRef]
- Kudo, M.; Takeuchi, A.; Nozaki, Y.; Endo, H.; Sumita, J. Forecasting electric power generation in a photovoltaic power system for an energy network. Electr. Energ. Jpn. 2009, 167, 16–23. [Google Scholar] [CrossRef]
- Gupta, M.K.; Kaushik, S.C.; Ranjan, K.R.; Panwar, N.L.; Reddy, V.S.; Tyagi, S.K. Thermodynamic performance evaluation of solar and other thermal power generation systems: A review. Renew. Sustain. Energy Rev. 2015, 50, 567–582. [Google Scholar] [CrossRef]
- McTigue, J.D.; Castro, J.; Mungas, G.; Kramer, N.; King, J.; Turchi, C.; Zhu, G. Hybridizing a geothermal power plant with concentrating solar power and thermal storage to increase power generation and dispatchability. Appl. Energy 2018, 228, 1837–1852. [Google Scholar] [CrossRef]
- Montes, M.J.; Abánades, A.; Martínez-Val, J.M. Performance of a direct steam generation solar thermal power plant for electricity production as a function of the solar multiple. Sol. Energy 2009, 83, 679–689. [Google Scholar] [CrossRef]
- Karthick, K.; Suresh, S.; Joy, G.C.; Dhanuskodi, R. Experimental investigation of solar reversible power generation in Thermoelectric Generator (TEG) using thermal energy storage. Energy Sustain. Dev. 2019, 48, 107–114. [Google Scholar] [CrossRef]
- Sioshansi, R.; Denholm, P. The Value of Concentrating Solar Power and Thermal Energy Storage. IEEE Trans. Sustain. Energy 2010, 1, 173–183. [Google Scholar] [CrossRef]
- Wang, R.L.; Zhang, Z.C.; Wei, X.X. Modelling and control of solar thermal power generation network in smart grid. Therm. Sci. 2021, 25, 2861–2870. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Moradi, M. A novel MCFC hybrid power generation process using solar parabolic dish thermal energy. Int. J. Hydrog. Energy 2019, 44, 8548–8565. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yuan, G.F. Analysis of distributed concentrating solar power technology and industry development. Bull. Chin. Acad. Sci. 2016, 31, 182–190. [Google Scholar]
- Qiu, T.; Miao, X.L.; Song, W.J.; Lou, D.; Zhang, S.F. Research progress on materials for perovskites solar cells. J. Mater. Eng. 2018, 46, 142–150. [Google Scholar]
- He, J. Application of molten salt and heat conducting oil thermal storage technology in photothermal power generation. Energy Conserv. Environ. Prot. 2019, 19, 100–101. [Google Scholar]
- Qin, C. Analysis and research on application of molten salt and heat transfer oil in solar power plant. Low Carbon World 2018, 12, 34–35. [Google Scholar]
- Wu, Y.T.; Ren, N.; Ma, C.F. Research and application of molten salts for sensible heat storage. Energy Storage Sci. Technol. 2013, 2, 586–592. [Google Scholar]
- Steinmann, W.D.; Tamme, R. Latent heat storage for solar steam systems. J. Sol. Eng. 2008, 130, 011004-1–011004-5. [Google Scholar] [CrossRef]
- Peng, Q.; Ding, J.; Wei, X.L.; Yang, J.P.; Yang, X.X. Research progress in application of molten nitrate salt in energy utilization. Mod. Chem. Ind. 2009, 29, 17–24. [Google Scholar]
- Yang, Z.; Garimella, S.V. Thermal analysis of solar thermal energy storage in a molten-salt thermocline. Sol. Energy 2010, 84, 974–985. [Google Scholar] [CrossRef]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Kearney, D.; Herrmann, U.; Nava, P.; Kelly, B.; Mahoney, R.; Pacheco, J.; Cable, R.; Potrovitza, N.; Blake, D.; Price, H. Assessment of a molten salt heat transfer fluid in a parabolic trough solar field. J. Sol. Energy Eng. Trans. ASME. 2002, 125, 293–299. [Google Scholar] [CrossRef]
- Myers, P.D.; Goswami, D.Y. Thermal energy storage using chloride salts and their eutectics. Appl. Therm. Eng. 2016, 109, 889–900. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Wang, W.; Ding, J.; Yang, J. Design and thermal properties of a novel ternary chloride eutectics for high-temperature solar energy storage. Appl. Energy 2015, 156, 306–310. [Google Scholar] [CrossRef]
- Araki, N.; Matsuura, M.; Makino, A.; Hirata, T.; Kato, Y. Measurement of thermophysical properties of molten salts: Mixture of alkaline carbonate Salts. Int. J. Thermophys. 1988, 9, 1071–1080. [Google Scholar] [CrossRef]
- Shin, B.C.; Sang, D.K.; Park, W.H. Ternary carbonate eutectic (lithium, sodium and potassium carbonates) for latent heat storage medium. Sol. Energy Mater. 1990, 21, 81–90. [Google Scholar] [CrossRef]
- Xing, Y.M.; Xu, X.; Yuan, X.G.; Cui, H.T.; Zhang, Y.H. Numerical simulation of high-temperature phase change heat storage system. Heat Transf.-Asian Res. 2002, 33, 32–41. [Google Scholar] [CrossRef]
- Jacobson, D.L.; Ponnappan, R. Performance of a cylindrical phase-change thermal energy storage unit. AIAA J. 1982, 21, 774–780. [Google Scholar]
- Ren, D.M.; Li, Z.S.; Hao, P.P. Chemistry of Carbon Nanotubes; Chemical Industry Press: Beijing, China, 2013. [Google Scholar]
- Zhang, Z.T.; Lin, Y.H.; Tang, Z.L.; Zhang, J.Y. Nanometer materials & nanotechology and their application prospect. J. Mater. Eng. 2000, 3, 42–48. [Google Scholar]
- Yao, S.W.; Zou, Y.; Zhang, W.G. Research progress of the properties, preparation and application of Au nanoparticles. Chem. Ind. Eng. Prog. 2007, 26, 310–314. [Google Scholar]
- Peng, H.; Liu, Y.; Zhang, J.S.; Zhang, J.S.; Zheng, H.L.; Ruan, R.S. Progress in utilization of silver nanoparticle material. Chem. Ind. Eng. Prog. 2017, 36, 2525–2532. [Google Scholar]
- Zhai, X.J.; Zhang, N. Research development of nanometer metal materials. Mater. Rev. 1999, 13, 22–24. [Google Scholar]
- Zhang, S.Q.; Wang, J.Y.; Wang, D.H.; Hou, X.G.; Zhang, M.; Huang, X. Recent progress on nano metallic materials. Mater. Rev. 2011, 1, 5–9. [Google Scholar]
- Zhong, Q.D.; Li, Y.G.; Zhou, S.Z.; Zhou, G.D. Development and status quo of nano-oxide research in the recent decade. J. Shanghai Univ. Electr. Power 2003, 19, 1–7. [Google Scholar]
- Jia, X.D. Synthesis and Application of Oxide Nanoparticles and Their Composites. Ph.D. Thesis, Nanjing University, Nanjing, China, 2011. [Google Scholar]
- WU, J. Thermodynamics of Metal Oxide Micro-Nanostructures. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2011. [Google Scholar]
- Trong Tam, N.; Viet Phuong, N.; Hong Khoi, P.; Ngoc Minh, P.; Afrand, M.; Van Trinh, P.; Hung Thang, B.; Żyła, G.; Estellé, P. Carbon Nanomaterial-Based Nanofluids for Direct Thermal Solar Absorption. Nanomaterials 2020, 10, 1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Q.Y. Novel progress on application of Carbon materials as counter electrode in dye-sensitized solar cells and perovskite solar cells. J. Mater. Eng. 2018, 46, 56–63. [Google Scholar]
- Gao, X.J.; Guo, Q.G.; Liu, L.; Song, J.R. The study progress on carbon materials with high thermal conductivity. Funct. Mater. 2006, 37, 173–177. [Google Scholar]
- Chen, Y.H. Manufacturing methods and application characters of carbon nano-materials. Carbon Tech. 2008, 27, 28–32. [Google Scholar]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- He, P.; Geng, H.Y. Research Progress of Advanced Thermal Management Materials. J. Mater. Eng. 2018, 46, 1–11. [Google Scholar]
- Yan, C.; Liang, J.; Zhong, X.; Li, C.; Chen, D.; Wang, Z.; Li, S.; Xu, J.; Wang, H.; Li, Y.; et al. BN white graphene well-dispersed solar salt nanofluids with significant improved thermal properties for concentrated solar power plants. Sol. Energy Mater. Sol. Cells 2022, 245, 111875. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Zhu, P.; Wang, C.; Li, X. Preparation, characterization and thermal properties of binary nitrate salts/expanded graphite as composite phase change material. Thermochim. Acta 2014, 587, 52–58. [Google Scholar] [CrossRef]
- Saranprabhu, M.K.; Chandini, D.; Bharathidasan, P.; Devaraj, S.; Rajan, K.S. Lowered total solidification time and increased discharge rate of reduced graphene oxide-solar salt composites: Potential for deployment in latent heat thermal energy storage system. Sol. Energy. 2020, 204, 466–475. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Li, J.L.; Wang, M.; Wang, H.Y.; Zhong, Y.; Zhao, Y.J.; Wei, M.; Li, Y. Solar salt doped by MWCNTs as a promising high thermal conductivity material for CSP. RSC Adv. 2018, 8, 1925–1926. [Google Scholar] [CrossRef]
- Hamdy, E.; Saad, L.; Abulfotuh, F.; Soliman, M.; Ebrahim, S. Enhancement of Molten Nitrate Thermal Properties by Reduced Graphene Oxide and Graphene Quantum Dots. ACS Omega 2020, 5, 21345–21354. [Google Scholar] [CrossRef]
- Yuan, F.; He, Y.; Li, M.; Li, X. Study on the theoretical calculation method for the effective thermal conductivity of carbon nanotube composite molten salt for solar energy application. Sol. Energy Mater. Sol. Cells 2022, 238, 111631. [Google Scholar] [CrossRef]
- Ueki, Y.; Fujita, N.; Kawai, M.; Shibahara, M. Molten salt thermal conductivity enhancement by mixing nanoparticles. Fus. Eng. Des. 2018, 136, 1295–1299. [Google Scholar] [CrossRef]
- El-Sayed, W.G.; Attia, N.F.; Ismail, I.; El-Khayat, M.; Nogami, M.; Abdel-Mottaleb, M.S.A. Innovative and cost-effective nanodiamond based molten salt nanocomposite as efficient heat transfer fluid and thermal energy storage media. Renew. Energy 2021, 177, 596–602. [Google Scholar] [CrossRef]
- Lyu, H.; Feng, D.; Feng, Y.; Zhang, X. Enhanced thermal energy storage of sodium nitrate by graphene nanosheets: Experimental study and mechanisms. J. Energy Storage 2022, 54, 105294. [Google Scholar] [CrossRef]
- Vaka, M.; Walvekar, R.; Khalid, M.; Jagadish, P.; Mubarak, N.M.; Panchal, H. Synthesis of Hybrid Graphene/TiO2 Nanoparticles Based High-Temperature Quinary Salt Mixture for Energy Storage Application. J. Energy Storage 2020, 31, 101540. [Google Scholar] [CrossRef]
- Vaka, M.; Walvekar, R.; Khalid, M.; Jagadish, P.; Low, J.H. Corrosion, rheology, and thermal ageing behaviour of the eutectic salt-based graphene hybrid nanofluid for high-temperature TES applications. J. Mol. Liq. 2021, 334, 116156. [Google Scholar] [CrossRef]
- Li, Z.; Wen, B.; Chen, H.Z.; Li, B.R.; Du, X.Z. Research status and progress of high-temperature molten salt-based nanofluids. Proc. CSEE 2021, 41, 2168–2186. [Google Scholar]
- Tian, H.Q.; Zhou, J.J.; Guo, C.X. Progress of specific heat enhancement of molten salt thermal energy storage materials. Chem. Ind. Eng. Prog. 2020, 39, 584–595. [Google Scholar]
- Huang, Y.; Cheng, X.; Li, Y.; Yu, G.; Xu, K.; Li, G. Effect of in-situ synthesized nano-MgO on thermal properties of NaNO3 -KNO3. Sol. Energy 2018, 160, 208–215. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Mohamed, T.Y.; Ahmed, I.S.; Samir, I. MgO nanostructure via a sol-gel combustion synthesis method using different fuels: An efficient nano-adsorbent for the removal of some anionic textile dyes. J. Mol. Liq. 2017, 225, 730–740. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Effect of Dispersion Homogeneity on Specific Heat Capacity Enhancement of Molten Salt Nanomaterials Using Carbon Nanotubes. Int. J. Sol. Energy Eng. 2015, 137, 011011. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Viscosity measurements of multi-walled carbon nanotubes-based high temperature nanofluids. Mater. Lett. 2014, 122, 212–215. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Effect of solvent on specific heat capacity enhancement of binary molten salt-based carbon nanotube nanomaterials for thermal energy storage. Int. J. Therm. Sci. 2015, 98, 219–227. [Google Scholar] [CrossRef]
- Tian, H.; Wang, W.; Ding, J.; Wei, X.; Huang, C. Preparation of binary eutectic chloride/expanded graphite as high-temperature thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2016, 149, 187–194. [Google Scholar] [CrossRef]
- Xie, Q.Z.; Zhu, Q.Z.; Li, Y. Thermal Storage Properties of Molten Nitrate Salt-Based Nanofluids with Graphene Nanoplatelets. Nanoscale Res. Lett. 2016, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.Z.; Zhu, Q.Z. Effect of doped graphene nanosheets on the specific heat capacity of molten salts of nitrate. J. Shanghai Electr. Power. 2017, 33, 285–288. [Google Scholar]
- Wu, D.; Liu, S. Effects of Multi-Walled Carbon Nanotubes on Enhancing the Thermodynamics Characteristic of Alkali Nitrate and Chlorate Salt for Concentration Solar Plants. Adv. Mater. Res. 2013, 805–806, 63–69. [Google Scholar] [CrossRef]
- Wang, Y. Experimental Study of the Physical Strengthening and Corrosion Characteristics of Mixed Molten Salts. Master’s Thesis, North China Electric Power University, Beijing, China, 2020. [Google Scholar]
- Kim, H.; Jo, B. Anomalous Increase in Specific Heat of Binary Molten Salt-Based Graphite Nanofluids for Thermal Energy Storage. Appl. Sci. 2018, 8, 1305. [Google Scholar] [CrossRef]
- Shin, D.; Jo, B. Investigation of high temperature nanofluids for solar thermal power conversion and storage applications. Int. J. Heat Transfer. 2010, 7, 583–591. [Google Scholar]
- Hamdy, E.; Ebrahim, S.; Abulfotuh, F.; Soliman, M. Effect of multi-walled carbon nanotubes on thermal properties of nitrate molten salts. In Proceedings of the 2016 International Renewable and Sustainable Energy Conference (IRSEC), Marrakech, Morocco, 14–17 November 2016; pp. 317–320. [Google Scholar]
- Yuan, F.; Li, M.; Qiu, Y.; Ma, Z.; Li, M. Specific heat capacity improvement of molten salt for solar energy applications using charged single-walled carbon nanotubes. Appl. Energy 2019, 250, 1481–1490. [Google Scholar] [CrossRef]
- Derbenev, I.N.; Filippov, A.V.; Stace, A.J.; Besley, E. Electrostatic interactions between charged dielectric particles in an electrolyte solution: Constant potential boundary conditions. Soft Matter 2018, 14, 5480–5487. [Google Scholar] [CrossRef]
- Derbenev, I.N.; Filippov, A.V.; Stace, A.J.; Besley, E. Electrostatic interactions between spheroidal dielectric particles. J. Chem. Phys. 2020, 152, 024121. [Google Scholar] [CrossRef]
- Siryk, S.V.; Rocchia, W. Arbitrary-Shape Dielectric Particles Interacting in the Linearized Poisson-Boltzmann Framework: An Analytical Treatment. J. Phys. Chem. B 2022, 126, 10400–10426. [Google Scholar] [CrossRef]
- Obolensky, O.I.; Doerr, T.P.; Yu, Y.-K. Rigorous treatment of pairwise and many-body electrostatic interactions among dielectric spheres at the Debye-Huckel level. Eur. Phys. J. E 2021, 44, 129. [Google Scholar] [CrossRef]
- Denton, A.R. Effective electrostatic interactions in colloid-nanoparticle mixtures. Phys. Rev. E 2017, 96, 062610. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Ding, Y.; Wen, D. Rheological Characteristics of Molten Salt Seeded with Al2O3 Nanopowder and Graphene for Concentrated Solar Power. Energies 2019, 12, 467. [Google Scholar] [CrossRef]
- Lee, D.; Jo, B. Thermal energy storage characteristics of binary molten salt nanofluids: Specific heat and latent heat. Int. J. Energy Res. 2021, 45, 3231–3241. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; Shin, D. Mechanism of heat capacity enhancement in molten salt nanofluids. Int. J. Heat Mass Tranf. 2020, 161, 120260. [Google Scholar] [CrossRef]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Preparation and thermal properties characterization of carbonate salt/carbon nanomaterial composite phase change material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Yuan, F.; He, Y.L.; Ma, Z.; Li, M.J. Study on the compressed ion layer and the specific heat of the phase change materials doping charged single-walled carbon nanotubes. Energy Procedia 2019, 158, 4909–4914. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Enhanced specific heat capacity of molten salt-based nanomaterials: Effects of nanoparticle dispersion and solvent material. Acta Mater. 2014, 75, 80–91. [Google Scholar] [CrossRef]
- Tariq; Chen, X.M.; Li, Y.Y.; Huang, Y. Effect of carbon nanotubes and alumina nanoparticles on the thermal storage properties of nitrate phase change materials. Energy Storage Sci. Technol. 2018, 7, 47–53. [Google Scholar]
- Liu, S.; Wu, D.; Liu, J.; Nian, Y.; Qiu, P.G. Development of a novel molten-salt filled with nanoparticles for concentration solar plants. Int. Energy Technol. 2013, 623, 1–4. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Zhang, Z.; Wen, D. Effect of Al2O3 nanoparticle dispersion on the specific heat capacity of a eutectic binary nitrate salt for solar power applications. Energy Convers. Manag. 2017, 142, 366–373. [Google Scholar] [CrossRef]
- Jung, J.; Koo, J.; Kang, Y.T. Model for predicting the critical size of aggregates in nanofluids. J. Mech. Sci. Technol. 2013, 27, 1165–1169. [Google Scholar] [CrossRef]
- Shukla, K.N.; Koller, T.M.; Rausch, M.H.; Fröba, A.P. Effective thermal conductivity of nanofluids-A new model taking into consideration Brownian motion. Int. J. Heat Mass Transf. 2016, 99, 532–540. [Google Scholar] [CrossRef]
- Vatani, A.; Woodfield, P.L.; Dao, D.V. A survey of practical equations for prediction of effective thermal conductivity of spherical-particle nanofluids. J. Mol. Liq. 2015, 211, 712–733. [Google Scholar] [CrossRef]
- Shende, R.; Sundara, R. Nitrogen doped hybrid carbon based composite dispersed nanofluids as working fluid for low-temperature direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2015, 140, 9–16. [Google Scholar] [CrossRef]
- Ibrahim, H.A. Experimental measurements of viscosity and thermal conductivity of single layer graphene based di-water nanofluid. J. Eng. 2017, 23, 142–161. [Google Scholar]
- Ahammed, N.; Asirvatham, L.G.; Titus, J.; Bose, J.R. Measurement of thermal conductivity of graphene-water nanofluid at below and above ambient temperatures. Int. Commun. Heat Mass Transf. 2016, 70, 66–74. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Estelle, P.; Halefadl, S.; Mare, T. Thermal conductivity of CNT based nanofluids: Experimental trends and models overview. Therm. Eng. 2015, 1, 381–390. [Google Scholar] [CrossRef]
- Hajjar, Z.; Rashidi, A.M.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Zhang, J.Y. Experimental and Mechanistic Study of the Thermal Conductivity of Nanocomposites (MWCNTs-Solar Salts). Master’s Thesis, North China Electric Power University, Beijing, China, 2020. [Google Scholar]
- Gorji, T.B.; Ranjbar, A.A.; Mirzababaei, S.N. Optical properties of carboxyl functionalized carbon nanotube aqueous nanofluids as direct solar thermal energy absorbers. Sol. Energy 2015, 119, 332–342. [Google Scholar] [CrossRef]
- Omrani, A.N.; Esmaeilzadeh, E.; Jafari, M.; Behzadmehr, A. Effects of multi walled carbon nanotubes shape and size on thermal conductivity and viscosity of nanofluids. Diam. Relat. Mater. 2019, 93, 96–104. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Zeng, L.; Ji, L.; Zhang, D. Research on the thermal conductivities of graphene and graphene based composite materials. Dev. Appl. Mater. 2010, 25, 94–100. [Google Scholar]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Mechanisms of heat flow in suspensions of nanosized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Ghosh, M.M.; Ghosh, S.; Pabi, S.K. Effects of Particle Shape and Fluid Temperature on Heat-Transfer Characteristics of Nanofluids. J. Mater. Eng. Perform. 2013, 22, 1525–1529. [Google Scholar] [CrossRef]
- Ghosh, M.M.; Roy, S.; Pabi, S.K.; Ghosh, S. A Molecular Dynamics-Stochastic Model for Thermal Conductivity of Nanofluids and Its Experimental Validation. J. Nanosci. Nanotechnol. 2011, 11, 2196–2207. [Google Scholar] [CrossRef]
- Timofeeva, E.; Routbort, J.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. Appl. Phys. 2009, 106, 014304. [Google Scholar] [CrossRef]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. A comprehensive study of effect of concentration, particle size and particle shape on thermal conductivity of titania/water based nanofluid. Appl. Therm. Eng. 2017, 119, 79–88. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.H.; Lee, J.H.; Jang, S. Effect of particle shape on suspension stability and thermal conductivities of water-based bohemite alumina nanofluids. Energy 2015, 90, 1290–1297. [Google Scholar] [CrossRef]
- Simpson, S.; Schelfhout, A.; Golden, C.; Vafaei, S. Nanofluid Thermal Conductivity and Effective Parameters. Appl. Sci. 2019, 9, 87. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Evans, W. Role of Brownian motion hydrodynamics on nanofluid thermal con-ductivity. Appl. Phys. Lett. 2006, 88, 219–482. [Google Scholar] [CrossRef]
- Cui, L.; Yu, Q.; Wei, G.; Du, X. Mechanisms for thermal conduction in molten salt-based nanofluid. Int. J. Heat Mass Tranf. 2022, 188, 122648. [Google Scholar] [CrossRef]
- Eastman, J.A.; Phillpot, S.R.; Choi, S.U.S.; Keblinski, P. Thermal transport in nanofluids. Annu. Rev. Mater. Res. 2004, 34, 219–246. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Xue, Q. Model for effective thermal conductivity of nanofluids. Phys. Lett. A 2003, 307, 313–317. [Google Scholar] [CrossRef]
- Teng, K.L.; Hsiao, P.Y.; Hung, S.W.; Chieng, C.C.; Liu, M.S.; Lu, M.C. Enhanced thermal conductivity of nanofluids diagnosis by molecular dynamics simulation. J. Nanosci. Nanotechnol. 2008, 8, 3710–3718. [Google Scholar] [CrossRef]
- Prasher, R.; Phelan, P.E.; Bhattacharya, P. Effect of aggregation kinetics on the thermal conductivity of nanoscale colloidal solutions(nanofluid). Nano Lett. 2006, 6, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.X.; Song, C.Y.; Yao, C.H.; Wang, H.H.; Wang, H.X.; Hu, Z.L.; Wu, Y.T.; Ding, Y.L. A review of nanofluid stability studies. Integr. Intell. Energy 2021, 43, 68–74. [Google Scholar]
- Cakmak, N.K. The impact of surfactants on the stability and thermal conductivity of graphene oxide de-ionized water nanofluids. J. Therm. Anal. Calorim. 2020, 139, 1895–1902. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, T.; Zeng, M.; Sundén, B. Effect of various surfactants on stability and thermophysical properties of nanofluids. J. Therm. Anal. Calorim. 2020, 143, 4057–4070. [Google Scholar] [CrossRef]
- Kumar, R.S.; Chaturvedi, K.R.; Iglauer, S.; Sharma, T. Impact of anionic surfactant on stability, viscoelastic moduli, and oil recovery of silica nanofluid in saline environment. J. Pet. Sci. Eng. 2020, 195, 107634. [Google Scholar] [CrossRef]
- Wang, L.H.; Xiang, J.; Li, J.X. Study on stability of nanofluid. Mater. Rep. 2011, 25, 17–20. [Google Scholar]
- Ding, J.; Wang, P.; Zhang, B.G.; Dong, S. Stability and heat transfer characteristics in automotive radiators of mixed-based nanofluids. Sci. Technol. Eng. 2019, 19, 196–206. [Google Scholar]
- Teng, T.; Fang, Y.; Hsu, Y.; Lin, L. Evaluating Stability of Aqueous Multiwalled Carbon Nanotube Nanofluids by Using Different Stabilizers. J. Nanomater. 2014, 2014, 203. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Synthesis, characterization and dispersion stability of water-based Cu–CNT hybrid nanofluid without surfactant. Microfluid. Nanofluid. 2021, 25, 1–14. [Google Scholar] [CrossRef]
- Suresh, S.; Venkitaraj, K.P.; Selvakumar, P.; Chandrasekar, M. Effect of Al2O3–Cu/water hybrid nanofluid in heat transfer. Exp. Therm. Fluid. Sci. 2012, 38, 54–60. [Google Scholar] [CrossRef]
- Batmunkh, M.; Tanshen, M.R.; Nine, M.J.; Myekhlai, M.; Choi, H.; Chung, H.; Jeong, H. Thermal Conductivity of TiO2 Nanoparticles Based Aqueous Nanofluids with an Addition of a Modified Silver Particle. Ind. Eng. Chem. Res. 2014, 53, 8445–8451. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced heat transfer and friction factor of MWCNT–Fe3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Ali, N.; Bahman, A.M.; Aljuwayhel, N.F.; Ebrahim, S.A.; Mukherjee, S.; Alsayegh, A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials 2021, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).