Solvothermal Liquefaction of Blackcurrant Pomace in the Water-Monohydroxy Alcohol Solvent System
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Solvothermal Liquefaction Procedure
2.3. Analysis Methods of STL Bioproducts
2.3.1. Elemental Analysis (EA)
2.3.2. Infrared Fourier-Transform Spectroscopy (FT-IR)
2.3.3. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
2.4. Calculations
- ERi—energy recovery in the form of STL group of products, where i means biocrude, biochar, gas phase, or water-soluble organics,
- HHVi—a higher heating value of i-group of products.
- Cshare,i—the content of C element in the i-group of products,
- Ci/feedstock—C element content in the i-group of products/feedstock, respectively.
3. Results and Discussion
3.1. Raw Material
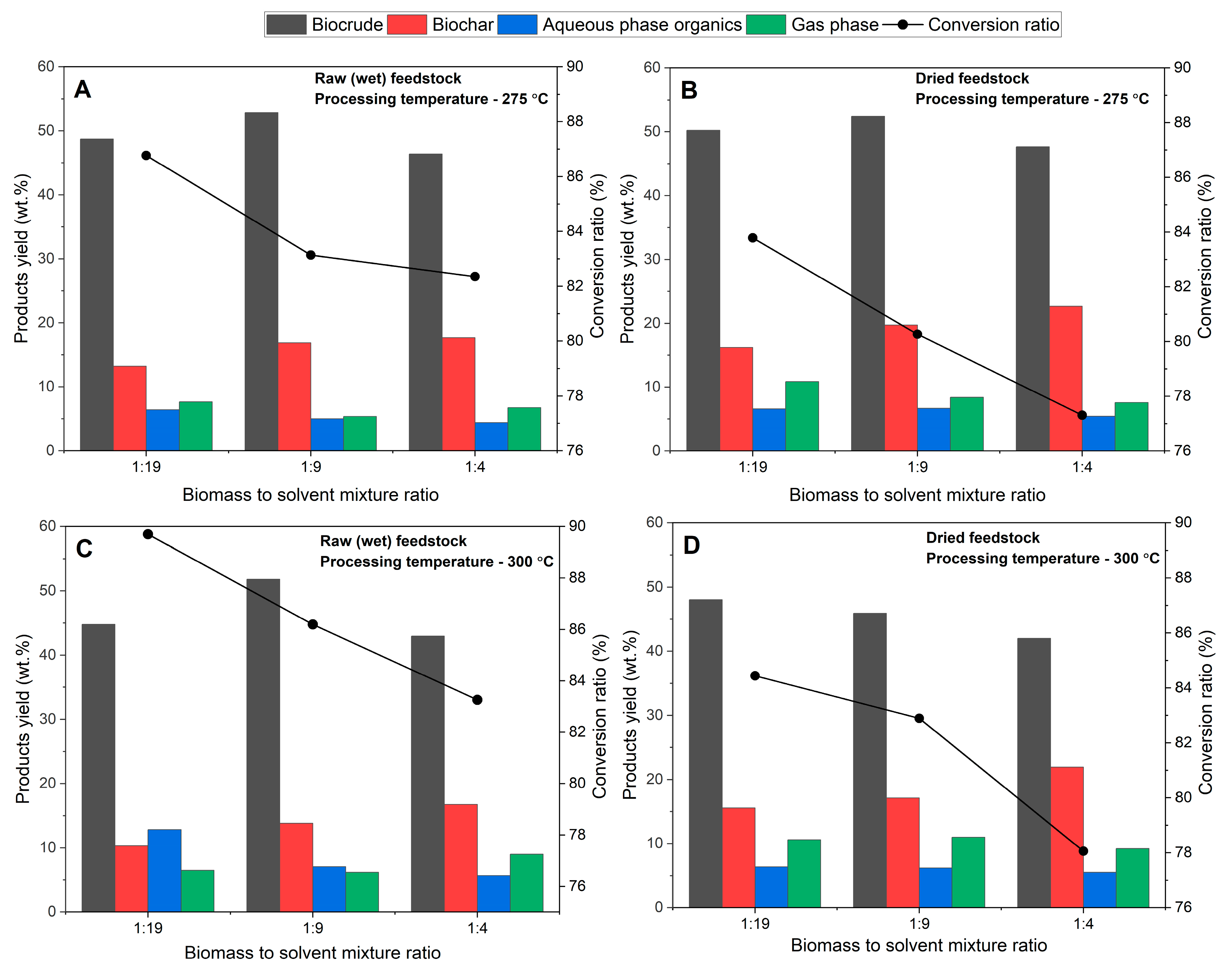
3.2. Effect of Process Variables on Bioproducts Yield Distribution
3.2.1. Temperature
3.2.2. Biomass-to-Solvent Ratio
3.3. Biocrude Composition
3.3.1. Elemental Composition
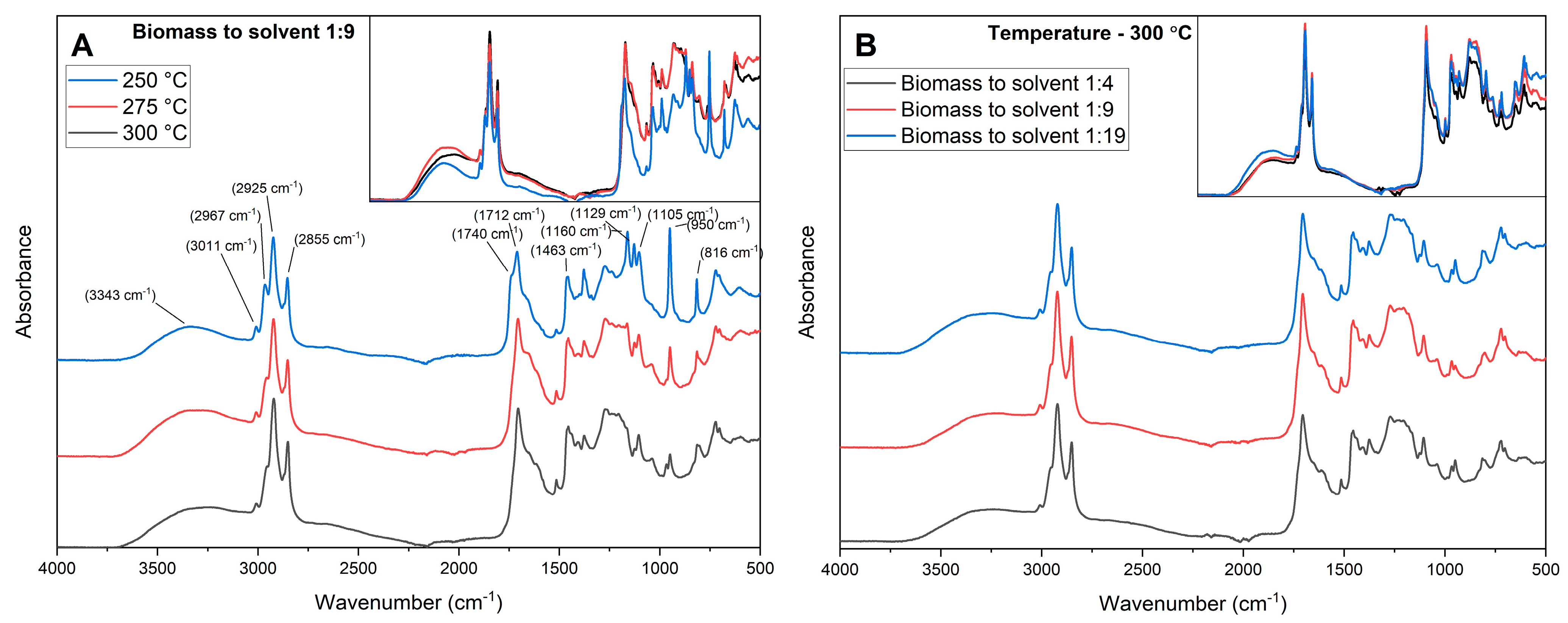
3.3.2. FT-IR
3.3.3. GC-MS
3.4. Other Groups of Bioproducts Composition
3.5. Energy Recovery and Biocrude Application Potential
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of Different Drying Techniques on Physical Properties, Total Polyphenols and Antioxidant Capacity of Blackcurrant Pomace Powders. LWT-Food Sci. Technol. 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Reißner, A.; Al-hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. SCI 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- FAOSTAT Global Crop Production-Currants. Available online: https://www.fao.org/faostat/en/#data (accessed on 30 September 2022).
- Jurgoński, A.; Juskiewicz, J.; Zduńczyk, Z.; Matusevicius, P.; Kołodziejczyk, K. Polyphenol-Rich Extract from Blackcurrant Pomace Attenuates the Intestinal Tract and Serum Lipid Changes Induced by a High-Fat Diet in Rabbits. Eur. J. Nutr. 2014, 53, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Optimisation of Bio-Oil Production by Hydrothermal Liquefaction of Agro-Industrial Residues: Blackcurrant Pomace (Ribes Nigrum L.) as an Example. Biomass and Bioenergy 2016, 95, 273–285. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Colombino, E.; Zduńczyk, Z.; Jankowski, J.; Cocolin, L.S.; Schiavone, A.; Biasato, I.; Prieto-Botella, D.; Karlińska, E.; Kosmala, M.; Ognik, K.; et al. Fibre-Phenolic Compound on Meat Quality, Blood Chemistry and Redox Status of Broilers. Animals 2020, 10, 1968. [Google Scholar] [CrossRef]
- Azman, E.M.; House, A.; Charalampopoulos, D.; Chatzifragkou, A. Effect of Dehydration on Phenolic Compounds and Antioxidant Activity of Blackcurrant (Ribes Nigrum L.) Pomace. Int. J. Food Sci. Technol. 2021, 56, 600–607. [Google Scholar] [CrossRef]
- Chojnacka, K.; Samoraj, M. New Micronutrient Biocomponents Based on Blackcurrant Seeds Pomace–Bench-Scale Kinetic Studies. Energy Environ. 2020, 32, 1–17. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Vos, M.P.; Brilman, D.W.F. Effect of Process Conditions on Bio-Oil Obtained through Continuous Hydrothermal Liquefaction of Scenedesmus Sp. Microalgae. J. Anal. Appl. Pyrolysis 2018, 134, 415–426. [Google Scholar] [CrossRef]
- López Barreiro, D.; Beck, M.; Hornung, U.; Ronsse, F.; Kruse, A.; Prins, W. Suitability of Hydrothermal Liquefaction as a Conversion Route to Produce Biofuels from Macroalgae. Algal Res. 2015, 11, 234–241. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Savage, P.E. Hydrothermal Liquefaction of Sewage Sludge under Isothermal and Fast Conditions. Bioresour. Technol. 2017, 232, 27–34. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Li, R.; Li, B.; Kai, X. Hydrothermal Liquefaction of Sewage Sludge to Produce Bio-Oil: Effect of Co-Pretreatment with Subcritical Water and Mixed Surfactants. J. Supercrit. Fluids 2019, 144, 28–38. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Grzywacz, P.; Janus, R.; Michalik, M. A Two-Stage Processing of Cherry Pomace via Hydrothermal Treatment Followed by Biochar Gasification. Renew. Energy 2021, 179, 248–261. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Berdel, M.; Janus, R.; Brilman, D.W.F. Hydrothermal Processing of Pine Wood: Effect of Process Variables on Bio-Oil Quality and Yield. E3S Web Conf. 2019, 108, 11p. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Drabik, N.; Wądrzyk, M. Hydrothermal Co-Liquefaction of Microalgae, Wood, and Sugar Beet Pulp. Biomass Convers. Biorefinery 2017, 7, 445–454. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Hydrothermal Liquefaction of Blackcurrant Pomace and Model Molecules: Understanding of Reaction Mechanisms. Sustain. Energy Fuels 2017, 1, 555–582. [Google Scholar] [CrossRef]
- Anouti, S.; Haarlemmer, G.; Déniel, M.; Roubaud, A. Analysis of Physicochemical Properties of Bio-Oil from Hydrothermal Liquefaction of Blackcurrant Pomace. Energy and Fuels 2016, 30, 398–406. [Google Scholar] [CrossRef]
- Missaoui, A.; Bostyn, S.; Belandria, V.; Cagnon, B.; Sarh, B. Hydrothermal Carbonization of Dried Olive Pomace: Energy Potential and Process Performances. J. Anal. Appl. Pyrolysis 2017, 128, 281–290. [Google Scholar] [CrossRef]
- Ji, C.; He, Z.; Wang, Q.; Xu, G.; Wang, S.; Xu, Z.; Ji, H. Effect of Operating Conditions on Direct Liquefaction of Low-Lipid Microalgae in Ethanol-Water Co-Solvent for Bio-Oil Production. Energy Convers. Manag. 2017, 141, 155–162. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zhu, W.W.; Wei, X.Y.; Fan, X.; Cao, J.P.; Dou, Y.Q.; Zong, Z.M.; Zhao, W. Synergic Effect of Methanol and Water on Pine Liquefaction. Bioresour. Technol. 2013, 142, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, Z.; Yang, T.; Li, B.; Wei, L.; Sun, Y. Sub–Supercritical Liquefaction of Municipal Wet Sewage Sludge to Produce Bio-Oil: Effect of Different Organic–Water Mixed Solvents. J. Supercrit. Fluids 2018, 138, 115–123. [Google Scholar] [CrossRef]
- Lai, F.; Chang, Y.; Huang, H.; Wu, G.; Xiong, J.; Pan, Z.; Zhou, C. Liquefaction of Sewage Sludge in Ethanol-Water Mixed Solvents for Bio-Oil and Biochar Products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, K.; Jena, U.; Das, P. Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies 2019, 13, 124. [Google Scholar] [CrossRef]
- Sebhat, W.; El Roz, A.; Fongarland, P.; Vilcocq, L.; Djakovitch, L. Catalytic Liquefaction of Kraft Lignin with Solvothermal Approach. Catalysts 2021, 11, 875. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Rządzik, B.; Lewandowski, M.; Budzyń, S. Pyrolysis Oil from Scrap Tires as a Source of Fuel Components: Manufacturing, Fractionation, and Characterization. Energy Fuels 2020, 34, 5917–5928. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal Liquefaction of Various Biomass and Waste Feedstocks for Biocrude Production: A State of the Art Review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Maziarka, P.A.; Schoder, K.A.; Pfersich, J.; Ronsse, F.; Kruse, A. Influence of Sequential HTC Pre-Treatment and Pyrolysis on Wet Food-Industry Wastes: Optimisation toward Nitrogen-Rich Hierarchical Carbonaceous Materials Intended for Use in Energy Storage Solutions. Sci. Total Environ. 2022, 816, 151648. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Wądrzyk, M.; Kruse, A. Py-GC-MS of Hydrochars Produced from Brewer’s Spent Grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Plata, M.; Zaborowska, K.; Janus, R.; Lewandowski, M. Py-GC-MS Study on Catalytic Pyrolysis of Biocrude Obtained via HTL of Fruit Pomace. Energies 2021, 14, 7288. [Google Scholar] [CrossRef]



| Parameter | Value [wt.%] |
|---|---|
| Moisture content | 67.5 |
| Ash content d | 3.1 |
| Volatile matter content d | 78.1 |
| Fixed carbon content d | 18.8 |
| C d | 50.5 |
| H d | 7.1 |
| N d | 2.7 |
| O d | 37.2 |
| Process. Cond. | 250 °C | 275 °C | 300 °C | |||
|---|---|---|---|---|---|---|
| Element/HHV | 1:9 | 1:19 | 1:9 | 1:4 | 1:9 | |
| C [wt.%] | 69.68 | 69.80 | 69.60 | 70.35 | 69.34 | |
| H [wt.%] | 10.29 | 8.81 | 9.63 | 9.55 | 8.97 | |
| N [wt.%] | 1.48 | 2.48 | 2.37 | 2.39 | 2.38 | |
| O [wt.%] | 18.55 | 18.91 | 18.40 | 17.71 | 19.31 | |
| HHV [MJ∙kg−1] | 34.5 | 32.8 | 33.8 | 34.0 | 32.8 | |
| Compound Group | RT [min] | Compound | Relative Share [%] | ||
|---|---|---|---|---|---|
| 250 °C | 275 °C | 300 °C | |||
| Carboxylic acids and derivatives | 3.3 | Acetic acid | 3.40 | 2.33 | 1.02 |
| 50.3 | N-Ethyl-2-isopropoxycarbonylazetidine | 3.55 | 2.11 | 0.93 | |
| 60.5 | n-Hexadecanoic acid | 6.27 | 8.43 | 11.29 | |
| 60.6 | isopropyl palmitate | - | - | 2.47 | |
| 66.5 | 9-Octadecenoic acid | 2.83 | 8.13 | 12.63 | |
| 66.9 | iso-Propyl 9,11-octadecadienoate | - | - | 2.47 | |
| 67.2 | 9,12-Octadecadienoic acid | 32.47 | 36.34 | 29.25 | |
| Other oxygen compounds | 20.8 | 2-Cyclopenten-1-one, 2-methyl- | - | - | 0.40 |
| 24.8 | 2-Vinylfuran | - | 0.38 | 0.45 | |
| 30.8 | Phenol, 2-methoxy- | - | - | 0.79 | |
| 34.2 | Phenol, 4-ethyl- | - | - | 1.22 | |
| 39.1 | Phenol, 4-ethyl-2-methoxy- | - | - | 0.63 | |
| 55.7 | Hexadecyl octyl ether | 1.31 | 1.45 | 1.39 | |
| 57.3 | Cyclododecanemethanol | 2.26 | - | 1.33 | |
| 57.5 | 1-Tetradecanol | 0.92 | 0.84 | - | |
| 57.7 | 2-Cyclopropen-1-one, 2,3-diphenyl- | 3.04 | 3.02 | - | |
| Nitrogen compounds | 15.1 | Pyrazine, methyl- | 0.73 | 0.65 | 0.56 |
| 33.0 | 3-Pyridinol | 3.49 | 3.82 | 3.80 | |
| 33.8 | 2-Pyrrolidinone | - | 0.66 | 1.11 | |
| 35.2 | 3-Pyridinol, 6-methyl- | 0.39 | 0.58 | 0.68 | |
| 50.0 | Quinoline, 6-methoxy-, 1-oxide | - | - | 2.09 | |
| 75.62 | 9-octadecenamide | 0.62- | 1.67 | 1.89 | |
| Element | 250 °C | 275 °C | 300 °C | ||||
|---|---|---|---|---|---|---|---|
| 1:9 | 1:19 | 1:9 | 1:4 | 1:19 | 1:9 | 1:4 | |
| C [wt.%] | 56.91 | 57.59 | 57.96 | 61.20 | 58.56 | 60.01 | 63.20 |
| H [wt.%] | 5.72 | 4.69 | 4.23 | 4.39 | 4.12 | 4.30 | 4.65 |
| N [wt.%] | 1.99 | 2.66 | 3.11 | 3.85 | 3.15 | 3.45 | 3.40 |
| O [wt.%] | 27.12 | 26.69 | 21.07 | 18.56 | 20.53 | 19.42 | 18.28 |
| HHV [MJ kg−1] | 22.60 | 21.44 | 21.91 | 23.69 | 22.05 | 22.99 | 24.79 |
| RT [min] | Compound | Formula | Relative Share [%] |
|---|---|---|---|
| 2.79 | 4-Pentyn-2-ol | C5H8O | 2.27 |
| 3.44 | Acetic acid | C2H4O2 | 29.06 |
| 4.21 | Hydrazinecarbothioamide | CH5N3S | 1.23 |
| 26.24 | Glycerin | C3H8O3 | 9.14 |
| 55.59 | N-Benzyl-1H-benzimidazole | C14H12N2 | 6.70 |
| 57.55 | Sulfurous acid, cyclohexylmethyl isobutyl ester | C11H22O3S | 1.29 |
| 58.24 | 1,2-Benzenediol, O-cyclobutanecarbonyl-O′-cyclopropanecarbonyl- | C15H16O4 | 20.13 |
| 60.36 | Isophthalic acid, di(2-methylprop-2-en-1-yl) ester | C16H18O4 | 1.93 |
| 60.87 | Sulfurous acid, di(cyclohexylmethyl) ester | C14H26O3S | 1.87 |
| 62.28 | 2-Buten-1-one, 1-(6,7,7-trimethyl-2,3-dioxabicyclo) | C13H18O3 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wądrzyk, M.; Korzeniowski, Ł.; Plata, M.; Janus, R.; Lewandowski, M.; Borówka, G.; Maziarka, P. Solvothermal Liquefaction of Blackcurrant Pomace in the Water-Monohydroxy Alcohol Solvent System. Energies 2023, 16, 1127. https://doi.org/10.3390/en16031127
Wądrzyk M, Korzeniowski Ł, Plata M, Janus R, Lewandowski M, Borówka G, Maziarka P. Solvothermal Liquefaction of Blackcurrant Pomace in the Water-Monohydroxy Alcohol Solvent System. Energies. 2023; 16(3):1127. https://doi.org/10.3390/en16031127
Chicago/Turabian StyleWądrzyk, Mariusz, Łukasz Korzeniowski, Marek Plata, Rafał Janus, Marek Lewandowski, Grzegorz Borówka, and Przemysław Maziarka. 2023. "Solvothermal Liquefaction of Blackcurrant Pomace in the Water-Monohydroxy Alcohol Solvent System" Energies 16, no. 3: 1127. https://doi.org/10.3390/en16031127
APA StyleWądrzyk, M., Korzeniowski, Ł., Plata, M., Janus, R., Lewandowski, M., Borówka, G., & Maziarka, P. (2023). Solvothermal Liquefaction of Blackcurrant Pomace in the Water-Monohydroxy Alcohol Solvent System. Energies, 16(3), 1127. https://doi.org/10.3390/en16031127






