Polymer Poly (Ethylene Oxide) Additive for High-Stability All-Inorganic CsPbI3−xBrx Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Solution
2.2. Experimental Procedure
2.3. Characterization
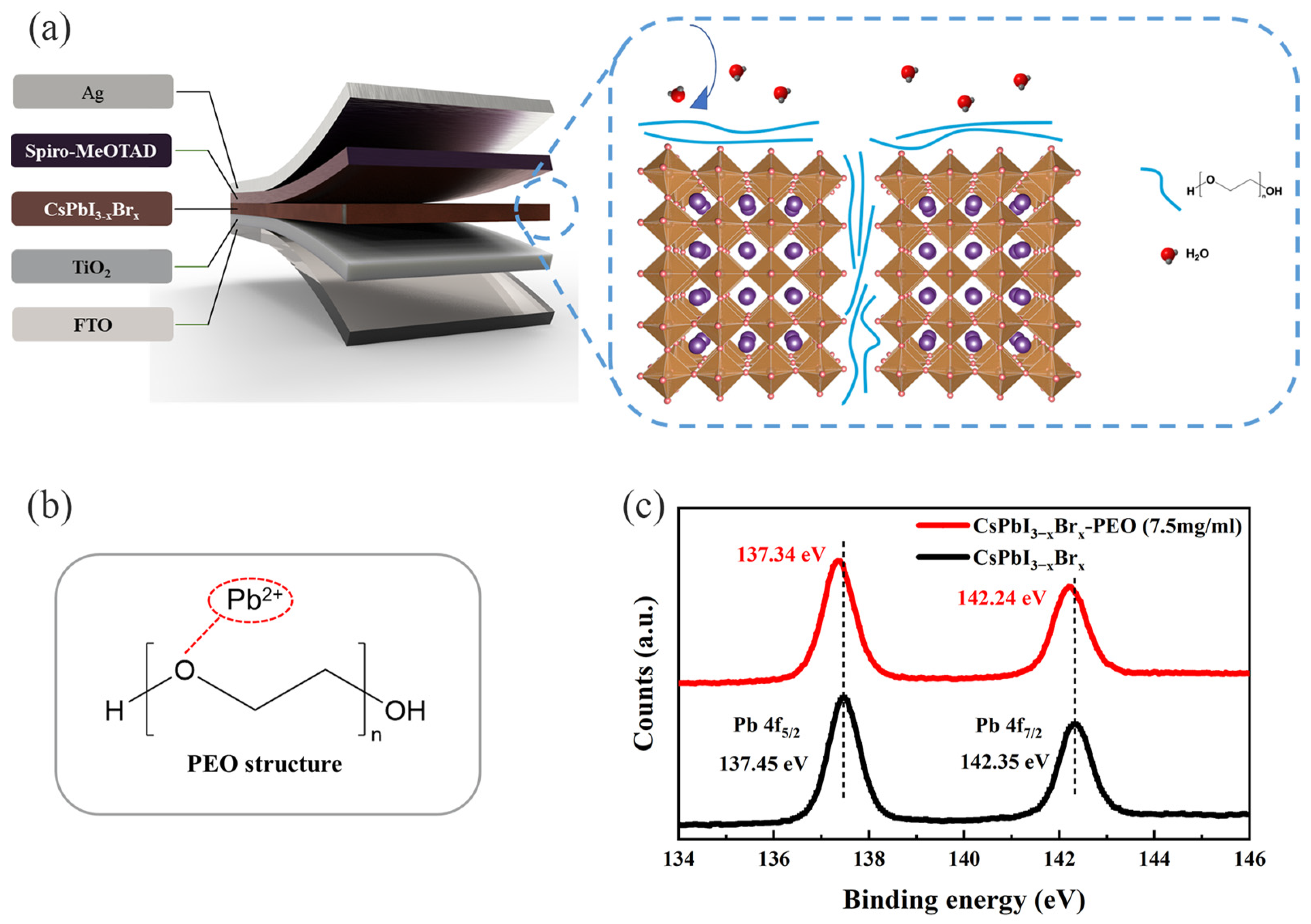
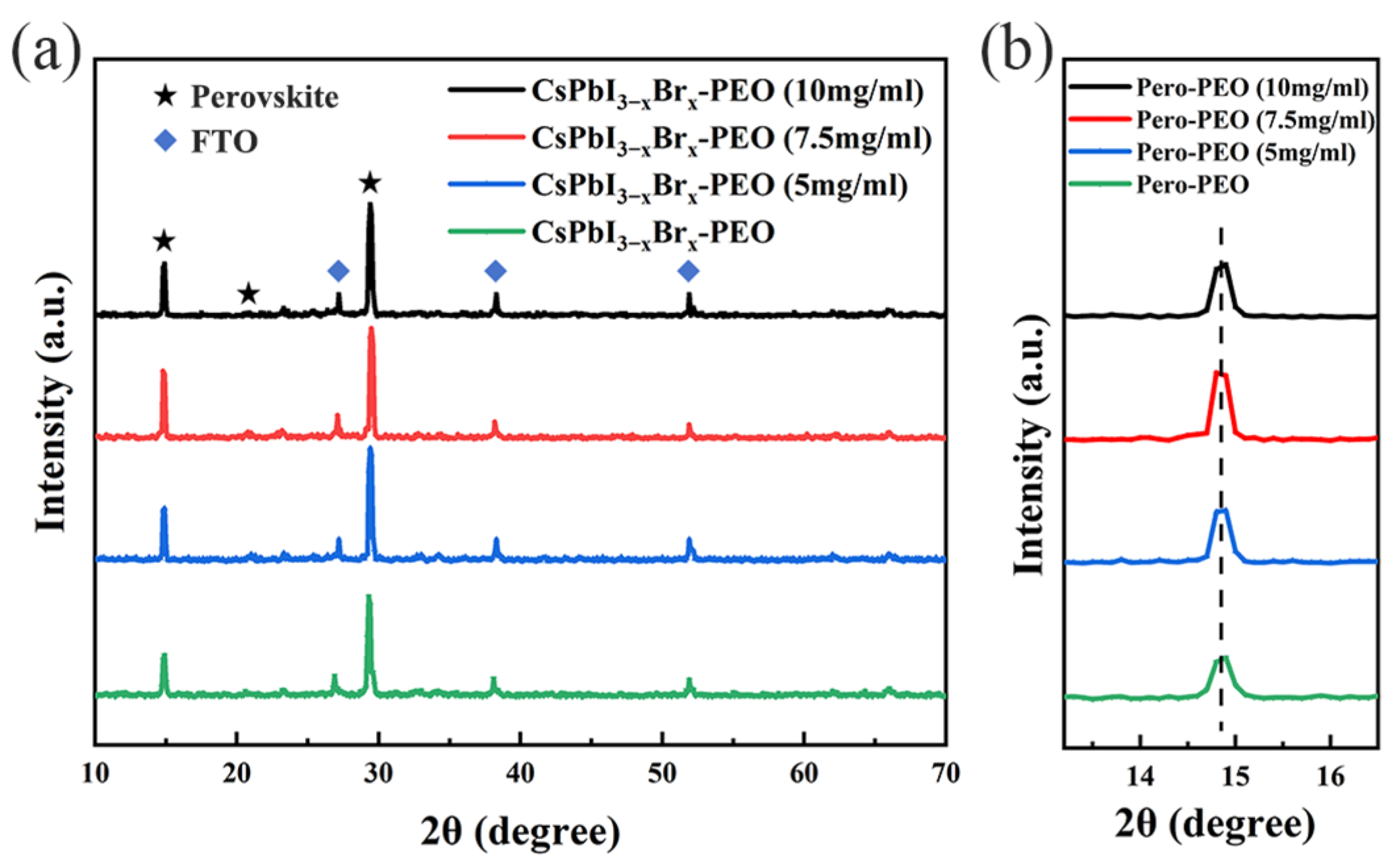
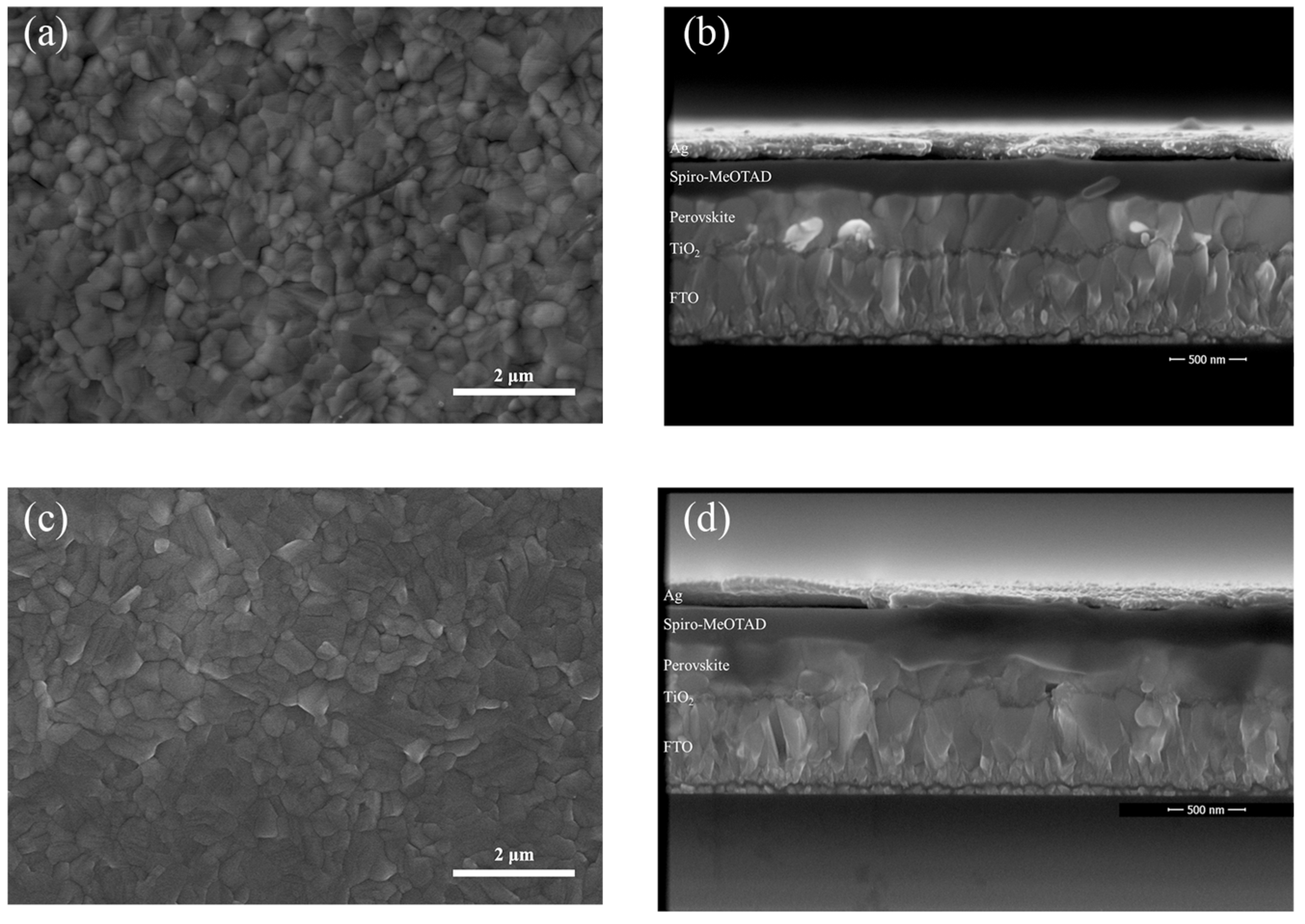
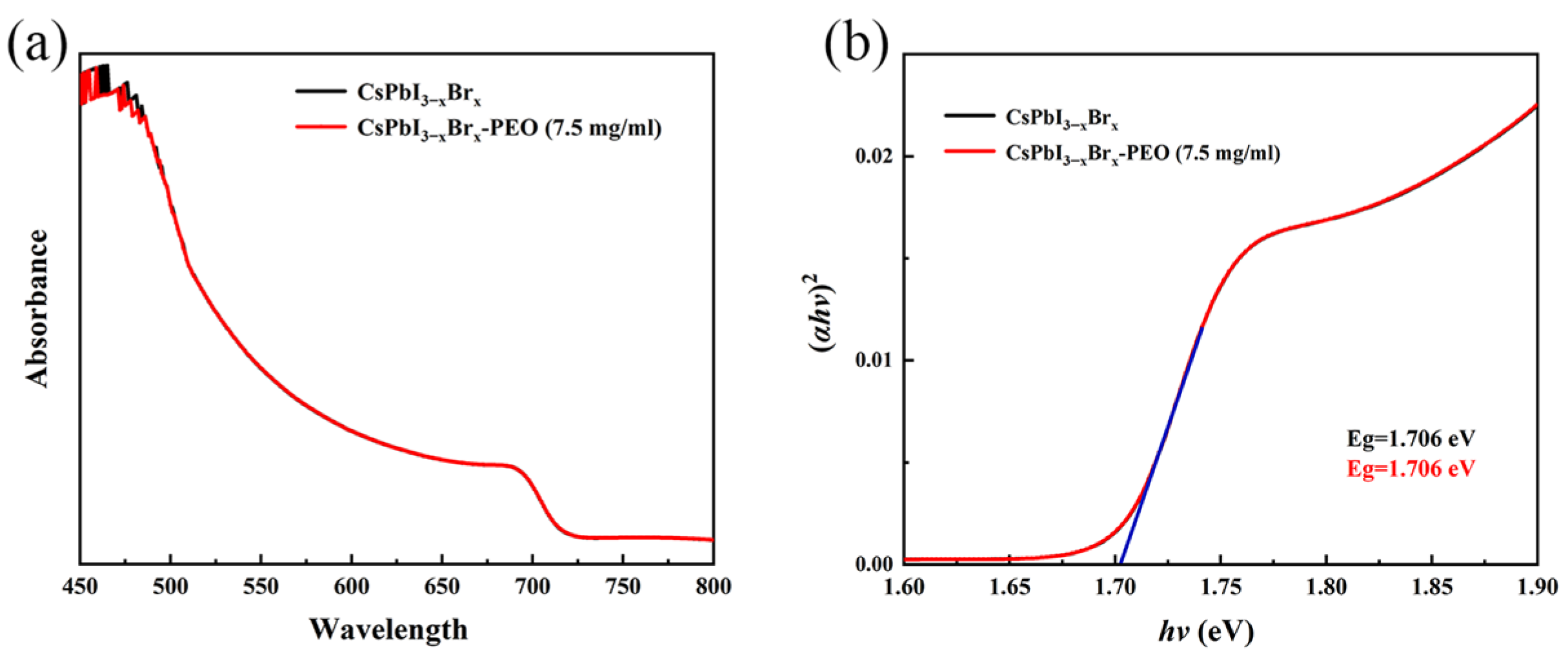
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayan, K.D.; Sebastian, V.; Kurian, J. Simulation and optimization studies on CsPbI3 based inorganic perovskite solar cells. Sol. Energy 2021, 221, 99–108. [Google Scholar] [CrossRef]
- Bai, D.; Zhang, J.; Jin, Z.; Bian, H.; Wang, K.; Wang, H.; Liang, L.; Wang, Q.; Liu, S. Interstitial Mn2+-driven high-aspect-ratio grain growth for low-trap-density microcrystalline films for record efficiency CsPbI2Br solar cells. ACS Energy Lett. 2018, 3, 970–978. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, R.; Yin, L. Slow-photon-effect-induced photoelectrical-conversion efficiency enhancement for carbon-quantum-dot-sensitized inorganic CsPbBr3 inverse opal perovskite solar cells. Adv. Mater. 2017, 29, 1703682. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Fu, L.; Yu, T.; Zhou, S.; Zhang, L.; Yin, L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018, 9, 1076. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, D.; Liu, F.; Lai, H.; Wan, X.; Zhang, X.; Liu, Y.; Chen, Y. Phase Distribution and Carrier Dynamics in Multiple-Ring Aromatic Spacer-Based Two-Dimensional Ruddlesden–Popper Perovskite Solar Cells. ACS Nano 2020, 14, 4871–4881. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.; Chen, B.; Uddin, M.A.; Ni, Z.; Yang, G.; Huang, J.-S. Solution-Processed Ternary Tin (II) Alloy as Hole-Transport Layer of Sn–Pb Perovskite Solar Cells for Enhanced Efficiency and Stability. Adv. Mater. 2022, 34, 2205769. [Google Scholar] [CrossRef]
- Yi, Z.; Ladi, N.H.; Shai, X.; Li, H.; Shen, Y.; Wang, M. Will organic–inorganic hybrid halide lead perovskites be eliminated from optoelectronic applications? Nanoscale Adv. 2019, 1, 1276–1289. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Peng, J.; Tang, J.; Zheng, K.; Liang, Z. 2D Ruddlesden–Popper Perovskites for Optoelectronics. Adv. Mater. 2017, 30, 1703487. [Google Scholar] [CrossRef]
- Huang, J.; Chen, C.; Li, Y.; Wang, H.; Zhang, F.; Zhang, D. BT-MA0.6FA0.4PbI3–xClx Unsymmetrical Perovskite for Solar Cells with Superior Stability and PCE over 23%. ACS Appl. Energy Mater. 2022, 5, 11058–11066. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, S.; Li, W.; Liu, H.; Zhu, L.; Yang, S. Inorganic Perovskite Solar Cells: A Rapidly Growing Field. Sol. RRL 2018, 2, 1700188. [Google Scholar] [CrossRef]
- Gao, C.; Shao, Z.; Sun, X.; Li, Z.; Rao, Y.; Lv, P.; Wei, Y.; Chen, C.; Liu, D.; Wang, X.; et al. Fabrication and Characterization of FAxCs1−xPbI3 Polycrystal Perovskite Solar Cells. Sol. RRL 2021, 5, 2100166. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xu, G.; Xue, R.; Wang, S.; Li, Y.; Li, Y. Precise Control of Crystal Growth for Highly Efficient CsPbI2Br Perovskite Solar Cells. Joule 2019, 3, 191–204. [Google Scholar] [CrossRef]
- Weerasinghe, H.C.; Dkhissi, Y.; Scully, A.D.; Caruso, R.A.; Cheng, Y.-B. Encapsulation for improving the lifetime of flexible perovskite solar cells. Nano Energy 2015, 18, 118–125. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Tang, Z.; Su, C.; Huang, W.; Li, Y.; Xing, G. Polymer strategies for high-efficiency and stable perovskite solar cells. Nano Energy 2021, 82, 105712. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, X.; Zhou, K.; Cai, C.; Hu, Z.; Zhang, J.; Zhu, Y. Amorphous polymer with C=O to improve the performance of perovskite solar cells. J. Mater. Chem. C 2017, 5, 9037–9043. [Google Scholar] [CrossRef]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, J.-W.; Choi, C.; Tan, S.; Lee, C.; Zhao, Y.; Dai, Z.; De Marco, N.; Lee, S.-J.; Bae, S.-H.; et al. Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells. Nat. Commun. 2019, 10, 520. [Google Scholar] [CrossRef]
- Wu, J.; Cui, Y.; Yu, B.; Liu, K.; Li, Y.; Li, H.; Shi, J.; Wu, H.; Luo, Y.; Li, D.; et al. A Simple Way to Simultaneously Release the Interface Stress and Realize the Inner Encapsulation for Highly Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1905336. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.; Yu, D.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Khan, J.I.; Liu, W.; Ugur, E.; Duong, T.; Wu, Y.; Shen, H.; Wang, K.; Dang, H.; Aydin, E.; et al. A Universal Double-Side Passivation for High Open-Circuit Voltage in Perovskite Solar Cells: Role of Carbonyl Groups in Poly(methyl methacrylate). Adv. Energy Mater. 2018, 8, 1801208. [Google Scholar] [CrossRef]
- Zhang, M.; Li, T.; Zheng, G.; Li, L.; Qin, M.; Zhang, S.; Zhou, H.; Zhan, X. An amino-substituted perylene diimide polymer for conventional perovskite solar cells. Mater. Chem. Front. 2017, 1, 2078–2084. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.; Zhang, M.; Chen, C.; Zhang, W.; Huang, J. Fluorine-containing organic ammonium salt-doped inverted inorganic perovskite solar cells. Semicond. Sci. Technol. 2022, 37, 095014. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhang, C.; Ma, Y.; Fan, J.; Mai, Y. All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%. J. Am. Chem. Soc. 2018, 140, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Chen, C.; Tang, Y.; Yang, H.; Wang, H.; Huang, J.; Zhang, D. Improve Perovskite Solar Cell Stability and Efficiency via Multifunctional Multiple-Ring Aromatic Dopant Passivation. Sol. RRL 2023, 7, 2300163. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, X.; Xu, S.; Liu, G.; Chen, S.; Zhang, X.; Chen, T.; Pan, X. The multiple effects of polyaniline additive to improve the efficiency and stability of perovskite solar cells. J. Mater. Chem. C 2019, 7, 4441–4448. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, K.; Zhu, T.; Yang, Y.; Chen, R.; Gu, K.; Liu, C.; Gong, X. High-Performance Perovskite Solar Cells by One-Step Self-Assembled Perovskite-Polymer Thin Films. ACS Appl. Energy Mater. 2020, 3, 5902–5912. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, J.; Li, J.; Daoud, W.A. Organic Dye Passivation for High-Performance All-Inorganic CsPbI1.5Br1.5 Perovskite Solar Cells with Efficiency over 14%. Adv. Energy Mater. 2020, 11, 2003585. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Li, R.; Jing, Y.; Zhang, H.; Wang, X.; Du, Z.; Wu, J.; Lan, Z. PbS/CdS heterojunction thin layer affords high-performance carbon-based all-inorganic solar cells. Nano Energy 2022, 95, 106973. [Google Scholar] [CrossRef]
- Wang, J.; Li, N.; Zhong, C.; Miao, J.; Huang, Z.; Yu, M.; Hu, Y.X.; Luo, S.; Zou, Y.; Li, K.; et al. Metal-Perturbed Multiresonance TADF Emitter Enables High-Efficiency and Ultralow Efficiency Roll-Off Nonsensitized OLEDs with Pure Green Gamut. Adv. Mater. 2022, 35, 2208378. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Guo, H.; deQuilettes, D.W.; Jariwala, S.; De Marco, N.; Dong, S.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M.; et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, e1700106. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qiao, W.; Xiong, S.; Yang, J.; Wang, X.; Ding, L.; Yao, Y.; Bao, Q. Surface Passivation and Energetic Modification Suppress Nonradiative Recombination in Perovskite Solar Cells. Nano-Micro Lett. 2022, 14, 108. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, P.; Luo, D.; Dar, M.I.; Eickemeyer, F.T.; Arora, N.; Hu, Q.; Luo, J.; Liu, Y.; Zakeeruddin, S.M.; et al. Enabling full-scale grain boundary mitigation in polycrystalline perovskite solids. Sci. Adv. 2022, 8, eabo3733. [Google Scholar] [CrossRef]
- Tan, X.; Liu, X.; Liu, Z.; Sun, B.; Li, J.; Xi, S.; Shi, T.; Tang, Z.; Liao, G. Enhancing the optical, morphological and electronic properties of the solution-processed CsPbIBr2 films by Li doping for efficient carbon-based perovskite solar cells. Appl. Surf. Sci. 2020, 499, 143990. [Google Scholar] [CrossRef]
- Tang, S.; Peng, Y.; Zhu, Z.; Zong, J.; Zhao, L.; Yu, L.; Chen, R.; Li, M. Simultaneous Bulk and Surface Defect Passivation for Efficient Inverted Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 5116–5122. [Google Scholar] [CrossRef]
- Liu, X.; Fu, S.; Zhang, W.; Xu, Z.; Li, X.; Fang, J.; Zhu, Y. A Universal Dopant-Free Polymeric Hole-Transporting Material for Efficient and Stable All-Inorganic and Organic–Inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 52549–52559. [Google Scholar] [CrossRef]
- Wang, K.; Gao, C.; Xu, Z.; Tian, Q.; Gu, X.; Zhang, L.; Zhang, S.; Zhao, K.; Liu, S. In-Situ Hot Oxygen Cleansing and Passivation for All-Inorganic Perovskite Solar Cells Deposited in Ambient to Breakthrough 19% Efficiency. Adv. Funct. Mater. 2021, 31, 2101568. [Google Scholar] [CrossRef]
- Ran, C.; Gao, W.; Li, J.; Xi, J.; Li, L.; Dai, J.; Yang, Y.; Gao, X.; Dong, H.; Jiao, B.; et al. Conjugated Organic Cations Enable Efficient Self-Healing FASnI3 Solar Cells. Joule 2019, 3, 3072–3087. [Google Scholar] [CrossRef]
- Chang, C.; Chu, C.; Huang, Y.; Huang, C.; Chang, S.; Chen, C.-A.; Chao, C.; Su, W. Tuning Perovskite Morphology by Polymer Additive for High Efficiency Solar Cell. ACS Appl. Mater. Interfaces 2015, 7, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
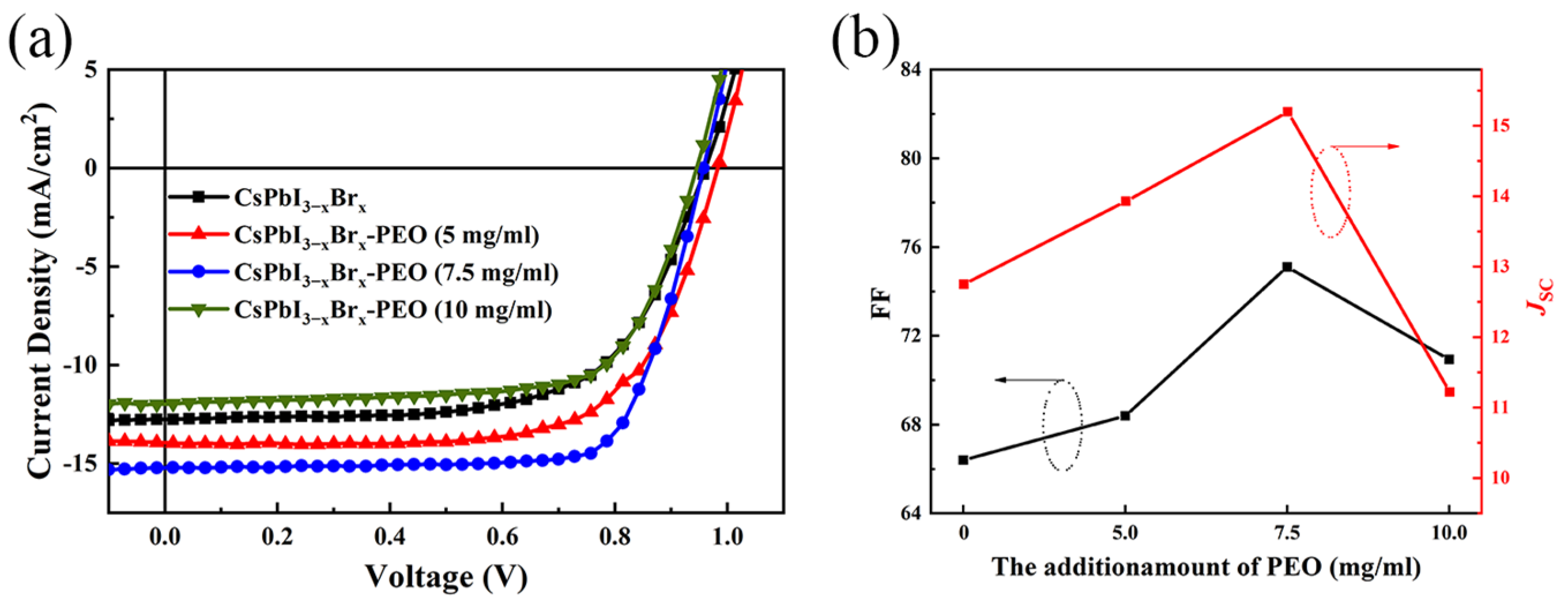
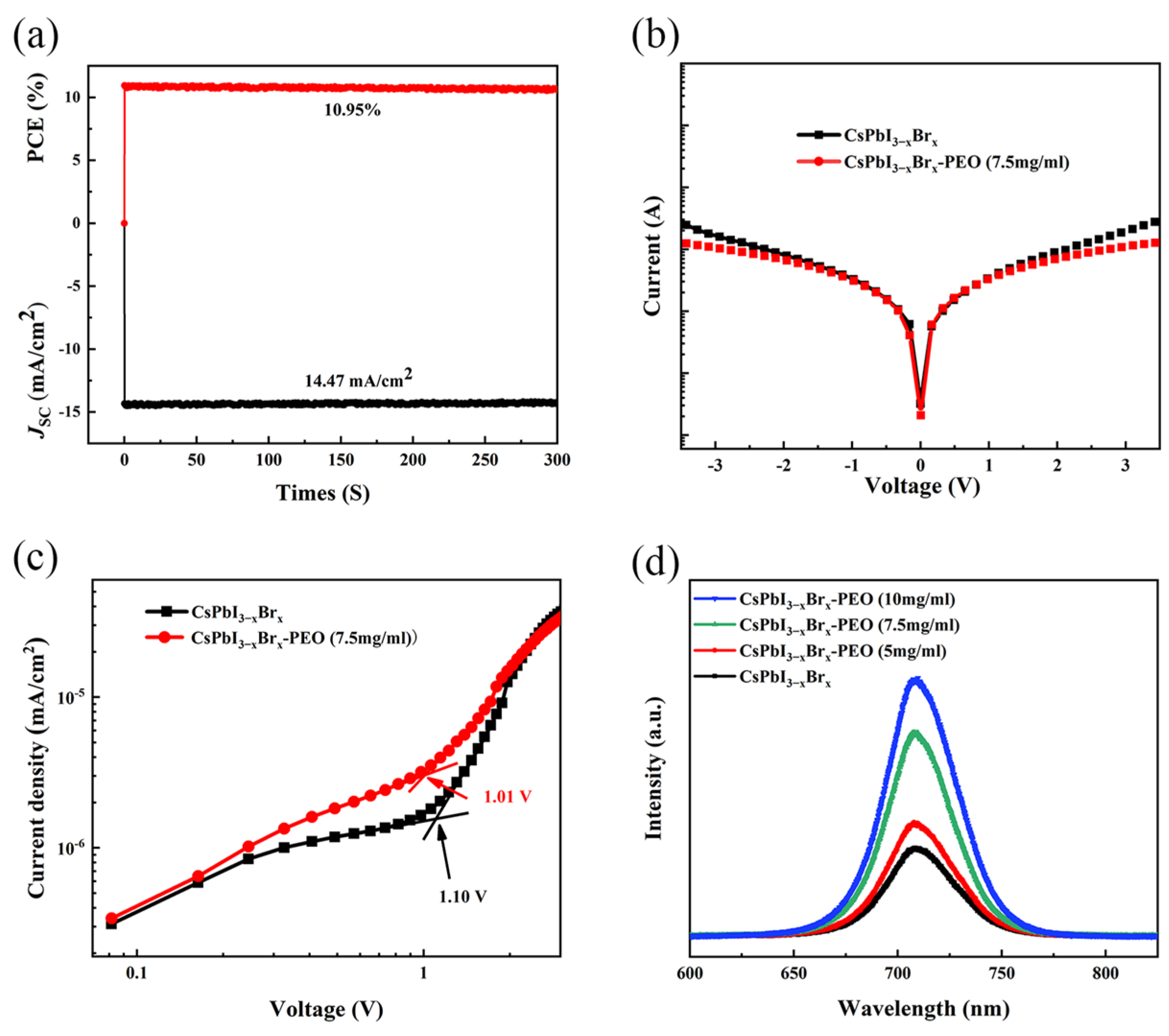
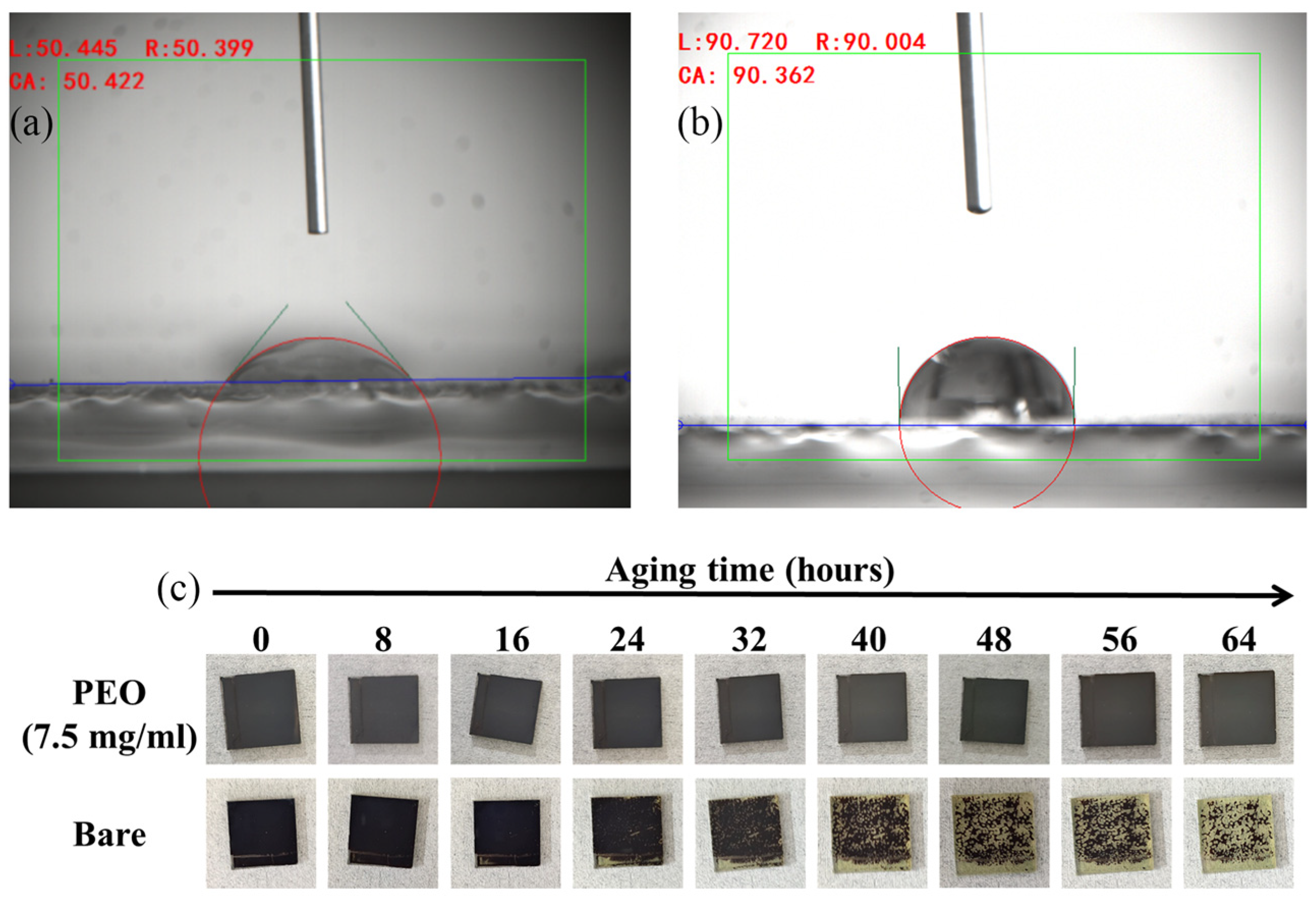
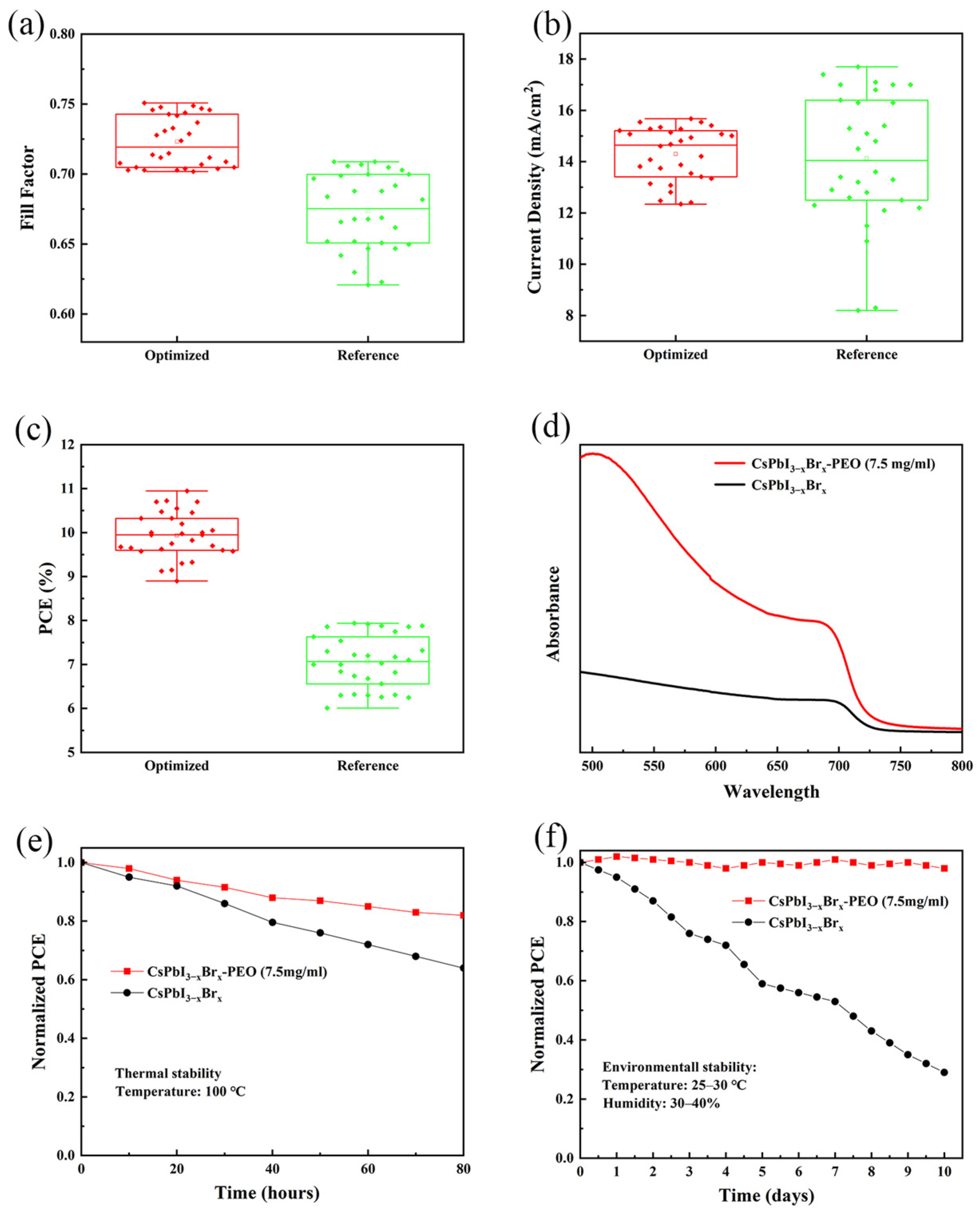
| Devices PEO Ratio | VOC (V) | JSC (mA cm−2) | Fill Factor | Rs/Rsh (Ω/cm−2) | PCE(%) Average | PCE(%) Best |
|---|---|---|---|---|---|---|
| (0 mg/mL) | 0.96 | 12.75 | 0.65 | 119.70/34,047.62 | 7.06 | 7.94 |
| (5 mg/mL) | 0.98 | 13.94 | 0.68 | 100.41/6651.16 | 8.26 | 9.37 |
| (7.5 mg/mL) | 0.96 | 15.20 | 0.75 | 82.41/16,627.91 | 9.93 | 10.95 |
| (10 mg/mL) | 0.95 | 11.22 | 0.70 | 101.05/8909.65 | 7.15 | 7.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Zhang, F.-H.; Huang, J.; Xue, T.; Wang, X.; Zheng, C.-F.; Wang, H.; Jia, C.-L. Polymer Poly (Ethylene Oxide) Additive for High-Stability All-Inorganic CsPbI3−xBrx Perovskite Solar Cells. Energies 2023, 16, 7849. https://doi.org/10.3390/en16237849
Chen C-Y, Zhang F-H, Huang J, Xue T, Wang X, Zheng C-F, Wang H, Jia C-L. Polymer Poly (Ethylene Oxide) Additive for High-Stability All-Inorganic CsPbI3−xBrx Perovskite Solar Cells. Energies. 2023; 16(23):7849. https://doi.org/10.3390/en16237849
Chicago/Turabian StyleChen, Chun-Yang, Fang-Hui Zhang, Jin Huang, Tao Xue, Xiao Wang, Chao-Fan Zheng, Hao Wang, and Chun-Liang Jia. 2023. "Polymer Poly (Ethylene Oxide) Additive for High-Stability All-Inorganic CsPbI3−xBrx Perovskite Solar Cells" Energies 16, no. 23: 7849. https://doi.org/10.3390/en16237849
APA StyleChen, C.-Y., Zhang, F.-H., Huang, J., Xue, T., Wang, X., Zheng, C.-F., Wang, H., & Jia, C.-L. (2023). Polymer Poly (Ethylene Oxide) Additive for High-Stability All-Inorganic CsPbI3−xBrx Perovskite Solar Cells. Energies, 16(23), 7849. https://doi.org/10.3390/en16237849







