The Effect of Organic Acid Dopants on the Specific Capacitance of Electrodeposited Polypyrrole-Carbon Nanotube/Polyimide Composite Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Preparation of Poly(amic acid)/SWCNT Composite
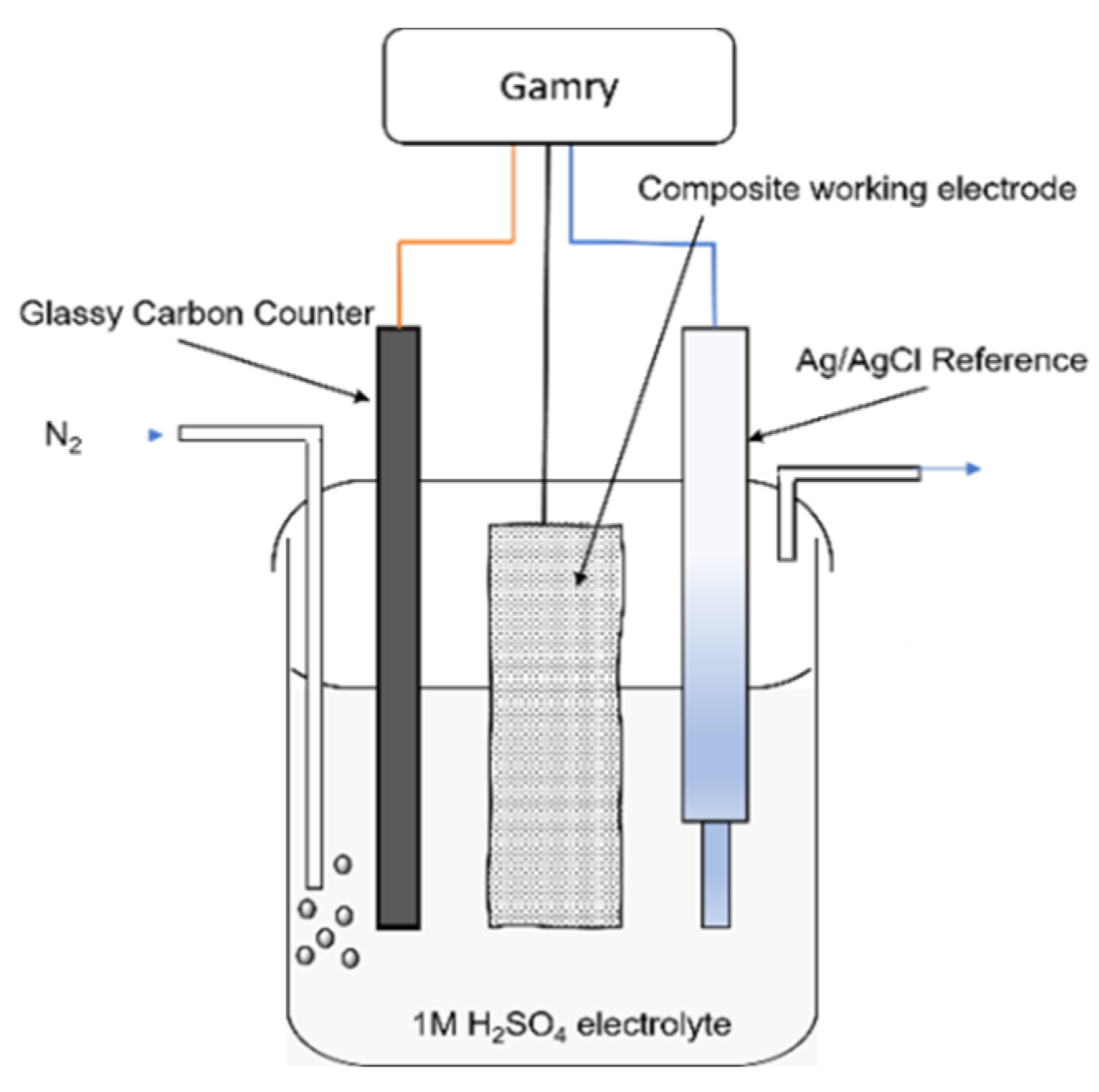
2.2.2. Electrodeposition of Polypyrrole
3. Characterization Techniques
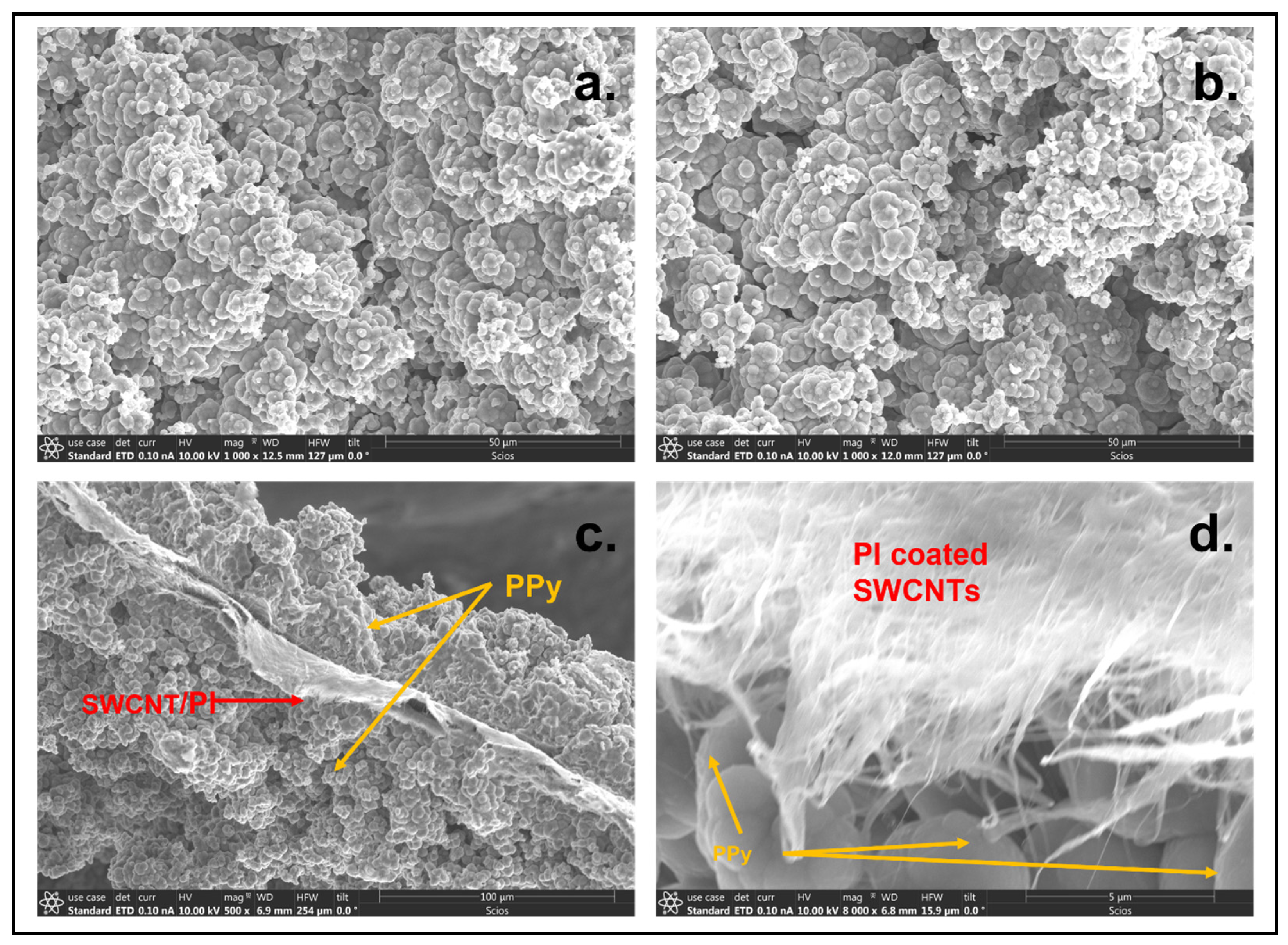
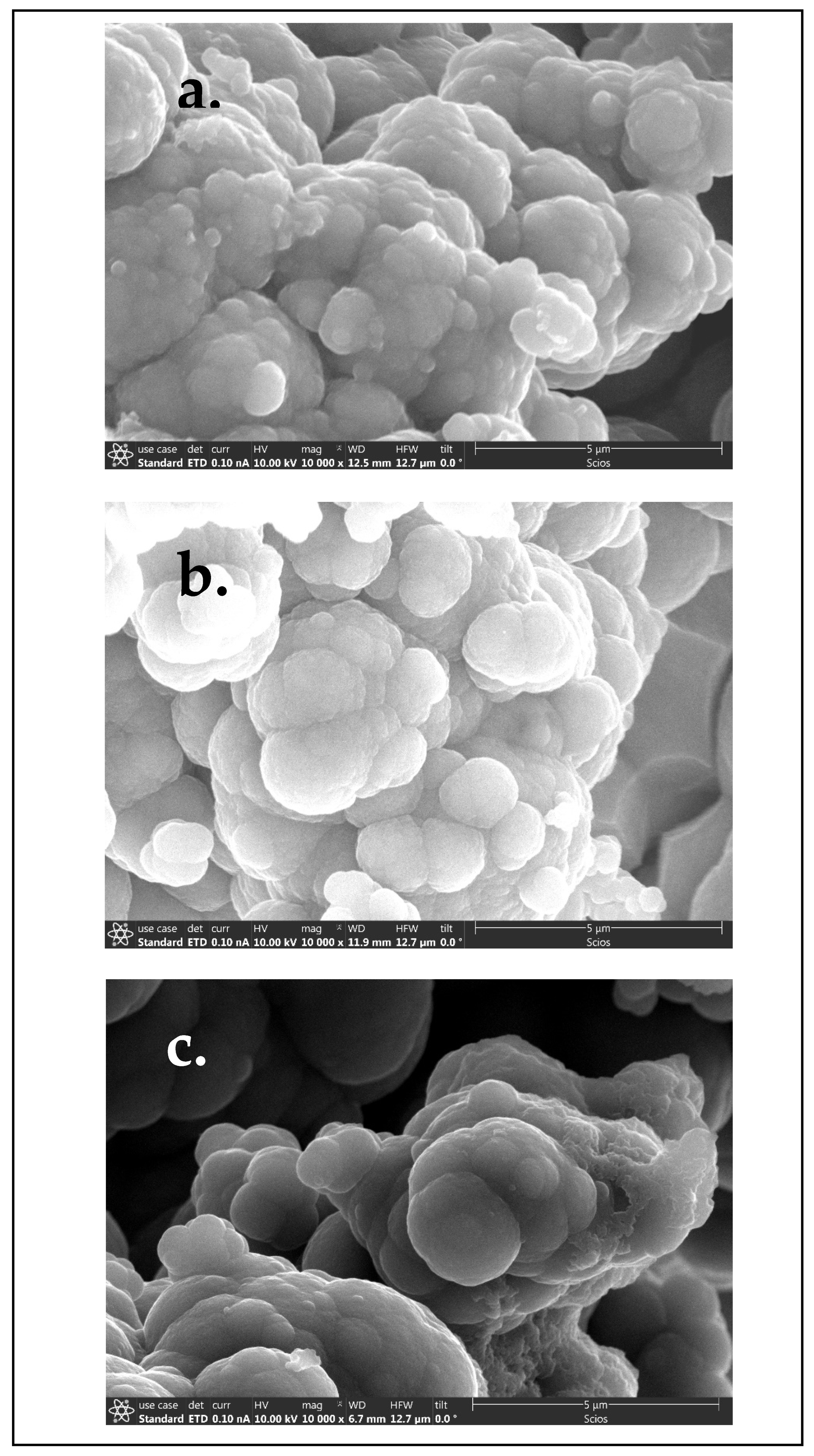
3.1. Scanning Electron Microscopy (SEM)
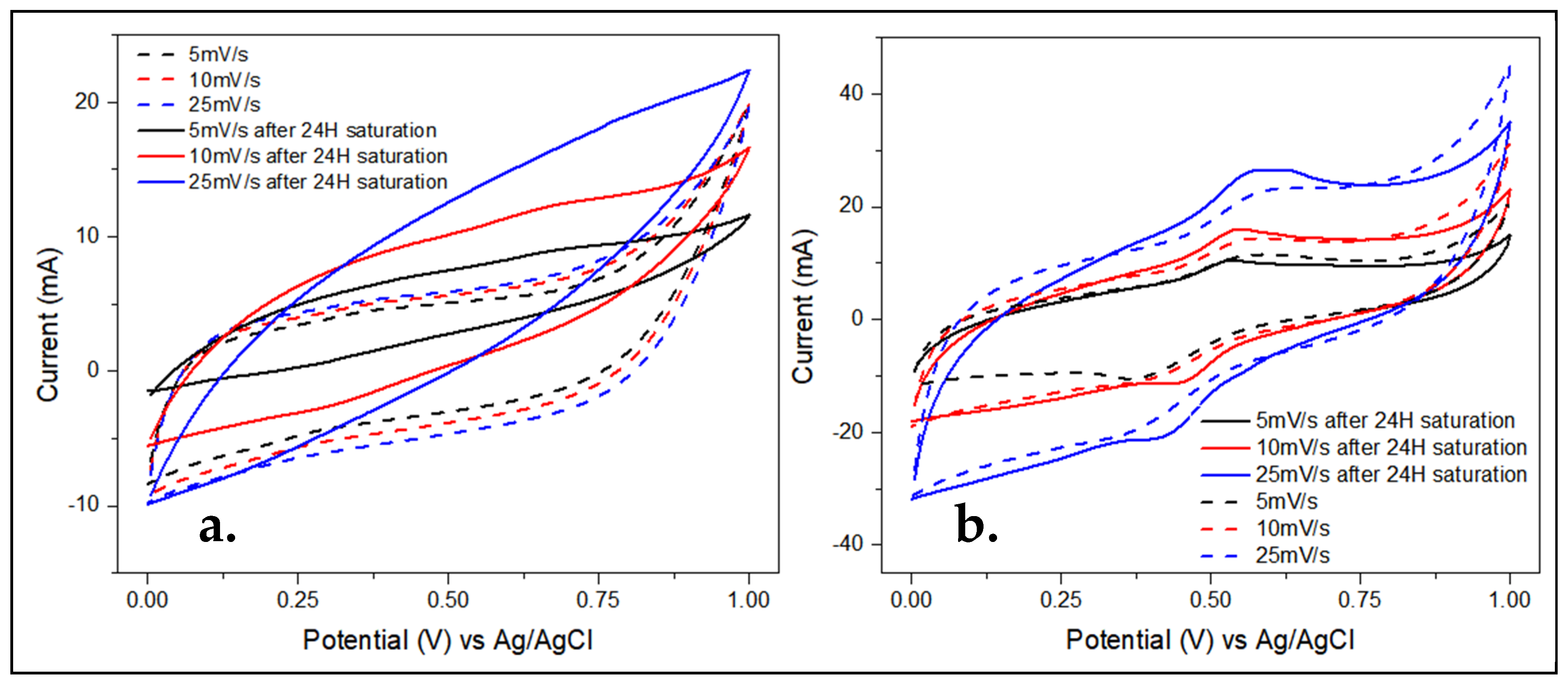
3.2. Cyclic Voltammetry
3.3. Galvanic Charge–Discharge Cycles
3.4. Electrochemical Impedance Spectroscopy, EIS
3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.6. Dynamic Mechanical Analysis
4. Results and Discussion
4.1. Scanning Electron Microscopy (SEM)
4.2. Cyclic Voltammetry, CV
4.3. Galvanic Charge–Discharge Curves
4.4. Electrochemical Impedance Spectroscopy
4.5. Fourier Transform Infrared Spectroscopy
4.6. Dynamic Mechanical Analysis, DMA
4.7. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Fu, X.; Zheng, M.; Zhong, W.H.; Cao, G. Strategies for Building Robust Traffic Networks in Advanced Energy Storage Devices: A Focus on Composite Electrodes. Adv. Mater. 2019, 31, 1804204. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, L.; Chen, D.; Yu, G. Intercalation Pseudocapacitance in Ultrathin VOPO4 Nanosheets: Toward High-Rate Alkali-Ion-Based Electrochemical Energy Storage. Nano Lett. 2016, 16, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xiong, P.; Ma, L.; Yuan, Y.; Zhu, Y.; Chen, D.; Luo, X.; Lu, J.; Amine, K.; Yu, G. Holey two-dimensional transition metal oxide nanosheets for efficient energy storage. Nat. Commun. 2017, 8, 15139. [Google Scholar] [CrossRef]
- Yuan, R.; Bi, W.; Zhou, T.; Zhang, N.; Zhong, C.; Chu, W.; Yan, W.; Xu, Q.; Wu, C.; Xie, Y. Two-Dimensional Hierarchical Fe-N-C Electrocatalyst for Zn-Air Batteries with Ultrahigh Specific Capacity. ACS Mater. Lett. 2020, 2, 35–41. [Google Scholar] [CrossRef]
- Zeng, Z.; Fu, G.; Yang, H.B.; Yan, Y.; Chen, J.; Yu, Z.; Gao, J.; Gan, L.Y.; Liu, B.; Chen, P. Bifunctional N-CoSe2/3D-MXene as Highly Efficient and Durable Cathode for Rechargeable Zn-Air Battery. ACS Mater. Lett. 2019, 1, 432–439. [Google Scholar] [CrossRef]
- Shi, B.; Shang, Y.; Pei, Y.; Pei, S.; Wang, L.; Heider, D.; Zhao, Y.Y.; Zheng, C.; Yang, B.; Yarlagadda, S.; et al. Low Tortuous, Highly Conductive, and High-Areal-Capacity Battery Electrodes Enabled by Through-thickness Aligned Carbon Fiber Framework. Nano Lett. 2020, 20, 5504–5512. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Plenum Press: New York, NY, USA, 1999; ISBN 9780306457364. [Google Scholar]
- Yu, D.; Dai, L. Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J. Phys. Chem. Lett. 2010, 1, 467–470. [Google Scholar] [CrossRef]
- Wu, G.; Tan, P.; Wang, D.; Li, Z.; Peng, L.; Hu, Y.; Wang, C.; Zhu, W.; Chen, S.; Chen, W. High-performance Supercapacitors Based on Electrochemical-induced Vertical-aligned Carbon Nanotubes and Polyaniline Nanocomposite Electrodes. Sci. Rep. 2017, 7, 43676. [Google Scholar] [CrossRef]
- Lewandowski, A.; Olejniczak, A.; Galinski, M.; Stepniak, I. Performance of carbon-carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J. Power Sources 2010, 195, 5814–5819. [Google Scholar] [CrossRef]
- Samantara, A.K.; Ratha, S. Materials Development for Active/Passive Components of a Supercapacitor Background, Present Status and Future Perspective; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Simpson, C. Characteristics of Rechargeable Batteries, Texas Instruments, Literature Number: SNVA533. Snva533. 2011. Available online: https://pdf4pro.com/view/characteristics-of-rechargeable-batteries-1f813a.html (accessed on 20 August 2023).
- Moyseowicz, A.; Gryglewicz, G. High-performance hybrid capacitor based on a porous polypyrrole/reduced graphene oxide composite and a redox-active electrolyte. Electrochim. Acta 2020, 354, 136661. [Google Scholar] [CrossRef]
- Khare, R.; Bose, S. Carbon Nanotube Based Composites—A Review. J. Miner. Mater. Charact. Eng. 2005, 4, 31–46. [Google Scholar] [CrossRef]
- He, H.; Tu, Y.; Li, J.; Lin, Y.; Chen, J. Porous Polyimide and Carbon Nanotubes: Solvent Vapor–Induced Transformation in the Nanochannels of Anodic Aluminum Oxide Templates. Macromol. Mater. Eng. 2019, 304, 1800700. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Thuau, D.; Koutsos, V.; Cheung, R. Electrical and mechanical properties of carbon nanotube-polyimide composites. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2009, 27, 3139. [Google Scholar] [CrossRef]
- Wang, H.; Yi, H.; Chen, X.; Wang, X. Asymmetric supercapacitors based on nano-architectured nickel oxide/graphene foam and hierarchical porous nitrogen-doped carbon nanotubes with ultrahigh-rate performance. J. Mater. Chem. A 2014, 2, 3223–3230. [Google Scholar] [CrossRef]
- Ansaldo, A.; Bondavalli, P.; Bellani, S.; Del Rio Castillo, A.E.; Prato, M.; Pellegrini, V.; Pognon, G.; Bonaccorso, F. High-power graphene–Carbon nanotube hybrid supercapacitors. ChemNanoMat 2017, 3, 436–446. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G. Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Kareem, A.A. Preparation and electrical properties of polyimide/carbon nanotubes composites. Mater. Sci. Pol. 2017, 35, 755–759. [Google Scholar] [CrossRef]
- Dong, Z.H.; Wei, Y.L.; Shi, W.; Zhang, G.A. Characterisation of doped polypyrrole/manganese oxide nanocomposite for supercapacitor electrodes. Mater. Chem. Phys. 2011, 131, 529–534. [Google Scholar] [CrossRef]
- Iroh, J.O.; Levine, K. Electrochemical synthesis of polypyrrole/polyimide conducting composite using a polyamic acid precursor. Eur. Polym. J. 2002, 38, 1547–1550. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Liu, L.; Zhang, Y.; Shi, Q.; Zhao, Q.; Cheng, Y.; Zhou, C.; Yang, S.; Song, X. Spongy p-Toluenesulfonic Acid-doped Polypyrrole with Extraordinary Rate Performance as Durable Anodes of Sodium-Ion Batteries at Different Temperatures. Langmuir 2020, 36, 15075–15081. [Google Scholar] [CrossRef] [PubMed]
- Neithalath, N.; Weiss, J.; Olek, J. Characterizing Enhanced Porosity Concrete using electrical impedance to predict acoustic and hydraulic performance. Cem. Concr. Res. 2006, 36, 2074–2085. [Google Scholar] [CrossRef]
- Tully-Dartez, S.; Cardenas, H.E.; Sit, P.F.S. Pore characteristics of chitosan scaffolds studied by electrochemical impedance spectroscopy. Tissue Eng.-Part C Methods 2010, 16, 339–345. [Google Scholar] [CrossRef]
- Stamatopoulos, K.M.; Chondrou, I.T.; Panteliou, S.D. Damping associated with porosity in porous rectangular plates. Civil-Comp Proc. 2008, 88, 115. [Google Scholar] [CrossRef]





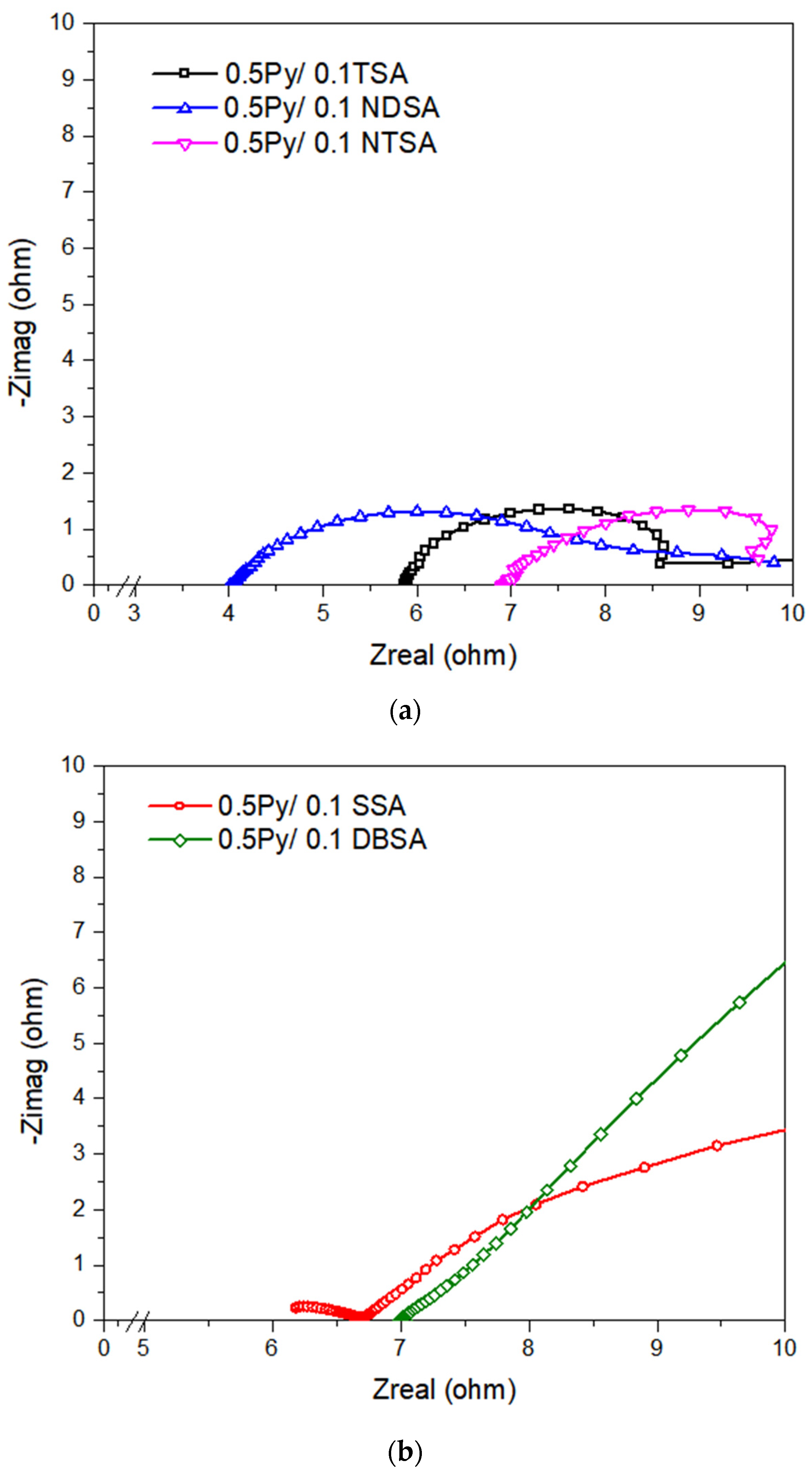



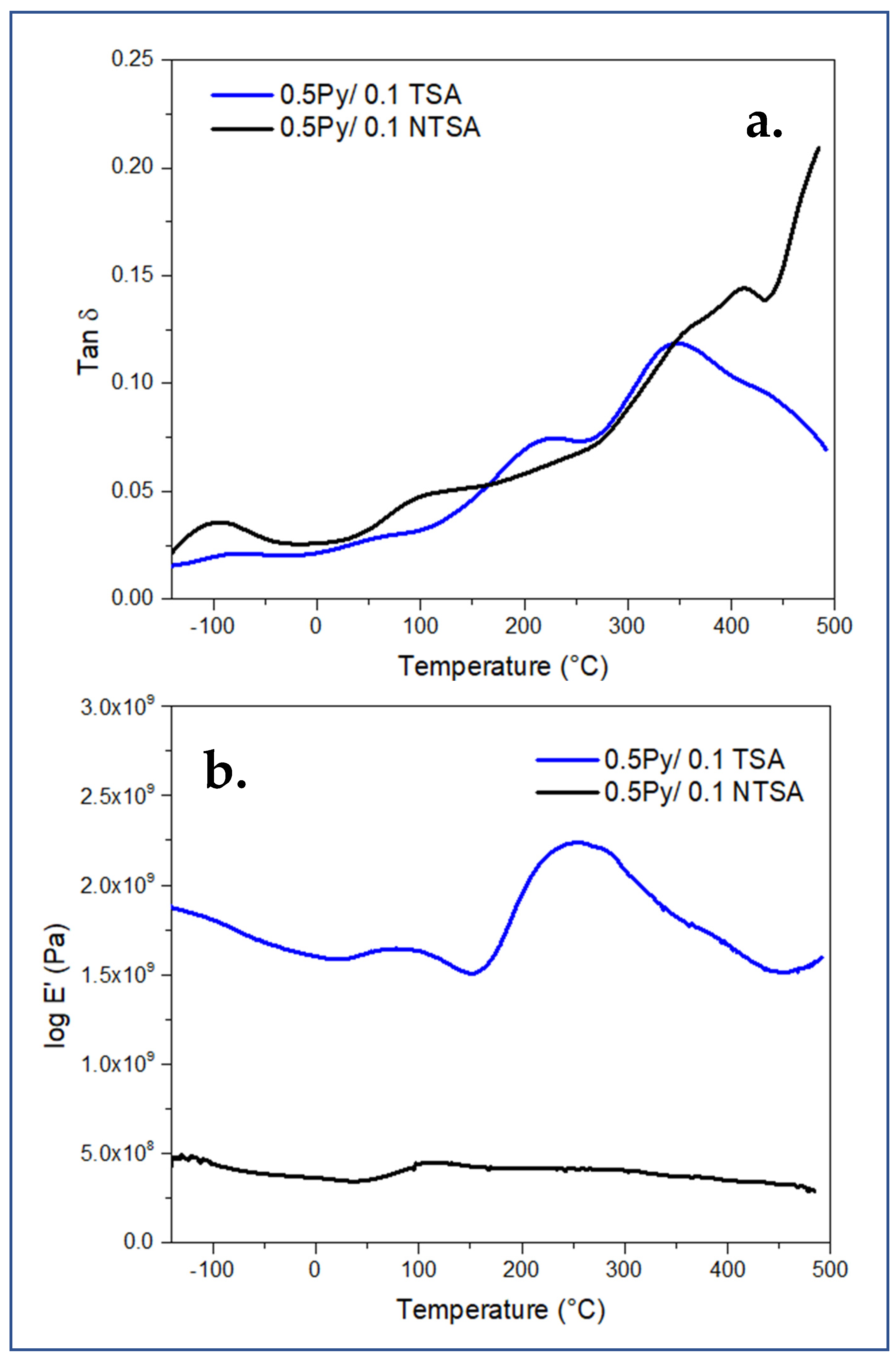
| Scan Rate (mV/s) | 30 min Saturation (F/g) | 24 h Saturation (F/g) |
|---|---|---|
| 5 | 84.88 | 96.10 |
| 10 | 50.37 | 99.53 |
| 25 | 22.95 | 49.90 |
| Sample | Theoretical Porosity (%) |
|---|---|
| 0.5 PPy/0.1 TSA | 34.68 |
| 0.5 PPy/0.1 TSA 24 h | 38.93 * |
| 0.5 PPy/0.1 NDSA | 31.60 |
| 0.5 PPy/0.1 NDSA 24 h | 43.10 * |
| 0.5 PPy/0.1 NTSA | 39.91 |
| 0.5 PPy/0.1 DBSA | 3.89 |
| 0.5 PPy/0.1 SSA | 13.22 |
| Characteristic Wavenumber (cm−1) | Functional Group |
|---|---|
| 3500–3300 | N–H amine stretch |
| 1700 | C=C aromatic bending |
| 1561–1531 | C=C/C–C stretching |
| 1300 | C–N stretching |
| 1170 | N–C stretch bending |
| 860 | C–H bending |
| 739 | C=O bending |
| Sample | Theoretical Porosity—EIS (%) | Tan Delta Peak Area | SEM Image Analysis (%) |
|---|---|---|---|
| 0.5 PPy 0.1 TSA | 34.68 | 11.05 | 15.01 |
| 0.5 PPy 0.1 NTSA | 39.91 | 12.87 | 20.79 |
| 0.5 PPy 0.1 SSA | 13.22 | 7.55 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gooneratne, R.; Iroh, J.O. The Effect of Organic Acid Dopants on the Specific Capacitance of Electrodeposited Polypyrrole-Carbon Nanotube/Polyimide Composite Electrodes. Energies 2023, 16, 7462. https://doi.org/10.3390/en16227462
Gooneratne R, Iroh JO. The Effect of Organic Acid Dopants on the Specific Capacitance of Electrodeposited Polypyrrole-Carbon Nanotube/Polyimide Composite Electrodes. Energies. 2023; 16(22):7462. https://doi.org/10.3390/en16227462
Chicago/Turabian StyleGooneratne, Ruchinda, and Jude O. Iroh. 2023. "The Effect of Organic Acid Dopants on the Specific Capacitance of Electrodeposited Polypyrrole-Carbon Nanotube/Polyimide Composite Electrodes" Energies 16, no. 22: 7462. https://doi.org/10.3390/en16227462
APA StyleGooneratne, R., & Iroh, J. O. (2023). The Effect of Organic Acid Dopants on the Specific Capacitance of Electrodeposited Polypyrrole-Carbon Nanotube/Polyimide Composite Electrodes. Energies, 16(22), 7462. https://doi.org/10.3390/en16227462







