Life Cycle Assessment of Poplar Biomass for High Value Products and Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aim and Scope of the LCA
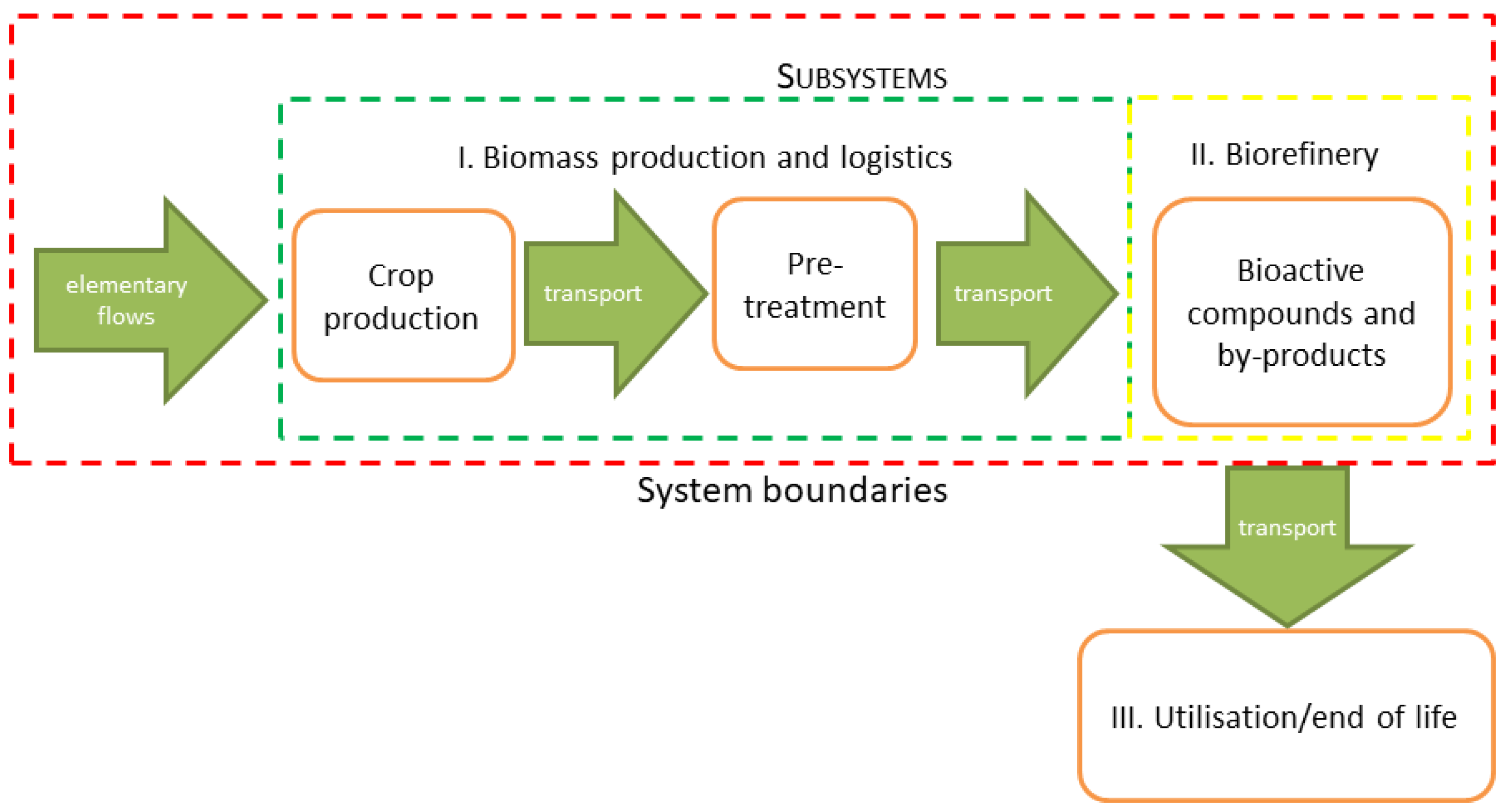
- Subsystem I: poplar biomass production and logistics, including pre-treatment.
- Subsystem II: acquisition of bioactive compounds and by-products in a biorefinery.
2.2. System Description
2.2.1. Subsystem I: Biomass Production and Logistics
2.2.2. Subsystem II: Production of Bioactive Substances from Poplar Biomass, and Conversion of Residual Biomass to Pellets
3. Results
3.1. Characterisation Scores at the Midpoint Level
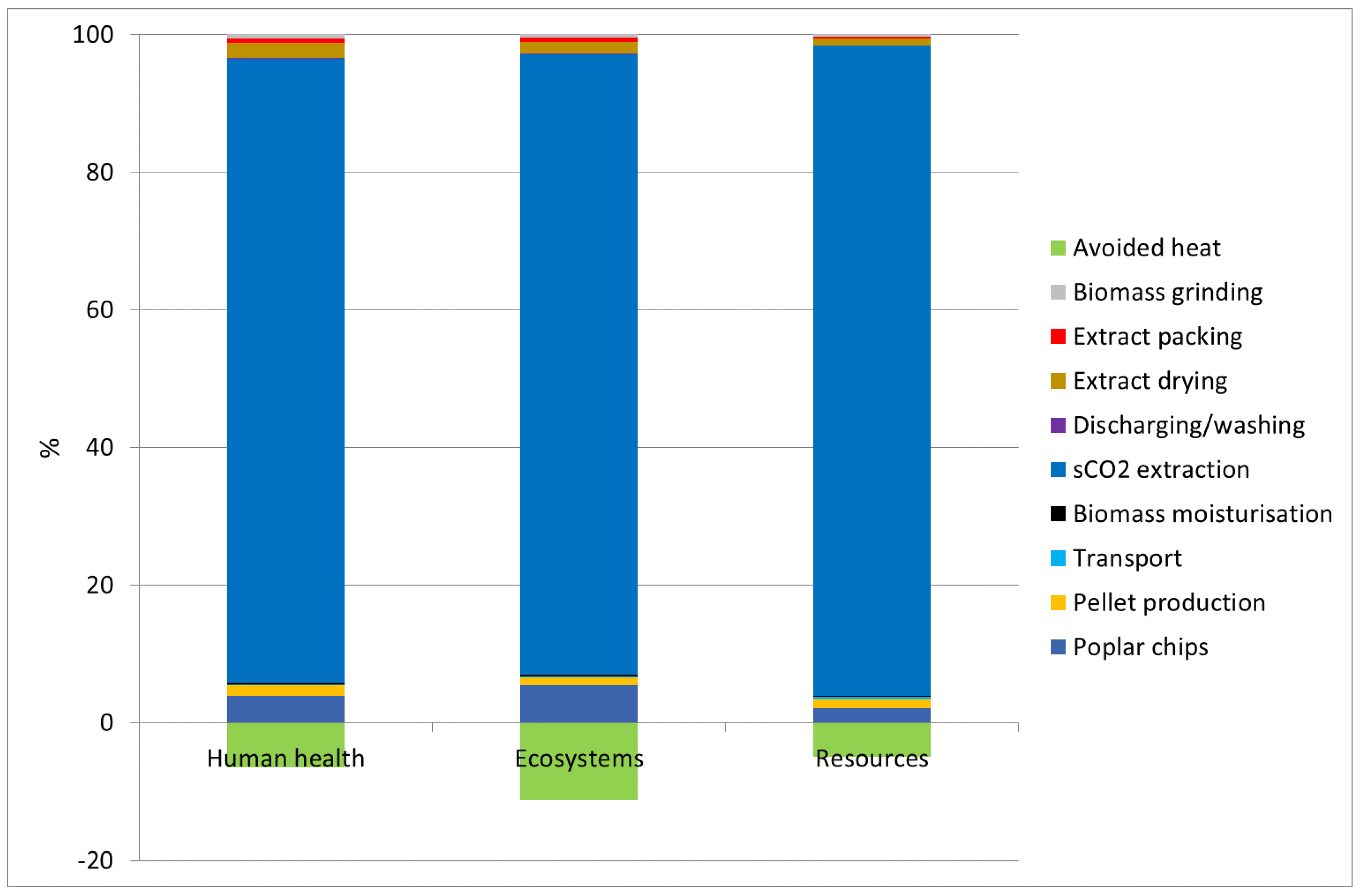
3.2. Characterisation Scores at the Endpoint Level
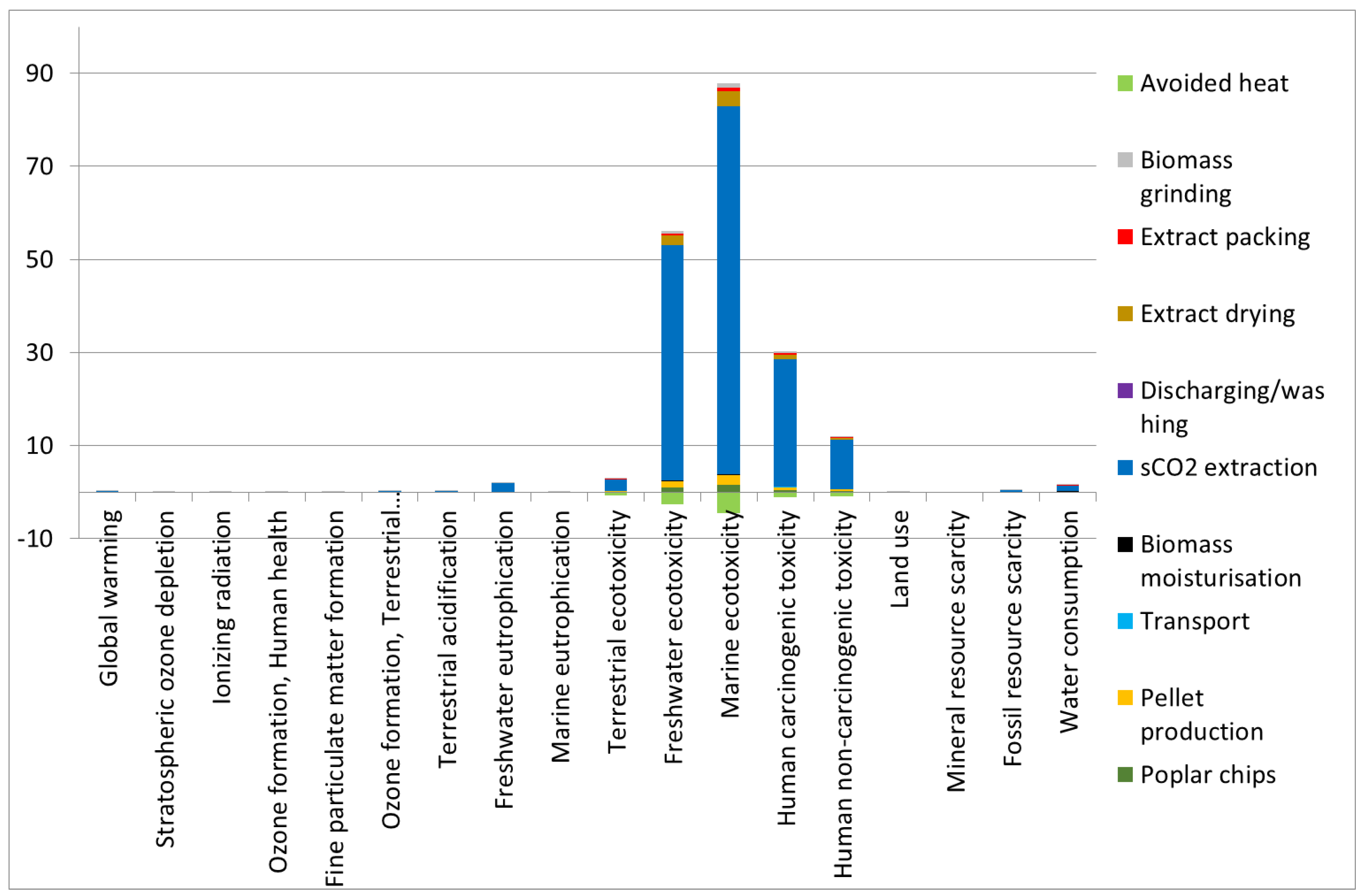
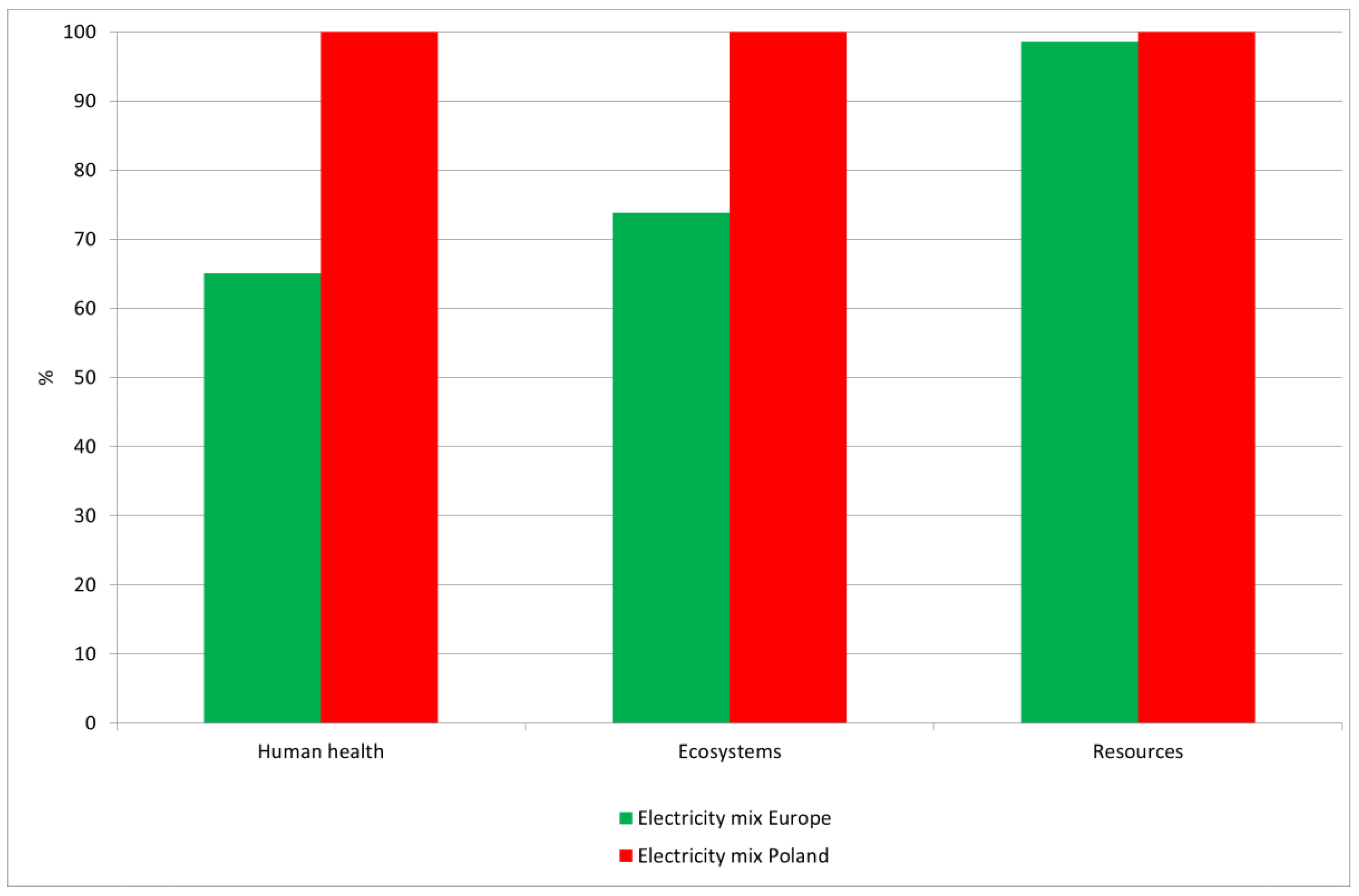
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. Communication from The Commission to The European Parliament, The European Council, The Council, The European Economic and Social Committee and The Committee of The Regions The European Green Deal. In COM/2019/640 Final; Commission, E., Ed.; European Commission: Brussels, Belgium, 2019; Volume COM/2019/640 final. [Google Scholar]
- Qin, Z.; Zhuang, Q.; Cai, X.; He, Y.; Huang, Y.; Jiang, D.; Lin, E.; Liu, Y.; Tang, Y.; Wang, M.Q. Biomass and biofuels in China: Toward bioenergy resource potentials and their impacts on the environment. Renew. Sustain. Energy Rev. 2018, 82, 2387–2400. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Aan den Toorn, S.I.; Worrell, E.; van den Broek, M.A. Meat, dairy, and more: Analysis of material, energy, and greenhouse gas flows of the meat and dairy supply chains in the EU28 for 2016. J. Ind. Ecol. 2020, 24, 601–614. [Google Scholar] [CrossRef]
- Dasgupta, S.; Robinson, E.J.Z. Attributing changes in food insecurity to a changing climate. Sci. Rep. 2022, 12, 4709. [Google Scholar] [CrossRef]
- Éliás, B.A.; Jámbor, A. Food security and COVID-19: A systematic review of the first-year experience. Sustainability 2021, 13, 5294. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Graban, Ł.; Lajszner, W. Short rotation coppices, grasses and other herbaceous crops: Biomass properties versus 26 genotypes and harvest time. Ind. Crops Prod. 2018, 119, 22–32. [Google Scholar] [CrossRef]
- Celma, S.; Sanz, M.; Ciria, P.; Maliarenko, O.; Prysiazhniuk, O.; Daugaviete, M.; Lazdina, D.; von Cossel, M. Yield Performance of Woody Crops on Marginal Agricultural Land in Latvia, Spain and Ukraine. Agronomy 2022, 12, 908. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Olba-Zięty, E. Cascaded use of perennial industrial crop biomass: The effect of biomass type and pre-treatment method on pellet properties. Ind. Crops Prod. 2022, 185, 115104. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Olba-Zięty, E.; Stolarski, M.J.; Krzyżaniak, M.; Rój, E.; Tyśkiewicz, K.; Łuczyński, M.K. Supercritical production of extract from poplar containing bioactive substances—An economic analysis. Ind. Crops Prod. 2022, 184, 115094. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Brussels, Belgium, 2006; Volume ISO 14040, p. 20.
- Carlqvist, K.; Wallberg, O.; Lidén, G.; Börjesson, P. Life cycle assessment for identification of critical aspects in emerging technologies for the extraction of phenolic compounds from spruce bark. J. Clean. Prod. 2022, 333, 130093. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.A.; Teodosiu, C.; Volf, I. Life cycle assessment of polyphenols extraction processes from waste biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef]
- Espada, J.J.; Pérez-Antolín, D.; Vicente, G.; Bautista, L.F.; Morales, V.; Rodríguez, R. Environmental and techno-economic evaluation of β-carotene production from Dunaliella salina. A biorefinery approach. Biofuels Bioprod. Biorefining 2020, 14, 43–54. [Google Scholar] [CrossRef]
- De Marco, I.; Riemma, S.; Iannone, R. Life cycle assessment of supercritical CO2 extraction of caffeine from coffee beans. J. Supercrit. Fluids 2018, 133, 393–400. [Google Scholar] [CrossRef]
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Brussels, Belgium, 2006; Volume ISO 14044, p. 46.
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K. Life cycle assessment of Virginia mallow production with different fertilisation options. J. Clean. Prod. 2018, 177, 824–836. [Google Scholar] [CrossRef]
- Parajuli, R.; Knudsen, M.T.; Djomo, S.N.; Corona, A.; Birkved, M.; Dalgaard, T. Environmental life cycle assessment of producing willow, alfalfa and straw from spring barley as feedstocks for bioenergy or biorefinery systems. Sci. Total Environ. 2017, 586, 226–240. [Google Scholar] [CrossRef]
- Parajuli, R.; Kristensen, I.S.; Knudsen, M.T.; Mogensen, L.; Corona, A.; Birkved, M.; Peña, N.; Graversgaard, M.; Dalgaard, T. Environmental life cycle assessments of producing maize, grass-clover, ryegrass and winter wheat straw for biorefinery. J. Clean. Prod. 2017, 142, 3859–3871. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Christensen, B.T.; Hutchings, N.J.; Vejlin, J.; Kätterer, T.; Glendining, M.; Olesen, J.E. C-TOOL: A simple model for simulating whole-profile carbon storage in temperate agricultural soils. Ecol. Model. 2014, 292, 11–25. [Google Scholar] [CrossRef]
- Petersen, B.M.; Knudsen, M.T.; Hermansen, J.E.; Halberg, N. An approach to include soil carbon changes in life cycle assessments. J. Clean. Prod. 2013, 52, 217–224. [Google Scholar] [CrossRef]
- Alaphilippe, A.; Boissy, J.; Simon, S.; Godard, C. Environmental impact of intensive versus semi-extensive apple orchards: Use of a specific methodological framework for Life Cycle Assessments (LCA) in perennial crops. J. Clean. Prod. 2016, 127, 555–561. [Google Scholar] [CrossRef]
- Vinther, F. SimDen—A simple empirical model for quantification of N2O emission and denitrification. Paper at: Manure—An agronomic and environmental challenge. In Proceedings of the NJF-Seminar No. 372, Nils Holgerssongymnasiet, Skurup, Sweden, 5–6 September 2005. [Google Scholar]
- Agency, E.E. EMEP/EEA Air Pollutant Emission Inventory Guidebook 2016; European Environment Agency: Copenhagen, Denmark, 2016. [Google Scholar]
- Iriarte, A.; Rieradevall, J.; Gabarrell, X. Life cycle assessment of sunflower and rapeseed as energy crops under Chilean conditions. J. Clean. Prod. 2010, 18, 336–345. [Google Scholar] [CrossRef]
- Fazio, S.; Monti, A. Life cycle assessment of different bioenergy production systems including perennial and annual crops. Biomass Bioenergy 2011, 35, 4868–4878. [Google Scholar] [CrossRef]
- Bacenetti, J.; González-García, S.; Mena, A.; Fiala, M. Life cycle assessment: An application to poplar for energy cultivated in Italy. J. Agric. Eng. 2012, 43, 72–78. [Google Scholar] [CrossRef]
- Krzyzaniak, M.; Stolarski, M.; Warminski, K. Life cycle assessment of poplar production.: Environmental impact of different soil enrichment methods. J. Clean. Prod. 2019, 206, 785–796. [Google Scholar] [CrossRef]
- Ding, T.; Bianchi, S.; Ganne-Chédeville, C.; Kilpeläinen, P.; Haapala, A.; Räty, T. Life cycle assessment of tannin extraction from spruce bark. iForest Biogeosci. For. 2017, 10, 807–814. [Google Scholar] [CrossRef]
- Poland, S. Energy Statistics in 2020 and 2021; Statistics Poland: Warsaw, Poland, 2022; p. 81.
- Gwee, Y.L.; Yusup, S.; Tan, R.R.; Yiin, C.L. Techno-economic and life-cycle assessment of volatile oil extracted from Aquilaria sinensis using supercritical carbon dioxide. J. CO2 Util. 2020, 38, 158–167. [Google Scholar] [CrossRef]




| Process | Diesel Oil (kg ha−1) | Quantity in the Plantation Life Cycle | Materials and Comments |
|---|---|---|---|
| Production of cuttings | 1.26 | 1 | 20,000 cuttings, data from Ecoinvent 3 |
| Spraying | 2.04 | 1 | Roundup 360 SL, 5 L ha−1 |
| Ploughing | 29.30 | 1 | 5-ridge plough, ploughing depth—30 cm |
| Harrowing | 11.20 | 1 | 2 operations |
| Marking planting locations | 24.44 | 1 | |
| Mechanical weeding | 21.09 | 1 | 3 operations |
| Fertilisation | 13.24 | 20 | Ammonium nitrate, triple superphosphate, potash salt: N—90; P2O5—30; K2O—60 kg ha−1 |
| Plantation closure | 117.21 | 1 | Rootstock grinding with a rototiller |
| Harvest | 57.22 | 20 | Self-propelled chip harvester, biomass yield: 22.2 Mg ha−1 year−1 f.m. |
| Field transport | - | 20 | Yield multiplied by distance (7 km): the functional unit for this input is 1 tonne-kilometre for a tractor with a trailer (Ecoinvent 3) |
| Drying | - | 20 | 958 MJ and 25 kWh Mg−1, wood drying (Ecoinvent 3) |
| Road transport | - | 20 | Yield multiplied by distance (100 km) for a semi-trailer truck (Euro 4) (Ecoinvent 3) |
| Source | Unit of Measure | Quantity | Emission Type |
|---|---|---|---|
| CO2 from soil organic carbon | kg ha−1 year−1 | −862 | sequestration |
| N2O | kg ha−1 year−1 | 1.58 | to air |
| NH3 | kg ha−1 year−1 | 4.79 | |
| NOx | kg NO2 ha−1 year−1 | 3.6 | |
| PM10 | kg ha−1 year−1 | 0.90 | |
| PM2.5 | kg ha−1 year−1 | 0.048 | |
| NMVOC | kg ha−1 year−1 | 0.86 | |
| PO43− | kg ha−1 year−1 | 0.30 | to water |
| Input/Output | Input (I)/Output (O) | Type | Quantity | Unit of Measure | Type/Source | Utilisation/Disposal | Comments |
|---|---|---|---|---|---|---|---|
| Grinding | |||||||
| Poplar biomass (chips) | I | biomass | 251.25 | kg | chip production | ||
| Grinding | I | electricity | 10 | kWh | 360 V | ||
| Ground poplar biomass | O | biomass | 1.25 | kg | chip production | waste | grinding loss |
| Ground poplar biomass | O | biomass | 250.0 | kg | hydration | ||
| Hydration | |||||||
| Ground poplar biomass | I | biomass | 250.0 | kg | biomass processing plant | ||
| Water | I | distilled water | 107.0 | kg | distilling unit and mains water | ||
| Stirrer | I | electricity | 3.00 | kWh | 230 V | ||
| Hydrated biomass | O | biomass | 357.0 | kg | extraction | ||
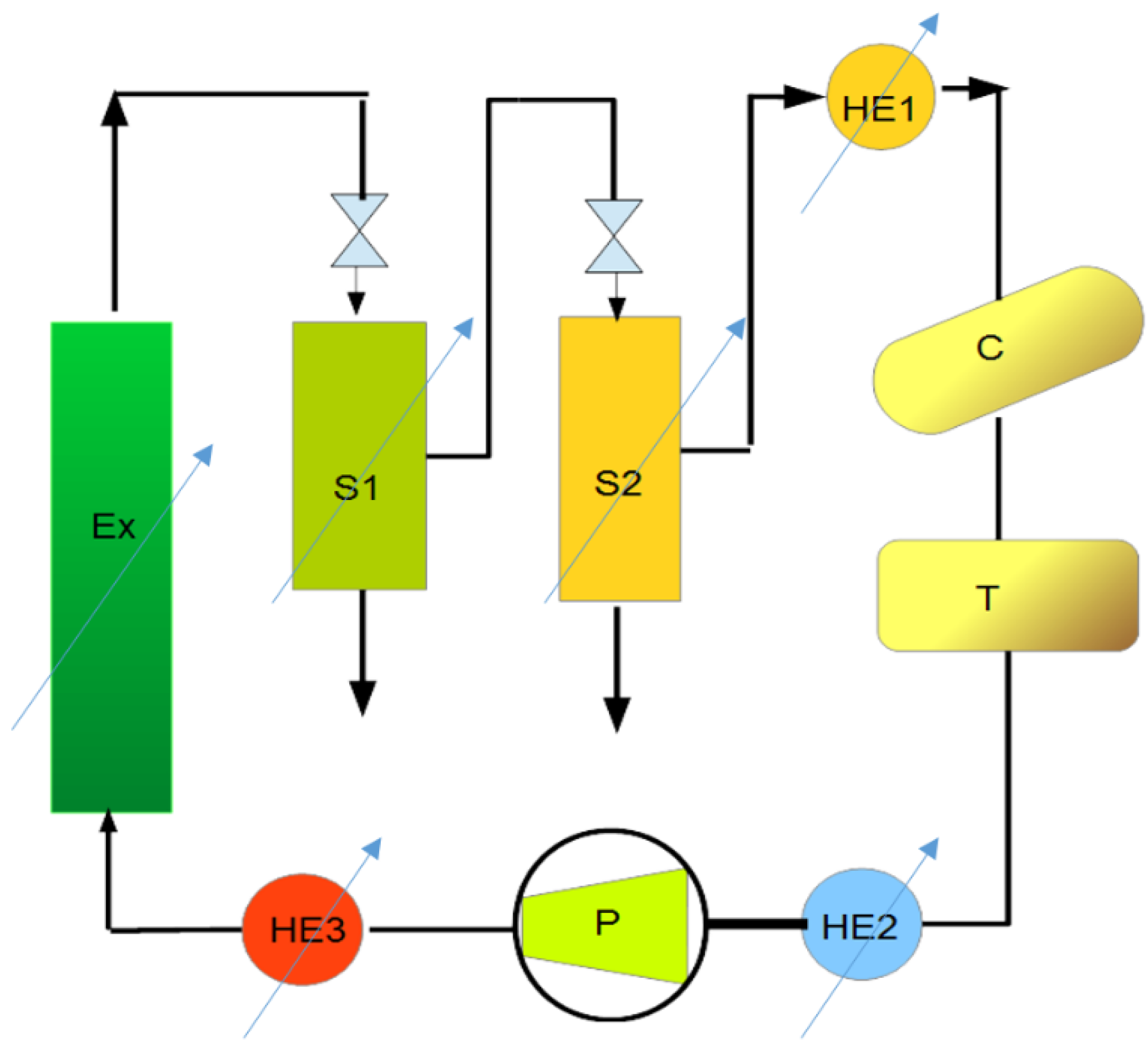
| Extraction | |||||||
| Hydrated biomass | I | biomass | 357.0 | kg | hydration | ||
| Coolant (HE1, HE2) | I | propylene glycol; total in circulation—5000 kg | 2.42 | kg | 1000 kg per 5 years | ||
| Heat exchangers (HE1 and HE2) | I | electricity = HE1 = 270 kW; HE2 = 205 kW | 656.6 | kWh | |||
| CO2 tank (T) | I | carbon dioxide | 125.0 | kg | |||
| Pump (P) | I | electricity | 175.9 | kWh | |||
| Heat exchanger (HE3) | I | steam | 437.0 | kWh | combined heat and power plant or energy from industrial heat exchangers | ||
| Extractor (Ex) | I | steam | 113.3 | kWh | |||
| Separator (S1) | I | steam | 232.7 | kWh | |||
| Separator (S2) | I | steam | 438.7 | kWh | |||
| Raw extract | O | biomass extract | 110.75 | kg | unloading/cleaning | ||
| Residual biomass | O | biomass | 246.25 | kg | unloading/cleaning | ||
| Unloading/cleaning | |||||||
| Raw extract | I | biomass extract | 110.75 | kg | extraction process | ||
| Residual biomass | I | biomass | 246.25 | kg | extraction process | ||
| Detergent | I | kitchen detergent | 10.0 | g | sewer | ||
| Ethanol | I | 96% ethanol v/v | 0.197 | kg | reagent disposal | ||
| Water | I | demineralised water | 10.0 | kg | sewer | ||
| Residual biomass | O | biomass | 246.25 | kg | pellet production | ||
| Raw extract | O | biomass extract | 110.75 | kg | drying | ||
| Dehydration | |||||||
| Raw extract | I | 110.75 | kg | unloading/cleaning | |||
| Evaporator | I | electricity | 17.80 | kWh | |||
| Freeze-dryer | I | electricity | 17.83 | kWh | |||
| Water | O | water from the drying process | 107.00 | kg | unloading/cleaning—water from the extract | sewer | |
| Dry extract | O | 3.75 | kg | packaging | |||
| Packaging | |||||||
| Dry extract | I | 3.75 | kg | packaging process | |||
| Bulk packaging | I | 0.4 | kg | 5 dm3 metal cans with a seal | Ecoinvent 3 database (materials: steel, zinc, rubber) | ||
| Packaged extract | O | extract | 4.15 | kg | |||
| Pellet production | |||||||
| Residual biomass | I | biomass | 246.25 | kg | |||
| Pellets produced from residual biomass | O | biomass | 246.25 | kg | heat generation | inputs and outputs for pellet production were selected from the Ecoinvent 3 database | |
| Heat generation | |||||||
| Pellets produced from residual biomass | I | biomass | 246.25 | kg | pellet production | ||
| Heat | O | heat | −3987 | MJ | avoided burden (heat) | LHV 18.42 MJ kg−1, biomass boiler efficiency—88% | |
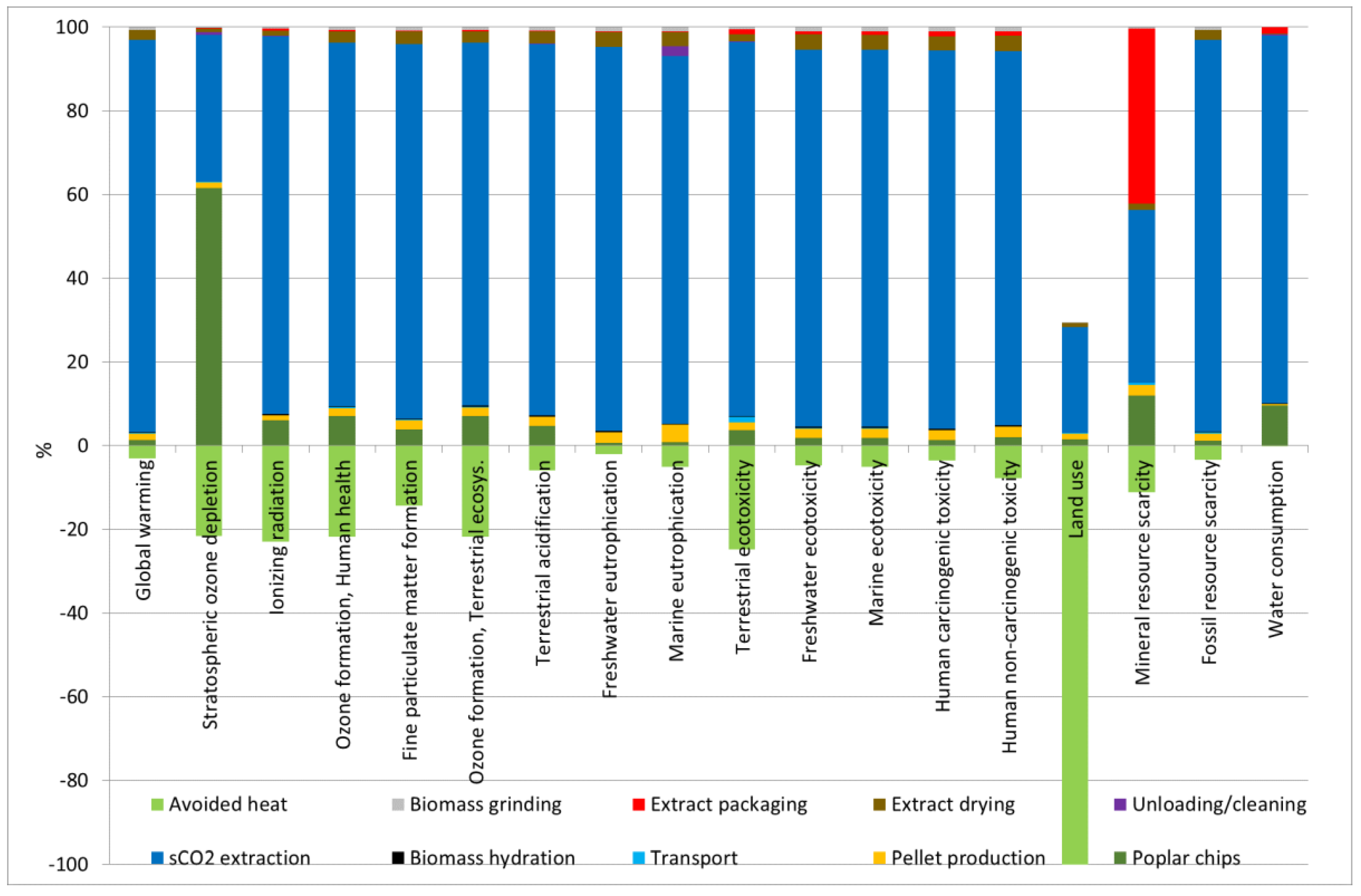
| Impact Category | Unit | Total | Poplar Chips | Biomass Grinding | Transport | Biomass Hydration | sCO2 Extraction | Unloading/Cleaning | Extract Drying | Extract Packaging | Pellet Production | Avoided Heat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global warming | kg CO2 eq | 440 | 5.71 | 2.84 | 1.11 | 0.87 | 425 | 0.121 | 10.1 | 0.53 | 7.24 | −13.8 |
| Stratospheric ozone depletion | kg CFC11 eq | 2.07 × 10−4 | 1.62 × 10−4 | 6.48 × 10−7 | 8.36 × 10−7 | 2.14 × 10−7 | 9.25 × 10−5 | 1.71 × 10−6 | 2.30 × 10−6 | 2.05 × 10−7 | 3.65 × 10−6 | −5.72 × 10−5 |
| Ionizing radiation | kBq Co-60 eq | 12.8 | 1.010 | 0.061 | 0.026 | 0.038 | 15.05 | 0.019 | 0.218 | 0.068 | 0.187 | −3.834 |
| Ozone formation, Human health | kg NOx eq | 0.59 | 0.052 | 0.006 | 0.004 | 0.002 | 0.649 | 3.31 × 10−4 | 0.020 | 0.002 | 0.015 | −0.163 |
| Fine particulate matter formation | kg PM2.5 eq | 0.51 | 0.023 | 0.005 | 0.001 | 0.002 | 0.533 | 2.12 × 10−4 | 0.018 | 0.001 | 0.013 | −0.085 |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | 0.59 | 0.053 | 0.006 | 0.004 | 0.002 | 0.657 | 3.40 × 10−4 | 0.020 | 0.002 | 0.015 | −0.165 |
| Terrestrial acidification | kg SO2 eq | 1.65 | 0.081 | 0.015 | 0.003 | 0.004 | 1.558 | 0.001 | 0.052 | 0.002 | 0.038 | −0.103 |
| Freshwater eutrophication | kg P eq | 0.35 | 0.003 | 0.004 | 8.14 × 10−5 | 0.001 | 0.324 | 2.90 × 10−5 | 0.013 | 3.52 × 10−4 | 0.008 | −0.007 |
| Marine eutrophication | kg N eq | 0.02 | 1.99 × 10−4 | 2.24 × 10−4 | 6.99 × 10−6 | 6.82 × 10−5 | 0.021 | 0.001 | 0.001 | 3.66 × 10−5 | 9.68 × 10−4 | −0.001 |
| Terrestrial ecotoxicity | kg 1,4-DCB | 601 | 29.6 | 3.85 | 19.78 | 1.21 | 703.1 | 0.86 | 13.7 | 9.06 | 14.3 | −194 |
| Freshwater ecotoxicity | kg 1,4-DCB | 17.5 | 0.344 | 0.191 | 0.026 | 0.057 | 16.52 | 0.003 | 0.679 | 0.132 | 0.409 | −0.883 |
| Marine ecotoxicity | kg 1,4-DCB | 22.9 | 0.448 | 0.249 | 0.044 | 0.074 | 21.75 | 0.006 | 0.884 | 0.184 | 0.541 | −1.243 |
| Human carcinogenic toxicity | kg 1,4-DCB | 21.5 | 0.280 | 0.218 | 0.023 | 0.067 | 20.17 | 0.003 | 0.775 | 0.260 | 0.525 | −0.811 |
| Human non-carcinogenic toxicity | kg 1,4-DCB | 435 | 9.66 | 4.79 | 0.811 | 1.43 | 421 | 0.526 | 17.0 | 5.06 | 11.56 | −36.6 |
| Land use | m2a crop eq | −23.7 | 0.486 | 0.086 | 0.047 | 0.026 | 8.51 | 0.002 | 0.304 | 0.019 | 0.480 | −33.6 |
| Mineral resource scarcity | kg Cu eq | 0.45 | 0.060 | 0.002 | 0.004 | 0.001 | 0.210 | 0 | 0.007 | 0.212 | 0.013 | −0.057 |
| Fossil resource scarcity | kg oil eq | 103 | 1.15 | 0.704 | 0.381 | 0.210 | 99.3 | 0 | 2.50 | 0.081 | 1.96 | −3.62 |
| Water consumption | m3 | 106 | 10.3 | 0.084 | 0.002 | 0.281 | 93.5 | 0.355 | 0.298 | 1.50 | 0.200 | −0.194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżaniak, M.; Stolarski, M.J.; Warmiński, K.; Rój, E.; Tyśkiewicz, K.; Olba-Zięty, E. Life Cycle Assessment of Poplar Biomass for High Value Products and Energy. Energies 2023, 16, 7287. https://doi.org/10.3390/en16217287
Krzyżaniak M, Stolarski MJ, Warmiński K, Rój E, Tyśkiewicz K, Olba-Zięty E. Life Cycle Assessment of Poplar Biomass for High Value Products and Energy. Energies. 2023; 16(21):7287. https://doi.org/10.3390/en16217287
Chicago/Turabian StyleKrzyżaniak, Michał, Mariusz J. Stolarski, Kazimierz Warmiński, Edward Rój, Katarzyna Tyśkiewicz, and Ewelina Olba-Zięty. 2023. "Life Cycle Assessment of Poplar Biomass for High Value Products and Energy" Energies 16, no. 21: 7287. https://doi.org/10.3390/en16217287
APA StyleKrzyżaniak, M., Stolarski, M. J., Warmiński, K., Rój, E., Tyśkiewicz, K., & Olba-Zięty, E. (2023). Life Cycle Assessment of Poplar Biomass for High Value Products and Energy. Energies, 16(21), 7287. https://doi.org/10.3390/en16217287








