Abstract
Using carbon dioxide as a gasification agent for underground coal gasification (UCG) can not only reduce carbon dioxide emissions but is also expected to lead to a new natural gas technology revolution and ensure national energy security. To explore the effect of the oxygen content in oxygen-enriched carbon dioxide gasification agents on the results of gasification experiments, underground gasification experiments under different oxygen-enrichment conditions were designed, and quantitative parameters were used to analyze and evaluate the gas produced in the gasification experiments. The results showed that as the oxygen content in the oxygen-enriched carbon dioxide gasification agent increased, the CO and H2 in the combustible gas gradually increased, and the calorific value of the combustible gas also slowly increased, reaching a peak value under the gasification condition of 60% oxygen concentration, and then decreased slightly; the product formation rate and the gas production per unit mass of coal fluctuated. The coal consumption rate increased with time and was relatively stable. According to theoretical calculations for the gasification energy recovery evaluation system, the overall energy recovery rate was 56.34%, and the energy utilization rate was relatively high. Research on quantitative indicators based on gas production data has good practical significance for evaluating the gasification efficiency of UCG, which can be used to better evaluate and control the reaction process of UCG.
1. Introduction
With the continuous development of China’s economy, the demand for oil and gas is increasing, the dependence on foreign oil and natural gas is increasing year by year, and the supply security situation is severe []. China is rich in coal resources, but coal mining causes great pollution to the environment, and good clean development methods are lacking. As a subversive technology, underground coal gasification (UCG) enables the controlled combustion of coal by creating appropriate process conditions underground and generates hydrogen, carbon monoxide, and methane through coal pyrolysis and a series of chemical reactions between coal, oxygen, and water vapor. It is a clean coal utilization technology integrating the three major processes of well construction, coal mining, and gasification. UCG technology can ensure sufficient oil and gas production and reduce carbon emissions from coal mining and is expected to lead to a new natural gas technology revolution []. According to estimates, China’s onshore coal resources buried at a depth of 1000–3000 m are 3.77 × 1012 t, and the equivalent methane resource is 272–332 × 1012 m3 based on a gasification production rate of 40%, which is a conventional natural gas resource. This is three times the amount of natural gas, which is greater than the total amount of natural gas resources that have been put into development (210 × 1012 m3). Calculated with a coal seam thickness of 5 m, the abundance of coal gasifiable resources is as high as 112 × 108 m3/km2 [].
The development of UCG technology was initiated more than 150 years ago when the German scientist William Siemens creatively proposed the underground gasification of coal in 1868 []. A large number of field experiments have been carried out in many countries, including the former Soviet Union, the United States, the United Kingdom, Poland, Belgium, Spain, Australia, and China []. There have also been many new developments and understandings in UCG in terms of technology, cost, and environmental impact [,]. Although there have been small breakthroughs, it is still in the stage of industrial testing, has not yet been fully commercially developed, and has not formed a complete industrial chain [,,]. Therefore, exploring cost-effective gasification processes will be one of the key directions for commercial development. Using indoor simulation experiments to simulate the gas production of different gasification agents and mastering the gasification operation rules will provide guidance for the exploration of high-efficiency gasification processes. Researchers have also carried out gasification simulation experiments on air, oxygen, oxygen-enriched air, oxygen-enriched CO2, and pure oxygen water vapor []. Air is used as a gasification agent, which eliminates the cost of air separation when the initial gasification agent is inflated; however, air gasification leads to a lower temperature, reaction intensity and gasification efficiency in the UCG reaction zone, especially in coal seams with a high ash content, and air gasification produces gas with a low calorific value [,]. Oxygen-enriched air as a gasification medium can reduce the operating cost of gasification agent separation and further ensure the stability of the flame in the UCG process. However, the generated gas still contains nitrogen, which must be separated in a subsequent process. It is generally believed that pure oxygen gasification is suitable for coal with a high ash content, but pure oxygen gasification requires an expensive gasification agent and is difficult to control [,]. Pure oxygen water vapor is used as a gasification agent. It reduces the temperature in the gasification chamber. However, due to the temperature reduction, some of the water vapor condenses into droplets; hence, the gasification agent cannot be evenly distributed onto the surface of the coal seam, which leads to gasification process instability and decreased gasification efficiency [,]. O2/CO2 is a better choice as a gasification agent; it can avoid the problem of transporting superheated steam to deep coal seams and can also resolve the carbon dioxide utilization issue. Chen et al. [] found experimentally that compared with the existing oxygen-enriched underground gasification model test results, adding CO2 to the gasification agent can inhibit the formation of CO2 in the process of underground gasification, and the temperature field under O2/CO2 gasification is relatively low, with a coal seam maximum temperature during gasification of only 1200 °C, which is favorable for generating effective components of coal gas. Zhao et al. [] compared the O2/CO2 and O2/air gasification methods and found that the presence of a high concentration of CO2 can prolong the gasification time and make the temperature field in the furnace distribute in the vertical direction. However, the high concentration of the CO2 gasification agent can cause the flame to cool down or even extinguish in the combustion air area of the underground gasifier. Therefore, in the process of oxygen-enriched gasification, maintaining the optimal O2/CO2 ratio in the gasification agent delivery process seems to be especially critical. Moreover, the gasification efficiency under different ratios has been rarely evaluated quantitatively.
In this paper, on the basis of previous studies, experiments on carbon dioxide gasification under different oxygen-enrichment conditions were conducted, and the optimal O2/CO2 injection ratio was determined. The gasification experimental method was compared and evaluated in stages to identify the influence laws of the oxygen concentration, gas-to-oxygen ratio, gas injection flow rate, and other parameters on the changes in the gas production components. Based on stoichiometric theory, the coal consumption, gas yield, combustible gas volume, calorific value of the gasification products, and energy recovery rate were evaluated by calculation. This study provides a theoretical basis for underground gasification field tests.
2. Experiments and Methods
2.1. Sample Information
The coal seam used in this experiment was mined in the northern part of the Ordos Basin. Before the start of the experiment, industrial analysis and elemental analysis were performed on the experimental coal. The results are shown in Table 1 and Table 2. The proximate analysis and elemental analysis results show that this area is of medium high volatile content and extremely low ash content, and has similar coal quality conditions to Swan Mountain [], which is suitable for underground gasification experiment.

Table 1.
Coal industry analysis.

Table 2.
Analysis of coal elements.
2.2. Experimental Setup
The equipment used in the experiment was self-developed equipment, including an oxygen generator, steam generator, gasifier, temperature sensor, filter box, and gas chromatograph. Figure 1 is a structural diagram of the device, and Figure 2 is a physical diagram. The size of the coal filling was 2150 mm long, 600 mm wide, and 550 mm high.

Figure 1.
Composition diagram of the underground gasification simulation device.

Figure 2.
Physical map of the underground gasification simulation device.
2.3. Experimental Design
In this experiment, oxygen-enriched carbon dioxide was used as the gasification agent. The volume fraction of oxygen and carbon dioxide was adjusted with the same total injection rate. Under the same condition, we investigate to the composition of the gas generated by the corresponding gasification process and the pattern of changes in the result under different oxygen and carbon ratios. Compared with the conventional oxygen-enriched coal UCG process, the main significant difference of the oxygen-enriched carbon dioxide UCG process is the addition of CO2 to the conventional oxygen-enriched gasification agent, which not only changes the balance of the three-zone reaction in the gasification zone but also promotes the reduction reaction, which in turn affects the gas composition of the gasification product. The variation in the experimental gasification agent injection flow rate is shown in Figure 3. The total gasification agent injection flow rate was 25 m3/h, and six kinds of oxygen-rich carbon gasification agents with different oxygen-to-carbon ratios with oxygen concentrations of 30%, 40%, 50%, 60%, 70%, and 80% were used for the gasification experiment. These gasification agents were utilized in sequence for 2 h, and the total length of the experiment was 12 h.
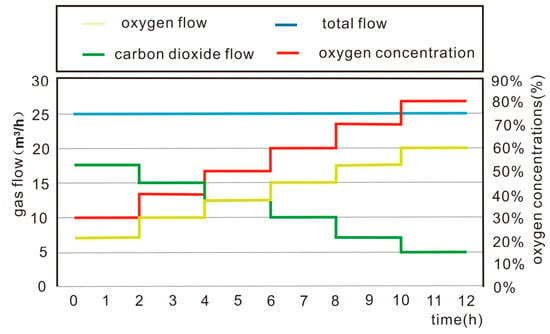
Figure 3.
Injection flow rate of the gasification agent.
2.4. Experimental Procedure
After the experiment started, the gasification agent was blown into the gasifier, and the ignition device was turned on. After successful ignition, the experiment was carried out according to the experimental parameter settings of each experimental stage, and the data for each experimental subsystem were recorded regularly. Throughout the gasification process, the generated gas was collected and stored regularly, the temperature of the gasification area was monitored in real time, and the gas composition of the gasification product was tested using a gas chromatograph.
By comparatively evaluating each stage of the experiment, the influence laws of parameters such as the oxygen concentration, gas-to-oxygen ratio, and gas injection flow rate on the produced gas components were obtained. Based on stoichiometric theory, an improved method for calculating the calorific value of the gasification gas was developed, which was used to obtain the pilot experimental parameters and evaluate the gasification efficiency. The coal consumption, gas yield, combustible gas volume, calorific value of the gasification products, and energy recovery rate were evaluated by calculation.
3. Experimental Results
The content of each component of the gas produced by oxygen-enriched carbon dioxide gasification is shown in Figure 4. The main gas components of the gasification coal gas included H2, CO, CH4, and CO2. With increasing oxygen concentration injected by the gasification agent, the CO2 content gradually decreased from 78.09% and reached its lowest value at approximately 8 h, at which time the CO content in the generated gas also reached its peak value of 29.73%. The H2 content reached its peak value of 27.63% at approximately 8.5 h, and the CH4 content was relatively stable without obvious fluctuations.
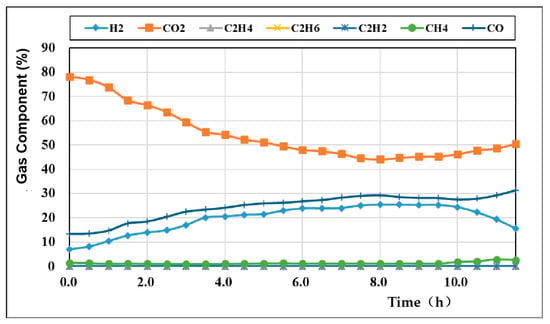
Figure 4.
Variation curves of the oxygen-rich carbon dioxide gas components.
During the gasification process, due to the low oxygen concentration in the initial period, the oxidation reaction intensity in the gasification reaction was also low, and the high concentration of CO2 hindered the forward progress of the oxidation reaction. Therefore, the production temperature of the oxidation reaction in the early stage did not reach the temperature required for the reduction reaction in the later stage, and the intensity of the entire gasification process was relatively weak, resulting in low levels (approximately 10%) of all major gas components except for CO2 in the initial stage. With increasing oxygen concentration, the contents of CO and H2 gradually increased and peaked under the gasification condition of 60% oxygen concentration. As the oxygen concentration continued to rise, the CO2 content began to increase, and the H2 content began to decrease.
The calorific value is an index for measuring the quality of the produced gas, and it also indirectly reflects the quality of the gasification effect []. The change in the calorific value of the gas produced in this experiment is shown in Figure 5. The average calorific value was 6.17 MJ/m3. During the initial period of this stage (oxygen concentration of 30%), the calorific value of the generated gas began to increase slowly, rising from 3.23 MJ/m3 to 4.32 MJ/m3 within 2 h. At 2 h, the oxygen concentration of the injected gasification agent was adjusted to 40%, and the calorific value of the generated gas began to rise rapidly, increasing by 1.96 MJ/m3 over 2 h, and increased nearly twice as much as that under the gasification condition of 30% oxygen concentration. In the subsequent 4 h gasification process, with increasing oxygen concentration, the calorific value of the generated gas also increased continuously, but the rate of increase gradually decreased. When the O2 concentration in the gasification agent component reached 60%, the flow of the combustible gas in the generated gas reached its peak value. At 8 h, the oxygen concentration changed from 60% to 70%, and the calorific value of the generated gas began to decrease slowly.
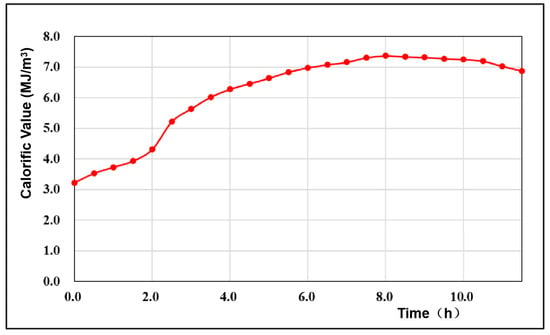
Figure 5.
Variation curve of the generated gas.
4. Discussion
4.1. Generation Rate of the Product Gas
The gas generation rate of UCG reflects the activity of the gasification process []. Figure 6 shows that the product generation rate fluctuated, mainly due to the change in the dominance of the oxidation reaction and the reduction reaction during the gasification process. When the oxidation reaction was dominant, the gas production was larger, and when the reduction reaction was dominant, the gas production decreased. In the early stage of the experiment (oxygen concentration of 30%), the production rate of the product gas was low and slowly increasing. Due to the low concentration of O2 injected into the gasification agent in the initial stage, the oxidation reaction in the gasification reaction was relatively gentle, and consequently, the overall temperature in the gasifier was low, resulting in the inability of CO2 to effectively participate in the reduction reaction; moreover, because the gasification process was relatively weak, the amount of gas generated at this time was low. At 2 h, the oxygen concentration of the injected gasification agent increased to 40%. At this time, the production rate of the product gas increased rapidly, rising to 39.11 m3/h within 0.5 h, and then began to decline. The decline stopped at 4 h, and within the following 4 h, the generation rate of the product gas had two large fluctuations again, decreasing overall. This overall decrease occurred because when the O2 concentration in the gasification agent reached 40%, the oxidation reaction in the gasification reaction was enhanced, resulting in an increase in the overall temperature in the gasifier; in addition, CO2 participated in the reduction reaction more effectively, and the gasification process was relatively active. The amount of gas produced by the gasification reaction peaked. Under oxygen concentrations of 40–70%, the gas volume of the generated gas decreased overall because the reduction reaction intensity at this time continued to increase, and the participation of CO2 in the reduction reaction decreased the generated gas volume. When the O2 concentration in the gasification agent exceeded 70%, the oxidation reaction in the gasification reaction was enhanced, the amount of CO2 gas in the generated gas increased, and then the amount of generated gas appeared to rebound, so the product gas generation rate started to increase after 8 h. The average production rate of the product gas was 34.65 m3/h.
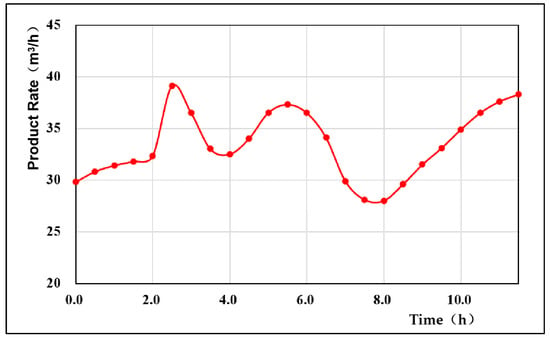
Figure 6.
Variation curve of the product rate.
4.2. Coal Consumption Rate
The coal consumption rate, as a basic variable for evaluating the gasification effect, directly reflects the coal consumption in the gasification process and indirectly reflects the coal gasification rate []. The overall coal consumption rate of the experiment fluctuated minimally, the coal consumption rate increased with time, and the average coal consumption rate was 15.46 kg/h (Figure 7). During the initial 2 h of the experiment, the coal consumption rate was stable at approximately 13.7 kg/h without a significant change, because in the early stage of the experiment, under 30% oxygen concentration gasification, the oxidation reaction was weak, the temperature field in the gasification zone was low, and the reaction intensity was low. At 2 h, the oxygen concentration of the gasification agent rose to 40%, the oxidation reaction in the gasification reaction began to increase in intensity, and the coal consumption rate began to rise slowly. In the subsequent gasification process, with the continuous increase in the injected oxygen concentration of the gasification agent, the oxidation reaction became increasingly intense, and the coal consumption per unit time also increased continuously. When the oxygen concentration of the gasification agent exceeded 70%, the coal consumption rate also increased correspondingly under gasification conditions with an excessively high oxygen concentration; thus, the coal consumption rate started to increase rapidly after 8 h.
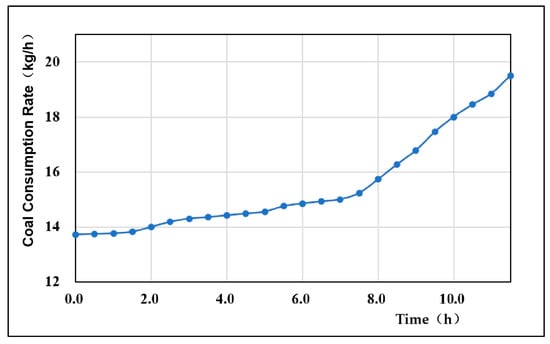
Figure 7.
Variation curve of the coal consumption rate.
4.3. Gas Production Per Unit Mass of Coal
As an important index for evaluating the quality of UCG, the gas production per unit mass of coal can indirectly reflect the change trend of the gasification efficiency in the gasification process []. The gas production rate per unit mass of coal fluctuates but is generally in the range of 2–3 m3/kg. In this experiment, the average gas production rate of coal was 2.35 m3/kg. Figure 8 shows that at the initial stage of the reaction, since the oxygen concentration of the injected gasification agent was not high at this time, the oxidation reaction intensity was low, and the gas production per unit mass of coal was low. When the oxygen concentration of the injected gasification agent was adjusted to 40%, the gas production per unit mass of coal increased rapidly to a maximum value of 2.82 m3/kg and then began to decline due to the increase in the oxygen concentration of the gasification agent, which provided more oxygen for the oxidation reaction. As the oxidation reaction intensified, the temperature of the gasification zone increased, and the concentration of CO2 also increased, which further promoted the reduction reaction. As the reduction reaction intensity increased, the temperature field in the gasification zone became increasingly low and could not provide the temperature environment required for the reduction reaction, which in turn promoted the intensification of the oxidation reaction. Thus, the gas production per unit mass of coal declined. Therefore, in the gasification process, there is a cyclic process in which the gas production initially increases, then decreases, and finally increases again.
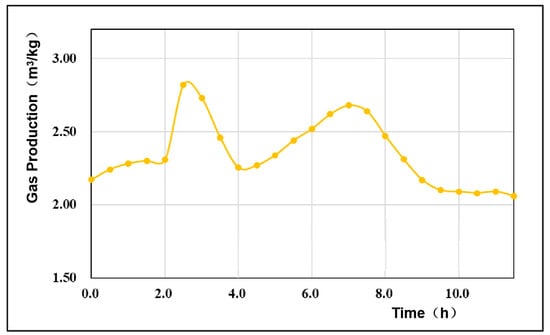
Figure 8.
Variation curve of the gas production per unit mass of coal.
4.4. Energy Recovery Rate
As an important indicator in the gasification energy recovery evaluation system, the gas production per unit mass of coal can indirectly reflect the quality of the gasification effect in the gasification process. In this paper, it is proposed that the energy recovery rate is the ratio of the actual gas energy produced to the heat generated by the coal itself. This experiment was evaluated according to the theoretical calculation of the gasification energy recovery evaluation system [,]. The calculated energy recovery rate was 56.34%. The energy utilization rate was high.
5. Conclusions
- (1)
- In the oxygen-enriched carbon dioxide gasification experiment, with increasing oxygen content, the effective product and calorific value of the gasification product increased. The best gas production effect was obtained when the oxygen concentration was 50–60%. The content of active components in the generated gas was the highest under gasification conditions, with a hydrogen content of 25.1%, CO content of 29.6%, and calorific value of 7.3 MJ/m3, indicating that the redox reaction in the gasification reaction was close to equilibrium.
- (2)
- The gas production rate and the gas production per unit mass of coal fluctuated, mainly due to the change in the dominance of the oxidation reaction and the reduction reaction during the gasification process. When the oxidation reaction was dominant, the gas production was larger, and when the reduction reaction was dominant, the gas production decreased. The overall coal consumption rate in the experiment fluctuated minimally, the coal consumption rate increased with time, and the average coal consumption rate was 15.46 kg/h. The gas production rate of coal in this experiment showed a cyclic process of gas production increasing first and then decreasing, and then increasing again, with an average value of 2.35 m3/kg. According to the theoretical calculation of the gasification energy recovery evaluation system, the overall energy recovery rate was 56.34%. The energy utilization rate was high.
- (3)
- Various quantitative parameters, namely, the product generation rate, coal consumption rate, gas production per unit mass of coal, and energy recovery rate, have good practical significance for evaluating the gasification efficiency of UCG. The energy recovery evaluation method of the gasification process can be used to better evaluate the reaction process of UCG.
Author Contributions
Conceptualization, Y.Q. and Y.C.; methodology, H.C.; investigation, H.C.; resources, Z.D.; data curation, H.C.; writing—original draft preparation, H.C.; writing—review and editing, S.C.; visualization, J.X. and Y.Z.; supervision, M.Z.; project administration, Y.C.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
Key Technologies R&D Programme of PetroChina Company Limited (2019E-25).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, L.; Zhang, N.; Kan, J.; Wang, Y. Concept, model and prediction of green coal resources in China. J. China Univ. Min. Technol. 2018, 47, 1–8. [Google Scholar]
- Zhang, J.; Li, H. Commercialization path of medium deep underground coal gasification in China. Nat. Gas Ind. 2020, 40, 156–165. [Google Scholar]
- Zou, C.; Chen, Y.; Kong, L.; Sun, F.; Chen, S.; Dong, Z. Underground coal gasification and its strategic significance to the development of natural gas industry in China. Pet. Explor. Dev. 2019, 46, 195–204. [Google Scholar] [CrossRef]
- Khan, M.M.; Mmbaga, J.P.; Shirazi, A.S.; Trivedi, J.; Liu, Q.; Gupta, R. Modelling Underground Coal Gasification-A Review. Energies 2015, 8, 12603–12668. [Google Scholar] [CrossRef]
- Liu, S.; Chang, Z.; Liu, J. Key technologies and prospect for in-situ gasification mining of deep coal resources. J. Min. Sci. Technol. 2021, 6, 261–270. [Google Scholar]
- Feng, L.; Dong, M.; Wang, B.; Qin, B. Gas production performance of underground coal gasification with continuously moving injection: Effect of direction and speed. Fuel 2023, 347, 128425. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Thomas, H.R.; Zagorščak, R.; Sadasivam, S.; Masum, S.; Kempka, T.; Otto, C.; Basa, W.; Szyja, M.; et al. Experimental simulations of methane-oriented underground coal gasification using hydrogen—The effect of coal rank and gasification pressure on the hydrogasification process. Int. J. Hydrogen Energy 2023, 48, 921–932. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification-Part I: Field demonstrations and process performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Vyas, D.U.; Singh, R.P. Worldwide Developments in UCG and Indian Initiative. Procedia Earth Planet. Sci. 2015, 11, 29–37. [Google Scholar] [CrossRef]
- Derbin, Y.; Walker, J.; Wanatowski, D.; Marshall, A. Soviet experience of underground coal gasification focusing on surface subsidence. J. Zhejiang Univ.-Sci. A 2015, 16, 12. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification—Part II: Fundamental phenomena and modeling. Prog. Energy Combust. Sci. 2018, 67, 234–274. [Google Scholar] [CrossRef]
- Stańczyk, K.; Howaniec, N.; Smoliński, A.; Świądrowski, J.; Kapusta, K.; Wiatowski, M.; Grabowski, J.; Rogut, J. Gasification of lignite and hard coal with air and oxygen enriched air in a pilot scale ex situ reactor for underground gasification. Fuel 2011, 90, 1953–1962. [Google Scholar] [CrossRef]
- Pang, X.; Pan, X.; Liu, H.; Yang, L.; Chen, F. Experimental study on underground gasification model of lean coal. Coal Sci. Technol. 2011, 39, 120–124. [Google Scholar]
- Liang, J.; Xi, J.; Sun, J.; Liang, X.; Lou, Y. Model test of oxygen-enriched underground gasification of thin coal seam in Ezhuang. J. China Coal Soc. 2007, 10, 1031–1035. [Google Scholar]
- Huang, W.; Wang, Z.; Duan, T.; Duan, T.; Xin, L. Study on underground gasification characteristics of Huating coal air, oxygen-rich and pure oxygen. Clean Coal Technol. 2011, 17, 71–74. [Google Scholar]
- Bai, Y.; Zhu, W.; Zuo, Y.; Li, F. Research Progress and Application Prospect of Coal Coke Water Vapor and CO2 Cogasification Technology. Coal Chem. Ind. 2013, 41, 13–15. [Google Scholar]
- Sadasivam, S.; Zagorščak, R.; Thomas, H.; Kapusta, K.; Stańczyk, K. Experimental study of methane-oriented gasification of semi-anthracite and bituminous coals using oxygen and steam in the context of underground coal gasification (UCG): Effects of pressure, temperature, gasification reactant supply rates and coal rank. Fuel 2020, 268, 117330. [Google Scholar]
- Chen, F.; Pan, X.; Liu, H.; Yao, K. O2/CO2 underground coal gasification model test. J. China Coal Soc. 2013, 38, 495–500. [Google Scholar]
- Zhao, J.; Liu, H.; Pan, X.; Chen, F. Experimental Study on O2/CO2 underground gasification under different Oxygen-enriched Conditions. Coal Sci. Technol. 2017, 45, 214–220. [Google Scholar]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Stanczyk, K.; Stanczyk, K. Efficiency assessment of underground gasification of ortho-and meta-lignite: High-pressure ex situ experimental simulations. Fuel 2019, 236, 221–227. [Google Scholar] [CrossRef]
- Mishra, A.; Gautam, S.; Sharma, T. Effect of operating parameters on coal gasification. Int. J. Coal Sci. Technol. 2018, 5, 113–125. [Google Scholar] [CrossRef]
- Guo, W.; Liu, H.; Chang, Z.; Cao, D.; Liu, S. Study on Effects of Thermal Resistance and Thermal Buoyancy on Oxygen Flow Patterns during Underground Coal Gasification. ACS Omega 2021, 6, 32977–32986. [Google Scholar]
- Su, F.; Jing, S.; Gao, X.; Pu, H.; Fan, W.; Yu, G.; Wu, J.; Deng, Q.; Zhang, T. Evaluation of gasification cavity growth and gas energy recovery in underground coal gasification. J. China Coal Soc. 2021, 46, 3682–3691. [Google Scholar]
- Hamanaka, A.; Su, F.-Q.; Itakura, K.-I.; Takahashi, K.; Kodama, J.-I.; Deguchi, G. Experimental study on evaluation of underground coal gasification with a horizontal hole using two different coals. Fuel 2021, 305, 121556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).