Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digesters
2.2. Analytical Testing
2.3. Substrates
2.4. Experimental Procedure
2.4.1. Phase 1: Bedding Mixture with WS and WC
2.4.2. Phase 2: Bedding Mixture with WS
3. Results and Discussion
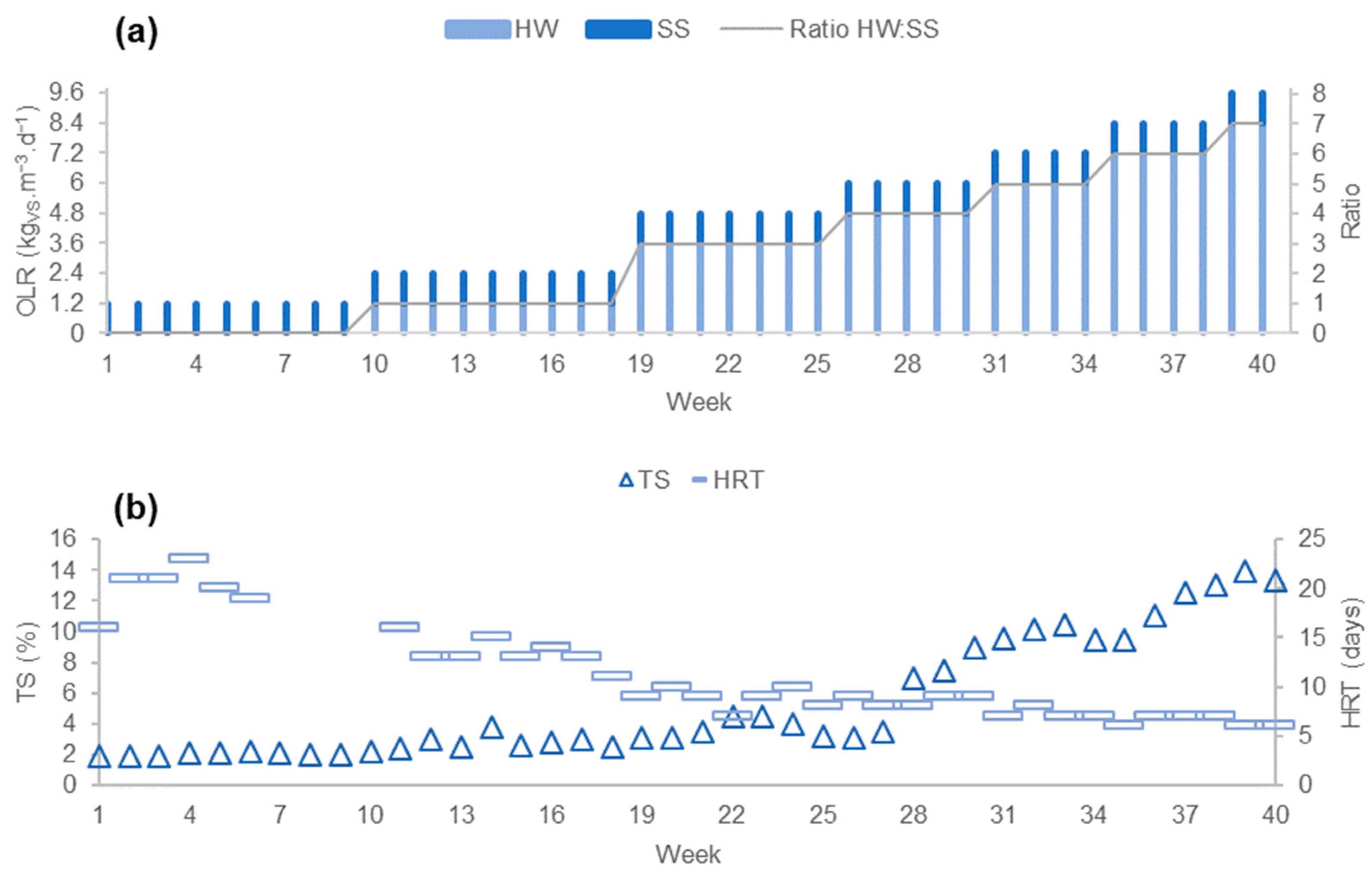
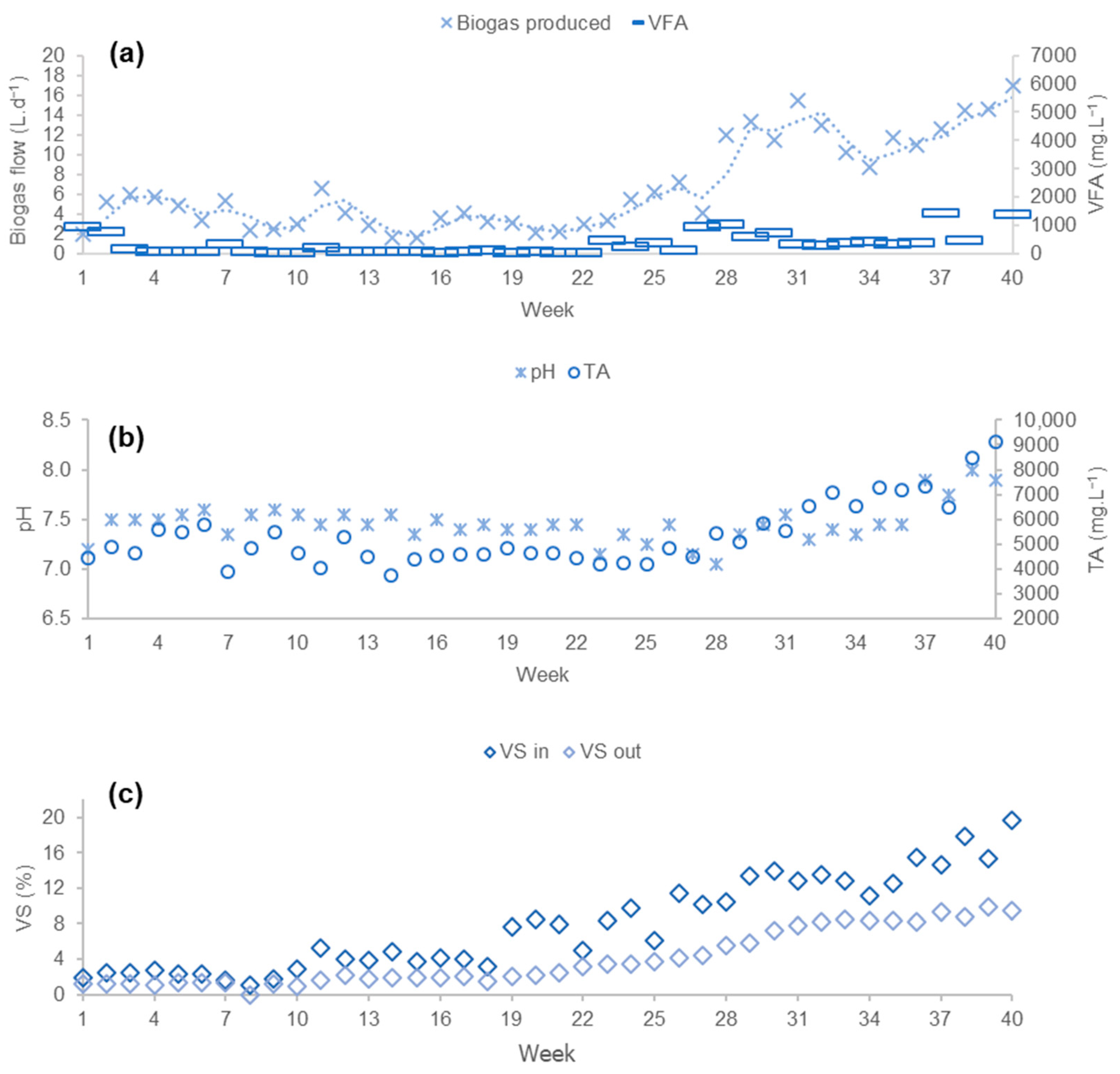
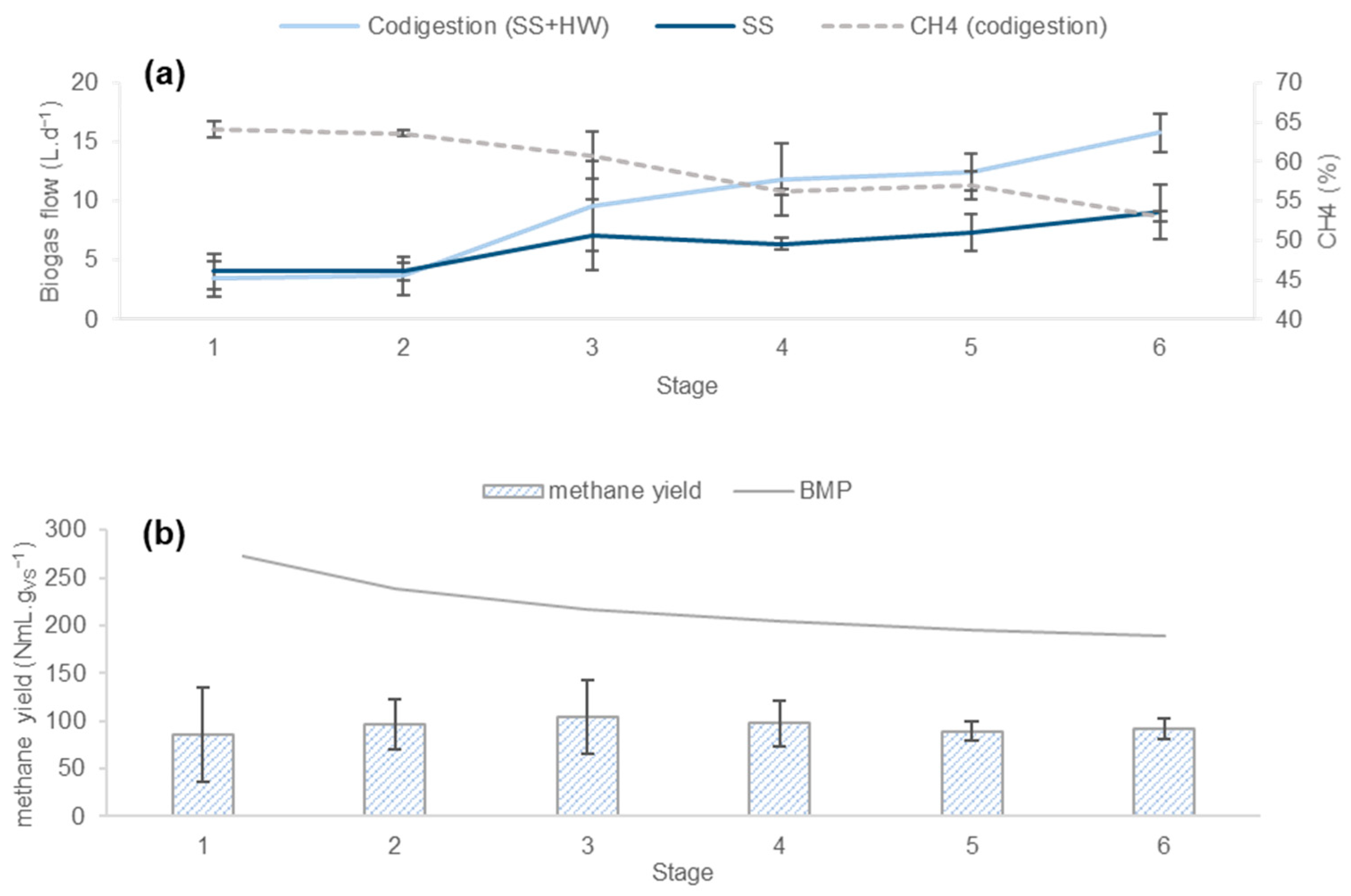
3.1. Results of Phase 1
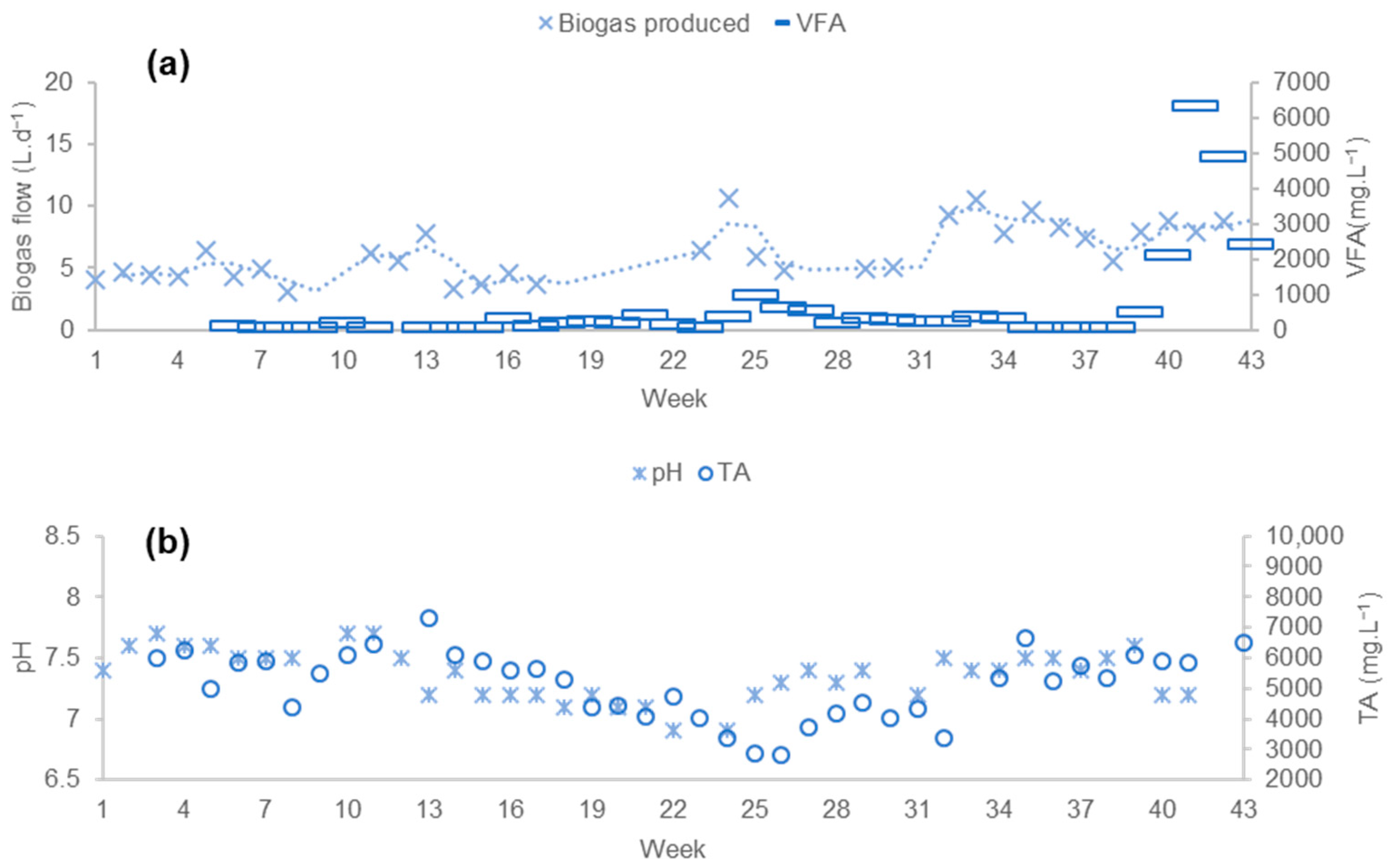
3.1.1. Process Stability
3.1.2. Productivity of the Process
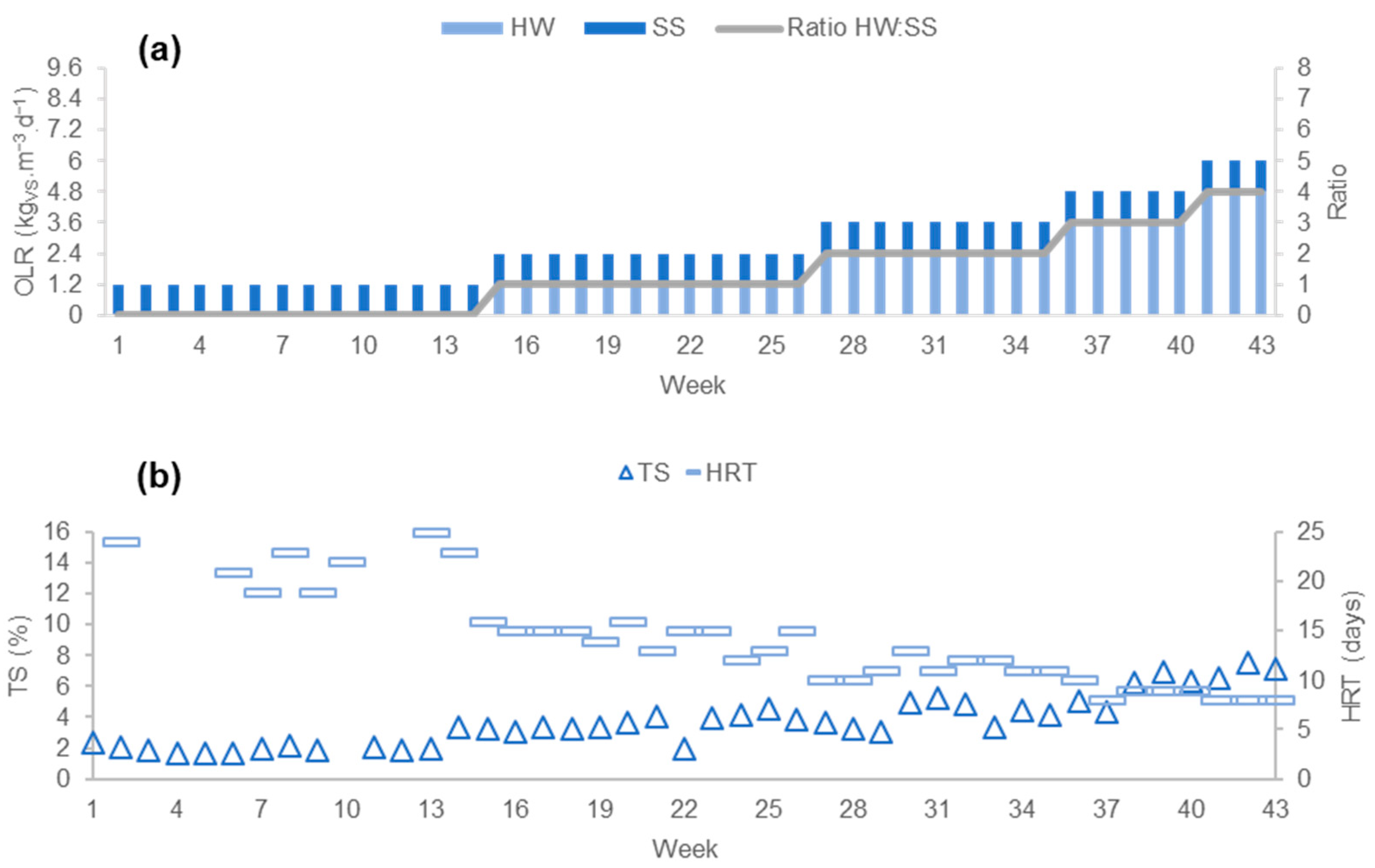
3.2. Results of Phase 2
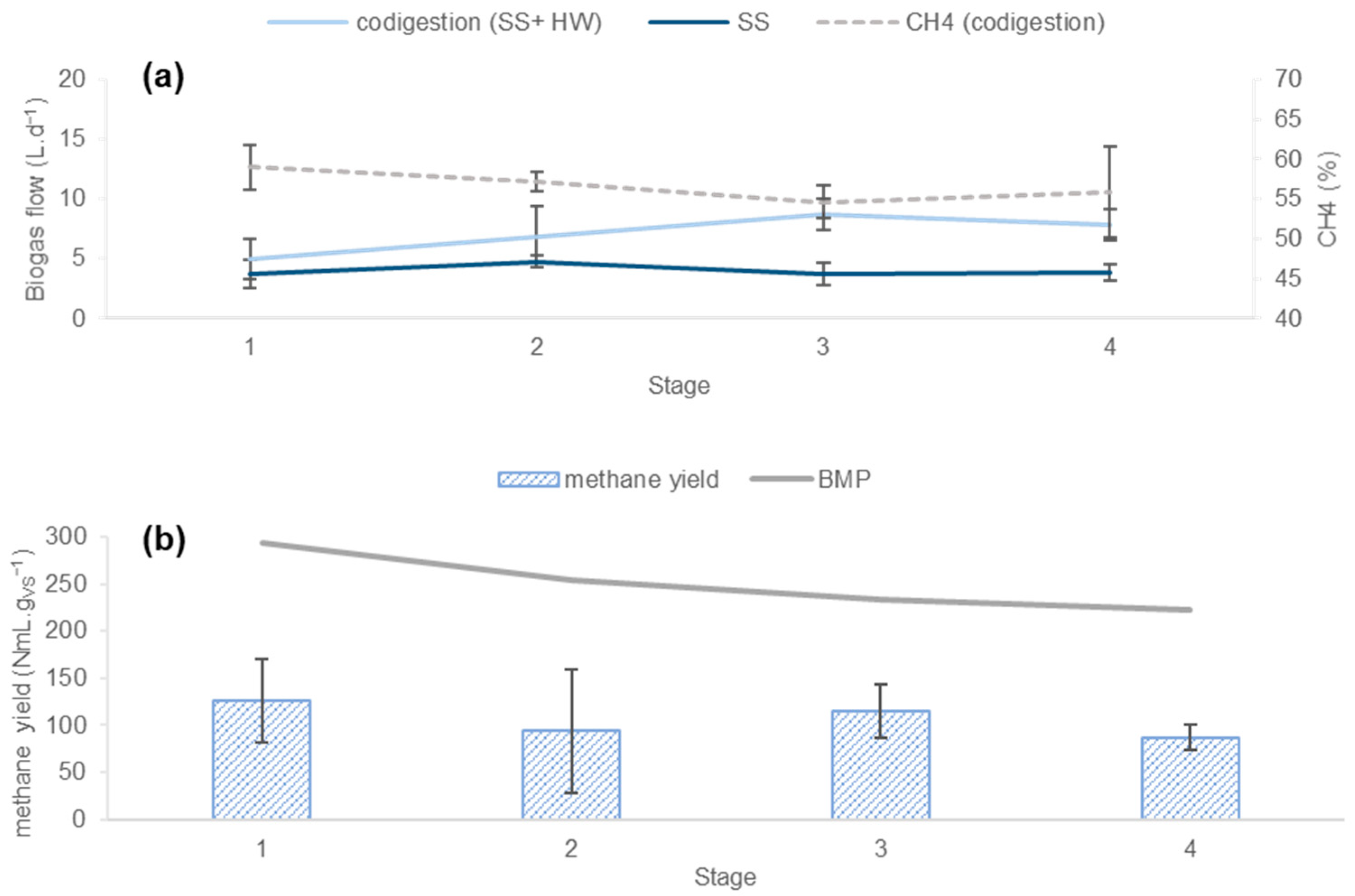
3.2.1. Process Stability
3.2.2. Productivity of the Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haberl, H.; Sprinz, D.; Bonazountas, M.; Cocco, P.; Desaubies, Y.; Henze, M.; Hertel, O.; Johnson, R.K.; Kastrup, U.; Laconte, P.; et al. Correcting a Fundamental Error in Greenhouse Gas Accounting Related to Bioenergy. Energy Policy 2012, 45, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The Future of Anaerobic Digestion and Biogas Utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and Perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Abdeen, F.R.H.; Mel, M.; Jami, M.S.; Ihsan, S.I.; Ismail, A.F. A Review of Chemical Absorption of Carbon Dioxide for Biogas Upgrading. Chin. J. Chem. Eng. 2016, 24, 693–702. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. A Review on Anaerobic Digestion of Lignocellulosic Wastes: Pretreatments and Operational Conditions. Appl. Sci. 2019, 9, 4655. [Google Scholar] [CrossRef]
- Benyahya, Y.; Fail, A.; Alali, A.; Sadik, M. Recovery of Household Waste by Generation of Biogas as Energy and Compost as Bio-Fertilizer—A Review. Processes 2021, 10, 81. [Google Scholar] [CrossRef]
- Brown, D.; Shi, J.; Li, Y. Comparison of Solid-State to Liquid Anaerobic Digestion of Lignocellulosic Feedstocks for Biogas Production. Bioresour. Technol. 2012, 124, 379–386. [Google Scholar] [CrossRef]
- Massanetnicolau, J.; Dinsdale, R.; Guwy, A. Hydrogen Production from Sewage Sludge Using Mixed Microflora Inoculum: Effect of PH and Enzymatic Pretreatment. Bioresour. Technol. 2008, 99, 6325–6331. [Google Scholar] [CrossRef]
- Naji, A.; Rechdaoui, S.G.; Jabagi, E.; Lacroix, C.; Azimi, S.; Rocher, V. Horse Manure and Lignocellulosic Biomass Characterization as Methane Production Substrates. Fermentation 2023, 9, 580. [Google Scholar]
- NF ISO 11465; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. ISO: Geneva, Switzerland, 1993.
- NF U44-160; Organic Soil Conditioners and Organic Material for Soil Improvement—Determination of total organic matter—Calcination method. AFNOR: Paris, France, 1985.
- NF EN ISO 9963-1; Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity. ISO: Geneva, Switzerland, 1994.
- Prusisz, B.; Mulica, K.; Pohl, P. Ion Exchange and Ion Exclusion Chromatographic Characterization of Wines Using Conductivity Detection. J. Food Drug Anal. 2020, 16, 13. [Google Scholar] [CrossRef]
- Shelor, C.P.; Dasgupta, P.K.; Liao, H. Conductometric Gradient Ion Exclusion Chromatography for Volatile Fatty Acids. Anal. Chem. 2016, 88, 12323–12329. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, S.; Luste, S.; Sillanpää, M. Increased Biogas Production at Wastewater Treatment Plants through Co-Digestion of Sewage Sludge with Grease Trap Sludge from a Meat Processing Plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.P.; Carrère, H. Benefit of Sodium Hydroxide Pretreatment of Ensiled Sorghum Forage on the Anaerobic Reactor Stability and Methane Production. Bioresour. Technol. 2013, 144, 149–155. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Rajendran, K.; Taherzadeh, M.J. A Comparative Study between Single- and Two-Stage Anaerobic Digestion Processes: Effects of Organic Loading Rate and Hydraulic Retention Time. Int. Biodeterior. Biodegrad. 2014, 95, 181–188. [Google Scholar] [CrossRef]
- Demirer, G.N.; Chen, S. Two-Phase Anaerobic Digestion of Unscreened Dairy Manure. Process Biochem. 2005, 40, 3542–3549. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Zhang, H.; Xu, S.; Lichtfouse, E.; Yun, Y. Anaerobic Digestion and Recycling of Kitchen Waste: A Review. Environ. Chem. Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Hadin, Å.; Eriksson, O. Horse Manure as Feedstock for Anaerobic Digestion. Waste Manag. 2016, 56, 506–518. [Google Scholar] [CrossRef]
- Yadvika; Santosh; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of Biogas Production from Solid Substrates Using Different Techniques––A Review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic Co-Digestion Process for Biogas Production: Progress, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Bio-Energy Recovery from High-Solid Organic Substrates by Dry Anaerobic Bio-Conversion Processes: A Review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Kabbashi, N. Sewage Sludge Composting Simulation as Carbon/Nitrogen Concentration Change. J. Environ. Sci. 2011, 23, 1925–1928. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and Methane Production by Co-Digestion of Waste Activated Sludge and Food Waste in the Two-Stage Fermentation Process: Substrate Conversion and Energy Yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, Y.; Mahar, R.; Wang, Z.; Nie, Y. Effects and Model of Alkaline Waste Activated Sludge Treatment. Bioresour. Technol. 2008, 99, 5140–5144. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.-P.; Steyer, J.-P.; Escudié, R. Total Solids Content Drives High Solid Anaerobic Digestion via Mass Transfer Limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry Anaerobic Digestion of Organic Waste: A Review of Operational Parameters and Their Impact on Process Performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Rechdaoui, S.; Azimi, S.; Bernier, J.; Rocher, V.; Mottelet, S.; Pauss, A.; Ribeiro, T. Cartographie des boues de STEP et réduction du temps de mesure par un couplage «expérimentation en réacteur/modélisation». L’EAU L’INDUSTRIE LES NUISANCES 2016, 9. Available online: www.revue-ein.com (accessed on 7 September 2023).
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Jayaraj Enhancement of Biogas Production by Pretreatment: A Review. In Proceedings of the IV th International Conference on Advances in Energy Research, Mumbai, India, 10–12 December 2013. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and Opportunities of Lignocellulosic Biomass for Anaerobic Digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Anaerobic Digestion of Solid Agroindustrial Waste in Semi-Continuous Mode: Evaluation of Mono-Digestion and Co-Digestion Systems. Waste Manag. 2017, 68, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; D’Imporzano, G.; Malagutti, L.; Fragali, E.; Ruboni, G.; Adani, F. Evaluating Inhibition Conditions in High-Solids Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Bioresour. Technol. 2010, 101, 5728–5732. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile Fatty Acids as Indicators of Process Imbalance in Anaerobic Digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Bi, S.; Hong, X.; Yang, H.; Yu, X.; Fang, S.; Bai, Y.; Liu, J.; Gao, Y.; Yan, L.; Wang, W.; et al. Effect of Hydraulic Retention Time on Anaerobic Co-Digestion of Cattle Manure and Food Waste. Renew. Energy 2020, 150, 213–220. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Xie, D. Insights into the Production Potential and Trends of China’s Rural Biogas: A Review of China’s Rural Biogas. Int. J. Energy Res. 2015, 39, 1068–1082. [Google Scholar] [CrossRef]
- Wartell, B.A.; Krumins, V.; Alt, J.; Kang, K.; Schwab, B.J.; Fennell, D.E. Methane Production from Horse Manure and Stall Waste with Softwood Bedding. Bioresour. Technol. 2012, 112, 42–50. [Google Scholar] [CrossRef]
- Ajeej, A.; Thanikal, J.V.; Narayanan, C.M.; Yazidi, H. Study on the Influence of the Characteristics of Substrates in Biogas Production. Int. J. Curr. Res. 2016, 8, 39795–39799. [Google Scholar]
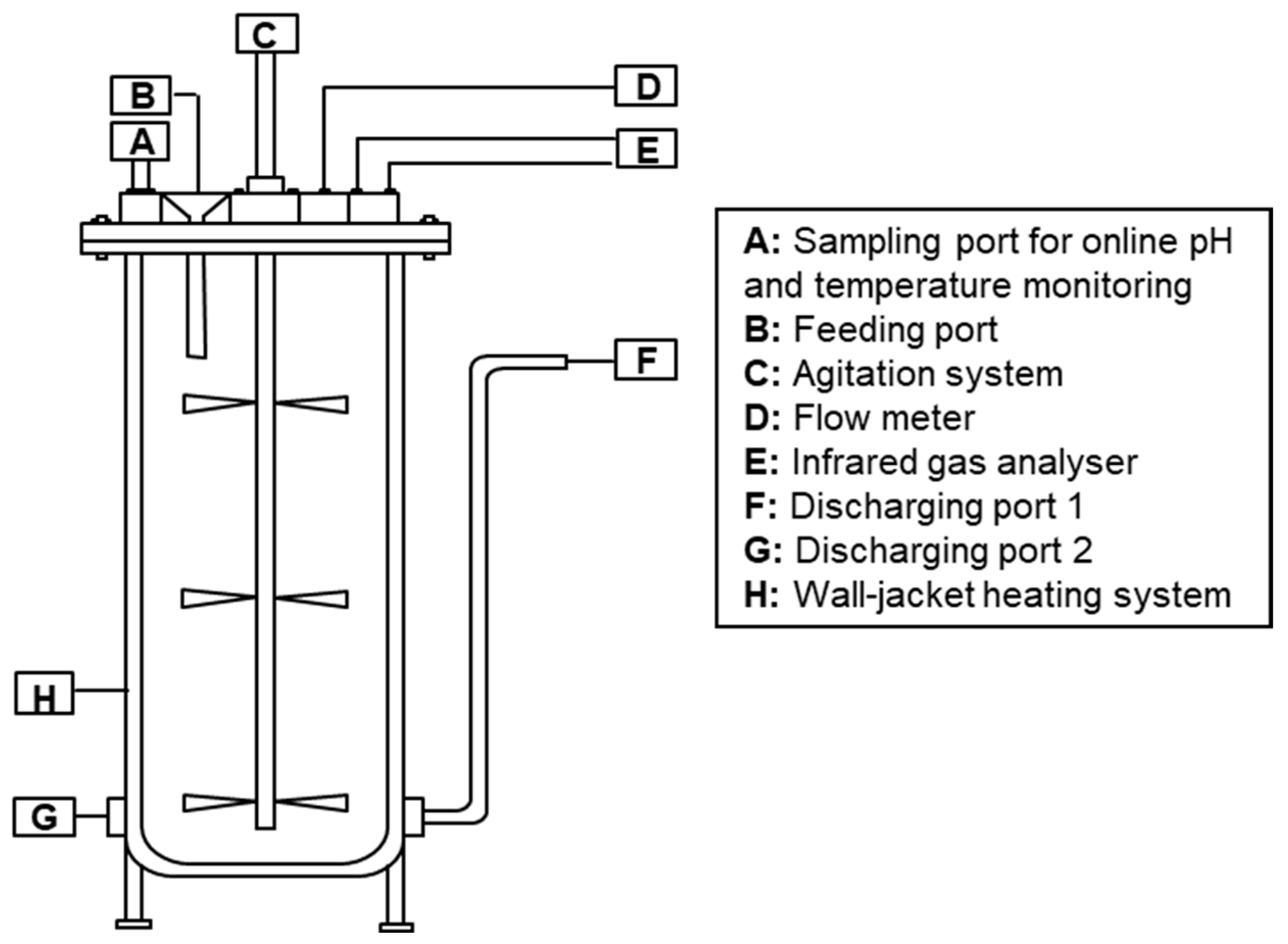
| Stage | Ratio (HW:SS) | OLR (kgvs·m−3·d−1) | HRT (Days) |
|---|---|---|---|
| 1 | 1:1 | 2.4 | 13 |
| 2 | 3:1 | 4.8 | 9 |
| 3 | 4:1 | 6 | 9 |
| 4 | 5:1 | 7.2 | 7 |
| 5 | 6:1 | 8.4 | 7 |
| 6 | 7:1 | 9.6 | 6 |
| Stage | Ratio (HW:SS) | OLR (kgvs·m−3·d−1) | HRT (Days) |
|---|---|---|---|
| 1 | 1:1 | 2.4 | 15 |
| 2 | 2:1 | 3.6 | 11 |
| 3 | 3:1 | 4.8 | 9 |
| 4 | 4:1 | 6 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naji, A.; Rechdaoui, S.G.; Jabagi, E.; Lacroix, C.; Azimi, S.; Rocher, V. Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits. Energies 2023, 16, 6595. https://doi.org/10.3390/en16186595
Naji A, Rechdaoui SG, Jabagi E, Lacroix C, Azimi S, Rocher V. Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits. Energies. 2023; 16(18):6595. https://doi.org/10.3390/en16186595
Chicago/Turabian StyleNaji, Amar, Sabrina Guérin Rechdaoui, Elise Jabagi, Carlyne Lacroix, Sam Azimi, and Vincent Rocher. 2023. "Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits" Energies 16, no. 18: 6595. https://doi.org/10.3390/en16186595
APA StyleNaji, A., Rechdaoui, S. G., Jabagi, E., Lacroix, C., Azimi, S., & Rocher, V. (2023). Pilot-Scale Anaerobic Co-Digestion of Wastewater Sludge with Lignocellulosic Waste: A Study of Performance and Limits. Energies, 16(18), 6595. https://doi.org/10.3390/en16186595





