Abstract
This paper aims to perform a systematic review, with a bibliometric approach, of the techno-economic evaluation studies of hydrogen production. To achieve this objective, a comprehensive outline of hydrogen production processes from fossil and renewable sources is presented. The results reveal that electrolysis, classified as water splitting, is the most investigated process in the literature since it contributes to a reduction in greenhouse gas emissions and presents other advantages, such as maturity and applicability, energy efficiency, flexibility, and energy storage potential. In addition, the processes of gasification, classified as thermochemical, and steam reforming, classified as catalytic reforming, are worth mentioning. Regarding the biological category, there is a balance between research on photo fermentation and dark fermentation. The literature on the techno-economic evaluation of hydrogen production highlights significant gaps, including a scarcity of comprehensive studies, a lack of emphasis on commercial viability, an absence of sensitivity analysis, and the need for comparative analyses between production technologies.
1. Introduction
The large-scale deployment of renewable energy sources has been driven by the proposal of carbon neutrality targets in several countries to reduce the excessive consumption of fossil fuels, which has led to their depletion accompanied by increased global warming of the atmosphere [1,2,3,4]. Moreover, the intensified global efforts to address climate change and the acceleration of the green economy in the post-pandemic period have contributed to this advancement [2,5,6,7], seeking to achieve sustainable development goals [8]. However, the intermittent nature of renewable energy sources, such as solar photovoltaic and wind power, has been a major challenge to their large-scale adoption. These energy sources are not constant and predictable in their energy production [9]. In addition, the large-scale use of these intermittent energy sources will result in periods of overproduction, making it essential to convert this surplus electricity into a storable form of energy [10].
To overcome these limitations, building a hydrogen supply system has been increasingly accepted by a global consensus [1,11,12] as a secondary energy vector with increasing importance in the decarbonization progress [13,14,15,16]. Studies such as those by Parra et al. [17], Liu et al. [18], Weidner et al. [19], Capurso et al. [20], and Oliveira [21] demonstrate that hydrogen has a higher heating value and lower carbon emissions compared to fossil fuels during its life cycle, playing an important role in decarbonizing the power system in many ways across the entire energy value chain.
Currently, hydrogen production is mainly categorized into three types: (i) green hydrogen, which is produced through electrolysis from renewable sources such as solar PV and wind power; (ii) blue hydrogen, which is produced from fossil sources with carbon capture and storage (CCS) or carbon capture, utilization, and storage (CCUS); and (iii) gray hydrogen, which is produced from fossil sources without carbon mitigation measures. Currently, hydrogen production is still largely dependent on non-renewable sources. However, the development and promotion of the participation of renewable sources in this process must be actively pursued to achieve the full sustainability of hydrogen production [22].
The hydrogen production process must be techno-economically feasible and low-carbon to promote sustainable development [14], and the levelized cost of hydrogen (LCOH) is generally used to assess the competitiveness of a method [15,23]. A rigorous techno-economic evaluation of hydrogen production technologies can provide a critical cost comparison for future resource allocation, priorities, and trajectory [24]. This evaluation will have a great impact on future hydrogen production projects and the development of new approaches to reduce overall production costs and make it a cheaper fuel [25].
Thus, this paper aims to carry out a systematic literature review with a bibliometric approach through direct searches in the Web of Science and Scopus databases to identify the main gaps in the literature on the techno-economic evaluation studies of hydrogen production, with particular attention to renewable hydrogen. This review is of utmost importance because a comprehensive and systematic study can provide a detailed overview of the technical and economic aspects involved, including the challenges, opportunities, and prospects. In addition, based on the information obtained in the systematic review, it is possible to inform decision making related to hydrogen production, including the formulation of public policies, strategic investments, targeting of resources, and implementation of measures to promote the techno-economic viability of hydrogen.
Besides this introductory section, the paper is structured as follows: Section 2 presents the methodology used in the research; in Section 3, the main bibliometric results are pointed out; in Section 4, the main systematic results are discussed, giving special attention to the different types of hydrogen production; and, finally, in Section 5, the final considerations are presented, wherein reflections are made on the results obtained and possible paths for the advancement of hydrogen use are pointed out, as well as new studies suggested to deepen the theme.
2. Methodology
The methodology of this paper consists of a systematic review with a bibliometric approach for mapping the main studies regarding the techno-economic evaluation of hydrogen production. Bibliometric analyses have been widely used in several academic disciplines to track a field’s state of the art and analyze its evolution over time. Numerous studies have been devoted to exploring these techniques, providing valuable insights into the progress and development of specific areas of knowledge. In turn, systematic reviews play a vital role in identifying knowledge gaps, assessing the quality and consistency of existing studies, and providing a sound basis for decision making. By carefully combining and analyzing the results of multiple studies, systematic reviews provide an overview of the evidence, reduce the likelihood of bias and uncertainty, and contribute to the advancement of scientific knowledge in a reliable and trustworthy manner.
Thus, the terms used, the inclusion and qualification criteria, as well as the details for the search and extraction from the database are presented in Table 1, where the abbreviation TS corresponds to Topic (TS = Topic) and means the words that are searched in the titles, abstracts, and keywords of the studies included in the database. It was considered appropriate to use the English variations of the term techno-economic evaluation, these being ‘techno-economic’, ‘technical and economic’, and ‘technological and economic’, and terms referring to hydrogen production as ‘hydrogen production’ and ‘production of hydrogen’, to choose the research topic. Despite their simplicity and intuitiveness, these terms provided results that aligned with the research objectives. It is crucial to note that the terms ‘Evaluation’, ‘Analysis’, and ‘Feasibility’ were intentionally not linked with ‘techno-economic’ and ‘technical-economic’ to ensure a broader inclusion of studies directly relevant to the topic, thereby avoiding unnecessary limitations in the sample.

Table 1.
Description of search strategies.
It is also noteworthy that it was decided to use the Web of Science and Scopus databases as the main search tool due to their diffusion in the academic community and the reliability of their selection standards [26]. Moreover, these databases present satisfactory reach and coverage [27], meeting the requirements of this research.
Another aspect that needs to be mentioned is that while the inclusion criteria serve mainly for a more superficial screening of the study, not least because they consider factors related to the year, type of study, and journal of publication, the qualification criteria are used for a deeper screening of the studies, analyzing aspects of applicability and quality, which are only possible to determine through more a specific analysis and reading of the studies.
The process of screening the studies from the preliminary database and creating the final database was carried out following the guidelines and criteria established for inclusion and qualification. The methodology employed for this process adhered to the framework outlined in Figure 1, which served as a guiding principle.
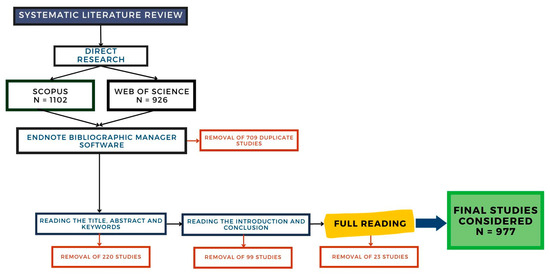
Figure 1.
Screening of the studies included in the database.
During the screening process, articles that met the inclusion criteria were selected for further analysis, while those that did not meet the criteria were excluded. This step aimed to ensure that only relevant and high-quality articles were included in the final database.
After completing the screening process, the selected articles were further examined to extract relevant information and data. This information might have included key findings, methodologies employed, and other pertinent details. This additional analysis contributed to creating a comprehensive final database containing articles meeting the inclusion and qualification criteria.
It is crucial to consider the search date during the bibliometric literature review because it indicates when the research was conducted. This may affect the results presented, especially if there are studies published after the initial search. For example, if the review was conducted in late 2023, new studies would be included in the search, which could alter the statistics related to the number of publications and citations per year.
While acknowledging the study’s limitations, it is essential to note that the databases utilized in the research exhibit satisfactory scope and coverage in the field of science and engineering. However, it is worth mentioning that they might not encompass studies focusing on business and economics.
3. Bibliometric Results
This section may be divided into subheadings. It should provide a concise and precise description of the experimental results, their interpretation, and the experimental conclusions that can be drawn.
From the search conducted in the Web of Science and Scopus databases, it was possible to verify that 977 publications were suitable to be included in the research repository, i.e., they met the inclusion and qualification criteria (quality and applicability). Thus, Figure 2 presents the evolution of publications on the theme over the years. This analysis is fundamental to evaluate the level of expansion of the theme, as well as new opportunities for studies.
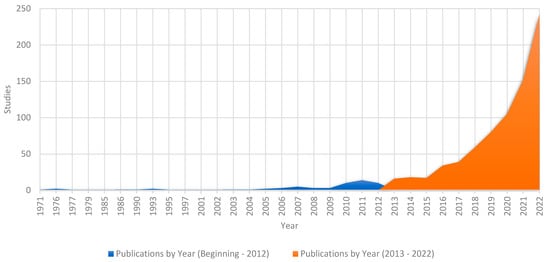
Figure 2.
Growth of publications per year.
Figure 2 illustrates the history of publications on the technical-economic viability of hydrogen production over the last five decades. It can be observed that interest in this subject has been an object of study since 1971, evidencing a continuous research trajectory over time. However, it is important to note that a constancy in publications occurred after 2001, i.e., after this year, there were publications for all subsequent years, indicating a greater interest and dedication of researchers in this area.
Looking at the last complete decade (2013–2022), marked in red in Figure 2, we see an exponential growth in publications, representing an impressive 94% of the total considered publications up to 2022. One noteworthy year is 2022, which recorded the peak in the number of publications, corresponding to 29% of the total in Figure 2. It should also be noted that 2023, although still in progress and therefore not included in Figure 2, already has 153 publications on the subject. This shows a significant increase in the interest in and production of scientific knowledge on the subject. This recent concentration of studies suggests a favorable environment for the development of additional research and the exploration of new perspectives in the context of hydrogen production.
It also becomes pertinent to evaluate the articles by journal of publication to identify which journals are most interested in the subject, as well as the impact factor of each of them. This allows researchers to direct their publication efforts to journals that directly focus on the subject studied, avoiding untargeted submissions and a considerable waste of time. Thus, Table 2 presents the journals with publication volumes higher than 20, where P (Publications) refers to the number of articles published in the journal on the area of interest investigated, and IF (Impact Factor) evaluates the importance of the scientific journals in their respective areas. It is important to note that the values presented in the IF (2022) column corresponds to 2022, the year available in the Web of Science database. In addition, the average for the last five years (2018–2022) is given in IF (mean) column.

Table 2.
Main journals.
In Table 2, it is possible to identify the journals that stand out when addressing the techno-economic evaluation of hydrogen production. The three leading journals in this area are the International Journal of Hydrogen Energy, with 202 publications, Energy Conversion and Management, with 92 publications, and Applied Energy, with 55 publications, which together account for approximately 36% of the total number of publications. These journals play a relevant role in the dissemination of scientific knowledge in this field. In addition, it is important to note that 221 different information sources published studies on the subject, indicating the diversity and breadth of academic interest in this area.
By ordering the journals according to impact factor, it is possible to identify those considered most relevant in this context. Renewable and Sustainable Energy Reviews, Bioresource Technology, Applied Energies, and the Journal of Cleaner Production stand out, all with an impact factor higher than 11, indicating an excellent score in this metric. These high-impact journals play a crucial role in disseminating relevant and influential research in the field of renewable hydrogen.
Based on the results presented in Table 2, it can be seen that the most relevant journals on this theme are focused on the investigation of energy solutions and clean fuels, with a focus on renewable hydrogen, aiming to minimize the environmental impacts related to the emissions of greenhouse gases and air pollutants. In addition, these journals also seek to address the issue of natural resource depletion, highlighting the importance of sustainability in the production of renewable hydrogen. This approach highlights the relevance of not only the techno-economic evaluation but also the environmental and sustainable aspects related to hydrogen production. The mentioned journals play a crucial role in encouraging and promoting research that contributes to a cleaner and more sustainable energy future.
Based on the results presented in Table 2 it can be seen that the most relevant journals on this theme are focused on investigating the application areas. It is pertinent to analyze those that show more interest in the techno-economic evaluation of hydrogen production. It is worth noting that the categorization of studies based on areas of application follows the division established by the Web of Science database. This approach enables us to efficiently identify, retrieve, and analyze documents from various databases that are interconnected and pertain to the same subject.
Observing Figure 3, one can see that Energy and Fuels is the area with the highest number of publications, corresponding to 32% of the total. Next are Engineering and Chemistry, with 14% and 13% of the total publications, respectively. These areas play a significant role in the research and development of solutions related to the production of hydrogen because:

Figure 3.
Division of studies by application area.
- Energy and Fuels: This area is directly relevant as it covers studies related to the production, storage, distribution, and use of energy, including hydrogen as an energy source. Understanding the properties and energy potential of hydrogen is crucial to its techno-economic evaluation as well as its application in different sectors, such as transportation, industry, and power generation [11];
- Engineering: Engineering plays an essential role in the techno-economic evaluation of hydrogen production as it is involved in the design and development of processes, systems, and infrastructure related to this technology. Engineering is responsible for designing and optimizing hydrogen production processes, considering technical, economic, and sustainability aspects. In addition, engineers play a crucial role in implementing practical and efficient solutions for the use of hydrogen in various applications;
- Chemistry: Chemistry plays a significant role in analyzing and understanding the chemical properties of hydrogen as well as developing catalysts and chemical processes associated with its production. Chemical research is critical to improve the efficiency of hydrogen production processes, explore new feedstock sources, develop storage technologies, and advance the use of hydrogen as an energy carrier. Understanding the chemical reactions involved in the production and utilization of hydrogen is critical to improving its technical and economic viability.
Besides the areas mentioned, other vital areas such as Thermodynamics, Biotechnology and Applied Microbiology, Nuclear Science and Technology, and Computer Science are worth mentioning. These complementary areas also play a relevant role in the investigation of the techno-economic evaluation of hydrogen, bringing multidisciplinary perspectives to the subject. The area of Thermodynamics contributes to the study of the thermodynamic processes involved in hydrogen production, including energy efficiency and the properties of the materials used. Biotechnology and Applied Microbiology provides insights into possible biotechnological applications in hydrogen production and storage.
Nuclear Science and Technology explores the potential of using nuclear energy as an energy source for hydrogen production, and Computer Science contributes technological advances, such as modeling and computer simulation, to optimize processes and systems related to hydrogen. These highlighted areas show the comprehensiveness and interdisciplinarity of the study of the techno-economic evaluation of renewable hydrogen production. Collaboration between different fields of knowledge is crucial in driving research and development in this promising field, allowing for a holistic and integrated approach to the search for sustainable energy solutions.
One can also observe the importance of evaluating the main keywords found in the articles in the research repository, as identified in the interconnection network between the keywords presented in Figure 4, developed utilizing the VOSviewer software (version 1.6.19). This strategy allows researchers to more easily find studies directly related to the subject investigated, and to identify new research directions that can be taken. The network represented in Figure 3 comprises 4938 items, 72 clusters, and 103,420 links/connections, with a total link size of 129,713. In it, it is possible to identify the most used keywords (according to the size of the sphere under its representation), the interconnections between them (according to the connections between spheres), and the clusters between keywords (according to the color of each sphere).

Figure 4.
Network of interconnection between keywords.
From the analysis of Figure 4, it is possible to observe the most frequent keywords related to the topic of the techno-economic evaluation of hydrogen production. Among the most common keywords are: “hydrogen production”, “techno-economic analysis”, and “hydrogen”. These keywords emerged most frequently due to the search terms used in the creation of the database and reflect the importance of these concepts in the field of hydrogen production research.
In addition to the keywords mentioned above, it is relevant to highlight the importance and significance of the additional keywords identified in the bibliometric analysis. These less intuitive keywords provide valuable insights into the different perspectives and applications of hydrogen. Among the other relevant keywords, it is important to mention those related to the different hydrogen production processes, such as “Electrolysis”, “Gasification”, “Steam reforming”, “Pyrolysis”, and “Fermentation”. These terms indicate specific approaches used to obtain hydrogen, highlighting the diversity of technologies employed in this field. It is worth noting that special attention is given to this in Section 4.
Another term to highlight is “sensitivity analysis”. This term is extremely relevant in the assessment of the techno-economic evaluation of hydrogen production, as it refers to an assessment of the impact of different variables and parameters on the results of the study. Sensitivity analysis assists in understanding the factors that influence feasibility and provides crucial information for informed decision making [28].
Sensitivity analysis plays a crucial role in evaluating the operational aspects of a plant. It provides valuable insights that aid in decision making before making an investment. One of the key factors to consider is the sensitivity to capital costs, which can be determined by calculating the return on investment. This analysis serves as a vital tool in assessing the overall growth trajectory of the plant from its inception to its completion as well as determining the level of investment return at different stages of the plant’s development [25].
However, it is important to note that only 18% of the publications found in the research repository use this analysis to study the techno-economic evaluation of hydrogen production processes. This reveals a significant gap in the approach researchers take in scientific papers. Sensitivity analysis allows one to examine how changes in critical variables affect feasibility indicators such as production costs, rate of return, payback time, and others. By performing this analysis, you can identify the most sensitive variables and understand their impact on project viability, which provides a more complete and robust view of the risks and uncertainties associated with this venture.
While sensitivity analysis is undeniably crucial for establishing a robust techno-economic analysis of hydrogen production studies, it is essential to recognize that other strategies can be employed to augment the assessment’s reliability and tackle potential gaps in the evaluation process, such as a Monte Carlo-based simulation tool, as discussed in the following sections.
4. Systematic Results
Studies on the techno-economic evaluation of hydrogen production processes were divided into categories and sub-categories to facilitate the discussion of the systematic results, as shown in Figure 5.
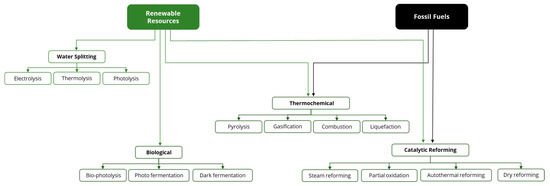
Figure 5.
Methods of hydrogen production. Source: based on Nikolaidis and Poullikkas [29].
Thus, initially, the studies included in the database were classified in a more general way: (i) Water splitting; (ii) Biological; (iii) Thermochemical; and (iv) Catalytic reforming, as described in Table 3.

Table 3.
Definition of the main hydrogen production processes.
Figure 6 presents the distribution of studies found for different hydrogen production processes, while Figure 7 correlates each of the categories with their corresponding subcategories. The “Water splitting” category stands out, accounting for 36% of the total publications. This category encompasses processes that aim to obtain hydrogen from the breakdown of a water molecule [16]. Within this category, the subcategory “Electrolysis” is the most investigated for reasons such as maturity and applicability, energy efficiency, flexibility, and energy storage potential, corresponding to 33% of the publications on hydrogen production in general and 92% of the publications related to this specific process.

Figure 6.
Percentage of studies by general process type for hydrogen production.

Figure 7.
Alluvial diagram of correlation between hydrogen production categories and subcategories.
Another relevant category for hydrogen production is the “Thermochemical” category, which corresponds to 31% of the publications. Within this category, the subcategory “Gasification” has a significant representation (see Figure 7), covering 17% of the total publications and 55% of the publications referring to the “Thermochemical” subcategory, which also presents representations referring to pyrolysis (9% of this total), liquefaction (3% of this total), and combustion (2% of this total). The “Catalytic reforming” category also stands out, comprising 19% of the total publications, which is mostly relative to the “Steam reforming” subcategory, accounting for 15% of the total publications. Finally, the “Biological” category also deserves attention, comprising 14% of the total, driven by the “dark fermentation” and “photo fermentation” processes, accounting for 8% and 5% of the total publications, respectively.
In order to address each of the hydrogen production categories and subcategories related to the techno-economic feasibility studies of renewable hydrogen production, the following subsections are intended to clarify questions, fill in gaps, and highlight the potential of each of these processes. In addition, they will provide insights into the technical and economic considerations involved in hydrogen production.
4.1. Water Splitting
The water splitting process may be divided into three processes: (i) electrolysis; (ii) thermolysis; and (iii) photolysis, as shown in Table 4.

Table 4.
Definition of the main processes of the water splitting category.
When considering water splitting, it is noted that electrolysis is by far the most addressed hydrogen production process in the literature. This is because water electrolysis presents many advantages, such as high energy conversion efficiency and the production of hydrogen with high purity [1]. In addition, it is a well-established technology and perfectly compatible with renewable energies [33].
Due to economic constraints, water electrolysis currently represents only a small share (4%) of global hydrogen production. However, it is considered one of the most promising and sustainable methods for hydrogen production due to the associated environmental benefits. By using renewable water as a feedstock and generating pure oxygen as a by-product, water electrolysis contributes to the reduction in greenhouse gas emissions and provides a clean source of energy [34]. Studies such as the one conducted by Kumar and Himabindu [35] reinforce the importance of this method as a viable and environmentally friendly solution for hydrogen production.
In addition to being a viable alternative to fossil fuels, since it uses water as the raw material, electrolysis has the added advantage of being able to use electricity from renewable sources, such as solar or wind power, to drive the reaction, obtaining in this case, renewable hydrogen [36]. The choice of electrolysis as a focus area for most studies is related to its technological maturity and ability to integrate with renewable energy systems [37]. Electrolysis offers a versatile solution to store and utilize hydrogen generated from renewable sources, allowing for better demand management and contributing sustainably to the energy transition [38].
A comprehensive and informed understanding of the subject can be achieved by comparing the electrolysis production process with various primary energy sources. Shaner et al. [24] conducted a techno-economic comparative analysis of renewable hydrogen production by utilizing solar energy. The results revealed that integrated photoelectrochemical hydrogen production and hydrogen production by discrete photovoltaic electrolysis are functionally equivalent systems, enabling a direct comparison based on cost. The trade-offs related to building a single integrated unit, which has potentially fewer components and generates hydrogen directly, in contrast to the increased operational flexibility afforded by the discrete photovoltaic electrolysis configuration, were instrumental in choosing the most cost-effective technology capable of delivering the specific quality and quantity of energy desired.
Shaner et al. [24] also compared the costs of solar hydrogen with other approaches that offer similar functionalities as part of a low-carbon energy system. Carbon-neutral energy production and storage technologies, such as electricity from nuclear fission or solar power, in conjunction with battery storage, pumped hydroelectricity, or compressed air-based energy storage, represent alternative technology options to the use of solar hydrogen for grid storage and, in some cases, for the transportation sectors. These technologies mainly compete with the electrolysis unit, and each of them presents different operating efficiencies as well as distinct operating and capital costs. Many of these technologies are already established in the market, while hydrogen derived from photo electrochemistry is still at a fundamental stage of research.
Regarding the feed sources, according to KABIR et al. [7], proton exchange membrane electrolysis (PEM) is deemed the most suitable technology for hydrogen production using pure water. In contrast, microbial electrolysis cells (MECs) are considered the most appropriate technology for hydrogen production from wastewater. When considering seawater as the feed source, electrodialysis (ED) and reverse electrodialysis (RED) emerge as the most competitive technologies for simultaneous desalination and hydrogen production. It is worth noting that all of these hydrogen production technologies are currently in their research and development stage [7].
For instance, according to TUFA et al. [39], the primary constraint of RED in its current state of the art is the scarcity of low-resistance ion-conductive membrane materials that are cost-effective (<4 EUR/m2; ~4.4 USDm2) and exhibit high perm selectivity (>95%) for practical operation. The practical implementation of RED technology as an energy source for water electrolysis in hydrogen production necessitates thorough material and process optimization. It requires the development of highly conductive and stable membrane separators and highly active and durable electrodes. Additionally, the utilization of low-cost catalyst materials is essential for the successful deployment of alkaline polymer electrolyte water electrolysis (APEWE).
The most significant limiting factor in the development and expansion of hydrogen technology is price. One of the main challenges in developing a hydrogen economy lies in scaling up hydrogen production and transitioning from carbon-intensive to low-carbon methods. Currently, the costs associated with green hydrogen production remain high, making it economically less competitive than other energy sources. To ensure profitability, the price of hydrogen per kilogram should be approximately USD 1.5. However, a significant obstacle is the requirement for ultrapure water, as it takes 2.38 gallons or 9 L of water to produce 1 kg of hydrogen gas. This high water demand could potentially lead to water shortages in the future hydrogen economy [7].
Although the literature addresses fewer thermolysis studies than electrolysis studies, Lee et al. [15] conducted a study on a two-step thermochemical water-splitting method for green hydrogen production. They evaluated the economic feasibility of two hydrogen production systems: (i) one utilizing high-temperature solar concentration power systems and (ii) the other employing microwaves with advanced nuclear power plants. The study assessed the LCOH, considering various cost factors to determine the required technological development level for achieving the ultimate hydrogen production target of 2 USD/kg H2. The analysis highlighted the importance of heat recuperators in the hydrogen production system, as indicated by the cost allocation to LCOH. The authors also identified essential research areas for the commercialization of the method, including catalyst development for high oxygen partial pressure, the optimization of the heat recuperation system, the primary heat source, and the power conversion system. By analyzing the LCOH with different values for advanced technology, the study confirmed the comparative value and emphasized the significance of these research fields in utilizing microwave heating and advanced nuclear power plants for hydrogen production.
Budama et al. [40] investigated the potential of maximizing the value of the two-step thermochemical metal oxide water-splitting cycle using ceria. They focused on utilizing the excess heat generated in this cycle for the co-production of electricity alongside hydrogen. Their techno-economic study analyzed a hybrid ceria cycle, where the surplus heat was converted into electricity through an organic Rankine cycle. Despite this co-generation approach, the estimated cost of hydrogen remained relatively high at 4.55 USD/kg. Sensitivity analyses identified various opportunities and challenges to achieve the target cost of 2 USD/kg, including improving solar field efficiency, increasing revenue from electricity sales, and reducing the capital recovery factor. The study emphasized the challenges of reaching the desired cost target using ceria as the active material, suggesting the need to develop new materials.
Another hydrogen production process in the water splitting category that is not well verified in terms of technical-economic analysis is photolysis. Oladipo et al. [41] conducted a techno-economic analysis of hydrogen production from hydrogen sulfide (H2S) using photocatalysis and utilized Aspen Plus software V8.4. to design and simulate the proposed plant. An economic analysis was performed using CapCost software to estimate capital expenditure (CAPEX), operational expenditure (OPEX), and the cost of hydrogen production. The results indicated that at a hydrogen production rate of 0.485 t/h, the process CAPEX was approximately USD 105 million, with an annual OPEX of USD 143 million. The LCOH was calculated to be USD 1860 per ton of hydrogen. According to the analysis, the proposed plant would break even at the end of its operational life. The sensitivity analysis revealed that the process’s OPEX and hydrogen price were significantly influenced by the cost of aqueous NaOH. These findings are particularly valuable for oil and gas industries interested in integrating emerging technologies, such as the one presented in the study, into their refineries.
4.2. Biological
According to Table 5, the Biological category may be divided into three processes: (i) bio-photolysis; (ii) photo fermentation; and (iii) dark fermentation.

Table 5.
Definition of the main processes of the Biological category.
Both dark fermentation and photo fermentation are considered effective routes for hydrogen production, and each method comes with its unique advantages and challenges. Photo fermentation relies on the use of light as an energy source, typically provided by solar radiation. However, the constant need for a light source can increase the cost of this process. On the other hand, dark fermentation does not require light and is known for its high efficiency in hydrogen production. However, one drawback of dark fermentation is the production of organic acids during the fermentation process, which can affect the purity of the hydrogen produced. Additionally, microbial contaminants and other toxic compounds can also be generated [43].
Dark fermentation is a well-established and widely employed technique for hydrogen production from diverse renewable biomass sources. These sources include crop residues, lignocellulosic biomass, organic waste, and algal biomass [25]. The utilization of these biomass feedstocks not only provides a renewable and sustainable resource but also offers an opportunity for recycling organic waste materials, contributing to waste management and environmental sustainability.
Compared to thermochemical processes, biological processes, such as dark fermentation and photo fermentation, are typically performed at lower temperatures (around 30–60 °C) and pressures (approximately 1 atm), which results in reduced energy costs. This lower energy demand makes biological processes more environmentally friendly and economically viable options for hydrogen production.
However, the complexity of organic and lignocellulosic materials can influence the efficiency and yield of hydrogen production in biological processes. The composition and structure of biomass feedstocks, as well as the presence of inhibitors and recalcitrant components, can impact the overall performance and economics of the process. Therefore, further research and development efforts are needed to optimize the conversion of biomass feedstocks and improve the efficiency and cost-effectiveness of biological hydrogen production methods [44].
Nikolaidis and Poullikkas [29] suggest that biological methods hold promising potential for hydrogen production. However, more extensive research studies and technological advances are essential to enhance their production rates and overcome their limitations, such as low conversion efficiencies and high investment costs. It is crucial to continue exploring and refining these biological processes to increase hydrogen production rates, improve cost competitiveness, and promote their widespread adoption as sustainable alternatives to conventional hydrogen production methods.
Concerning bio-photolysis, a process still little treated in the literature when compared to dark fermentation and photo fermentation, Frowijn and Van Sark [42] conducted an analysis comparing the technical-economic and general performance of various solar hydrogen generation and electrolysis methods, including bio-photolysis, based on photovoltaic energy in the Netherlands. The study focused on the production costs associated with different methods.
The results of Frowijn and Van Sark [42] indicated that the production costs for photo-catalytic water splitting, direct bio-photolysis, and photoelectrochemical water splitting were found to be 18.32 USD/kgH2, 18.45 USD/kgH2, and 18.98 USD/kgH2, respectively. These costs are expected to decrease significantly in the future. Notably, direct bio-photolysis and photo-catalytic water splitting show potential costs of 3.10 USD/kgH2 and 3.12 USD/kgH2, respectively, which could make them more affordable than photovoltaics-based electrolysis. These findings suggest that direct bio-photolysis and photo-catalytic water splitting methods hold promise for cost-effective hydrogen production and may outperform photovoltaics-based electrolysis in terms of cost efficiency.
4.3. Thermochemical
Thermochemical processes to produce hydrogen can employ fossil fuels as well as renewable sources as raw materials and may be divided into (see Table 6): (i) pyrolysis; (ii) gasification; (iii) combustion; and (iv) liquefaction.

Table 6.
Definition of the main processes of the Thermochemical category.
Regarding the above thermochemical methods, pyrolysis and gasification are the most employed processes, taking into account the low hydrogen yield obtained by liquefaction and combustion, in addition to the high pollutant gas emissions by the latter, making the process unfavorable in terms of sustainability [47].
Coal gasification is one of the main processes used to produce hydrogen in the industry, which may be attributed to the low cost and abundance of coal reserves. However, this process presents significant environmental problems since it releases large amounts of carbon dioxide and other pollutant gases. Furthemore, the mining of coal may cause topographical and ecological changes. In this sense, coal gasification followed by carbon sequestration may minimize the impacts. Carbon sequestration is usually performed by conventional pressure swing adsorption (PSA) or advanced membrane technology to remove the carbon from the gas. In addition, the hydrogen plants also co-produce electricity to improve the overall economics of the plant [48].
Due to the scarcity of fossil fuels, there is an increasing demand for developing technologies that use renewable sources, such as biomass. Nevertheless, hydrogen production from renewable sources is still more expensive than from fossil fuels, as shown by Bartels et al. [48]. The main thermochemical processes used to convert biomass into hydrogen are pyrolysis and gasification. The authors reported a promising cost of hydrogen from biomass in the range of 1.44–2.83 USD/kgH2. Furthermore, the increase in the fossil fuel feedstock cost, along with the development of technologies for renewable sources, may make renewable sources more economical soon.
Hoang et al. [13] conducted a study to investigate the current state of research on biomass steam gasification, focusing on the generation of hydrogen-enriched gas. The paper discussed various aspects, such as the reaction conditions, types of gasifiers, and catalysts used in the process. The implementation of biomass gasification offers significant potential for the cost-effective synthesis of hydrogen from biomass. The findings suggest that economic analysis and the development of more efficient and cost-effective catalysts could effectively tackle the technical challenges associated with gas conditioning and utilization. One of the main obstacles in biomass gasification is the presence of tar, which poses significant issues even when secondary catalysts are employed. Therefore, optimizing reaction conditions and catalysts is crucial to increasing hydrogen yield and reducing investment costs.
Vuppaladadiyam et al. [45] highlighted that biomass pyrolysis has gained attention as a thermochemical conversion method for obtaining value-added products due to the continuous advancement in the development of innovative, efficient, and economically viable pyrolysis processes that allow adjustments to maximize the production of high-quality hydrogen. These advancements represent a promising perspective for the broader utilization of biomass pyrolysis as a sustainable and efficient alternative. Nikolaidis and Poullikkas [29] and Lepage et al. [44] also reported that, in addition to pyrolysis, biomass gasification has also emerged as an economically viable approach that offers the greatest potential to become competitive on a large scale in the near future, for reasons including its suitability for both wet and dry biomass and its unnecessity for an oxidizing agent.
Shahabuddin et al. [49] reviewed techno-economic analyses of hydrogen production from biomass and residual wastes through gasification and pyrolysis followed by reforming. The authors found a LCOH from biomass ranging from ~2.3 to 5.2 USD/kgH2 at feedstock processing scales of 10 MWth and ~2.8–3.4 USD/kgH2 at scales above 250 MWth. The estimated LCOH from residual wastes were in the range of ~2.6–4.8 USD/kg at scales of 75 MWth and ~1.4–3.5 USD/kgH2 at 150 MWth. The main difficulties to produce hydrogen from residual wastes are the residue pre-treatment, technology development and competitiveness, and absence of incentive policies. Furthermore, the LCOH is more sensitive to the plant’s overall efficiency, feedstock cost, processing scale, and installed capital cost.
4.4. Catalytic Reforming
Catalytic reforming is one of the main routes used to produce hydrogen in the industry, mainly from hydrocarbons (natural gas and oil) but also from renewable sources, and comprises four different processes: (i) steam reforming; (ii) partial oxidation; (iii) autothermal reforming; and (iv) dry reforming. These are described in Table 7.

Table 7.
Definition of the main processes of the Catalytic reforming category.
It is noteworthy that steam reforming is the process of hydrogen production within the hydrocarbon reforming category that has received the most extensive investigations regarding its techno-economic feasibility. Furthermore, even in a general context, it is also the most widely used technique for large-scale hydrogen production [41,50]. It accounts for approximately 48% of the global hydrogen demand [53]. About 90% of hydrogen is primarily distributed at retail through the steam reforming of natural gas and a fair solution for glycerol [12].
Several studies have been conducted, approaching various forms of reforming and employing different compounds such as methane (CH4), methanol (CH3OH), ethanol (C2H5OH), glycerol (C3H8O3), acetic acid (CH3COOH), and toluene (C7H8) [54]. This procedure has been extensively investigated to assess the effectiveness and viability of these compounds as raw materials.
However, although methane steam reforming is currently the most cost-effective and preferred pathway for commercial hydrogen generation, it emits a considerable amount of CO2, thereby negating the advantage of using hydrogen as a clean energy vector [55]. Thus, the energy used in methane steam reforming must be derived from green sources, and the development of catalysts aiming to increase conversion and stability, reducing production costs, is also required [12].
Kannah et al. [25] indicate that, from an economic point of view, the steam reforming of natural gas is efficient, inexpensive, and represents the best method for hydrogen production. The steam reforming of natural gas for hydrogen production has attracted the attention of many researchers and policymakers due to its high efficiency in hydrogen production (70 to 85%) with low operating load (0.3 USD/kg H2) and production (1.25 to 3.50 USD/kg H2) costs. However, a considerable amount of carbon dioxide is emitted in the steam reforming process, which requires further research to reduce the emission and production costs.
Due to the scarcity of fossil fuels, there is an effort for the development of steam reforming from renewable sources, especially employing ethanol, an abundant and suitable raw material, to obtain hydrogen. Some techno-economic studies can be found in the literature aiming to investigate the feasibility of this process [56,57,58,59,60,61,62,63].
Compagnoni et al. [59] performed a techno-economic evaluation and sensitivity analysis of hydrogen production from the steam reforming of bioethanol, determining conventional economic indicators such as the net present value, internal rate of return, and pay-out period of the plant. Three scenarios were investigated by changing the fuel that heats the furnace of the reformer, which showed the economic feasibility of the process. The results revealed that the process is OPEX (operational expenditure) sensitive and strongly influenced by the ethanol cost and H2 selling price.
As mentioned above, biogas is a gaseous mixture composed mainly of CH4 and CO2, produced by the anaerobic digestion of organic matter. Interest in biogas production has increased in Europe and the United States in recent years, the main biogas producers. In Brazil, there is an increasing interest in producing energy through biogas obtained from landfills and agriculture [64]. As mentioned above, the growing interest in the dry reforming of methane is related to the interest in using biogas.
Da Silva et al. [64] performed a techno-economic evaluation of a process for energy production through a proton exchange membrane fuel cell using biogas reforming. The sensitivity analysis revealed that the biogas flow rate strongly influenced the price of the H2 produced to feed the membrane fuel cell and, consequently, the selling price of the produced energy. Using the highest biogas flow rate led to a higher H2 production, a lower amount of H2O consumed, and a lower energy selling price, showing the sustainability and economic feasibility of the process.
The dry reforming of methane presents some disadvantages in relation to the steam reforming since the H2/CO ratio obtained is 1:1, which corresponds to a lower hydrogen yield. In addition, the intense catalyst deactivation causes operational problems in the process. Likewise, the main drawbacks of partial oxidation are the high oxygen separation cost, high operating temperatures (1300–1500 °C) and pressures (3–8 MPa), and also the H2/CO ratio of 2:1 [65].
Ongis et al. [66] investigated the performance of a fluidized-bed membrane reactor for pure hydrogen production through the autothermal reforming of biogas and biomethane. Different pressures, reactor diameters, and numbers of membranes were employed to investigate their influence on the efficiency parameters, and the best design to minimize the LCOH was identified through an economic evaluation. In addition, the results pointed out that the LCOH is higher for biomethane than for biogas (~5.09 USD/kgH2@20 bar vs. ~4.84 USD/kgH2@20 bar, respectively) due to the lower price of biogas.
5. Discussion
The main gaps in the literature on techno-economic assessment studies of hydrogen production can be identified as follows:
- Although an increasing number of publications addressing hydrogen production from renewable sources through different processes have been identified, only 4% of the hydrogen produced in the world comes from renewable sources, such as biomass, waste, solar, wind, and hydropower [38,49,67].
- The techno-economic assumptions of the models vary significantly, leading to a very broad levelized cost of electricity. This finding underscores the need to use comprehensive techno-economic assumptions that can accurately predict hydrogen costs [68].
- Many studies have focused only on the technical feasibility of hydrogen production, which can be private (or business synonyms) or social [23], leaving aside an in-depth analysis of its commercial viability. It is essential to investigate the economic aspects, such as large-scale production costs, market prices, potential demand, and viable business models, to drive the adoption and implementation of renewable hydrogen in various sectors.
- Few case studies perform systematic comparisons between different hydrogen production technologies and processes from different categories (Water splitting, Biological, Thermochemical, and Catalytic reforming). Most of the studies that perform this procedure are literature reviews, such as Mohideen et al. [69], which compares the levelized cost of different hydrogen colors to provide a clear view of the cost-related challenges and limitations in hydrogen production routes and discusses cost-effective hydrogen routes. Technical and economic comparisons between different production routes, such as water electrolysis, photolysis, and biomass reforming, among others, are fundamental to identifying the most efficient and economically viable options.
- Most existing studies focus on short-term analyses, leaving gaps regarding the long-term effects of hydrogen production and use. It is essential to consider factors such as the durability of production systems, changes in the energy market, public policies, and socio-economic implications over longer time horizons.
- While there is a growing awareness of the importance of sustainability and reducing carbon emissions, there are gaps in the literature regarding the comprehensive assessment of environmental impacts associated with renewable hydrogen production. Studies that analyze life cycles, greenhouse gas emissions, natural resource consumption, and other environmental impacts are essential to informed and sustainable decision-making.
- Climate and energy policies play a crucial role in developing a sustainable economy based on equally sustainable energy systems. Thus, significant efforts by policymakers and technology developers to create innovative strategies to reduce costs per scale of production, stimulate standardization, and develop new market structures and regulatory frameworks that promote large-scale hydrogen use are still lacking [17].
The main parameters discussed in the papers are the levelized cost of hydrogen, the feedstock cost, plant size, taxes, operational expenditure (OPEX), and capital expenditure (CAPEX), for evaluating conventional economic indicators such as the net present value, internal rate of return, and pay-out period of the plant. Sensitivity analyses are generally performed to investigate which parameters most influence the economic performance of the process [48,49,59].
Another relevant gap in the literature on techno-economic evaluation studies of hydrogen production is the limited use of sensitivity analysis. Sensitivity analysis is an important tool used to assess the impact of different variables and parameters on a hydrogen production project’s economic and technical results. However, many studies do not adequately explore this approach, limiting themselves to a deterministic analysis or not considering the uncertainty associated with the input parameters. The lack of a sensitivity analysis can lead to a limited understanding of the risks and uncertainties involved in hydrogen production as well as in economic viability projections because it enables identifying which parameters have the most significant influence on outcomes and helps improve decision-making by considering different scenarios and possible changes in market conditions, input costs, government policies, and other relevant factors [25].
It should be noted that, although sensitivity analysis plays a pivotal role in the development of a robust techno-economic analysis of hydrogen production studies, there are various other strategies that can be adopted to further mitigate uncertainties and address the potential gaps in the evaluation process. One such strategy involves conducting thorough scenario analyses, exploring a range of plausible input parameters and market conditions. By systematically varying key variables, researchers can gain a comprehensive understanding of the sensitivity of the techno-economic model and its implications under different scenarios. This not only enhances the reliability of the analysis but also provides valuable insights into the system’s flexibility and adaptability to changing circumstances.
Furthermore, adopting a multi-model approach can also prove beneficial. By utilizing different techno-economic models or employing complementary methodologies, researchers can cross-validate the results and reduce their reliance on a single model’s outcomes. Diversifying the analytical approach can strengthen the overall study and inspire confidence in the findings [70]. Incorporating uncertainty analysis techniques can be another valuable addition [71]; Monte Carlo simulations, probabilistic modeling, or Bayesian statistics can help quantify the uncertainties associated with the input parameters and provide a more nuanced understanding of the potential range of outcomes [72,73,74,75]. By acknowledging and addressing uncertainty explicitly, decision makers can make better-informed choices and devise robust risk management strategies.
6. Conclusions
Considering the several advantages of hydrogen to boost sustainable development in various areas of the economy, such as industry, transportation, and energy, as well as the challenges faced for its consolidation as a widespread and low-cost technology, this paper aimed to conduct a systematic literature review with a bibliometric approach to identify the main gaps in the literature on techno-economic evaluation studies of hydrogen production, with particular attention given to renewable hydrogen.
The results show that studies on the subject are expanding as well as the publication of studies in important scientific journals in several areas of knowledge such as Energy and Fuels, Engineering, and Chemistry. The most predominant category in the publications is “Water splitting”, representing 36% of the total. Within this category, the most investigated subcategory is “Electrolysis”, corresponding to 33% of the publications on hydrogen production. The category “Thermochemistry” is also relevant, representing 31% of the publications, with emphasis on the subcategory “Gasification” (17% of the publications). The category “Catalytic reforming” represents 19% of the publications, mainly related to the subcategory “Steam reforming” (15% of the publications). Finally, the “Biological” category has 14% of the total publications, being influenced by the “dark fermentation” and “photo fermentation” processes (8% and 5% of the publications, respectively).
The literature on the techno-economic evaluation studies of hydrogen production presents some significant gaps that can possibly guide future research. These gaps include the scarcity of comprehensive studies that integrate technical and economic aspects, the lack of emphasis on the commercial viability of renewable hydrogen, the need for comparative analyses between different production technologies, the lack of long-term studies that consider the long-term effects of renewable hydrogen production and use, as well as the lack of comprehensive assessment of the environmental impacts associated with such production. Addressing these gaps is crucial to promoting the sustainable development and adoption of renewable hydrogen, providing valuable information to guide policy, investment, and technological advances in this area.
Furthermore, future studies on hydrogen production must include a comprehensive sensitivity analysis, exploring different scenarios and key variables to provide a more robust and informed assessment of the techno-economic feasibility of hydrogen production. This approach will help to identify the main factors impacting the profitability and sustainability of hydrogen production projects, contributing to the development of more robust and efficient strategies in this field. It is also worth noting that the barriers to hydrogen penetration in the energy market must be overcome through effective policy frameworks developed by government agencies. It is also advised that further reviews be conducted with a focus on databases centered around business and economics. This strategy may lead to more robust considerations of the economic analyses in the reviewed literature.
Author Contributions
Conceptualization, V.H.S.d.A. and V.G.F.P.; data curation, V.H.S.d.A. and V.G.F.P.; formal analysis, V.H.S.d.A.; investigation, V.H.S.d.A. and V.G.F.P.; methodology, V.H.S.d.A.; project administration, L.F.C.P., F.S.T. and A.S.S.; resources, A.S.S.; supervision, L.F.C.P., F.S.T. and A.S.S.; validation, V.H.S.d.A., V.G.F.P., L.F.C.P., F.S.T. and A.S.S.; visualization, V.H.S.d.A.; writing—original draft, V.H.S.d.A. and V.G.F.P.; writing—review and editing, V.H.S.d.A. and V.G.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was funded by the German Federal Ministry for Economic Cooperation and Development (BMZ).
Acknowledgments
This publication was made possible due to UFRJ’s partnership with the H2Brasil project. The H2Brasil project is part of the German-Brazilian Cooperation for Sustainable Development and is implemented by the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH and the Ministry of Mines and Energy (MME) and funded by the German Federal Ministry for Economic Cooperation and Development (BMZ).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Touili, S.; Alami Merrouni, A.; Azouzoute, A.; El Hassouani, Y.; Amrani, A. Illah A Technical and Economical Assessment of Hydrogen Production Potential from Solar Energy in Morocco. Int. J. Hydrogen Energy 2018, 43, 22777–22796. [Google Scholar] [CrossRef]
- Santos, A.S.; de Abreu, V.H.S.; de Assis, T.F.; Ribeiro, S.K.; Ribeiro, G.M. An Overview on Costs of Shifting to Sustainable Road Transport: A Challenge for Cities Worldwide. In Carbon Footprint Case Studies. Environmental Footprints and Eco-Design of Products and Processes; Springer: Singapore, 2021; pp. 93–121. [Google Scholar] [CrossRef]
- de Assis, T.F.; Monteiro, T.G.M.; de Abreu, V.H.S.; D’Agosto, M.d.A.; Santos, A.S. Enabling the Green Bonds Market for Sustainable Transport Projects Based on the Measure/Monitoring, Reporting and Verification Method. In Carbon Footprint Case Studies. Environmental Footprints and Eco-Design of Products and Processes; Springer: Singapore, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- de Assis, T.F.; Ricci, L.M.; Monteiro, T.G.M.; de Abreu, V.H.S.; D’Agosto, M.d.A.; Santos, A.S. Sustainable Transport Indicators and Mitigation Actions Applied to the Green Bond Principles; Springer: Singapore, 2022; ISBN 9789811972263. [Google Scholar]
- Akhtar, M.S.; Dickson, R.; Niaz, H.; Hwang, D.W.; Liu, J.J. Comparative Sustainability Assessment of a Hydrogen Supply Network for Hydrogen Refueling Stations in Korea–a Techno-Economic and Lifecycle Assessment Perspective. Green Chem. 2021, 23, 9625–9639. [Google Scholar] [CrossRef]
- Zhang, J.; Ling, B.; He, Y.; Zhu, Y.; Wang, Z. Life Cycle Assessment of Three Types of Hydrogen Production Methods Using Solar Energy. Int. J. Hydrogen Energy 2022, 47, 14158–14168. [Google Scholar] [CrossRef]
- Kabir, M.M.; Akter, M.M.; Huang, Z.; Tijing, L.; Shon, H.K. Hydrogen Production from Water Industries for a Circular Economy. Desalination 2023, 554, 116448. [Google Scholar] [CrossRef]
- United Nations. Paris Agreement; United Nations: New York, NY, USA, 2015; Available online: https://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 10 June 2020).
- Gulotta, T.M.; Salomone, R.; Lanuzza, F.; Saija, G.; Mondello, G.; Ioppolo, G. Life Cycle Assessment and Life Cycle Costing of Unitized Regenerative Fuel Cell: A Systematic Review. Environ. Impact Assess. Rev. 2022, 92, 106698. [Google Scholar] [CrossRef]
- Collet, P.; Flottes, E.; Favre, A.; Raynal, L.; Pierre, H.; Capela, S.; Peregrina, C. Techno-Economic and Life Cycle Assessment of Methane Production via Biogas Upgrading and Power to Gas Technology. Appl. Energy 2017, 192, 282–295. [Google Scholar] [CrossRef]
- Moran, C.; Moylan, E.; Reardon, J.; Gunawan, T.A.; Deane, P.; Yousefian, S.; Monaghan, R.F.D. A Flexible Techno-Economic Analysis Tool for Regional Hydrogen Hubs—A Case Study for Ireland. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Arham Khan, M.; Ibrahim, H.; Ekeoma, B.C.; Kamyab, H.; Rahman, M.M.; Nadda, A.K.; Chelliapan, S. A State-of-The-Art Review on the Latest Trends in Hydrogen Production, Storage, and Transportation Techniques. Fuel 2023, 340, 127574. [Google Scholar] [CrossRef]
- Hoang, A.T.; Huang, Z.H.; Nižetić, S.; Pandey, A.; Nguyen, X.P.; Luque, R.; Ong, H.C.; Said, Z.; Le, T.H.; Pham, V.V. Characteristics of Hydrogen Production from Steam Gasification of Plant-Originated Lignocellulosic Biomass and Its Prospects in Vietnam. Int. J. Hydrogen Energy 2022, 47, 4394–4425. [Google Scholar] [CrossRef]
- Ganeshan, P.; Vigneswaran, V.S.; Gowd, S.C.; Kondusamy, D.; Sanjay kumar, C.; Krishnamoorthy, N.; Kumar, D.; Juneja, A.; Paramasivan, B.; Raju, N.N.; et al. How Does Techno-Economic Analysis and Lifecycle Assessment Help in Commercializing the Biohydrogen Supply Chain? Fuel 2023, 341, 127601. [Google Scholar] [CrossRef]
- Lee, S.Y.; Na, U.J.; Jo, H.J. Techno-Economic Assessment of Green Hydrogen Production via Two-Step Thermochemical Water Splitting Using Microwave. Int. J. Hydrogen Energy 2023, 48, 10706–10723. [Google Scholar] [CrossRef]
- Abbas, M.K.; Hassan, Q.; Tabar, V.S.; Tohidi, S.; Jaszczur, M.; Abdulrahman, I.S.; Salman, H.M. Techno-Economic Analysis for Clean Hydrogen Production Using Solar Energy under Varied Climate Conditions. Int. J. Hydrogen Energy 2023, 48, 2929–2948. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A Review on the Role, Cost and Value of Hydrogen Energy Systems for Deep Decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Liu, W.; Wan, Y.; Xiong, Y.; Gao, P. Green Hydrogen Standard in China: Standard and Evaluation of Low-Carbon Hydrogen, Clean Hydrogen, and Renewable Hydrogen. Int. J. Hydrogen Energy 2022, 47, 24584–24591. [Google Scholar] [CrossRef]
- Weidner, T.; Tulus, V.; Guillén-Gosálbez, G. Environmental Sustainability Assessment of Large-Scale Hydrogen Production Using Prospective Life Cycle Analysis. Int. J. Hydrogen Energy 2023, 48, 8310–8327. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- de Oliveira, R.C. Panorama Do Hidrogênio No Brasil; IPEA—Instituto de Pesquisa Econômica Aplicada: Brasilia, Brazil, 2022; pp. 1–61. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A Sustainable Fuel for Future of the Transport Sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Kuckshinrichs, W.; Koj, J.C. Levelized Cost of Energy from Private and Social Perspectives: The Case of Improved Alkaline Water Electrolysis. J. Clean. Prod. 2018, 203, 619–632. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A Comparative Technoeconomic Analysis of Renewable Hydrogen Production Using Solar Energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-Economic Assessment of Various Hydrogen Production Methods—A Review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Ameen, W.; Ghaleb, A.M.; Alatefi, M.; Alkhalefah, H.; Alahmari, A. An Overview of Selective Laser Sintering and Melting Research Using Bibliometric Indicators. Virtual Phys. Prototyp. 2018, 13, 282–291. [Google Scholar] [CrossRef]
- Chen, X. The Declining Value of Subscription-Based Abstracting and Indexing Services in the New Knowledge Dissemination Era. Ser. Rev. 2010, 36, 79–85. [Google Scholar] [CrossRef]
- Bellotti, D.; Rivarolo, M.; Magistri, L. A Comparative Techno-Economic and Sensitivity Analysis of Power-to-X Processes from Different Energy Sources. Energy Convers. Manag. 2022, 260, 115565. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Levene, J.I.; Mann, M.K.; Margolis, R.M.; Milbrandt, A. An Analysis of Hydrogen Production from Renewable Electricity Sources. Sol. Energy 2007, 81, 773–780. [Google Scholar] [CrossRef]
- Cumpston, J.; Herding, R.; Lechtenberg, F.; Offermanns, C.; Thebelt, A.; Roh, K. Design of 24/7 Continuous Hydrogen Production System Employing the Solar-Powered Thermochemical S–I Cycle. Int. J. Hydrogen Energy 2020, 45, 24383–24396. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and Economic Feasibility of Centralized Facilities for Solar Hydrogen Production via Photocatalysis and Photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Dokhani, S.; Assadi, M.; Pollet, B.G. Techno-Economic Assessment of Hydrogen Production from Seawater. Int. J. Hydrogen Energy 2023, 48, 9592–9608. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; García-Gusano, D.; Iribarren, D. Long-Term Production Technology Mix of Alternative Fuels for Road Transport: A Focus on Spain. Energy Convers. Manag. 2020, 226, 113498. [Google Scholar] [CrossRef] [PubMed]
- Burdack, A.; Duarte-Herrera, L.; López-Jiménez, G.; Polklas, T.; Vasco-Echeverri, O. Techno-Economic Calculation of Green Hydrogen Production and Export from Colombia. Int. J. Hydrogen Energy 2023, 48, 1685–1700. [Google Scholar] [CrossRef]
- Meier, K. Hydrogen Production with Sea Water Electrolysis Using Norwegian Offshore Wind Energy Potentials: Techno-Economic Assessment for an Offshore-Based Hydrogen Production Approach with State-of-the-Art Technology. Int. J. Energy Environ. Eng. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Tufa, R.A.; Pawlowski, S.; Veerman, J.; Bouzek, K.; Fontananova, E.; di Profio, G.; Velizarov, S.; Goulão Crespo, J.; Nijmeijer, K.; Curcio, E. Progress and Prospects in Reverse Electrodialysis for Salinity Gradient Energy Conversion and Storage. Appl. Energy 2018, 225, 290–331. [Google Scholar] [CrossRef]
- Budama, V.K.; Johnson, N.G.; Ermanoski, I.; Stechel, E.B. Techno-Economic Analysis of Thermochemical Water-Splitting System for Co-Production of Hydrogen and Electricity. Int. J. Hydrogen Energy 2021, 46, 1656–1670. [Google Scholar] [CrossRef]
- Oladipo, H.; Yusuf, A.; Al-Ali, K.; Palmisano, G. Techno-Economic Evaluation of Photocatalytic H2S Splitting. Energy Technol. 2021, 9, 2100196. [Google Scholar] [CrossRef]
- Frowijn, L.S.F.; van Sark, W.G.J.H.M. Analysis of Photon-Driven Solar-to-Hydrogen Production Methods in the Netherlands. Sustain. Energy Technol. Assess. 2021, 48, 101631. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.H.; Parthiban, A.; Edwin Geo, V.; Brindhadevi, K.; Pugazhendhi, A. Insights on Biological Hydrogen Production Routes and Potential Microorganisms for High Hydrogen Yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass Pyrolysis: A Review on Recent Advancements and Green Hydrogen Production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J. Hydrogen Applications and Research Activities in Its Production Routes through Catalytic Hydrocarbon Conversion. Rev. Chem. Eng. 2017, 34, 43–72. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Bartels, J.R.; Pate, M.B.; Olson, N.K. An Economic Survey of Hydrogen Production from Conventional and Alternative Energy Sources. Int. J. Hydrogen Energy 2010, 35, 8371–8384. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the Thermo-Chemical Production of Hydrogen from Biomass and Residual Wastes: Summary of Recent Techno-Economic Analyses. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic Analysis of Hydrogen Production from Steam Reforming Process: A Literature Review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Vozniuk, O.; Tanchoux, N.; Millet, J.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Spinel Mixed Oxides for Chemical-Loop Reforming: From Solid State to Potential Application, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 178, ISBN 9780444641274. [Google Scholar]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-Art Catalysts for CH4 Steam Reforming at Low Temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogen-Fueled Internal Combustion Engines. In Compendium of Hydrogen Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 117–217. [Google Scholar]
- Basu, S.; Pradhan, N.C. Selective Production of Hydrogen by Acetone Steam Reforming over Ni–Co/Olivine Catalysts. React. Kinet. Mech. Catal. 2019, 127, 357–373. [Google Scholar] [CrossRef]
- Ali Khan, M.H.; Daiyan, R.; Neal, P.; Haque, N.; MacGill, I.; Amal, R. A Framework for Assessing Economics of Blue Hydrogen Production from Steam Methane Reforming Using Carbon Capture Storage & Utilisation. Int. J. Hydrogen Energy 2021, 46, 22685–22706. [Google Scholar] [CrossRef]
- Ciambelli, P.; Palma, V.; Ruggiero, A.; Iaquaniello, G. Platinum Catalysts for the Low Temperature Catalytic Steam Reforming of Ethanol. Chem. Eng. Trans. 2009, 17, 19–24. [Google Scholar] [CrossRef]
- Rossetti, I.; Tripodi, A. Catalytic Production of Renewable Hydrogen for Use in Fuel Cells: A Review Study. Top. Catal. 2022, 1–20. [Google Scholar] [CrossRef]
- Roldán, R. Technical and Economic Feasibility of Adapting an Industrial Steam Reforming Unit for Production of Hydrogen from Renewable Ethanol. Int. J. Hydrogen Energy 2015, 40, 2035–2046. [Google Scholar] [CrossRef]
- Compagnoni, M.; Mostafavi, E.; Tripodi, A.; Mahinpey, N.; Rossetti, I. Techno-Economic Analysis of a Bioethanol to Hydrogen Centralized Plant. Energy Fuels 2017, 31, 12988–12996. [Google Scholar] [CrossRef]
- Moraes, T.S.; Cozendey da Silva, H.N.; Zotes, L.P.; Mattos, L.V.; Pizarro Borges, L.E.; Farrauto, R.; Noronha, F.B. A Techno-Economic Evaluation of the Hydrogen Production for Energy Generation Using an Ethanol Fuel Processor. Int. J. Hydrogen Energy 2019, 44, 21205–21219. [Google Scholar] [CrossRef]
- Khamhaeng, P.; Laosiripojana, N.; Assabumrungrat, S.; Kim-Lohsoontorn, P. Techno-Economic Analysis of Hydrogen Production from Dehydrogenation and Steam Reforming of Ethanol for Carbon Dioxide Conversion to Methanol. Int. J. Hydrogen Energy 2021, 46, 30891–30902. [Google Scholar] [CrossRef]
- Tippawan, P.; Arpornwichanop, A. Performance and Economic Assessments of a Solid Oxide Fuel Cell System with a Two-Step Ethanol-Steam-Reforming Process Using CaO Sorbent. J. Power Sources 2016, 306, 124–134. [Google Scholar] [CrossRef]
- Wang, B.; Yu, X.; Chang, J.; Huang, R.; Li, Z.; Wang, H. Techno-Economic Analysis and Optimization of a Novel Hybrid Solar-Wind-Bioethanol Hydrogen Production System via Membrane Reactor. Energy Convers. Manag. 2022, 252, 115088. [Google Scholar] [CrossRef]
- Cozendey da Silva, H.N.; Prata, D.M.; Alves Lima, G.B.; Zotes, L.P.; Mattos, L.V. A Techno-Economic Evaluation of the Energy Generation by Proton Exchange Membrane Fuel Cell Using Biogas Reforming. J. Clean. Prod. 2018, 200, 598–608. [Google Scholar] [CrossRef]
- Hamid, U.; Rauf, A.; Ahmed, U.; Selim Arif Sher Shah, M.; Ahmad, N. Techno-Economic Assessment of Process Integration Models for Boosting Hydrogen Production Potential from Coal and Natural Gas Feedstocks. Fuel 2020, 266, 117111. [Google Scholar] [CrossRef]
- Ongis, M.; Di Marcoberardino, G.; Manzolini, G.; Gallucci, F.; Binotti, M. Membrane Reactors for Green Hydrogen Production from Biogas and Biomethane: A Techno-Economic Assessment. Int. J. Hydrogen Energy 2023, 48, 19580–19595. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Mukelabai, M.D.; Wijayantha, K.G.U.; Blanchard, R.E. Hydrogen for Cooking: A Review of Cooking Technologies, Renewable Hydrogen Systems and Techno-Economics. Sustainability 2022, 14, 16964. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Subramanian, B.; Sun, J.; Ge, J.; Guo, H.; Radhamani, A.V.; Ramakrishna, S.; Liu, Y. Techno-Economic Analysis of Different Shades of Renewable and Non-Renewable Energy-Based Hydrogen for Fuel Cell Electric Vehicles. Renew. Sustain. Energy Rev. 2023, 174, 113153. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Kim, C.H.; Ryi, S.K.; Lim, H. Integrated Techno-Economic Analysis under Uncertainty of Glycerol Steam Reforming for H2 Production at Distributed H2 Refueling Stations. Energy Convers. Manag. 2019, 180, 250–257. [Google Scholar] [CrossRef]
- Rezaei, M.; Dampage, U.; Das, B.K.; Nasif, O.; Borowski, P.F.; Mohamed, M.A. Investigating the Impact of Economic Uncertainty on Optimal Sizing of Grid-Independent Hybrid Renewable Energy Systems. Processes 2021, 9, 1468. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.; Heo, J.; Moon, C.; Moon, S.; Lim, H. Stochastic Techno-Economic Analysis of H2 Production from Power-to-Gas Using a High-Pressure PEM Water Electrolyzer for a Small-Scale H2 Fueling Station. Sustain. Energy Fuels 2019, 3, 2521–2529. [Google Scholar] [CrossRef]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost Benefits of Optimizing Hydrogen Storage and Methanation Capacities for Power-to-Gas Plants in Dynamic Operation. Appl. Energy 2020, 257, 113967. [Google Scholar] [CrossRef]
- Khodabandehloo, M.; Larimi, A.; Khorasheh, F. Comparative Process Modeling and Techno-Economic Evaluation of Renewable Hydrogen Production by Glycerol Reforming in Aqueous and Gaseous Phases. Energy Convers. Manag. 2020, 225, 113483. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Singh, K.K.; Bhanja, K.; Grover, R.B. Assessing Techno-Economic Uncertainties in Nuclear Power-to-X Processes: The Case of Nuclear Hydrogen Production via Water Electrolysis. Int. J. Hydrogen Energy 2023, 48, 14149–14169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).