Release Characteristics of Potassium during Biomass Combustion
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Material
2.2. Experiment
2.3. Analysis
3. Results and Discussion
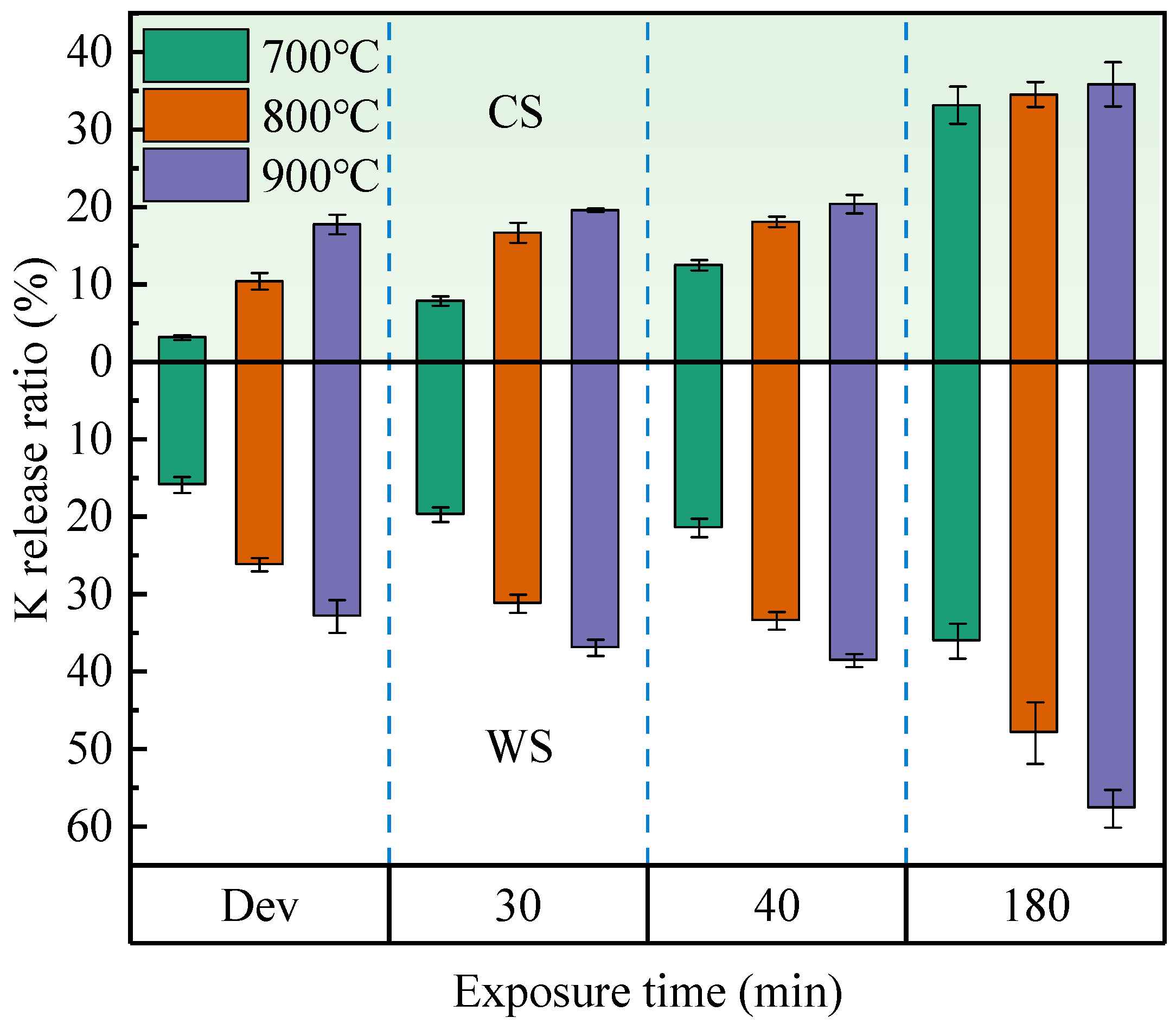
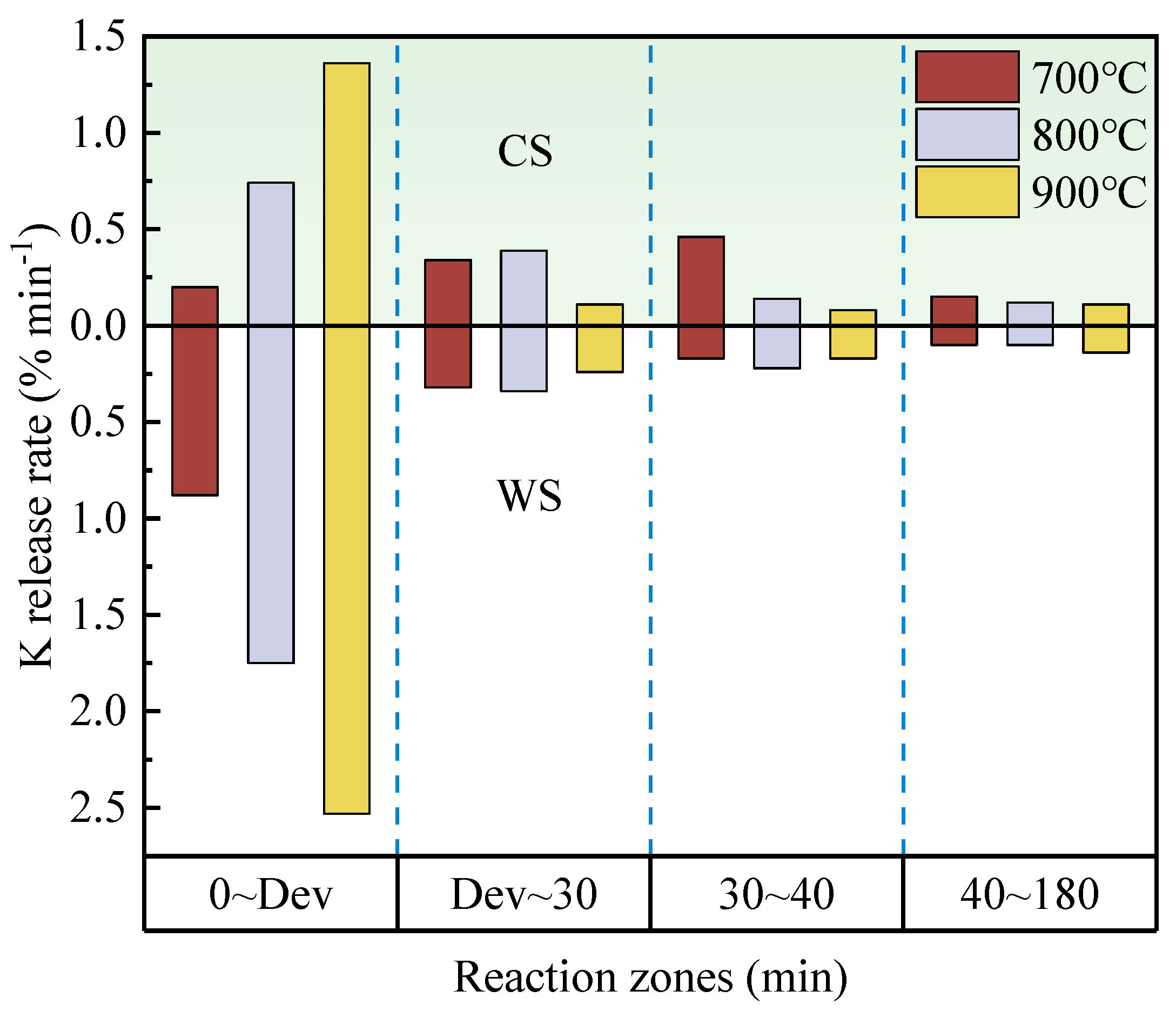
3.1. Effect of Exposure Time on K Release
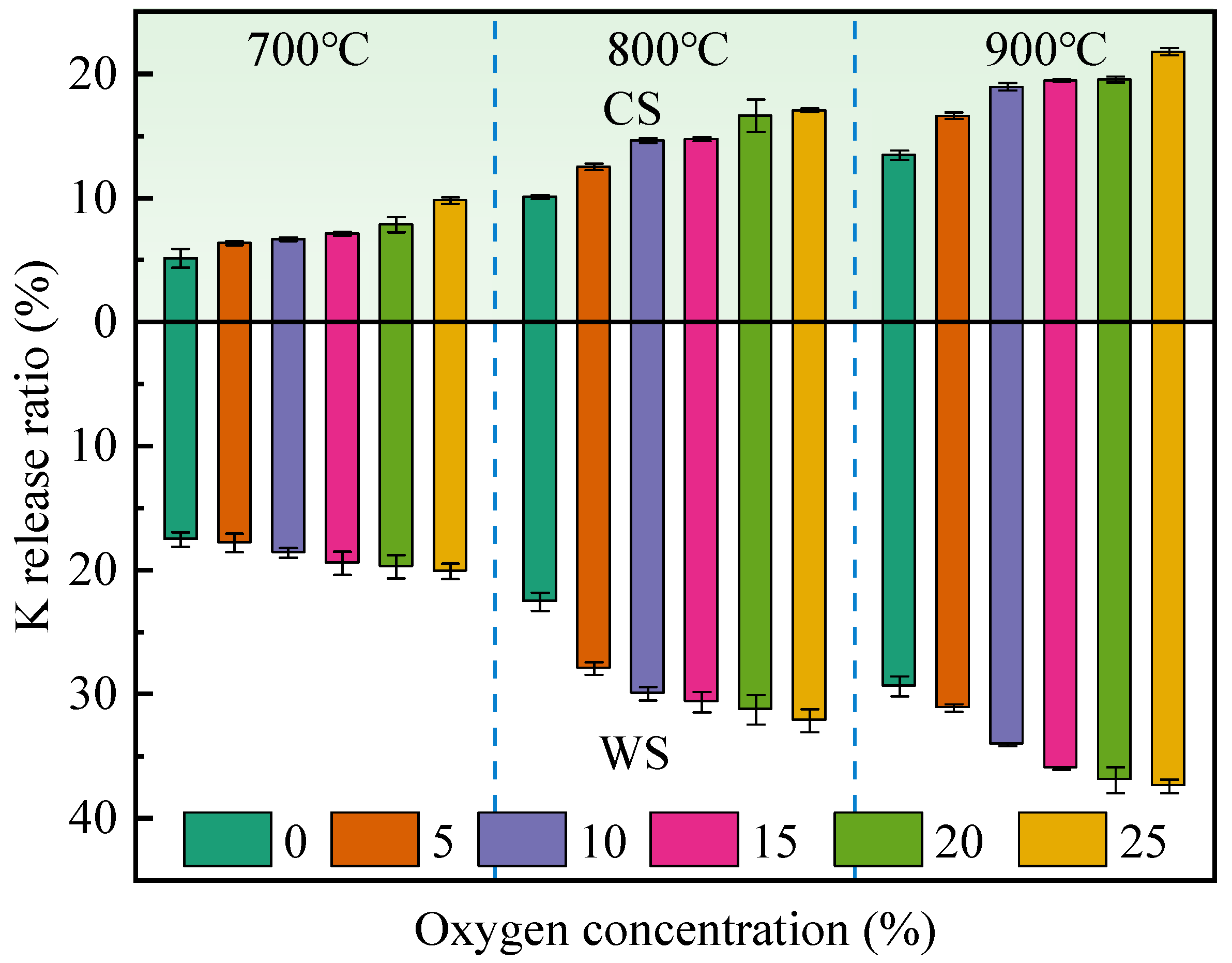
3.2. Effect of Oxygen Concentration on K Release
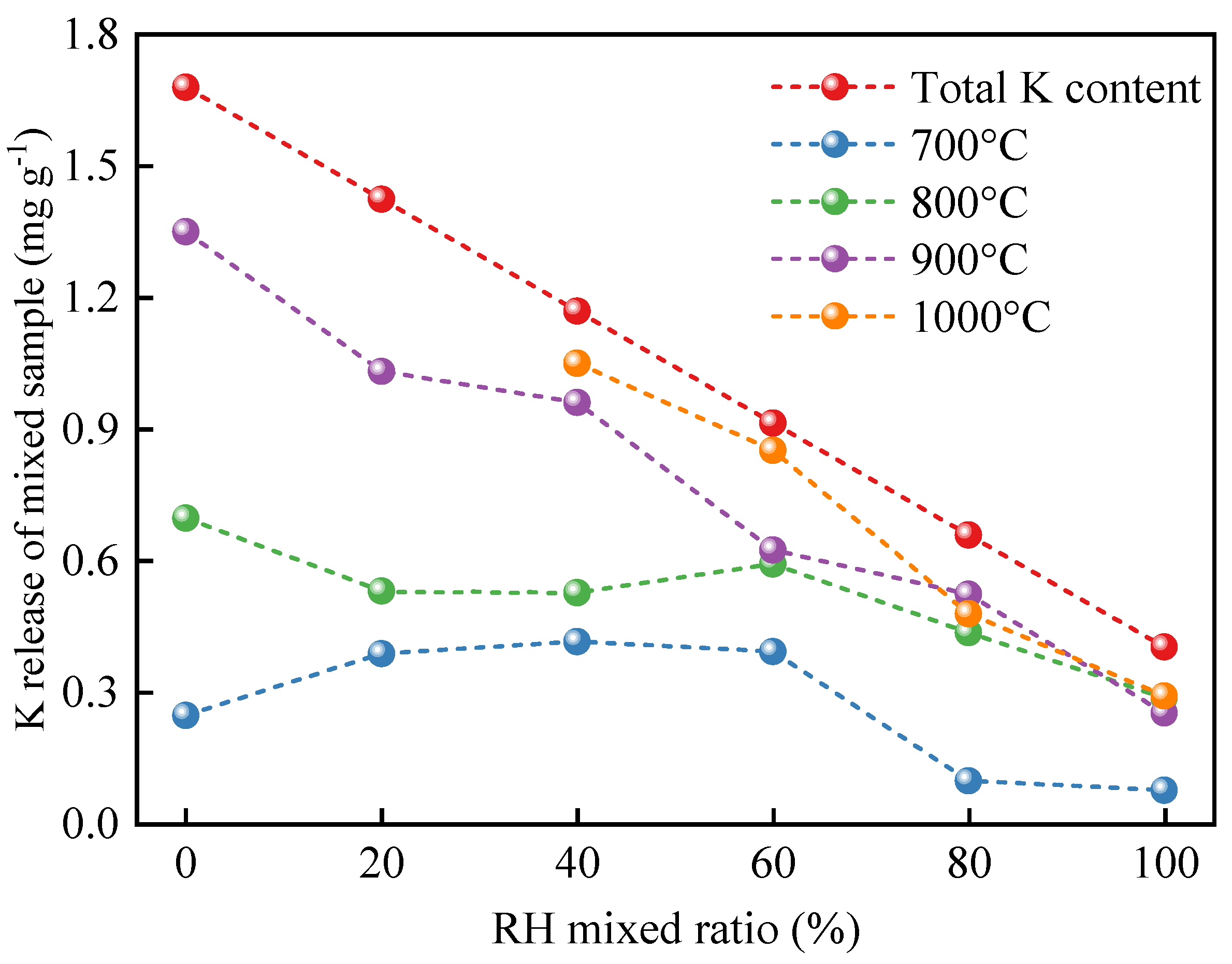
3.3. Effect of Co-Combustion on K Release
3.4. Combined Effect of Water Washing and Co-Combustion
4. Conclusions
- (1)
- When burning at 700–900 °C, the K release ratio in both CS and WS increases as exposure time rises. However, when the exposure time is long enough, the K release ratio of CS is close to the same value at different temperatures. For WS, the upper limit of the release ratio is different at different temperatures. After the volatile fraction burns out, 700 °C is not sufficient to convert water-soluble K to other fugitive forms in the solid phase. The production of insoluble K is significantly promoted above 800 °C.
- (2)
- With augmentation of the oxygen concentration, the K release ratio increases. The enhancement of the K release ratio is more significant when increasing the combustion temperature by 100 °C than with an increment in the oxygen concentration of 5%. The K release ratio decreases overall versus the RH mixing ratio, which improves and becomes more pronounced up to higher temperatures.
- (3)
- The oxidizing atmosphere facilitates K release from the water-washed samples. RH mixing has essentially no effect on K release from CS. Water-washing pretreatment significantly reduces K release in the samples with different mixing ratios at different combustion temperatures. In addition, water-washing pretreatment not only reduces the K content in the samples but also removes the K with release potential.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, I.I.; Gupta, A.K. Sugarcane bagasse gasification: Global reaction mechanism of syngas evolution. Appl. Energy 2012, 91, 75–81. [Google Scholar] [CrossRef]
- Long, J.; Deng, L.; Che, D. Analysis on organic compounds in water leachate from biomass. Renew. Energy 2020, 155, 1070–1078. [Google Scholar] [CrossRef]
- Jiang, J.; Tie, Y.; Deng, L.; Che, D. Influence of water-washing pretreatment on ash fusibility of biomass. Renew. Energy 2022, 200, 125–135. [Google Scholar] [CrossRef]
- Jun, L. Strategy for building a digital management system of carbon assets in group enterprises. Xinjiang Oil Gas 2022, 18, 10–15. [Google Scholar]
- Cheng, Y.; Tiemei, L.; Xiang, L.; Kecheng, L.; Xuyang, Y.; Jiaqin, G. Ecological utilization and development trend of environment-friendly drilling fluid. Xinjiang Oil Gas 2021, 17, 25–29. [Google Scholar]
- Liao, Y.; Wu, S.; Chen, T.; Cao, Y.; Ma, X. The Alkali Metal Characteristic during Biomass Combustion with Additives. Energy Procedia 2015, 75, 124–129. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Karatas, H.; Akgun, F. Experimental results of gasification of walnut shell and pistachio shell in a bubbling fluidized bed gasifier under air and steam atmospheres. Fuel 2018, 214, 285–292. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Osmieri, L.; Specchia, S.; Yusup, S.; Tavasoli, A.; Zamaniyan, A. H2-rich syngas production through mixed residual biomass and HDPE waste via integrated catalytic gasification and tar cracking plus bio-char upgrading. Chem. Eng. J. 2017, 308, 578–587. [Google Scholar] [CrossRef]
- Deng, L.; Jin, X.; Zhang, K.; Jiang, J.; Zhu, Z.; Che, D. Catalytic conversion of toluene by modified biochar from oak. J. Energy Inst. 2022, 102, 374–383. [Google Scholar] [CrossRef]
- Deng, L.; Ma, S.; Jiang, J.; Tie, Y.; Zhang, Y.; Zhu, Z.; Belošević, S.; Tomanović, I.; Che, D. Numerical Investigation on Cofiring Characteristics of Biomass Syngas and Coal in a 660-MW Tower Boiler. J. Energy Eng. 2022, 148, 04022014. [Google Scholar] [CrossRef]
- Song, H.; Zhen, H. Study on mechanism of high temperature superheater corrosion of biomass fired boiler. Boiler. Manufacturing 2010, 5, 14–18. [Google Scholar]
- Jensen, P.A.; Frandsen, F.J.; Hansen, J.; Dam-Johansen, K.; Henriksen, N.; Hörlyck, S. SEM Investigation of Superheater Deposits from Biomass-Fired Boilers. Energy Fuel 2004, 18, 378–384. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuel 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, K.; Sander, B. Experimental Investigation of the Transformation and Release to Gas Phase of Potassium and Chlorine during Straw Pyrolysis. Energy Fuel 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuel 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Q.; Wu, X.; Yao, Q. Study on the Transformation of Inherent Potassium during the Fast-Pyrolysis Process of Rice Straw. Energy Fuel 2015, 29, 6404–6411. [Google Scholar] [CrossRef]
- Michelsen, H.P.; Frandsen, F.; Dam-Johansen, K.; Larsen, O.H. Deposition and high temperature corrosion in a 10 MW straw fired boiler. Fuel Process. Technol. 1998, 54, 95–108. [Google Scholar] [CrossRef]
- Jin, X.; Ye, J.; Deng, L.; Che, D. Condensation Behaviors of Potassium during Biomass Combustion. Energy Fuel 2017, 31, 2951–2958. [Google Scholar] [CrossRef]
- Keown, D.M.; Favas, G.; Hayashi, J.; Li, C.Z. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass: Differences between sugar cane bagasse and cane trash. Bioresource Technol. 2005, 96, 1570–1577. [Google Scholar] [CrossRef]
- Lane, D.J.; van Eyk, P.J.; Ashman, P.J.; Kwong, C.W.; de Nys, R.; Roberts, D.A.; Cole, A.J.; Lewis, D.M. Release of Cl, S, P, K, and Na during Thermal Conversion of Algal Biomass. Energy Fuel 2015, 29, 2542–2554. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, J.; Tie, Y.; Ma, S.; Fan, G.; Zhu, T.; Che, D. Potassium transformation and release during biomass combustion. Can. J. Chem. Eng. 2023, 101, 337–346. [Google Scholar] [CrossRef]
- Ng, J.; DeMartini, N. Effect of Steam on the Release of K and Cl during Biomass and Black Liquor Combustion. Energy Fuel 2022, 36, 7733–7743. [Google Scholar] [CrossRef]
- Cao, W.H.; Li, J.; Li, L.; Zhang, X. Release of potassium in association with structural evolution during biomass combustion. Fuel 2021, 287, 119524. [Google Scholar] [CrossRef]
- Wang, M.; Xu, D.; Bai, Y.; Yu, G.; Zhang, J.; Zhang, S.; Xu, J.; Zhang, H.; Zhang, S.; Wei, J. Dynamic investigation on potassium migration and transformation during biochar combustion and its correlation with combustion reactivity. Fuel 2023, 340, 127540. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Korsgren, J.G.; Pettersson, J.B.C.; Jäglid, U. The effects of fuel washing techniques on alkali release from biomass. Fuel 2002, 81, 137–142. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Q.; Yao, Q.; Yang, R. Influence of the Atmosphere on the Transformation of Alkali and Alkaline Earth Metallic Species during Rice Straw Thermal Conversion. Energy Fuel 2012, 26, 1892–1899. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, W.; Zhou, J.; Yu, Z. Effects of S and Al on K Migration and Transformation during Coal and Biomass Co-combustion. ACS Omega 2022, 7, 15880–15891. [Google Scholar] [CrossRef]
- Patel, B.; Gami, B.; Patel, P. The Leaching of Soluble Chloride from Terrestrial and Water-based Biomass. Energy sources. Part A Recovery Util. Environ. Eff. 2012, 34, 2280–2286. [Google Scholar]
- Wu, H.; Yip, K.; Kong, Z.; Li, C.; Liu, D.; Yu, Y.; Gao, X. Removal and Recycling of Inherent Inorganic Nutrient Species in Mallee Biomass and Derived Biochars by Water Leaching. Ind. Eng. Chem. Res. 2011, 50, 12143–12151. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- Deng, L.; Che, D. Chemical, Electrochemical and Spectral Characterization of Water Leachates from Biomass. Ind. Eng. Chem. Res. 2012, 51, 15710–15719. [Google Scholar] [CrossRef]
- Huang, X.; Tie, Y.; Jiang, J.; Deng, L.; Che, D. Water washing of biomass and biochar. Sustain. Energy Techn. 2023, 56, 103066. [Google Scholar] [CrossRef]
- Deng, L.; Long, J.; Wu, Y.; Che, D. A study on benzene release during water washing of biomass. Asia-Pac. J. Chem. Eng. 2020, 15, e2536. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Wei, R.R.B.A. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- He, Z.; Mao, J.; Honeycutt, C.W.; Ohno, T.; Hunt, J.F.; Cade-Menun, B.J. Characterization of plant-derived water extractable organic matter by multiple spectroscopic techniques. Biol. Fert. Soils 2009, 45, 609–616. [Google Scholar] [CrossRef]


| Sample | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | O * | N | S | Cl | |
| WS | 6.80 | 75.29 | 17.91 | 44.11 | 4.97 | 43.67 | 0.20 | 0.25 | 0.183 |
| RH | 14.23 | 69.67 | 16.10 | 42.17 | 1.86 | 41.30 | 0.37 | 0.06 | 0.076 |
| CS | 7.09 | 74.89 | 18.02 | 46.18 | 4.89 | 40.50 | 1.09 | 0.25 | 0.055 |
| WCS | 4.32 | 80.95 | 14.74 | 46.54 | 5.10 | 43.44 | 0.57 | 0.02 | 0.024 |
| Sample | Element Composition | Molar Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Al | Ca | Mg | P | K | K/Sid | (Ca + Mg)/Si | Cl/K | |
| WS | 1.22 | 0.05 | 0.39 | 0.11 | 0.04 | 1.046 ± 0.016 | 0.615 | 0.328 | 0.193 |
| RH | 5.99 | - | 0.09 | - | 0.03 | 0.201 ± 0.001 | 0.024 | 0.010 | 0.416 |
| CS | 1.19 | 0.11 | 0.35 | 0.19 | 0.03 | 1.221 ± 0.016 | 0.737 | 0.387 | 0.050 |
| WCS | 1.20 | 0.07 | 0.31 | 0.14 | 0.02 | 0.185 ± 0.002 | 0.111 | 0.321 | 0.144 |
| Reaction Temperature (°C) | 400 | 500 | 600 | 700 | 800 | 900 | 1000 |
|---|---|---|---|---|---|---|---|
| CS | 0.08 | 0.07 | 0.08 | 0.53 | 0.65 | 0.45 | 0.18 |
| WCS | 6.46 | 2.99 | 3.43 | 0.55 | 0.52 | 0.39 | 0.55 |
| RH Mixed Ratio (%) | 0 | 20 | 40 | 60 | 80 | 100 |
|---|---|---|---|---|---|---|
| 700 °C | 5.94 | 10.19 | 13.12 | 11.82 | 3.97 | 7.24 |
| 800 °C | 13.28 | 12.47 | 11.21 | 18.45 | 18.92 | 19.10 |
| 900 °C | 13.68 | 14.89 | 14.76 | 12.10 | 16.34 | 19.10 |
| 1000 °C | - | - | 12.38 | 13.77 | 12.14 | 15.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Hou, X.; Xue, X.; Ren, J.; Dong, L.; Wei, X.; Jian, L.; Deng, L. Release Characteristics of Potassium during Biomass Combustion. Energies 2023, 16, 4107. https://doi.org/10.3390/en16104107
Zhang F, Hou X, Xue X, Ren J, Dong L, Wei X, Jian L, Deng L. Release Characteristics of Potassium during Biomass Combustion. Energies. 2023; 16(10):4107. https://doi.org/10.3390/en16104107
Chicago/Turabian StyleZhang, Feng, Xiuqin Hou, Xingchang Xue, Jiyun Ren, Lingxiao Dong, Xumeng Wei, Lin Jian, and Lei Deng. 2023. "Release Characteristics of Potassium during Biomass Combustion" Energies 16, no. 10: 4107. https://doi.org/10.3390/en16104107
APA StyleZhang, F., Hou, X., Xue, X., Ren, J., Dong, L., Wei, X., Jian, L., & Deng, L. (2023). Release Characteristics of Potassium during Biomass Combustion. Energies, 16(10), 4107. https://doi.org/10.3390/en16104107








