Biodegradation Kinetics of Organic Matter in Water from Sludge Dewatering after Autothermal Thermophilic Aerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
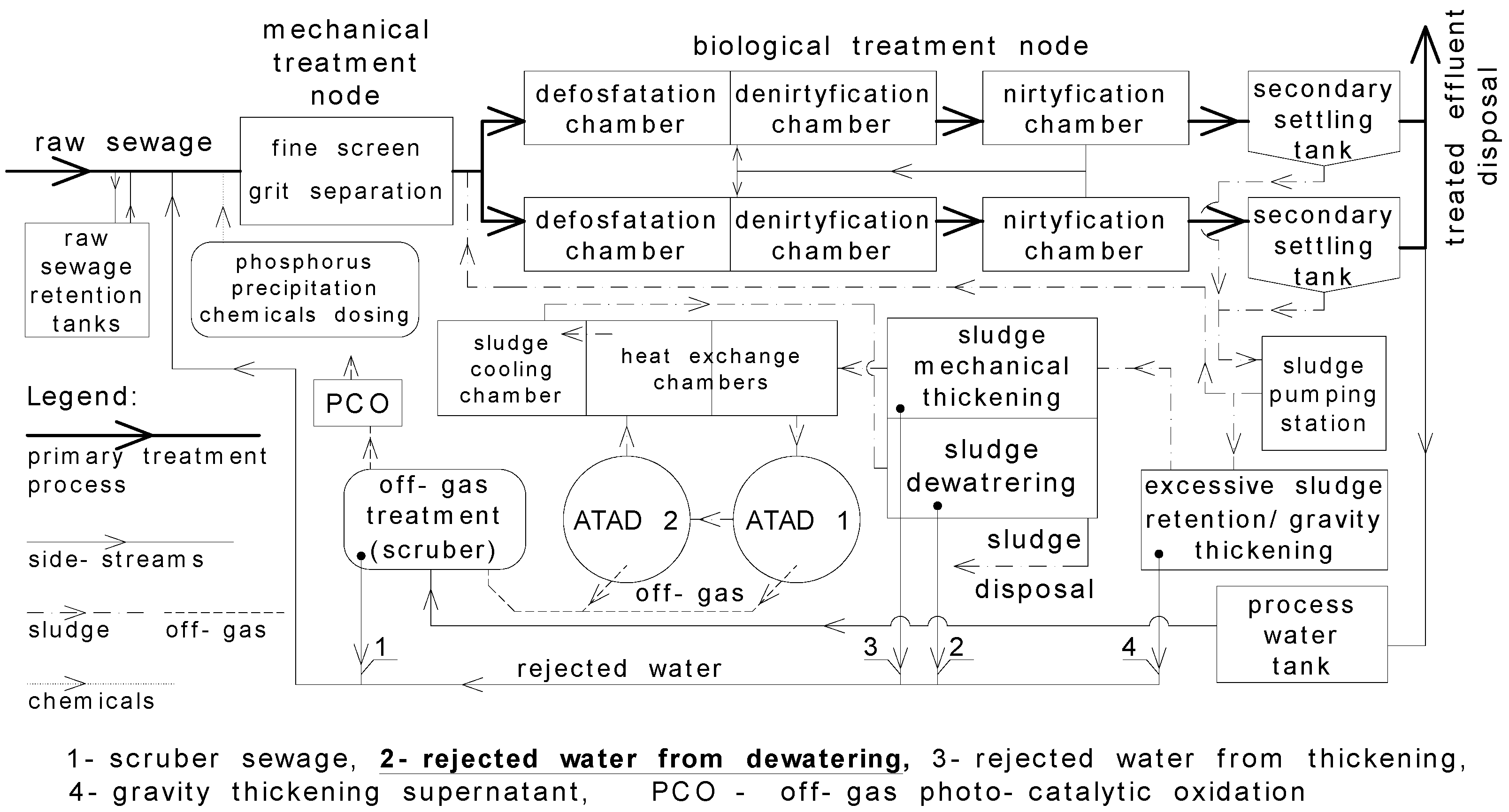
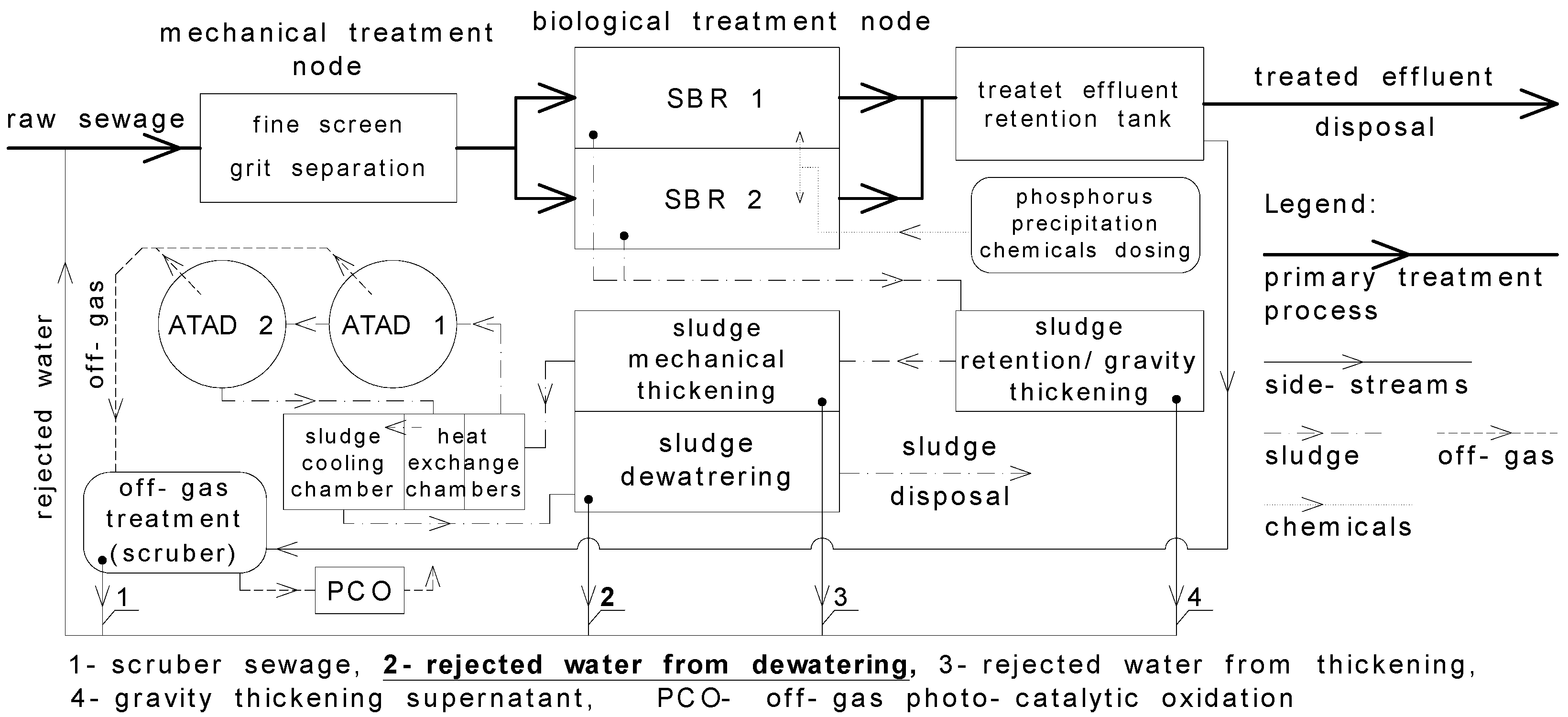
2.1. Characteristics of Analysed Wastewater Treatment Plants (WWTPs)
2.1.1. Pisz (WWTP1)
2.1.2. Dąbrowa Białostocka (WWTP2)
2.1.3. Wysokie Mazowieckie (WWTP3)
2.2. Analytical Methods
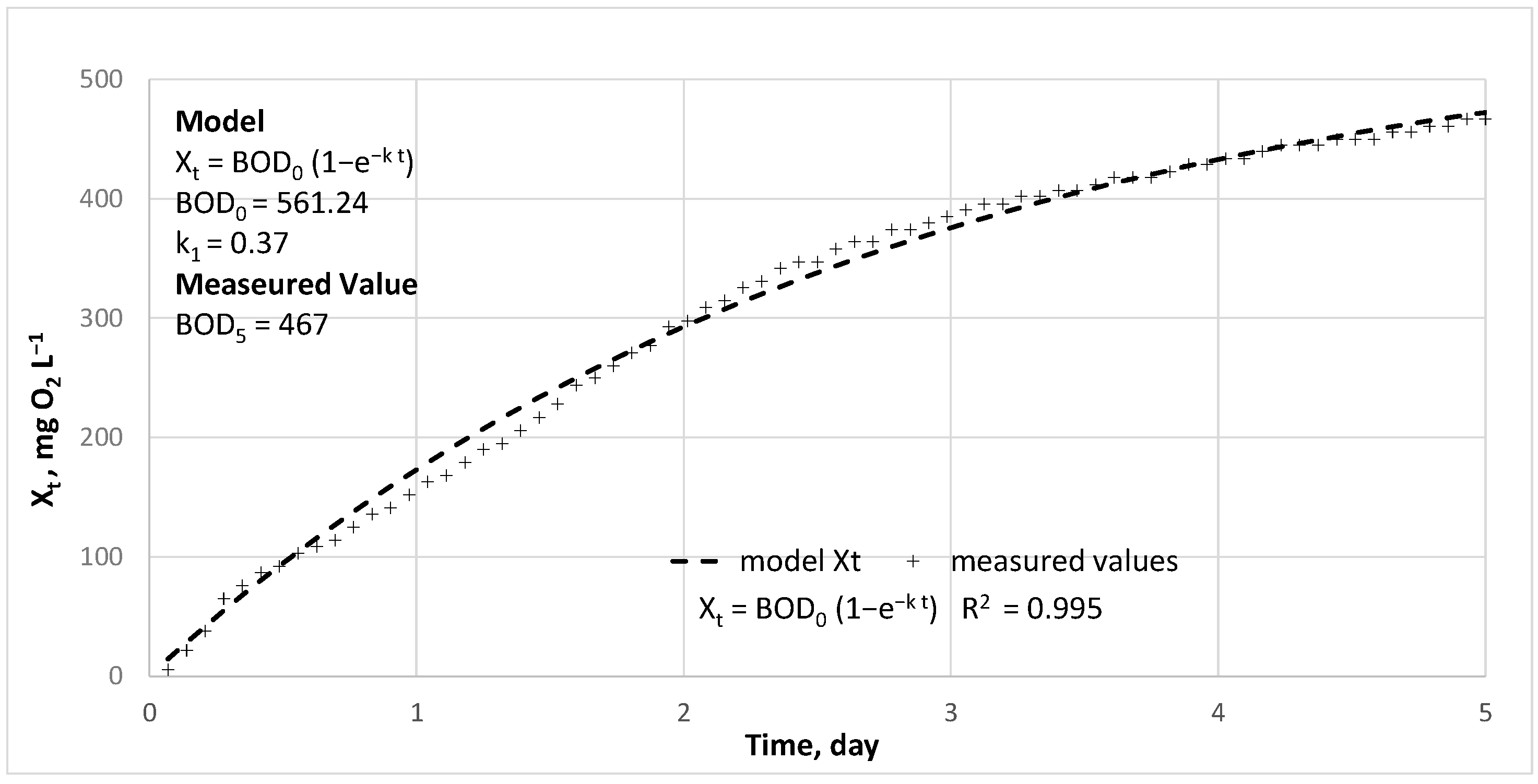
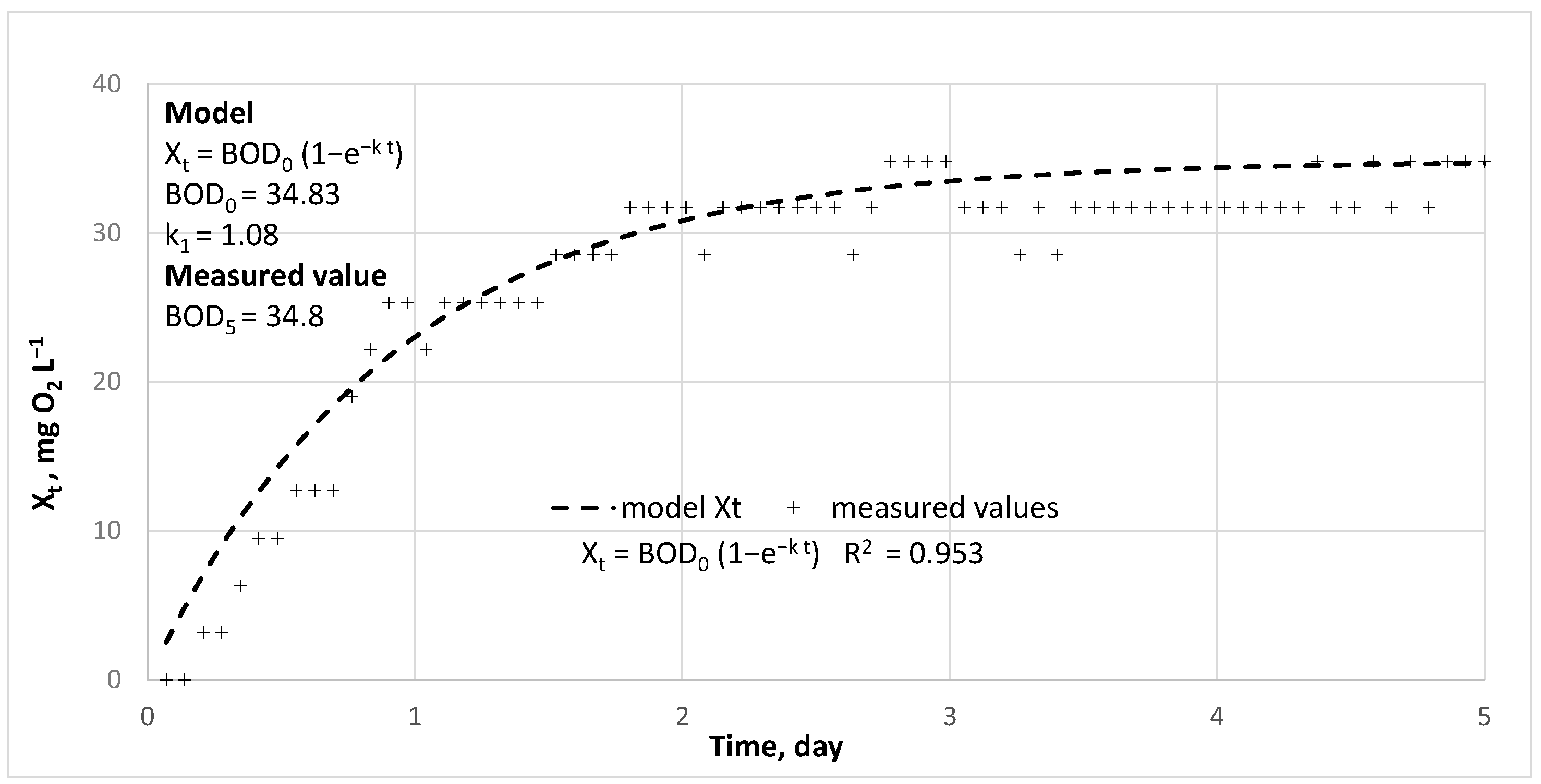
2.3. Calculations
- [BOD]—content of an organic matter undergoing biochemical degradation, expressed as biochemical oxygen demand, mg O2 L−1
- t—reaction time, d
- k—reaction rate constant, d−1
- [BOD]0—content of the organic substance undergoing biochemical decomposition in the initial incubation time, total BOD,
- [BOD]t—content of remaining organic matter undergoing biochemical decomposition over time t.
- Xt—oxygen consumption in the process of biochemical decomposition of organic matter over time t, mg O2 L−1.
- Y—dependent variable,
- X—predictor,
- N—number of observations,
- —arithmetical mean of the value.
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Statistics Poland, Spatial and Environmental Surveys Department. Environment 2021; Statistics Poland: Warsaw, Poland, 2021; pp. 68–71. (In Polish) [Google Scholar]
- Bartkowska, I.; Dzienis, L. Utilization of Sewage Sludge after the Process of Autothermal Digestion. J. Ecol. Eng. 2019, 20, 44–49. [Google Scholar] [CrossRef]
- Poblete, I.B.S.; Araujo, O.d.Q.F.; de Medeiros, J.L. Sewage-Water Treatment and Sewage-Sludge Management with Power Production as Bioenergy with Carbon Capture System: A Review. Processes 2022, 10, 788. [Google Scholar] [CrossRef]
- Bartkowska, I.; Dzienis, L. Technical and economic aspects of autothermal thermophilic aerobic digestion exemplified by sewage treatment plant in Giżycko. Environ. Prot. Eng. 2007, 33, 17–24. [Google Scholar]
- Rojas, J.; Zhelev, T. Energy efficiency optimisation of wastewater treatment: Study of ATAD. Comput. Chem. Eng. 2012, 38, 52–63. [Google Scholar] [CrossRef]
- Bartkowska, I. Autothermal Thermophilic Aerobic Digestion; Wydawnictwo Seidel-Przywecki Sp. z o.o.: Warsaw, Poland, 2017. (In Polish) [Google Scholar]
- Zupancic, G.D.; Ros, M. Thermophilic aerobic digestion of waste activated sludge. Acta Chim. Slov. 2002, 49, 931–943. [Google Scholar]
- Piterina, A.V.; Bartlett, J.; Pembroke, T.J. Evaluation of the Removal of Indicator Bacteria from Domestic Sludge Processed by Autothermal Thermophilic Aerobic Digestion (ATAD). Int. J. Environ. Res. Public Health 2010, 7, 3422–3441. [Google Scholar] [CrossRef]
- USEPA 40 CFR PART 503; Standards for the Use or Disposal of Sewage Sludge. United States Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 1993.
- Pembroke, J.T.; Ryan, M.P. Autothermal Thermophilic Aerobic Digestion (ATAD) for Heat, Gas, and Production of a Class A Biosolids with Fertilizer Potential. Microorganisms 2019, 7, 215. [Google Scholar] [CrossRef]
- Zhang, M.; Tashiro, Y.; Ishida, N.; Sakai, K. Application of autothermal thermophilic aerobic digestion as a sustainable recycling process of organic liquid waste: Recent advances and prospects. Sci. Total Environ. 2022, 828, 154187. [Google Scholar] [CrossRef]
- Dabrowski, W.; Malinowski, P.; Karolinczak, B. Application of SS-VF Bed for the Treatment of High Concentrated Reject Water from Autothermal Termophilic Aerobic Sewage Sludge Digestion. J. Ecol. Eng. 2018, 19, 103–110. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Galvagno, G.; Cimon, C. Approaches and processes for ammonia removal from side-streams of municipal effluent treatment plants. Bioresour. Technol. 2018, 268, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Morras, M.; Dosta, J.; García-Heras, J.L. Aerobic/anoxic post-treatment of anaerobically digested sewage sludge as an alternative to biological nitrogen removal from reject water. Bioprocess Biosyst. Eng. 2015, 38, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, I.; Wawrentowicz, D.; Dzienis, L. Analysis of aerobic and anaerobic sewage sludge disposal concepts. Ekon. Sr. 2022, 2, 203–221. [Google Scholar] [CrossRef]
- Janus, H.M.; van der Roest, H.F. Do not reject the idea of treating reject water. Water Sci. Technol. 1997, 35, 27–34. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Karolinczak, B.; Gajewska, M.; Wojciechowska, E. Application of subsurface vertical flow constructed wetlands to reject water treatment in dairy wastewater treatment plant. Environ. Technol. 2017, 38, 175–182. [Google Scholar] [CrossRef]
- Kim, I.-T.; Lee, Y.-E.; Jeong, Y.; Yoo, Y.-S. A novel method to remove nitrogen from reject water in wastewater treatment plants using a methane- and methanol-dependent bacterial consortium. Water Res. 2020, 172, 115512. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec-Liśniewska, A.; Chrobot, P.; Skrzypczak, D. Removal of ammonium and orthophosphates from reject water generated during dewatering of digested sewage sludge in municipal wastewater treatment plant using adsorption and membrane contactor system. J. Clean. Prod. 2017, 161, 277–287. [Google Scholar] [CrossRef]
- Tuszynska, A.; Czerwionka, K. Nutrient recovery from deammonification effluent in a pilot study using two-step reject water treatment technology. Water Resour. Ind. 2021, 25, 100148. [Google Scholar] [CrossRef]
- Radechovska, H.; Svehla, P.; Radechovsky, J.; Pacek, L.; Bali, J. High-performance system for partial nitritation of reject water resistant to temperature fluctuation. Chem. Pap. 2017, 71, 1657–1668. [Google Scholar] [CrossRef]
- Szaja, A.; Lagód, G.; Drewnowski, J.; Sabba, F. Bioaugmentation of a Sequencing Batch Reactor with Archaea for the Treatment of Reject Water. J. Water Chem. 2016, 38, 238–243. [Google Scholar] [CrossRef]
- Galí, A.; Dosta, J.; van Loosdrecht, M.C.M.; Mata-Alvarez, J. Two ways to achieve an anammox influent from real reject water treatment at lab-scale: Partial SBR nitrification and SHARON process. Process Biochem. 2007, 42, 715–720. [Google Scholar] [CrossRef]
- Zekker, I.; Rikmann, E.; Tenno, T.; Lemmiksoo, V.; Menert, A.; Loorits, L.; Vabamäe, P.; Tomingas, M.; Tenno, T. Anammox enrichment from reject water on blank biofilm carriers and carriers containing nitrifying biomass: Operation of two moving bed biofilm reactors (MBBR). Biodegradation 2012, 23, 547–560. [Google Scholar] [CrossRef]
- Quan, L.M.; Khanh, D.P.; Hira, D.; Fujii, T.; Furukawa, K. Reject water treatment by improvement of whole cel anammox entrapment using polyvinyl alcohol/alginate gel. Biodegradation 2011, 22, 1155–1167. [Google Scholar] [CrossRef]
- Wang, G.; Dai, X.; Zhao, S.; Zhang, D. Research on Ammonia Removal from Reject Water Produced from Anaerobic Digestion of Thermally Hydrolyzed Sludge Through Partial Nitrification—Anammox. Water Air Soil Pollut. 2022, 233, 106. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Ding, L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. J. Clean. Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Borowski, S. Aerobic thermophilic sewage sludge stabilization. Ochr. Srodowiska 2000, 4, 21–25. (In Polish) [Google Scholar]
- Liebig, T.; Wagner, M.; Bjerrum, L.; Denecke, M. Nitrification performance and nitrifier community composition of a chemostat and a membrane-assisted bioreactor for the nitrification of sludge reject water. Bioprocess Biosyst. Eng. 2001, 24, 203–210. [Google Scholar] [CrossRef]
- Ryzińska, J. Problems of reject water and possibility of its treatment in Poland. Gaz Woda Tech. Sanit. 2006, 7–8, 58–62. (In Polish) [Google Scholar]
- Bartkowska, I. Drop in dry mass and organic substance content in the process of autothermal thermophilic aerobic digestion. Process Saf. Environ. Prot. 2015, 98, 170–175. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment; IWA publishing: London, UK, 2008. [Google Scholar] [CrossRef]
- Abdallaa, K.Z.; Hammam, G. Correlation between Biochemical Oxygen Demand and Chemical Oxygen Demand for Various Wastewater Treatment Plants in Egypt to Obtain the Biodegradability Indices. Int. J. Sci. Basic Appl. Res. 2014, 13, 42–48. [Google Scholar]
- Al-Sulaiman, A.M.; Khudair, B.H. Correlation between BOD5 and COD for Al-Diwaniyah wastewater treatment plants to obtain the biodigrability indices. Pak. J. Biotechnol. 2018, 15, 423–427. [Google Scholar]
- Shokoohi, R.; Asgari, G.; Leili, M. Modelling of moving bed biofilm reactor (MBBR) efficiency on hospital wastewater (HW) treatment: A comprehensive analysis on BOD and COD removal. Int. J. Environ. Sci. Technol. 2017, 14, 841–852. [Google Scholar] [CrossRef]
- McKinney, R.E. Mathematics of complete mixing activated sludge. J. Sanit. Eng. ASCE 1962, 88, 87–113. [Google Scholar] [CrossRef]
- Fenu, A.; Guglielmi, G.; Jimenez, J.; Spèrandio, M.; Saroj, D.; Lesjean, B.; Brepols, C.; Thoeye, C.; Nopens, I. Activated sludge model (ASM) based modelling of membrane bioreactor (MBR) processes: A critical review with special regard to MBR specificities. Water Res. 2010, 44, 4272–4294. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; McNevin, D. Alternative analysis of bod removal in subsurface flow constructed wetlands employing monod kinetics. Wat. Res. 2001, 35, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Pahlavanzadeh, S.; Benis, K.Z.; Shakerkhatibi, M.; Jashni, A.K.; Beydokhti, N.T.; Kordkandi, S.A. Performance and kinetic modeling of an aerated submerged fixed-film bioreactor for BOD and nitrogen removal from municipal wastewater. J. Environ. Chem. Eng. 2018, 6, 6154–6164. [Google Scholar] [CrossRef]
- Thomann, R. Systems Analysis and Water Quality Management; McGraw Hill: New York, NY, USA, 1974. [Google Scholar]
- Sun, G.; Saeed, T. Kinetic modelling of organic matter removal in 80 horizontal flow reed beds for domestic sewage treatment. Process Biochem. 2009, 44, 717–722. [Google Scholar] [CrossRef]



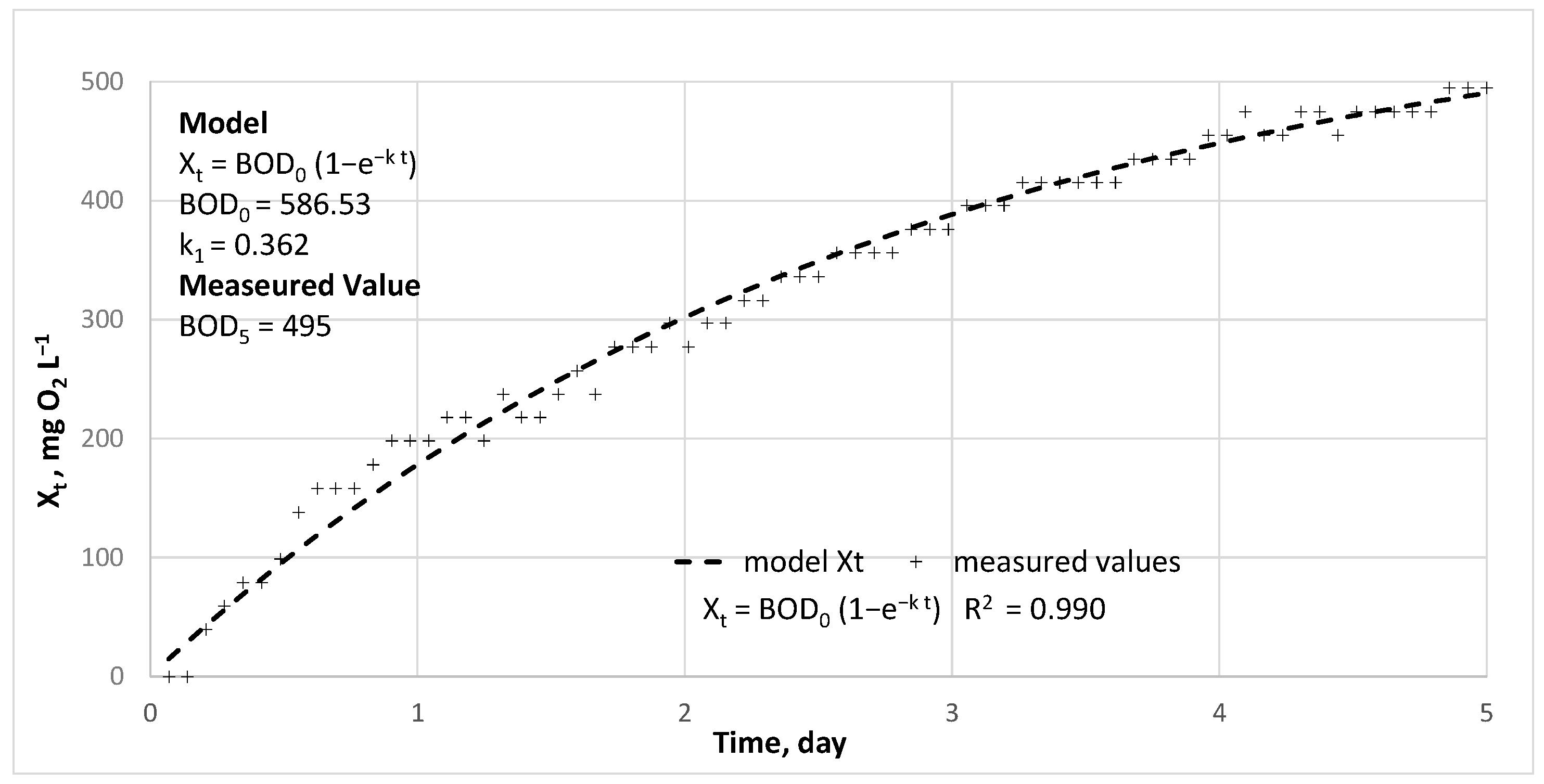
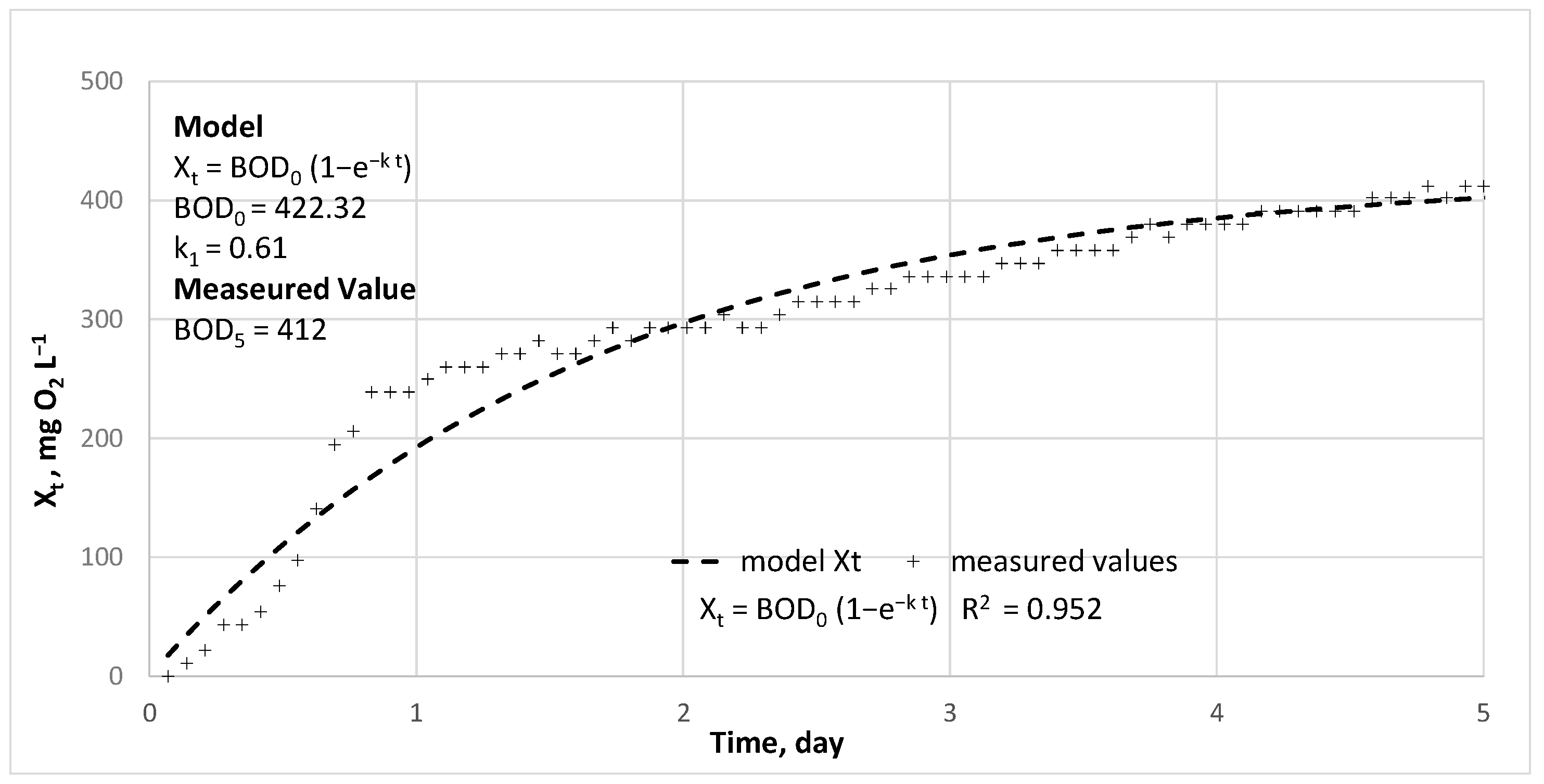

| COD | BOD5 | TKN | N-NH4+ | N-NO3− | TP | P-PO43− | |
|---|---|---|---|---|---|---|---|
| mg O2 L−1 | mg O2 L−1 | mg N L−1 | mg N L−1 | mg N L−1 | mg P L−1 | mg P L−1 | |
| Mean | 1424 | 251 | 485 | 318 | 1.20 | 202 | 168 |
| Minimum | 473 | 135 | 290 | 144 | 0.18 | 130 | 74 |
| Maximum | 2868 | 467 | 1033 | 811 | 2.52 | 420 | 390 |
| Std. dev. | 688 | 105 | 242 | 214 | 0.79 | 90 | 91 |
| COD | BOD5 | TKN | N-NH4+ | N-NO3− | TP | P-PO43− | |
|---|---|---|---|---|---|---|---|
| mg O2 L−1 | mg O2 L−1 | mg N L−1 | mg N L−1 | mg N L−1 | mg P L−1 | mg P L−1 | |
| Mean | 767 | 205 | 581 | 321 | 1.85 | 96 | 72 |
| Minimum | 219 | 35 | 183 | 114 | 0.18 | 50 | 19 |
| Maximum | 1840 | 449 | 1157 | 648 | 4.86 | 146 | 126 |
| Std. dev. | 530 | 154 | 324 | 184 | 1.83 | 39 | 39 |
| COD | BOD5 | TKN | N-NH4+ | N-NO3− | TP | P-PO43− | |
|---|---|---|---|---|---|---|---|
| mg O2 L−1 | mg O2 L−1 | mg N L−1 | mg N L−1 | mg N L−1 | mg P L−1 | mg P L−1 | |
| Mean | 4884 | 730 | 1573 | 736 | 1.72 | 281 | 172 |
| Minimum | 2064 | 288 | 887 | 351 | 0.14 | 142 | 49 |
| Maximum | 8040 | 2061 | 3140 | 1541 | 8.65 | 484 | 254 |
| Std. dev. | 2419 | 603 | 667 | 359 | 2.65 | 105 | 54 |
| WWTP1 | WWTP2 | WWTP3 | |
|---|---|---|---|
| sewage flow, m3 d−1 | 3260 | 1500 | 1573 |
| sewage sludge mass, kg d.m. d−1 | 2000 | 1200 * | 835 |
| rejected water, % | |||
| gravity thickening | 3.1 | 4.0 | 3.1 |
| mechanical thickening | 1.8 | 2.4 | 1.9 |
| mechanical dewatering | 1.0 | 1.4 | 1.1 |
| off-gas treatment | 2.9 | 4.8 | 2.9 |
| TOTAL, % | 8.8 | 12.6 | 9.0 |
| WWTP 1 | WWTP 2 | WWTP 3 | |
|---|---|---|---|
| Mean | 0.26 | 0.20 | 0.17 |
| Minimum | 0.08 | 0.09 | 0.14 |
| Maximum | 0.63 | 0.36 | 0.27 |
| Std. dev. | 0.21 | 0.09 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biedka, P. Biodegradation Kinetics of Organic Matter in Water from Sludge Dewatering after Autothermal Thermophilic Aerobic Digestion. Energies 2023, 16, 203. https://doi.org/10.3390/en16010203
Biedka P. Biodegradation Kinetics of Organic Matter in Water from Sludge Dewatering after Autothermal Thermophilic Aerobic Digestion. Energies. 2023; 16(1):203. https://doi.org/10.3390/en16010203
Chicago/Turabian StyleBiedka, Paweł. 2023. "Biodegradation Kinetics of Organic Matter in Water from Sludge Dewatering after Autothermal Thermophilic Aerobic Digestion" Energies 16, no. 1: 203. https://doi.org/10.3390/en16010203
APA StyleBiedka, P. (2023). Biodegradation Kinetics of Organic Matter in Water from Sludge Dewatering after Autothermal Thermophilic Aerobic Digestion. Energies, 16(1), 203. https://doi.org/10.3390/en16010203







