Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater
Abstract
1. Introduction
2. Materials and Methods
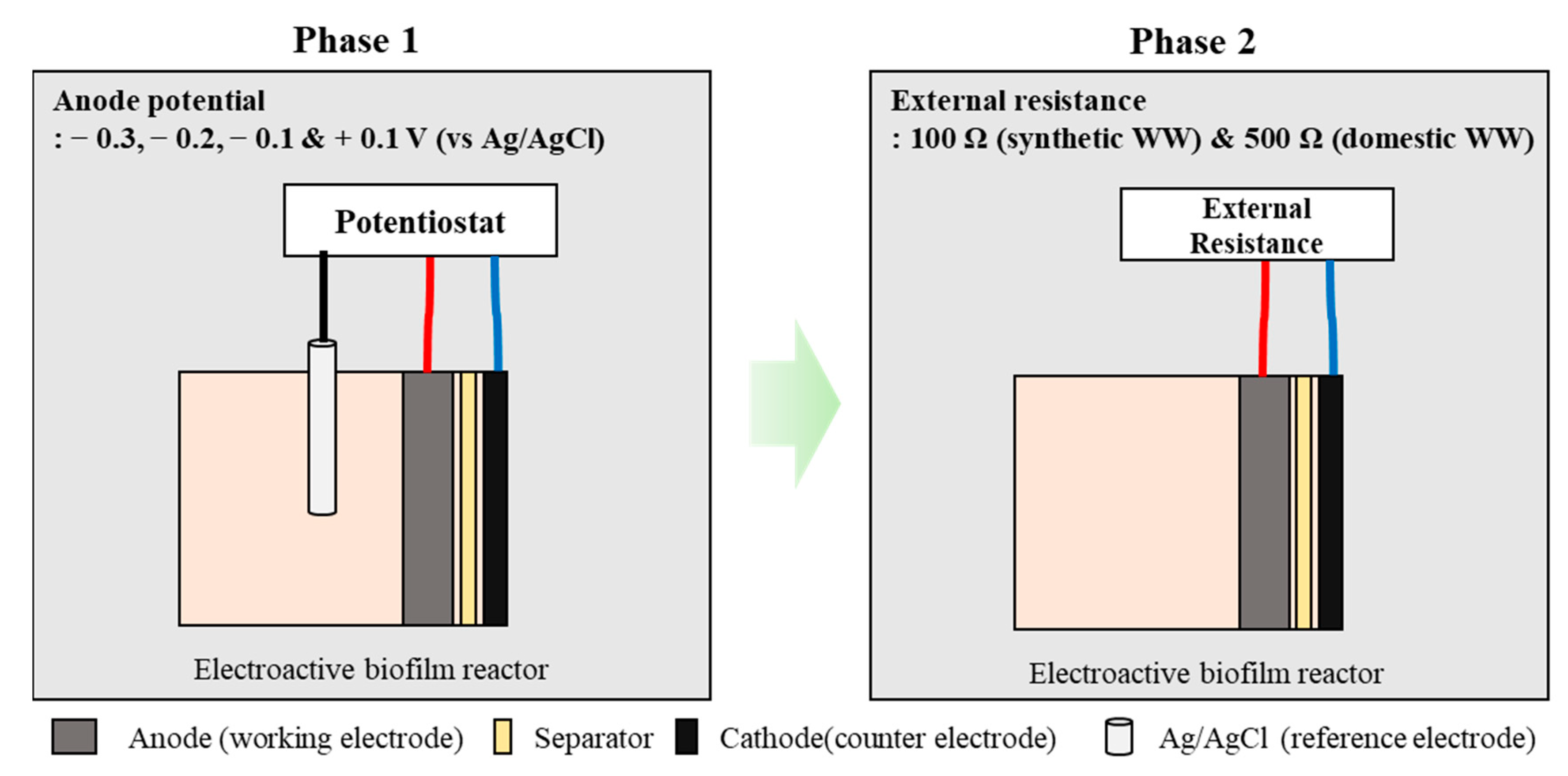
2.1. EBR Construction
2.2. Batch Test
2.3. Analysis
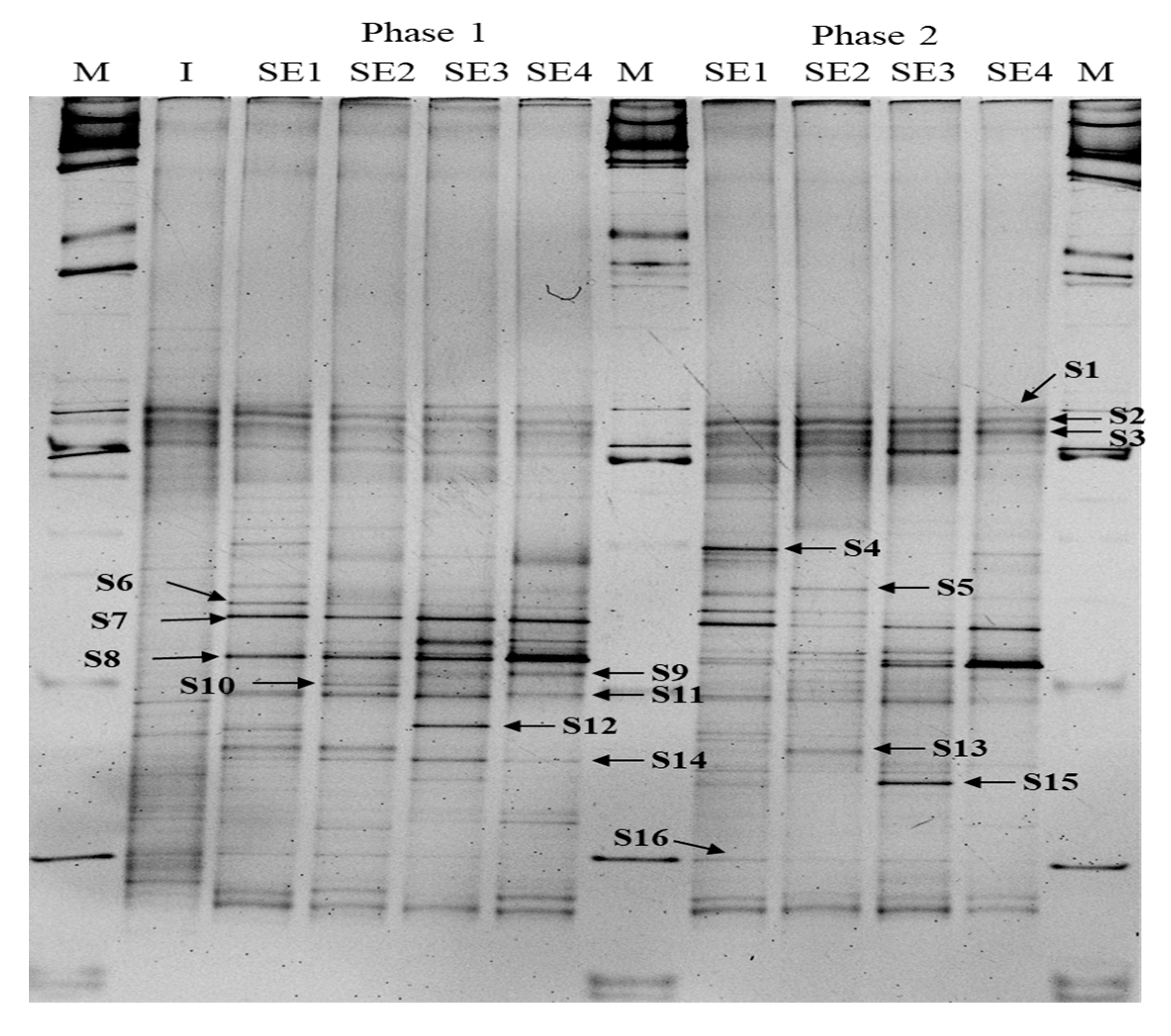
2.4. Microbial Community Analysis
3. Results and Discussion
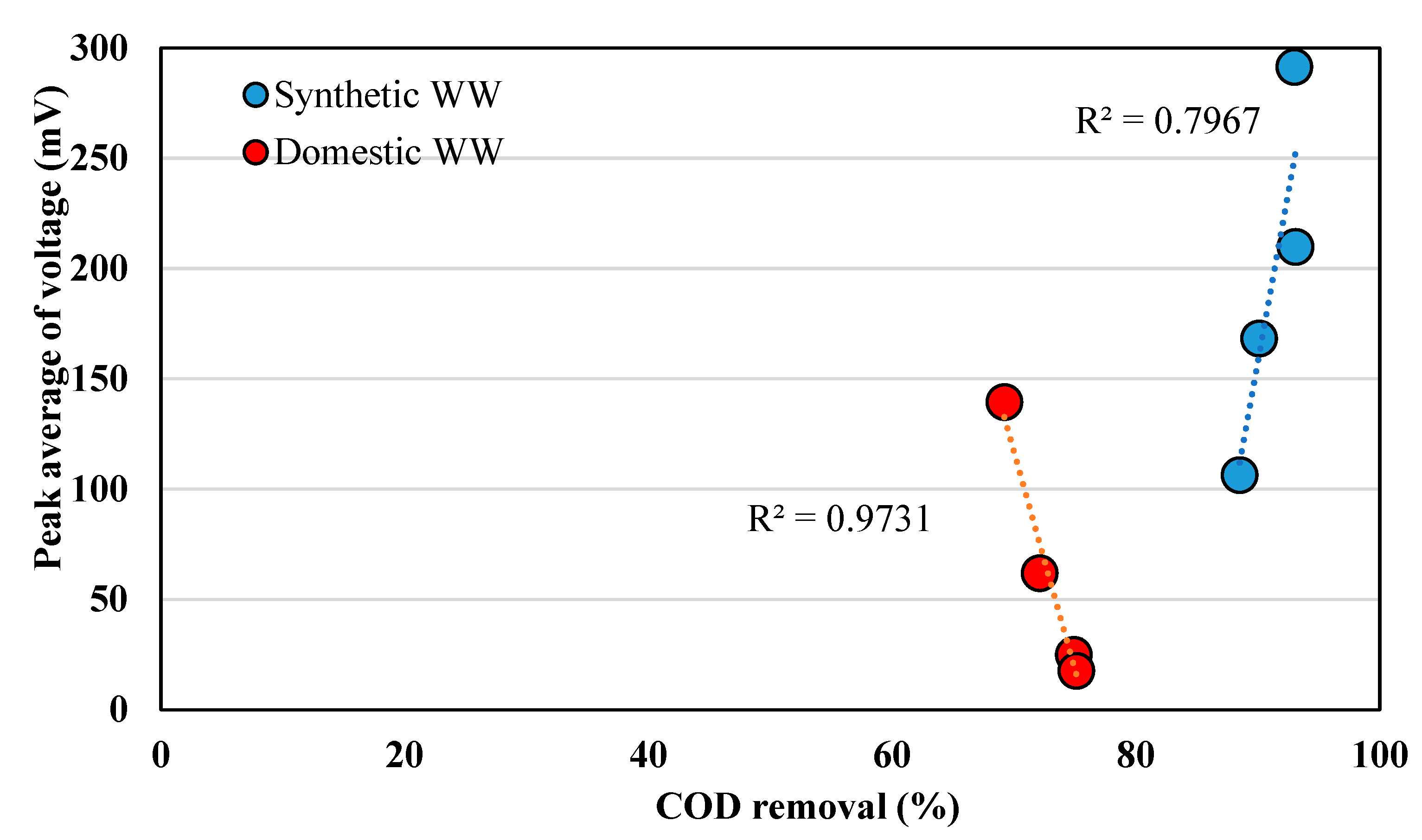
3.1. Voltage Generations in EBRs According to Initially Applied Voltages
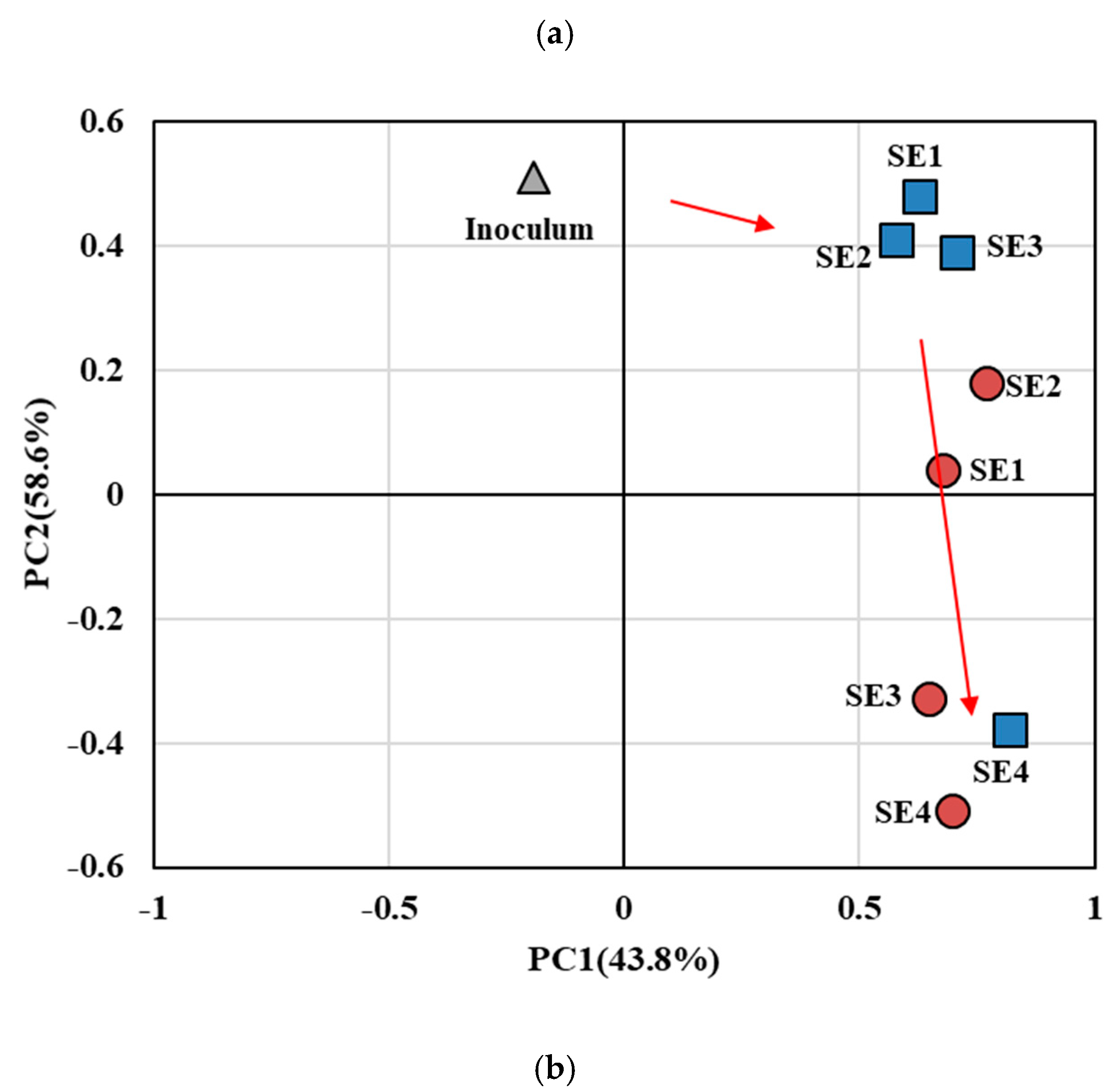
3.2. Anodic Microbial Community in EBRs Treating Synthetic WW
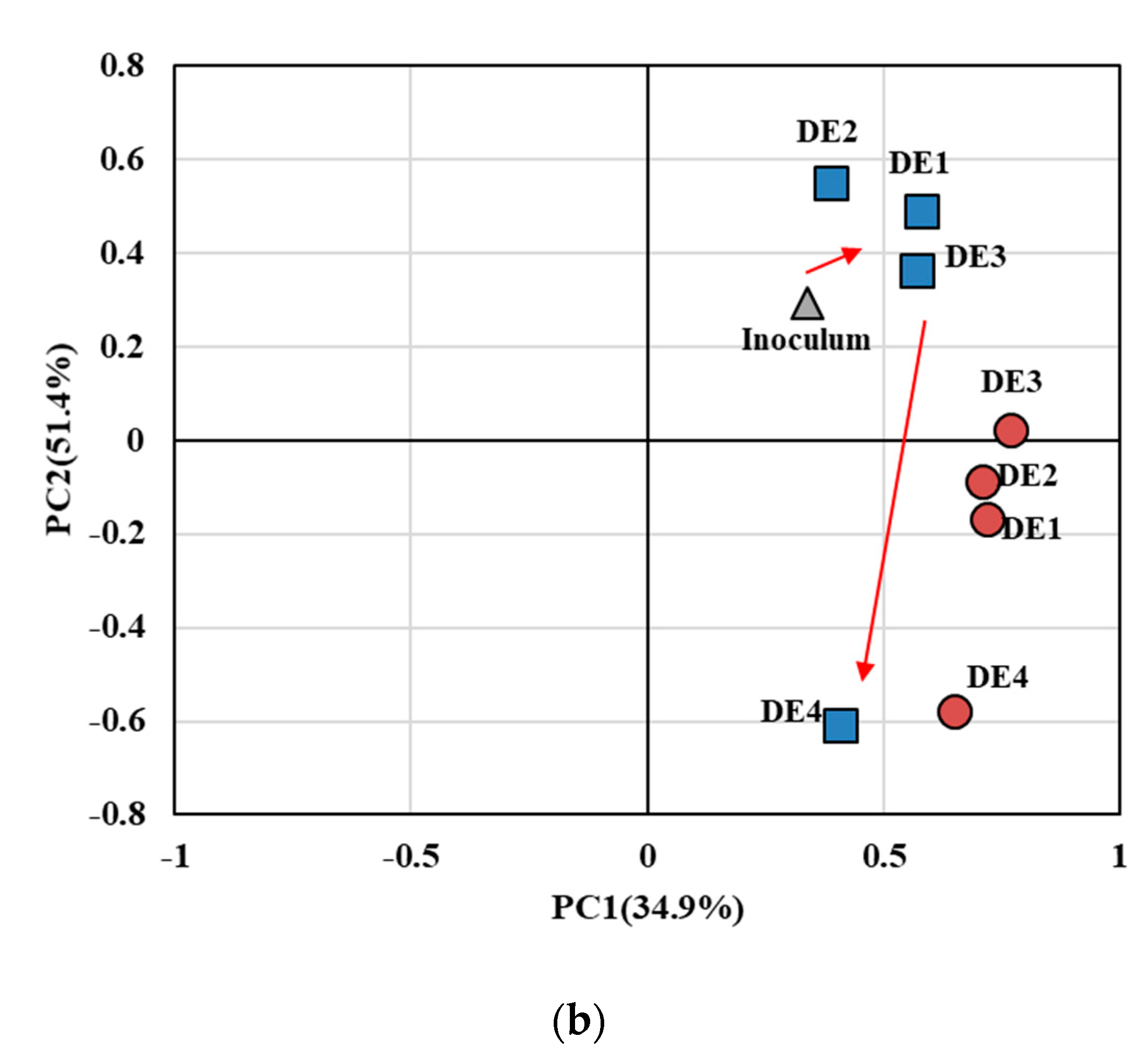
3.3. Anodic Microbial Community in EBRs Treating Domestic WW
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Widyaningsih, E.; Park, Y.; Lee, T. Nitrogen removal and microbial community diversity in single-chamber electroactive biofiom reactors with different ratios of the cathode surface area to reactor volume. Sci. Total Environ. 2021, 758, 143677. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Effects of a Hydraulic Series Connection and Flow Direction on Electricity Generation in a Stack Connected with Different Volume MFCs. Appl. Sci. 2021, 11, 1019. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Widyaningsih, E.; Lee, T. Microbial fuel cells L: Devices for real wastewater treatment, rather than elelctricity production. Sci. Total Environ. 2021, 775, 145904. [Google Scholar] [CrossRef]
- Catania, C.; Karbelkar, A.A.; Furst, A.L. Engineering the interface between electroactive bacteria and electrodes. Joule 2021, 5, 743. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Aelterman, P.; Freguia, S.; Keller, J.; Verstraete, W.; Rabaey, K. The anode potential regulates bacterial activity in microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 409–418. [Google Scholar] [CrossRef]
- Wager, R.C.; Cal, D.F.; Logan, B.E. Optimal set anode potentials vary in bioelectrochemical systems. Environ. Sci. Technol. 2010, 44, 6036–6041. [Google Scholar] [CrossRef]
- Cho, E.J.; Ellington, A.D. Optimization of the biological component of a bioelectrochemical cell. Bioelectrochemicstry 2007, 70, 165–172. [Google Scholar] [CrossRef]
- Duman, C.; Basseguy, R.; Bergel, A. Electrochmical activity of Geobacter sulfurreducens biofilms on stainless steel anodes. Electrochim. Acta 2008, 53, 5235–5241. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Torres, C.I.; Krajmalnik-Brown, R.; Rarameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting anode-respiring bacteria based on anode potential: Phylogentic, Electrochemical, and Microscopic characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ji, G.; Liu, H.; Yang, M.; Xu, S.; Ye, M.; Lichtfouse, E.K. Accelerated start-up and improved performance of wastwater microbial fuel cells in four circuit modes: Role of anodic potential. J. Power Sources 2022, 535, 231403. [Google Scholar] [CrossRef]
- Yu, J.; Park, Y.; Cho, H.; Chun, J.; Seon, J.; Cho, S.; Lee, T. Variations of electron flux and microbial community in air-cathode microbial fuel cells fed with different substrates. Water. Sci. Technol. 2012, 66, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, Y.; Lee, T. Effect of separator and inoculum type on electricity generation and microbial community in single-chamber microbial fuel cells. Bioprocess Biosyst. Eng. 2014, 37, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhou, S.; Zhuang, L.; Zhang, J.; Ni, J. Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Lee, J.W.; Kim, K.-Y.; Kim, I.-S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef]
- Najera, M.C.; Verea, L.; Lastres, O.; Mejía-López, M.; Hernández-Romano, J.; Sebastian, P.J. Electricity production in a two chamber microbial fuel cell with bioanodes and biocathodes catalyzed with gold. Fuel Cells 2020, 20, 762–768. [Google Scholar] [CrossRef]
- Yamamuro, A.; Kouzuma, A.; Abe, T.; Watanabe, K. Metagenomic analyses reveal the involvement of syntrophic consortia in methanol/electricity conversion in microbial cells. PLoS ONE 2014, 9, e98425. [Google Scholar] [CrossRef]
- Lay, C.-H.; Kokko, M.E.; Puhakka, J.A. Power generation in fed-batch and continuous up-flow microbial fuel cell from synthetic wastewater. Energy 2015, 91, 235–241. [Google Scholar] [CrossRef]
- Sun, M.; Tong, Z.-H.; Sheng, G.-P.; Chen, Y.-Z.; Zhang, F.; Mu, Z.-X.; Wang, H.-L.; Zeng, R.J.; Liu, X.-W.; Yu, H.-Q.; et al. Microbial communities involved in electricity generation from sulfide oxidation in a microbial fuel cell. Biosens. Bioelectron. 2010, 26, 470–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Peng, F.; Zhang, F.; Pang, Q.; Lian, J.; Zhang, Y.; Yang, F.; Zhu, Y.; Ding, C.; et al. Intensified nitrogen removal in the tidal flow constructed wetland-microbial fuel cell: Insight into evaluatino of denitrifying genes. J. Clean Prod. 2020, 264, 121580. [Google Scholar] [CrossRef]
- Zhu, X.M.; Tokash, J.C.; Hong, Y.; Logan, B.E. Controlling the occurrence of power overshoot by adapting microbial fuel cells to high anode potentials. Bioelectrochemistry 2013, 90, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.A.; Tender, L.M.; Zeikus, J.G. Effect of electrode potential on electrode-reducing Microbiota. Environ. Sci. Technol. 2006, 40, 6990–6995. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Mohan, S.V.; Sarma, P.N. Positive anodic poised potential regulates microbial fuel cell performance with the function of open and closed circuitry. Bioresour. Technol. 2010, 101, 5337–5344. [Google Scholar] [CrossRef] [PubMed]



| EBR | WW Type | Phase 1 | Phase 2 | ||
|---|---|---|---|---|---|
| Applied Anode Potential 1 (V) | External Resistance (Ω) | Applied Anode Potential (V) | External Resistance (Ω) | ||
| SE1 | Synthetic WW | −0.3 | No | No | 100 |
| SE2 | −0.2 | No | No | 100 | |
| SE3 | −0.1 | No | No | 100 | |
| SE4 | +0.1 | No | No | 100 | |
| DE1 | Domestic WW | −0.3 | No | No | 500 |
| DE2 | −0.2 | No | No | 500 | |
| DE3 | −0.1 | No | No | 500 | |
| DE4 | +0.1 | No | No | 500 | |
| Band | The Closet Sequence | Phylum | Similarity | Acc. No. |
|---|---|---|---|---|
| S1 | Uncultured Geobacter sp. | Proteobacteria | 97% | AB717104 |
| S2 | Uncultured bacterium clone MFC-GIST23 | Environmental samples | 97% | EU704538 |
| S3 | Uncultured Chloroflexi bacterium | Chloroflexi | 97% | JX023230 |
| S4 | Zoogloea sp. | Proteobacteria | 100% | HQ694764 |
| S5 | Uncultured Hyphomicrobiaceae bacterium | Proteobacteria | 98% | KF500830 |
| S6 | Acidovorax sp. | Proteobacteria | 99% | Y18617 |
| S7 | Zoogloea sp. | Proteobacteria | 100% | JQ751310 |
| S8 | Geobacter sp. | Proteobacteria | 99% | GQ463728 |
| S9 | Sphingomonas paucimobilis | Proteobacteria | 99% | HE800592 |
| S10 | Uncultured Pseudoxanthomonas sp. | Proteobacteria | 99% | JQ328218 |
| S11 | Uncultured Shigella sp. | Gammproteobacteria | 99% | JF833726 |
| S12 | Uncultured bacterium clone MFC-GIST252 | Environmental samples | 995 | GQ463728 |
| S13 | Uncultured bacterium | Environmental samples | 98% | GU908879 |
| S14 | Uncultured bacterium | Environmental samples | 97% | JX086768 |
| S15 | Actinobacterium | Actinobacteria | 97% | FJ529700 |
| S16 | Uncultured bacterium | Environmental samples | 98% | AF255632 |
| Band | The Closet Sequence | Phylum | Similarity | Acc. No. |
|---|---|---|---|---|
| R1 | Chitinophaga sp. | Bacteroidota | 100% | JF710262 |
| R2 | Zoogloea sp. | Proteobacteria | 99% | HQ694764 |
| R3 | Uncultured Chloroflexi bacterium | Chloroflexi | 97% | JX023230 |
| R4 | Uncultured Hyphomicrobiaceae bacterium | Proteobacteria | 99% | KF500830 |
| R5 | Uncultured bacterium | Environmental samples | 96% | JQ096520 |
| R6 | Uncultured bacterium | Environmental samples | 97% | GU934266 |
| R7 | Uncultured bacterium clone MFC-GIST2 | Environmental samples | 99% | EU704531 |
| R8 | Variovorax paradoxus | Proteobacteria | 99% | AF508103 |
| R9 | Uncultured bacterium | Environmental samples | 99% | JN391943 |
| R10 | Uncultured bacterium | Environmental samples | 99% | FJ375463 |
| R11 | Uncultured beta proteobacterium | Proteobacteria | 98% | GU013679 |
| R12 | Uncultured bacterium clone MFC-GIST2 | Environmental samples | 99% | EU704531 |
| R13 | Uncultured bacterium | Environmental samples | 99% | DQ444005 |
| R14 | Uncultured bacterium | Environmental samples | 99% | JX023223 |
| R15 | Uncultured bacterium | Environmental samples | 99% | GQ996483 |
| R16 | Uncultured Thauera sp. | Proteobacteria | 99% | KX914702 |
| R17 | Uncultured bacterium | Environmental samples | 99% | GU083491 |
| R18 | Thauera sp. | Proteobacteria | 99% | AY570693 |
| R19 | Uncultuyed Aminanaerobia bacterium | Environmental samples | 99% | CU926332 |
| R20 | Desulforhabdus sp. | Proteobacteria | 99% | EF442978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Park, H.; Park, Y.; Lee, T. Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater. Energies 2022, 15, 9459. https://doi.org/10.3390/en15249459
Yu J, Park H, Park Y, Lee T. Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater. Energies. 2022; 15(24):9459. https://doi.org/10.3390/en15249459
Chicago/Turabian StyleYu, Jaecheul, Hana Park, Younghyun Park, and Taeho Lee. 2022. "Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater" Energies 15, no. 24: 9459. https://doi.org/10.3390/en15249459
APA StyleYu, J., Park, H., Park, Y., & Lee, T. (2022). Power Generation and Microbial Community Shift According to Applied Anodic Potential in Electroactive Biofilm Reactors Treating Synthetic and Domestic Wastewater. Energies, 15(24), 9459. https://doi.org/10.3390/en15249459






