Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery
Abstract
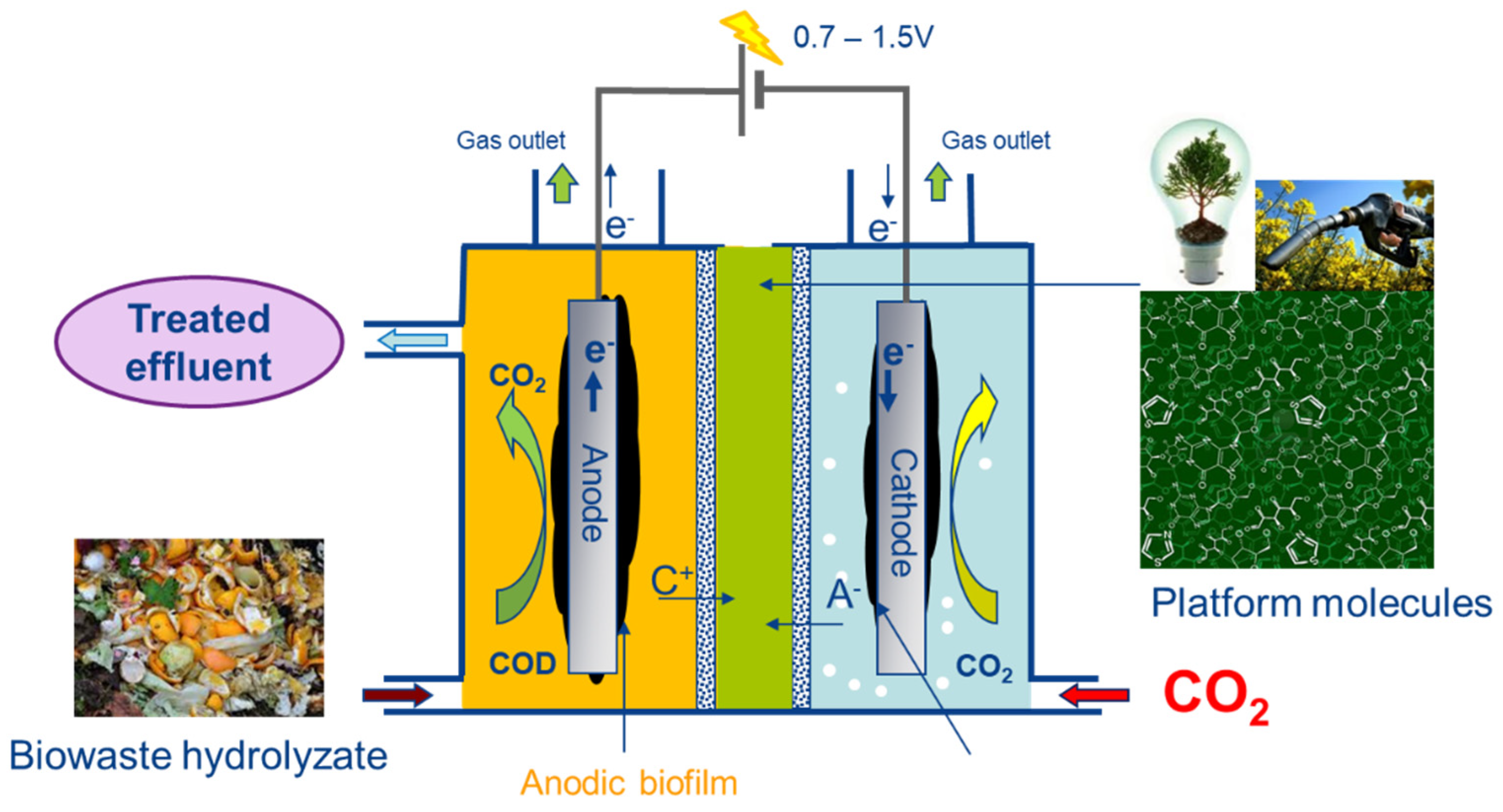
1. Introduction
2. Experimental Details
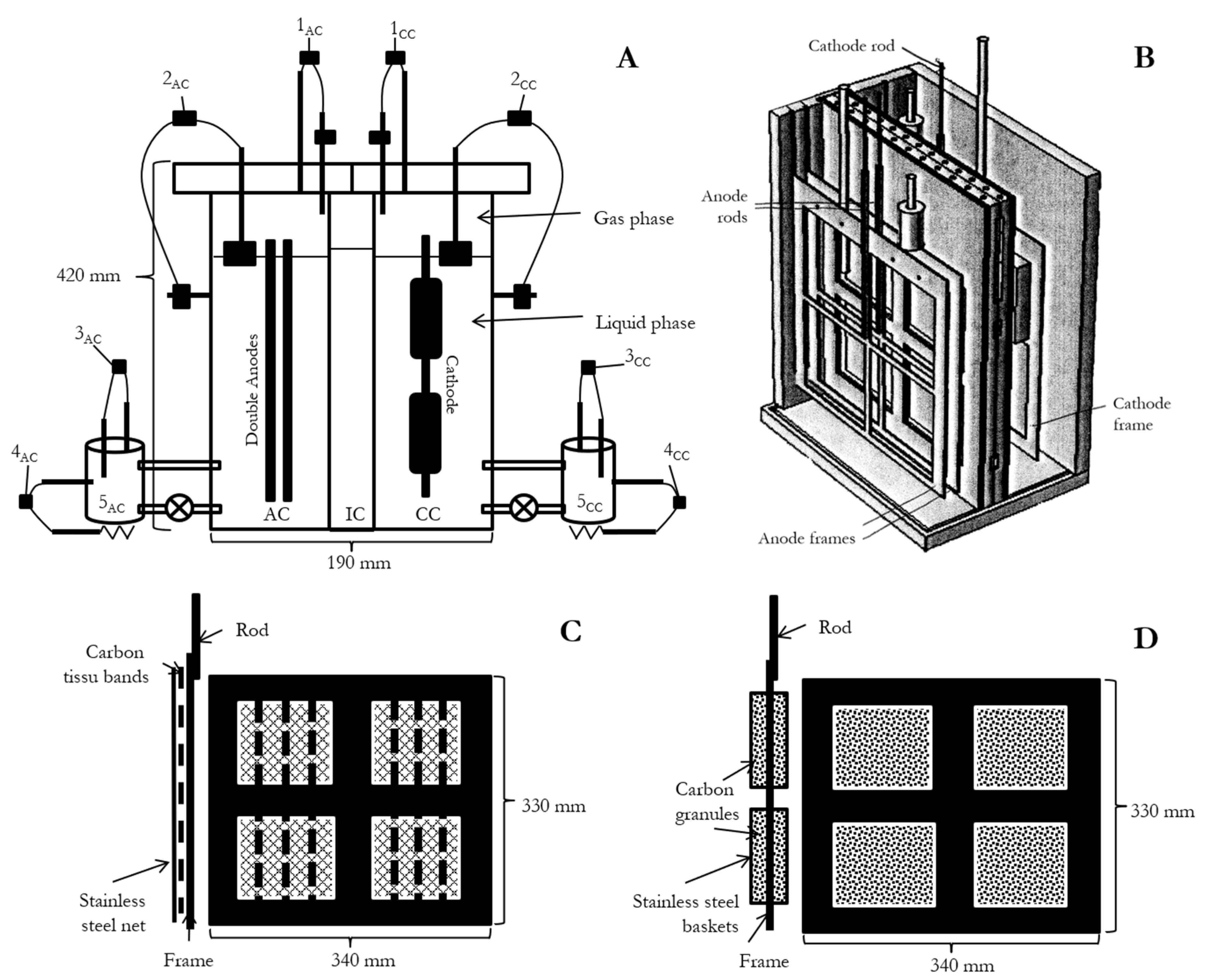
2.1. Reactor Design
2.2. Bioanode Performance Modeling
2.3. Reaction Setup
2.3.1. Inoculation
2.3.2. Start-Up Phase
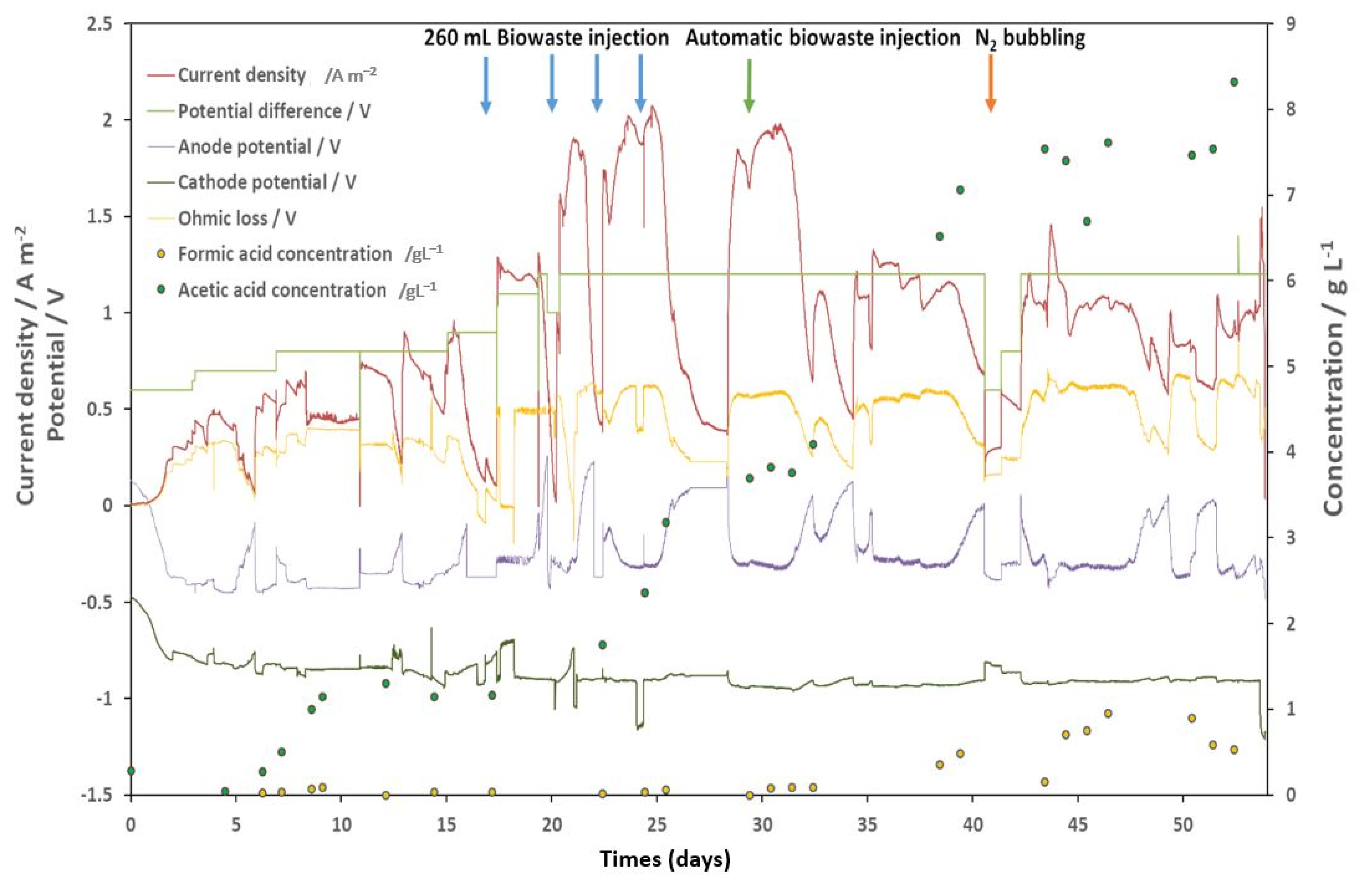
2.3.3. Biowaste Hydrolysate (BH) Feeding
2.3.4. Bioanode Activity Regeneration with N2 Gas Bubbling
2.3.5. Bioanode Activity Regeneration with the Double Anode Configuration
2.4. Chemical Analysis and Calculations
2.5. Microbial Community Analysis
3. Results and Discussion
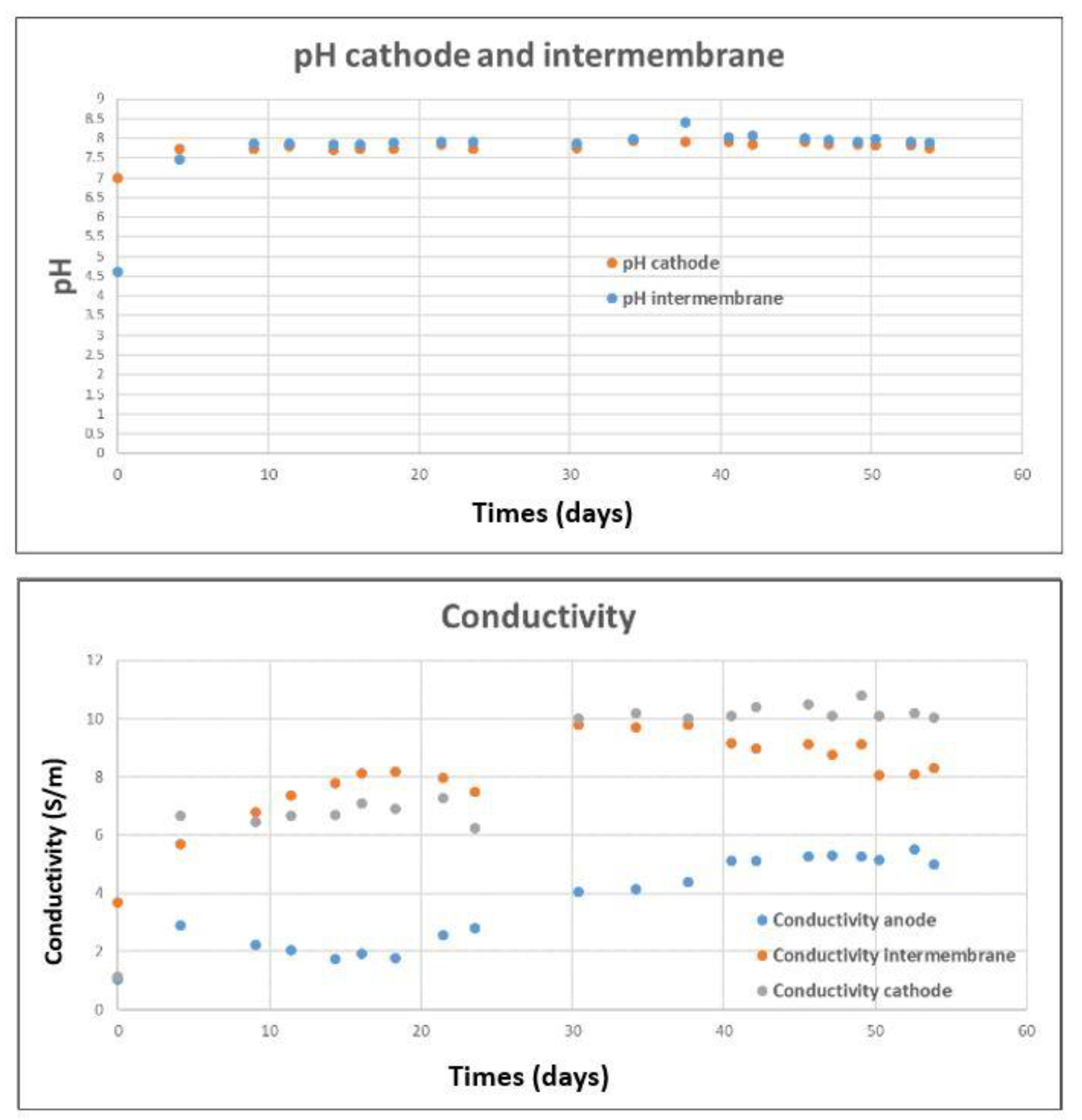
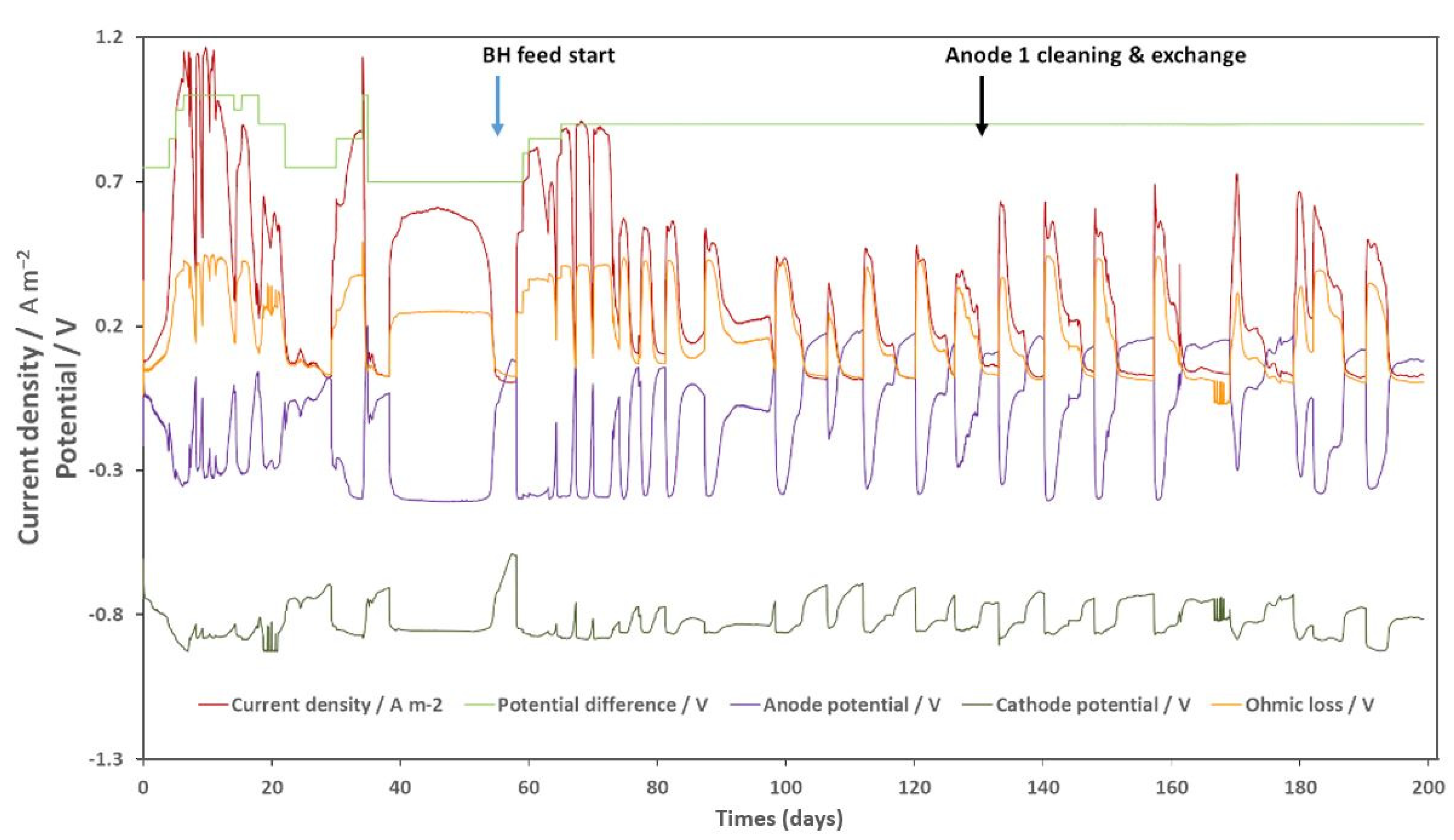
3.1. Pilot Operation and Bioanode Activity Regeneration with N2 Gas Bubbling
3.2. CV Measurements Reveal Complex Response of Bioanodes Facing Potential Change
3.3. Bioanode Activity Regeneration by Electrode Cleaning and Exchange
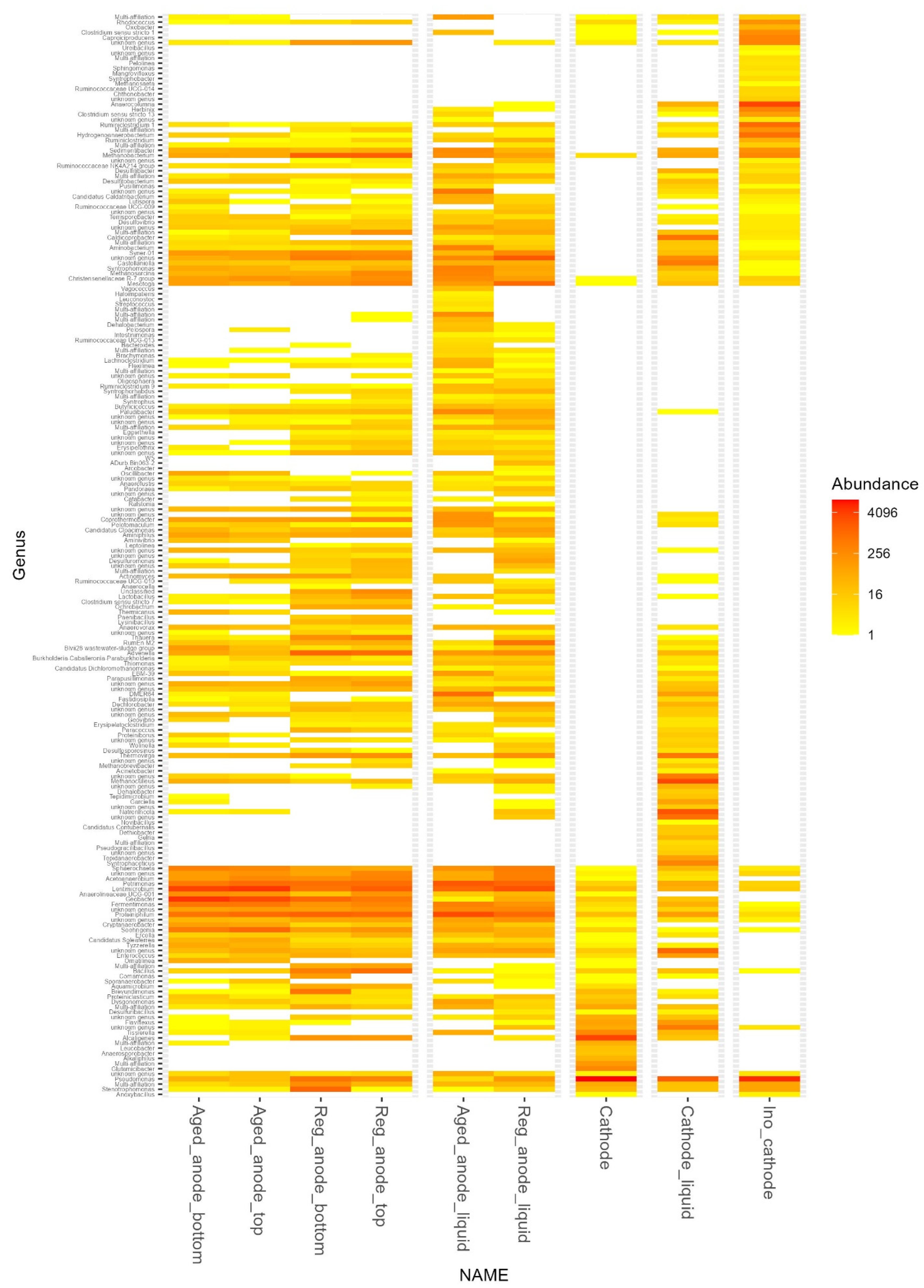
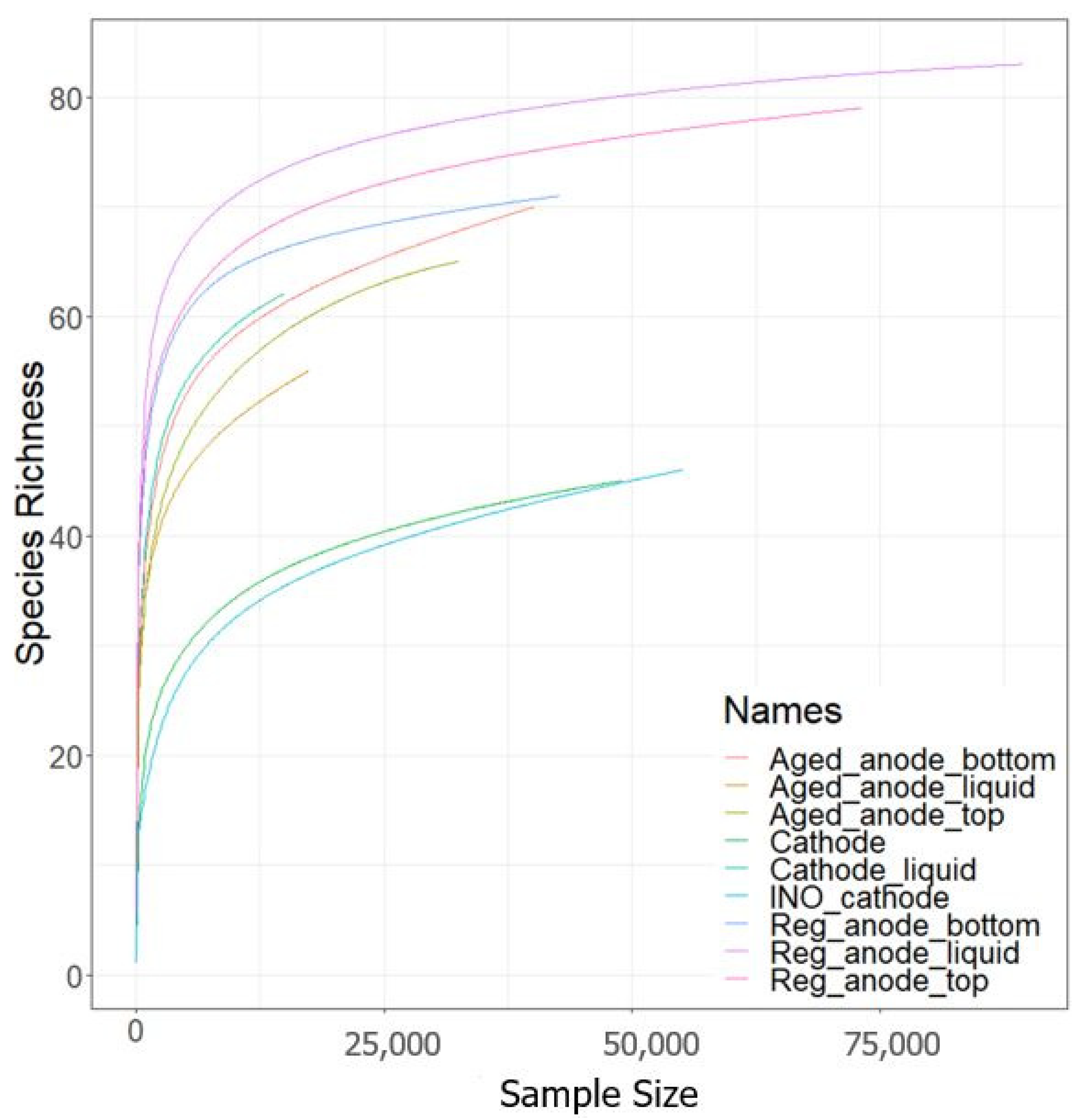
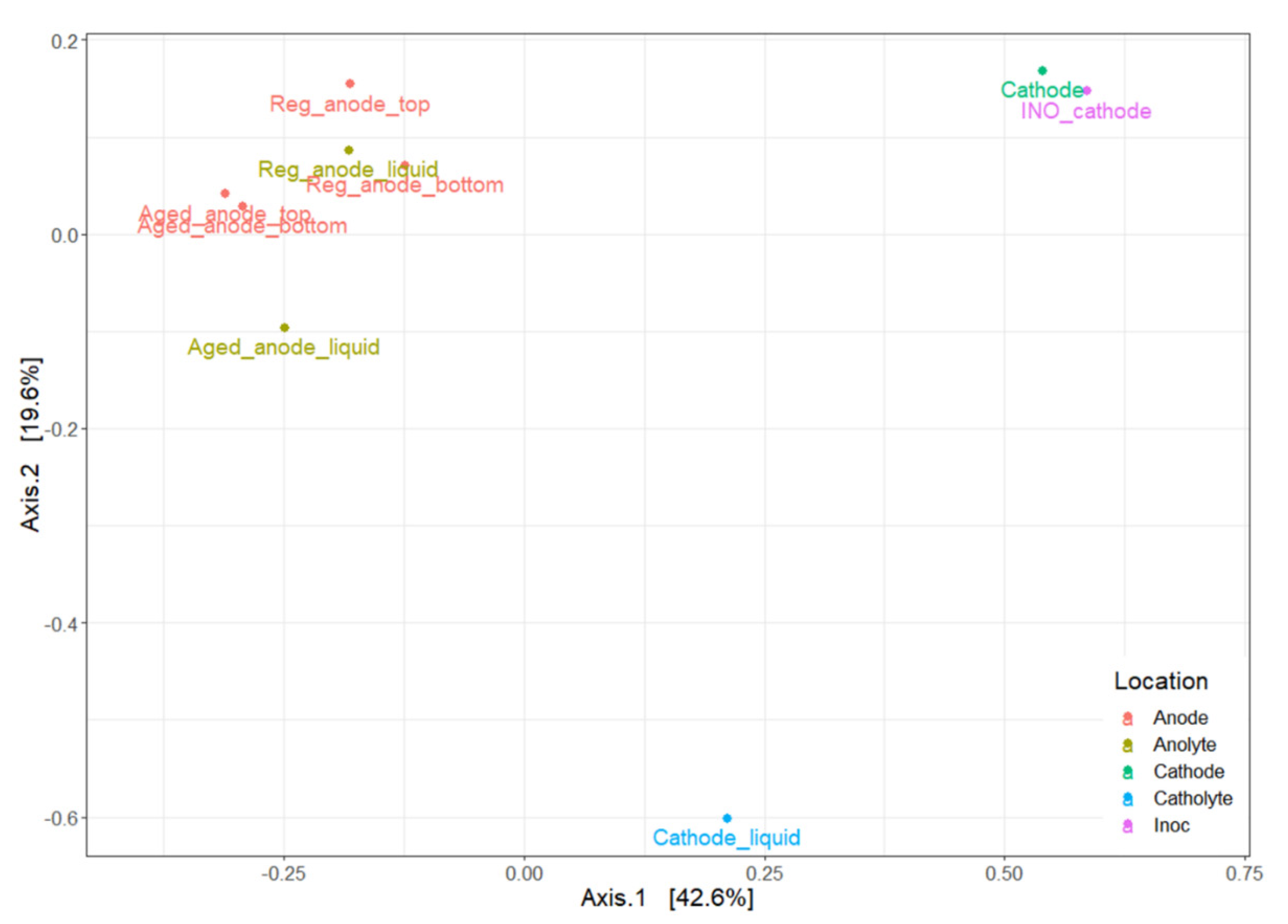
3.4. Microbial Community Evolution
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moscoviz, R.; Trably, E.; Bernet, N.; Carrère, H. The environmental biorefinery: State-of-the-art on the production of hydrogen and value-added biomolecules in mixed-culture fermentation. Green Chem. 2018, 20, 3159–3179. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Grando, R.L.; Antune, A.M.D.S.; da Fonseca, F.V.; Sánchez, A.; Barrena, R.; Font, X. Technology overview of biogas production in anaerobic digestion plants: A European evaluation of research and development. Renew. Sustain. Energy Rev. 2017, 80, 44–53. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Banja, M.; Motola, V. Renewable energy policy framework and bioenergy contribution in the European Union—An overview from National Renewable Energy Action Plans and Progress Reports. Renew. Sustain. Energy Rev. 2015, 51, 969–985. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Foulet, A.; Bouchez, T.; Desmond-Le Quéméner, E.; Giard, L.; Renvoisé, L.; Aissani, L. Life cycle assessment of a bioelectro-chemical system as a new technological platform for biosuccinic acid production from waste. Environ. Sci. Pollut. Res. 2018, 25, 36485–36502. [Google Scholar] [CrossRef]
- Sen, B.; Aravind, J.; Kanmani, P.; Lay, C.-H. State of the art and future concept of food waste fermentation to bioenergy. Renew. Sustain. Energy Rev. 2016, 53, 547–557. [Google Scholar] [CrossRef]
- Idris, M.O.; Noh, N.A.M. Sustainable microbial fuel cell functionalized with a bio-waste: A feasible route to formaldehyde bioremediation along with bioelectricity generation. Chem. Eng. J. 2022, 140781, 1–17. [Google Scholar]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Wainaina, S.; Horváth, I.S.; Taherzadeh, M.J. Biochemicals from food waste and recalcitrant biomass via syngas fermentation: A review. Bioresour. Technol. 2018, 248, 113–121. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Moscoviz, R.; Trably, E.; Bernet, N. Glucose electro-fermentation as main driver for efficient H2-producing bacteria selection in mixed cultures. Int. J. Hydrogen Energy 2019, 44, 2230–2238. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Ahmad, A. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl. Nanosci. 2021, 11, 1949–1961. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Tabassum, P.; Lokhat, D.; Siti, H. A glimpse into the microbial fuel cells for wastewater treatment with energy generation. Desalination Water Treat. 2021, 214, 379–389. [Google Scholar]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.; Euverink, G.J.; Metz, S.J.; Buisman, C.J. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy 2006, 31, 1632–1640. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. Mbio 2010, 1, e00103-10. [Google Scholar] [CrossRef]
- Blanchet, E.; Duquenne, F.; Rafrafi, Y.; Etcheverry, L.; Erable, B.; Bergel, A. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction. Energy Environ. Sci. 2015, 8, 3731–3744. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Long-term Operation of Microbial Electrosynthesis Systems Improves Acetate Production by Autotrophic Microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing biomass-based graphene oxide-polyaniline-Ag electrodes in microbial fuel cells to boost energy generation and heavy metal removal. Polymers 2022, 14, 845. [Google Scholar]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Genet. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Yaakop, A.S. Application of oil palm lignocellulosic derived material as an efficient anode to boost the toxic metal remediation trend and energy generation through microbial fuel cells. J. Clean. Prod. 2021, 314, 128062. [Google Scholar]
- Schröder, U. Discover the possibilities: Microbial bioelectrochemical systems and the revival of a 100-year–old discovery. J. Solid State Electrochem. 2011, 15, 1481–1486. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Rodríguez-Couto, S.; Ahmad, A. Preparation, characterization, and application of modified carbonized lignin as an anode for sustainable microbial fuel cell. Process Saf. Environ. Prot. 2021, 155, 49–60. [Google Scholar]
- Kumar, G.; Bakonyi, P.; Zhen, G.; Sivagurunathan, P.; Koók, L.; Kim, S.-H.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. Microbial electrochemical systems for sustainable biohydrogen production: Surveying the experiences from a start-up viewpoint. Renew. Sustain. Energy Rev. 2017, 70, 589–597. [Google Scholar] [CrossRef]
- Bridier, A.; Quemener, E.D.-L.; Bureau, C.; Champigneux, P.; Renvoise, L.; Audic, J.-M.; Blanchet, E.; Bergel, A.; Bouchez, T. Successive bioanode regenerations to maintain efficient current production from biowaste. Bioelectrochemistry 2015, 106, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-dimensional carbon cloth and three-dimensional carbon felt perform similarly to form bioanode fed with food waste. Electrochem. Commun. 2016, 66, 38–41. [Google Scholar] [CrossRef]
- Pocaznoi, D.; Calmet, A.; Etcheverry, L.; Erable, B.; Bergel, A. Stainless steel is a promising electrode material for anodes of microbial fuel cells. Energy Environ. Sci. 2012, 5, 9645–9652. [Google Scholar] [CrossRef]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of Commodity Chemicals by an Autotrophic Microbial Community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. General medium for the autotrophic cultivation of acetogens. Bioprocess Biosyst. Eng. 2016, 39, 1645–1650. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Bernet, N.; Steyer, J.-P.; Delgenès, J.-P.; Escudié, R. Cardboard proportions and total solids contents as driving factors in dry co-fermentation of food waste. Bioresour. Technol. 2018, 248, 229–237. [Google Scholar] [CrossRef]
- Idris, M.O.; Yaqoob, A.A.; Ibrahim, M.N.M.; Noh, N.A.M.; Daud, N.N.M. Electrochemical Measurements of Microbial Fuel Cells (MFCs). In Microbial Fuel Cells for Environmental Remediation; Springer: Singapore, 2022; Volume 1, pp. 41–64. [Google Scholar]
- Poirier, S.; Quéméner, E.D.-L.; Madigou, C.; Bouchez, T.; Chapleur, O. Anaerobic digestion of biowaste under extreme ammonia concentration: Identification of key microbial phylotypes. Bioresour. Technol. 2016, 207, 92–101. [Google Scholar] [CrossRef]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2017, 34, 1287–1294. [Google Scholar] [CrossRef]
- Midoux, C.; Rué, O.; Chapleur, O.; Mariadassou, M.; Bouchez, T.; Loux, V.; Bize, A. Easy16S: A User-Friendly Shiny Interface for Analysis and Visualization of Metagenomic Data. In Proceedings of the Journées Ouvertes Biologie, Informatique et Mathématiques (JOBIM), Marseille, France, 3–6 July 2018; Available online: https://hal.archives-ouvertes.fr/hal-01930383/document#page=539 (accessed on 24 July 2019).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, G.; Zhang, R.; Lu, Y.; Luo, H. High-efficient acetate production from carbon dioxide using a bioanode microbial electrosynthesis system with bipolar membrane. Bioresour. Technol. 2017, 233, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Borole, A.P.; Pavlostathis, S.G. Biotransformation of Furanic and Phenolic Compounds with Hydrogen Gas Production in a Microbial Electrolysis Cell. Environ. Sci. Technol. 2015, 49, 13667–13675. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Minteer, S.D. Nitrogenase Bioelectrocatalysis: From Understanding Electron-Transfer Mechanisms to Energy Applications. ACS Energy Lett. 2018, 3, 2736–2742. [Google Scholar] [CrossRef]
- Wang, A.; Liu, W.; Ren, N.; Zhou, J.; Cheng, S. Key factors affecting microbial anode potential in a microbial electrolysis cell for H2 production. Int. J. Hydrogen Energy 2010, 35, 13481–13487. [Google Scholar] [CrossRef]
- Reguera, G.; Pollina, R.B.; Nicoll, J.S.; Lovley, D.R. Possible Nonconductive Role of Geobacter sulfurreducens Pilus Nanowires in Biofilm Formation. J. Bacteriol. 2007, 189, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Toyonaga, M.; Ohashi, A.; Tourlousse, D.M.; Matsuura, N.; Meng, X.-Y.; Tamaki, H.; Hanada, S.; Cruz, R.; Yamaguchi, T.; et al. Lentimicrobium saccharophilum gen. nov., sp. nov., a strictly anaerobic bacterium representing a new family in the phylum Bacteroidetes and proposal of Lentimicrobiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 2635–2642. [Google Scholar] [CrossRef]
- Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar]
- Idris, M.O.; Kim, H.C. Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102183. [Google Scholar] [CrossRef]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef]



| Fully Developed Carbon Tissue | “Aged” Carbon Tissue | “Aged” TRL4 Reactor Anodes | |
|---|---|---|---|
| Equation | |||
| Jmax | 10.5 A m−2 | 6.0 A m−2 | 0.65 |
| α | 0.363 | 0.622 | 0.622 |
| R | 8.314 J.mol−1.K−1 | - | - |
| T | 298 K | - | - |
| E0.5 (vs Ag/AgCl) | −0.3 V | −0.35 V | −0.3 V |
| Number of Reads | Shannon Alpha Diversity | Methanogen Abundance/% | Most Abundant Family | |
|---|---|---|---|---|
| Ino_cathode | 55,122 | 1.60 | 1.33 | Pseudomonadaceae (37.5%) |
| Cathode | 48,945 | 1.09 | 0.03 | Pseudomonadaceae (64.9%) |
| Cathode_liquid | 14,746 | 2.64 | 23.50 | Dysgonomonadaceae (35.5%) |
| Aged_anode_bottom | 40,166 | 2.13 | 1.34 | Geobacteraceae (35.6%) |
| Aged_anode_top | 32,495 | 2.15 | 1.23 | Lentimicrobiaceae (33.1%) |
| Reg_anode_bottom | 42,708 | 2.99 | 10.40 | Dysgonomonadaceae (10.4%) |
| Reg_anode_top | 73,165 | 3.10 | 12.60 | Dysgonomonadaceae (16.1%) |
| Aged_anode_liquid | 21,627 | 1.98 | 4.38 | Dysgonomonadaceae (35.9%) |
| Reg_anode_liquid | 89,388 | 2.80 | 6.50 | Synergistaceae (17.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.-H.; Lacroix, R.; Yaqoob, A.A.; Bureau, C.; Midoux, C.; Desmond-Le Quéméner, E.; Bouchez, T. Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery. Energies 2023, 16, 591. https://doi.org/10.3390/en16020591
Tian J-H, Lacroix R, Yaqoob AA, Bureau C, Midoux C, Desmond-Le Quéméner E, Bouchez T. Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery. Energies. 2023; 16(2):591. https://doi.org/10.3390/en16020591
Chicago/Turabian StyleTian, Jiang-Hao, Rémy Lacroix, Asim Ali Yaqoob, Chrystelle Bureau, Cédric Midoux, Elie Desmond-Le Quéméner, and Théodore Bouchez. 2023. "Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery" Energies 16, no. 2: 591. https://doi.org/10.3390/en16020591
APA StyleTian, J.-H., Lacroix, R., Yaqoob, A. A., Bureau, C., Midoux, C., Desmond-Le Quéméner, E., & Bouchez, T. (2023). Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery. Energies, 16(2), 591. https://doi.org/10.3390/en16020591









