Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell
Abstract
1. Introduction
2. Materials and Methods
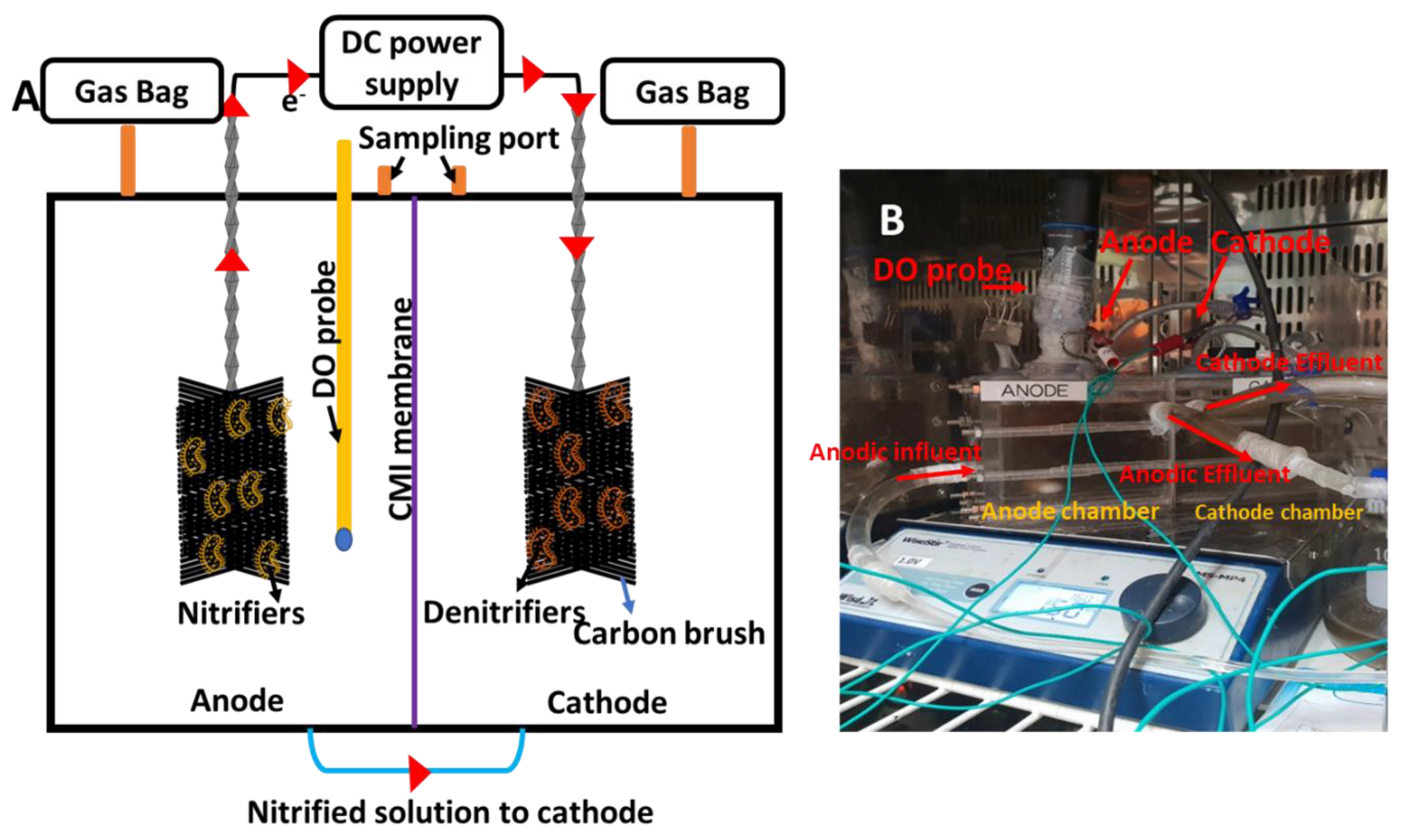
2.1. Fabrication and Inoculation of MEC for SND
2.2. Operation of MEC for SND
2.3. Analysis
3. Results and Discussion
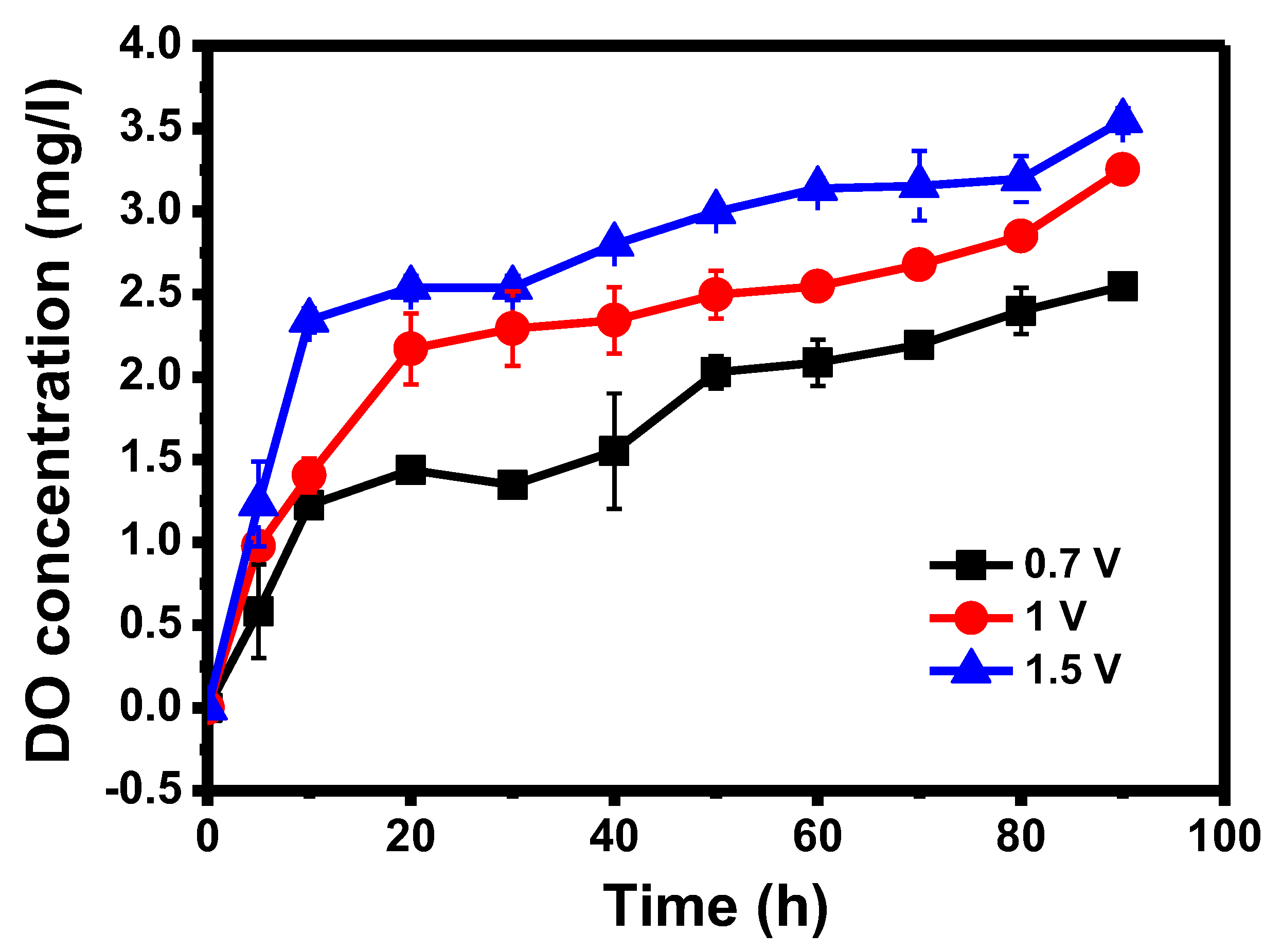
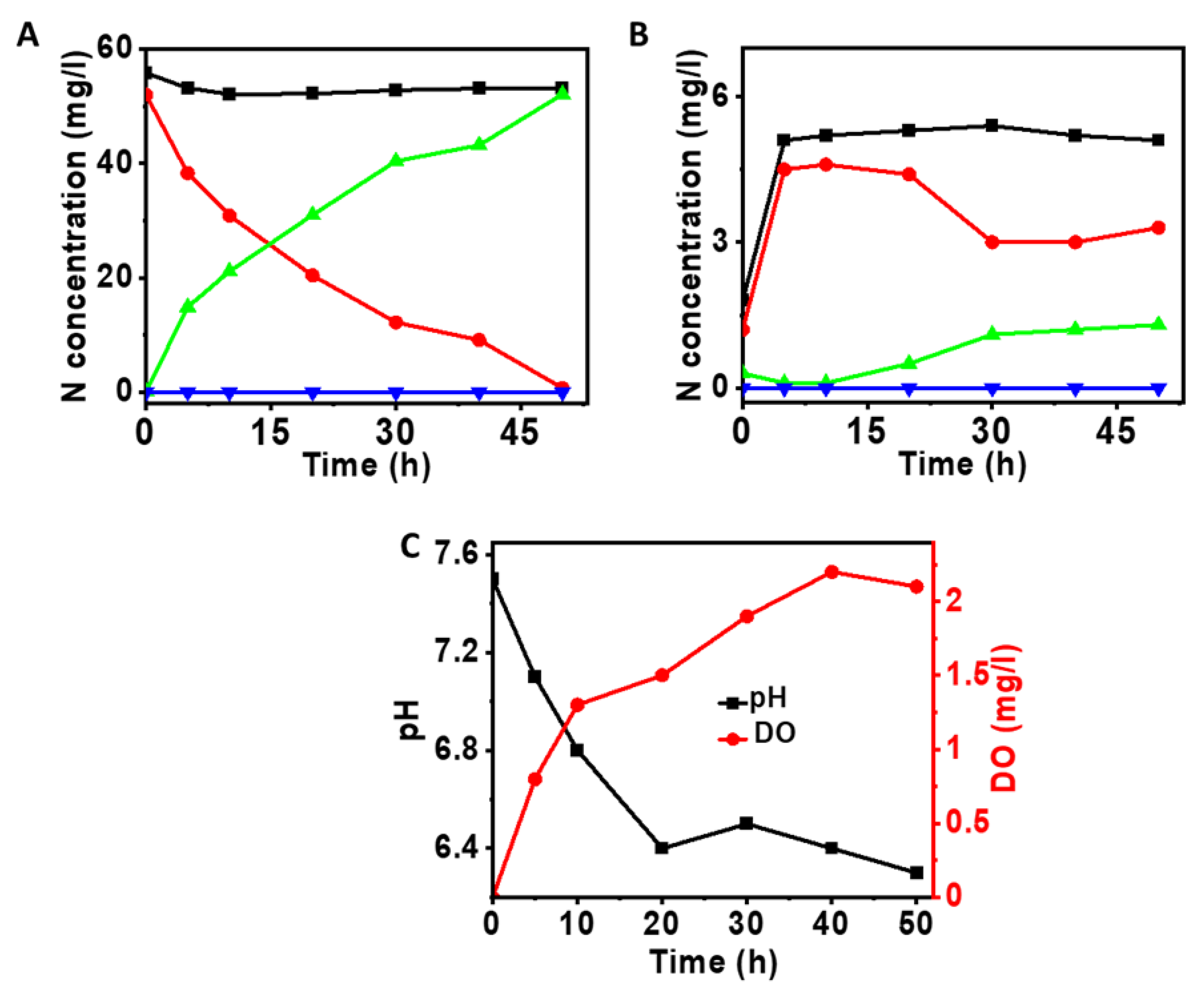
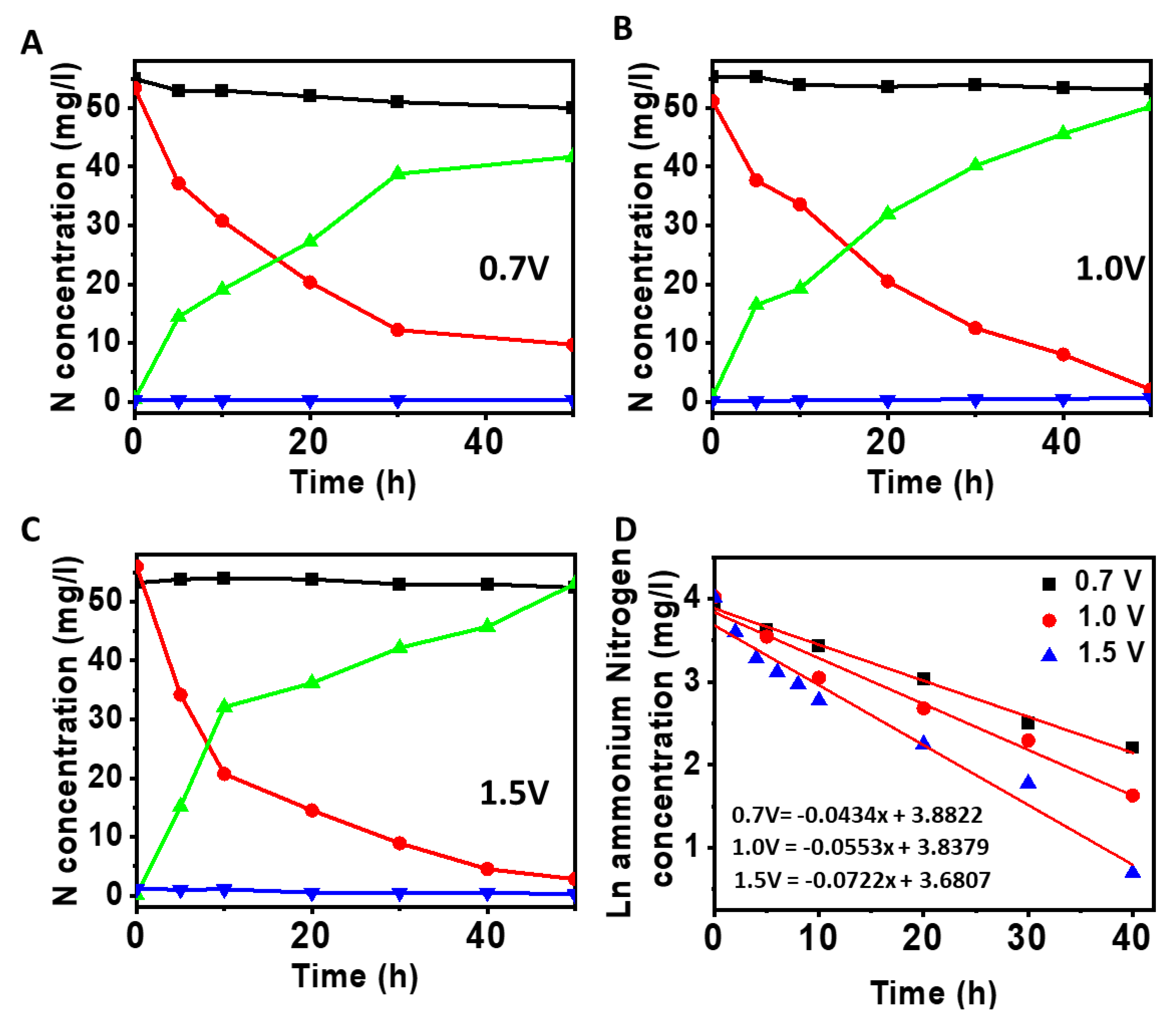
3.1. Oxygen Generation in the Anode Chamber at Different Applied Voltages
3.2. Nitrification at the Anode of MEC
3.3. Influence of Ammonium Nitrogen Loading on Nitrification
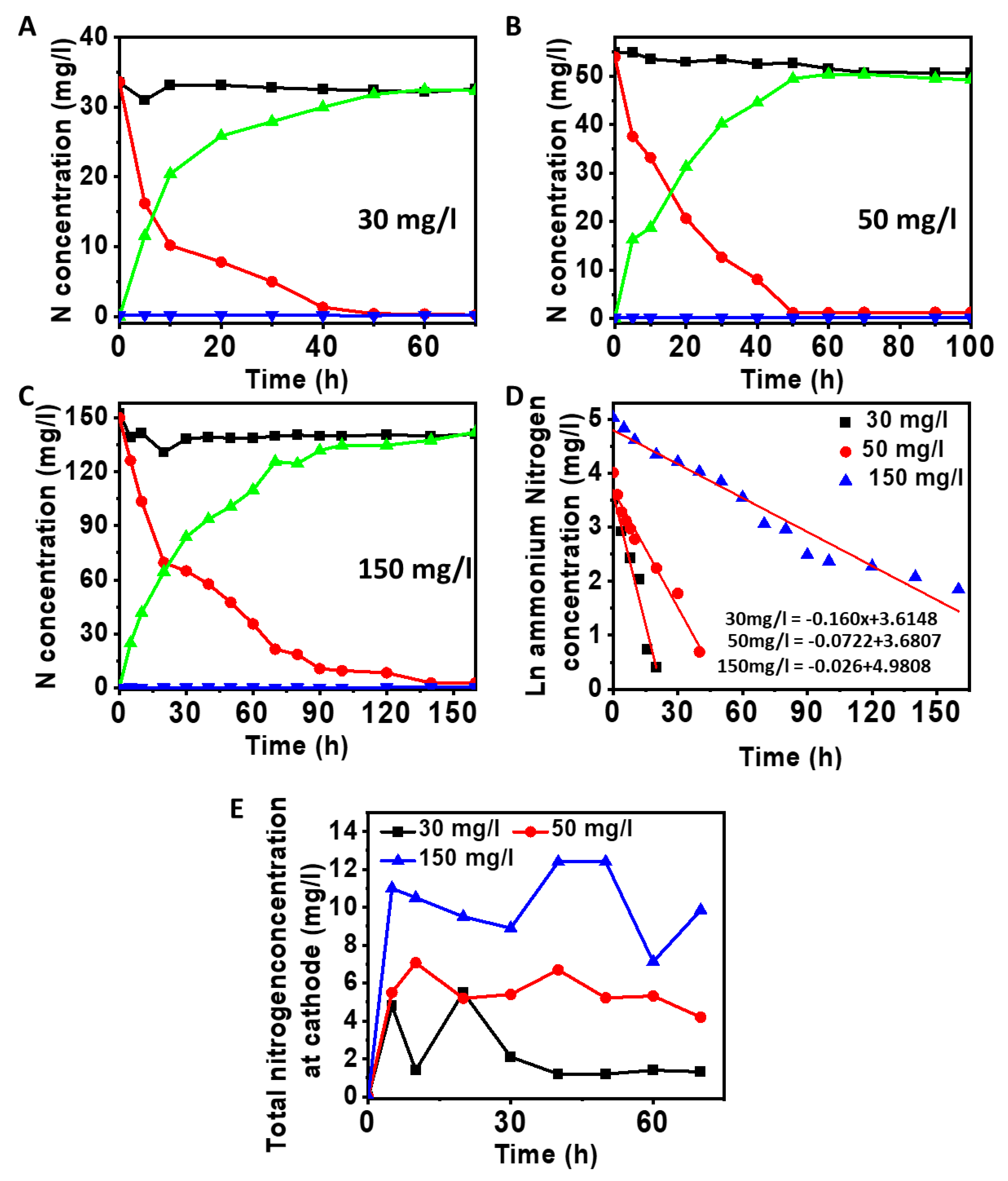
3.4. Denitrification at Cathode Using Nitrified Anode Solution
| BES Type | Volume, mL * | Main Substrate | Operating Conditions | Microbes | NH4-N rem. Rate (g-N/m3.d) | NH4-N rem. eff. (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | |||||||
| Dual chamber MFC | 336 | 336 | 0.393 g/L CH3COONa and 0.407 g/L NH4Cl | Continuous, 6.86 h HRT | Mixed bacteria enrichment | 104 | 94 | [46] |
| Dual chamber MFC | 4.1 L | 4.1 L | decomposed cyanobacteria solution of 800–1000 mg/L COD and 0.06–0.1 g/L NH4-N | Batch mode | Mixed bacteria enrichment | 64 | [47] | |
| Triple chamber with multianode design | 2.8 L | 2.8 L | 1 g/L CH3COONa and 0.06 g/L NH4−N | sequencing batch, 3-day HRT | Mixed bacteria | 12 | 78 | [48] |
| Triple chamber with multicathode design | 120 | 120 | 0.55 g/L CH3COONa and 0.05 g/L NH4-N | Batch mode, 6-day | Mixed bacteria | 6.4 | 96 | [49] |
| Single chamber air-cathode MFC | 150 | 1.2 g/L CH3COONa and 0.25 g/L NH4Cl | Continuous mode, 2.5 h HRT | Mixed bacteria | 620 | 94 | [50] | |
| Dual chamber MFC | 250 | 250 | 0.64 g/L CH3COONa and 0.23 g/L NH4Cl | Batch mode, 4-day | Mixed bacteria enrichment | 55.2 | ~100 | [51] |
| Dual Chamber MEC | 420 | 420 | 0.030–0.15 g/L NH4-N | Batch mode at 1.5 V applied potential, 50 h | Mixed bacteria enrichment | 15 | 85 | This study |
| Dual Chamber MEC | 30–150 mg/L NH4-N | Continuous mode at 1.5 V applied voltage, 15 h HRT | Mixed bacteria enrichment | 170 | 81 | This study | ||
3.5. MEC Operation in Continuous Mode for Ammonia Removal

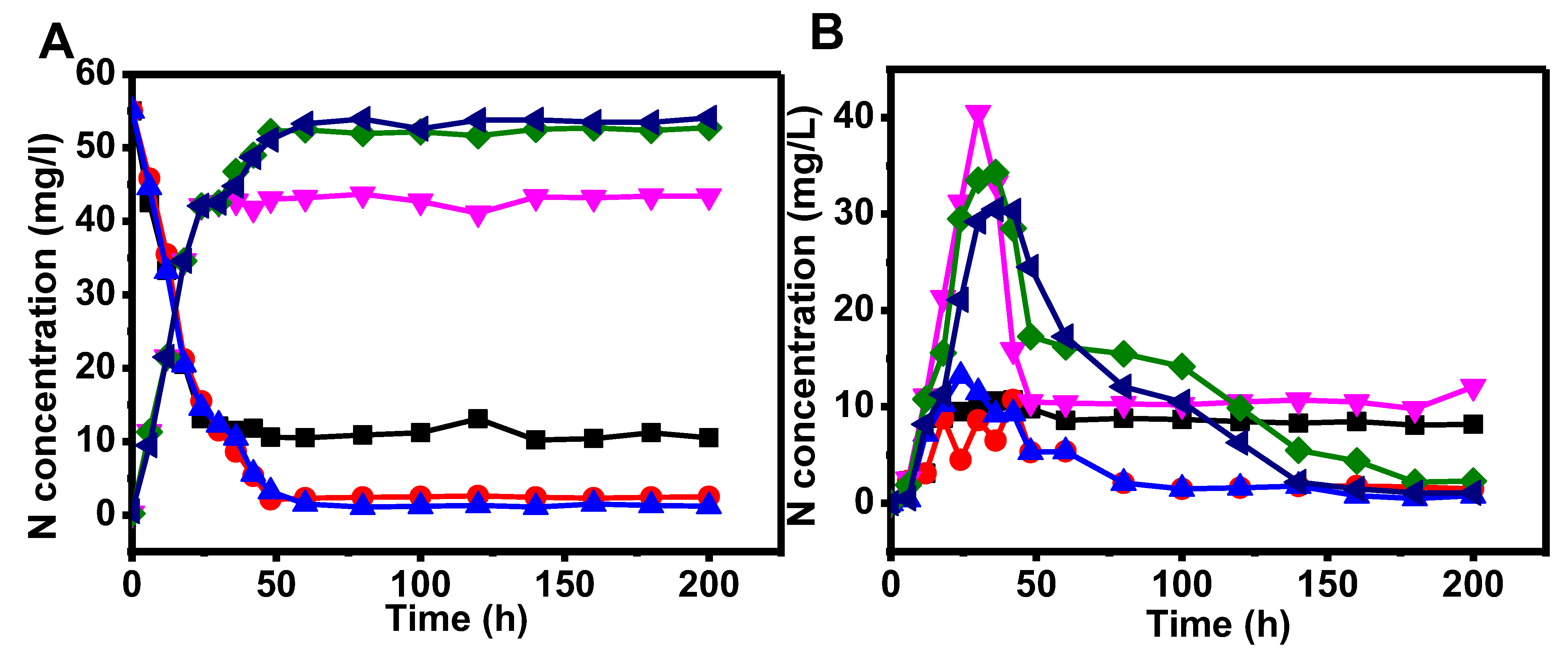
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, J.; Podola, B.; Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar] [CrossRef]
- Noori, T.; Ghangrekar, M.M.; Mitra, A.; Mukherjee, C.K. Conference Proceedings of the Second International Conference on Recent Advances in Bioenergy Research; Springer: Singapore, 2018; pp. 285–294. [Google Scholar] [CrossRef]
- Noori, M.T.; Chatterjee, P.; Ghangrekar, M.M.; Mukherjee, C.K. Low-Cost Solutions for Fabrication of Microbial Fuel Cells: Ceramic Separator and Electrode Modifications; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 978-0-444-64017-8. [Google Scholar]
- Clauwaert, P.; Desloover, J.; Shea, C.; Nerenberg, R.; Boon, N.; Verstraete, W. Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol. Lett. 2009, 31, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhang, L.; Li, D.; Su, W.; Tao, Y.; Qian, J. Autotrophic nitrogen removal from ammonium at low applied voltage in a single-compartment microbial electrolysis cell. Bioresour. Technol. 2012, 116, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Saito, T.; Regan, J.M. Nitrogen removal in a single-chamber microbial fuel cell with nitrifying biofilm enriched at the air cathode. Water Res. 2012, 46, 2215–2224. [Google Scholar] [CrossRef]
- Zhang, F.; He, Z. Integrated organic and nitrogen removal with electricity generation in a tubular dual-cathode microbial fuel cell. Process Biochem. 2012, 47, 2146–2151. [Google Scholar] [CrossRef]
- He, Z. Microbial fuel cells: Now let us talk about energy. Environ. Sci. Technol. 2013, 47, 332–333. [Google Scholar] [CrossRef]
- Han, X.; Qu, Y.; Dong, Y.; Chen, D.; Liang, D.; Liu, J.; Zhang, J.; Ren, N.; Feng, Y. Simultaneous electricity generation and eutrophic water treatment utilizing iron coagulation cell with nitrification and denitrification biocathodes. Sci. Total Environ. 2021, 782, 146436. [Google Scholar] [CrossRef]
- Noori, M.T.; Ganta, A.; Tiwari, B.R. Recent Advances in the Design and Architecture of Bioelectrochemical Systems to Treat Wastewater and to Produce Choice-Based Byproducts. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020023. [Google Scholar] [CrossRef]
- Noori, M.T.; Ezugwu, C.I.; Wang, Y.; Min, B. Robust bimetallic metal-organic framework cathode catalyst to boost oxygen reduction reaction in microbial fuel cell. J. Power Sources 2022, 547, 231947. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Yan, Q.; Zhang, G.; Chen, C. Simultaneous nitrogen removal and methane production from Taihu blue algae against ammonia inhibition using integrated bioelectrochemical system (BES). Sci. Total Environ. 2021, 777, 146144. [Google Scholar] [CrossRef]
- Lee, S.H.; Kondaveeti, S.; Min, B.; Park, H.D. Enrichment of Clostridia during the operation of an external-powered bio-electrochemical denitrification system. Process Biochem. 2013, 48, 306–311. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Kang, E.; Liu, H.; Min, B. Continuous autotrophic denitrification process for treating ammonium-rich leachate wastewater in bioelectrochemical denitrification system (BEDS). Bioelectrochemistry 2019, 130, 107340. [Google Scholar] [CrossRef]
- Huang, B.; Feng, H.; Wang, M.; Li, N.; Cong, Y.; Shen, D. How to ascertain the importance of autotrophic denitrification process in a bioelectrochemical system. Bioresour. Technol. 2013, 146, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Park, Y.; Jo, K.; Lee, T. Autotrophic denitrification performance and bacterial community at biocathodes of bioelectrochemical systems with either abiotic or biotic anodes. J. Biosci. Bioeng. 2015, 119, 180–187. [Google Scholar]
- Gavilanes, J.; Noori, M.T.; Min, B. Enhancing bio-alcohol production from volatile fatty acids by suppressing methanogenic activity in single chamber microbial electrosynthesis cells (SCMECs). Bioresour. Technol. Rep. 2019, 7, 100292. [Google Scholar] [CrossRef]
- Tabish Noori, M.; Min, B. Highly Porous FexMnOy Microsphere as an Efficient Cathode Catalyst for Microbial Electrosynthesis of Volatile Fatty Acids from CO2. ChemElectroChem 2019, 6, 5973–5983. [Google Scholar] [CrossRef]
- Noori, M.T.; Thatikayala, D.; Min, B. Bioelectrochemical Remediation for the Removal of Petroleum Hydrocarbon Contaminants in Soil. Energies 2022, 15, 8457. [Google Scholar] [CrossRef]
- Lim, S.S.; Fontmorin, J.M.; Izadi, P.; Wan Daud, W.R.; Scott, K.; Yu, E.H. Impact of applied cell voltage on the performance of a microbial electrolysis cell fully catalysed by microorganisms. Int. J. Hydrogen Energy 2020, 45, 2557–2568. [Google Scholar] [CrossRef]
- Yan, C.; Wang, M.; Ma, T.; Yang, S.; Kong, M.; Shen, J.; Yang, L.; Gao, Y. Study on the experimental performance by electrolysis-integrated ecological floating bed for nitrogen and phosphorus removal in eutrophic water. Sci. Rep. 2020, 10, 7619. [Google Scholar] [CrossRef]
- Thu, H.N.T.; Noori, M.T.; Min, B. Accelerating anaerobic digestion process with novel single chamber microbial electrochemical systems with baffle. Bioresour. Technol. 2022, 359, 127474. [Google Scholar]
- Noori, M.T.; Min, B. Fundamentals and recent progress in bioelectrochemical system assisted biohythane production. Bioresour. Technol. 2022, 361, 127641. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.T.; Noori, M.T.; Min, B. Magnetite/zeolite nanocomposite-modified cathode for enhancing methane generation in microbial electrochemical systems. Chem. Eng. J. 2020, 393, 124613. [Google Scholar] [CrossRef]
- Noori, M.T.; Mohan, S.V.; Min, B. Microbial electrosynthesis of multi-carbon volatile fatty acids under the influence of different imposed potentials. Sustain. Energy Technol. Assess. 2021, 45, 101118. [Google Scholar] [CrossRef]
- Ali, R.B.; Noori, M.T.; Lee, S.-H.; Park, H.-D.; Min, B. Enhancing biogas and electricity recovery using an iron-manganese oxide catalyzed bioanode in an integrated submersible microbial fuel cell-anaerobic digester. Sustain. Energy Technol. Assess. 2022, 52, 102276. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Lee, S.H.; Park, H.D.; Min, B. Bacterial communities in a bioelectrochemical denitrification system: The effects of supplemental electron acceptors. Water Res. 2014, 51, 25–36. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875530130. [Google Scholar]
- Kondaveeti, S.; Min, B. Nitrate reduction with biotic and abiotic cathodes at various cell voltages in bioelectrochemical denitrification system. Bioprocess Biosyst. Eng. 2013, 36, 231–238. [Google Scholar] [CrossRef]
- Hossini, H.; Rezaee, A.; Ayati, B.; Mahvi, A.H. Simultaneous nitrification and denitrification using a polypyrrole/microbial cellulose electrode in a membraneless bio-electrochemical system. RSC Adv. 2015, 5, 72699–72708. [Google Scholar] [CrossRef]
- Stenstrom, M.K.; Poduska, R.A. The effect of dissolved oxygen concentration on nitrification. Water Res. 1980, 14, 643–649. [Google Scholar] [CrossRef]
- Meyer, R.L.; Zeng, R.J.; Giugliano, V.; Blackall, L.L. Challenges for simultaneous nitrification, denitrification, and phosphorus removal in microbial aggregates: Mass transfer limitation and nitrous oxide production. FEMS Microbiol. Ecol. 2005, 52, 329–338. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Liu, X.; Chang, J. The impact of temperature and dissolved oxygen (DO) on the partial nitrification of immobilized fillers, and application in municipal wastewater. RSC Adv. 2020, 10, 37194–37201. [Google Scholar] [CrossRef]
- Watanabe, T.; Hashimoto, S.; Kuroda, M. Simultaneous nitrification and denitrification in a single reactor using bio-electrochemical process. Water Sci. Technol. 2002, 46, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhang, L.; Tao, Y.; Wang, Y.; Zhu, X.; Li, D. Anodic ammonia oxidation to nitrogen gas catalyzed by mixed biofilms in bioelectrochemical systems. Electrochim. Acta 2014, 135, 345–350. [Google Scholar] [CrossRef]
- Alabiad, I.; Ali, U.F.M.; Zakarya, I.A.; Ibrahim, N.; Radzi, R.W.; Zulkurnai, N.Z.; Azmi, N.H. Ammonia removal via microbial fuel cell (MFC) dynamic reactor. In Proceedings of the IOP Conference Series: Materials Science And engineering; IOP Publishing: Bristol, UK, 2017; Volume 206, p. 12079. [Google Scholar]
- Oh, J.; Silverstein, J. Oxygen inhibition of activated sludge denitrification. Water Res. 1999, 33, 1925–1937. [Google Scholar] [CrossRef]
- Sanjib, M.; Mohd, M.C.K.; Mukherjee, C.K. Evaluation of nitrification performance of a trickling filter with nylon pot scrubber as media. IJSN 2011, 2, 515. [Google Scholar]
- Gharasoo, M.; Centler, F.; Van Cappellen, P.; Wick, L.Y.; Thullner, M. Kinetics of substrate biodegradation under the cumulative effects of bioavailability and self-inhibition. Environ. Sci. Technol. 2015, 49, 5529–5537. [Google Scholar] [CrossRef]
- Princic, A.; Mahne, I.; Megušar, F.; Paul, E.A.; Tiedje, J.M. Effects of pH and oxygen and ammonium concentrations on the community structure of nitrifying bacteria from wastewater. Appl. Environ. Microbiol. 1998, 64, 3584–3590. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mazumder, D. Kinetic study on nitrification of ammonium nitrogen-enriched synthetic wastewater using activated sludge. Water Sci. Technol. 2020, 81, 62–70. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Nitrate removal from drinking water. J. Environ. Eng. 1997, 123, 371–380. [Google Scholar] [CrossRef]
- Park, H.I.; Kun Kim, D.; Choi, Y.-J.; Pak, D. Nitrate reduction using an electrode as direct electron donor in a biofilm-electrode reactor. Process Biochem. 2005, 40, 3383–3388. [Google Scholar] [CrossRef]
- Xia, S.; Wu, C.; Yang, X.; Zhou, Y.; Zhou, L.; Ran, Y.; Rittmann, B.E. Bioreduction of nitrate in high-sulfate water using a hydrogen-based membrane biofilm reactor equipped with a separate carbon dioxide module. Chem. Eng. J. 2020, 385, 123831. [Google Scholar] [CrossRef]
- Grommen, R.; Verhaege, M.; Verstraete, W. Removal of nitrate in aquaria by means of electrochemically generated hydrogen gas as electron donor for biological denitrification. Aquac. Eng. 2006, 34, 33–39. [Google Scholar] [CrossRef]
- Virdis, B.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 2010, 44, 2970–2980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, G.; Yu, R.; Li, H.; Wang, C. Enhanced simultaneous nitrification/denitrification in the biocathode of a microbial fuel cell fed with cyanobacteria solution. Process Biochem. 2016, 51, 80–88. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, S.; Lu, Y.; Gu, X. Simultaneous nitrification and denitrification in the bio-cathode of a multi-anode microbial fuel cell. Environ. Technol. 2021, 42, 1260–1270. [Google Scholar] [CrossRef]
- Zhang, F.; He, Z. Simultaneous nitrification and denitrification with electricity generation in dual-cathode microbial fuel cells. J. Chem. Technol. Biotechnol. 2012, 87, 153–159. [Google Scholar] [CrossRef]
- Park, Y.; Park, S.; Nguyen, V.K.; Yu, J.; Torres, C.I.; Rittmann, B.E.; Lee, T. Complete nitrogen removal by simultaneous nitrification and denitrification in flat-panel air-cathode microbial fuel cells treating domestic wastewater. Chem. Eng. J. 2017, 316, 673–679. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Ghangrekar, M.M. Application of bimetallic low-cost CuZn as oxygen reduction cathode catalyst in lab-scale and field-scale microbial fuel cell. Chem. Phys. Lett. 2020, 751, 137536. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Das, S.; Ghangrekar, M.M. Proficient Sanitary Wastewater Treatment in Laboratory and Field-Scale Microbial Fuel Cell with Anti-Biofouling Cu0.5Mn0.5Fe2O4 as Cathode Catalyst. J. Electrochem. Soc. 2021, 168, 054519. [Google Scholar] [CrossRef]
| Anode (water electrolysis) | 10H2O + 10e− → 5H2 + 10 OH− |
| 5H2O → 2.5O2 + 10H+ + 10e− | |
| Anode (Nitrification) | NH4+ + 3/2O2 → NO2− + 2H+ + H2O |
| NO2− + 1/2O2 →NO3− | |
| Cathode (Denitrification) | 2NO3− + 2H2 → 2NO2− + 2H2O |
| 2NO2− + 2H2 → N2O− + H2O + 2OH− | |
| N2O + H2 → N2 + H2O NO3− + 6H+ + 5e− → 1/2N2 +3H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondaveeti, S.; Choi, D.-H.; Noori, M.T.; Min, B. Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell. Energies 2022, 15, 9171. https://doi.org/10.3390/en15239171
Kondaveeti S, Choi D-H, Noori MT, Min B. Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell. Energies. 2022; 15(23):9171. https://doi.org/10.3390/en15239171
Chicago/Turabian StyleKondaveeti, Sanath, Dae-Hyeon Choi, Md Tabish Noori, and Booki Min. 2022. "Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell" Energies 15, no. 23: 9171. https://doi.org/10.3390/en15239171
APA StyleKondaveeti, S., Choi, D.-H., Noori, M. T., & Min, B. (2022). Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell. Energies, 15(23), 9171. https://doi.org/10.3390/en15239171






