Abstract
The development of efficient non-precious metal electrocatalysts through more economical and safe methods is consistent with the goals of sustainable development and accelerating the achievement of “carbon neutrality” in the 21st century but remains potentially challenging. Mott–Schottky heterojunction interfaces generated from metal/semiconductor have been a hot topic of recent research because of the unique built-in electric field effect which allows the preparation of more superior catalysts for water electrolysis. Herein, a glutinous rice potpourri-like Mott–Schottky two-dimensional (2D) nanosheet (abbreviated as Ni/CeO2 HJ-NSs) electrocatalyst composed of metal nickel (Ni) and cerium oxide (CeO2) hetero-nanoparticles was synthesized by a simple and scalable self-assembly and thermal reduction strategy. The experimental results and mechanistic analysis show that the Mott–Schottky heterojunction interface composed of metallic Ni and n-type semiconductor CeO2 with built-in electric field induces the electron redistribution at the interface to accelerate the dissociation of water and the binding of reaction intermediates, thus achieving lower water dissociation energy and more thermoneutral ΔGH* value to expedite the kinetics of the hydrogen evolution reaction (HER). Thus, the prepared Ni/CeO2 HJ-NSs exhibit excellent HER catalytic performance in 1 M KOH electrolyte with an overpotential of only 72 mV at 10 mA cm−2, as well as a moderate Tafel slope of 65 mV dec−1 and an extraordinary long-term stability over 50 h, laying a solid foundation for further in-depth investigation. The synthesis of splendid electrocatalysts by exploiting the metal/semiconductor interface effect provides an innovative way for the future generation of Mott–Schottky-based heterostructures with three or more heterocompositions with two or more heterojunction interfaces.
1. Introduction
Currently, the energy crisis and environmental pollution problems are becoming increasingly prominent due to the over-exploitation and consumption of fossil fuels worldwide [1]. The production and utilization of clean energy such as hydrogen is crucial. Hydrogen possesses the advantages of high calorific value and non-polluting combustion products, making it an ideal energy source for a sustainable society [2,3]. The use of excess energy generated from renewable sources (solar, wind, etc.) for electrocatalytic decomposition of water is an attractive way to produce hydrogen to meet energy demand and ameliorate environmental problems [4]. However, owing to the inherent energy barrier of the cathodic hydrogen evolution reaction (HER) in the water splitting, the cell voltage of actual commercial electrolyzers is usually greater than the theoretical value of 0 V, resulting in more drain of energy and resources [5]. Among all solutions, an effective approach is: designing high-performance electrocatalysts to reduce the overpotential to elevate the energy conversion efficiency. So far, platinum (Pt) and Pt-based materials are considered as perfect HER electrocatalysts on account of their negligible overpotential and outstanding kinetics [6]. Nevertheless, their expensive price, scarcity, and unsatisfactory durability limit the commercial production of clean energy on a large scale [7]. Non-precious metal catalysts, which are abundant, cheap, and efficient on earth, have been a hot topic of research in the field of electrocatalytic HER and have made great progress in the recent years [8]. In particular, Nickel alloy [9], nickel sulfide [10], nickel phosphide [11], and other transition metal Ni-based catalysts [12,13] for HER.
Metal nickel (Ni) which has the advantages of low price, high corrosion resistance, unexceptionable electrical conductivity, and allegro intermediate H* adsorption, is widely used in industry for water reduction of alkaline solutions [14]. However, then, the high overpotential and large Tafel slope as well as the lack of stability during the long-term testing will lead to a large gap in the catalytic performance compared to the Pt-based noble metal specimens [15,16]. In order to settle the existing problems, some corresponding solution measures have been taken. Up to now, a plethora of measures, including polymetallic alloying [17], single-atom loading substrates [18], single-atom doping [19], and heterojunction (HJ) interface engineering [20], have optimized the electrochemical properties of nickel from various aspects. Among these strategies, heterojunction interface engineering based on electron modulation has been one of the research focuses as a result of the composition of components with many different functions [21]. The structural advantages of heterostructures are reflected in the following three facets: (i) Synergistic effect: In heterostructures, bonding between the interfaces of different components can enhance the electron transfer rate. (ⅱ) Strain effect: Different chemical components and crystal structures in heterostructures can cause lattice strains, such as stretching and compression, which affect the adsorption energy of sites to intermediates and improve the catalytic activity of materials. (ⅲ) Electron interactions: Different energy band arrangements of different phases can lead to charge transfer at the interface, which facilitates surface electron modulation in heterostructures [22]. In particular, the Mott−Schottky heterojunction formed by coupling metals and semiconductors with different work function can further promote the electron flow across the interface, which increases the charge localizing density of states around phases with lager work functions as well as the rapid conversion of reaction intermediates, ultimately accelerating hydrogen production on a macroscopic scale. It is well known that CeO2 with semiconductor properties, including reversible conversion between Ce3+/Ce4+ oxidation states, admirable electron/ion conductivity, and luxuriant oxygen vacancies, is extensively introduced as a co-catalyst in alkaline HER electrocatalysts [23]. What is more worth considering is that the heterostructure prepared by CeO2 and other materials can easily generate strong electronic interactions at the interface sequentially promoting the water dissociation on the catalyst surface of the main electrocatalyst whilst prolonging the stability [24]. According to Sabatier principle, the |ΔGH*| value of the catalyst is closer to 0, indicating a rapid proceeding of HER [25]. Previous reports have shown −0.278 eV and −0.87 eV ΔGH* values for Ni and CeO2 [26,27], respectively. Therefore, the combination of metallic Ni and semiconducting CeO2 with different functional advantages and ΔGH* values will give full play to their synergistic effects through a similar “dilution effect” plus electron coupling effect, and it is expected that the interface created in the formed Ni/CeO2 heterojunction will facilitate the rapid adsorption and desorption of the active intermediate H* and ameliorate the HER performance of alkaline solutions.
Herein, a glutinous rice potpourri-like Mott–Schottky two-dimensional (2D) nanosheet (NSs) (Abbreviated as Ni/CeO2 HJ-NSs) electrocatalyst composed of Ni and CeO2 hetero-nanoparticles was constructed by a simple, economical, and safe self-assembly followed by urea thermal reduction strategy. The prepared nanosheets are characterized as ideal electrode materials because of short ion diffusion paths and nanoporous feature, which facilitate the contact between the active material and the electrolyte. Meanwhile, the self-assembly process further reduces the massive agglomeration phenomenon of metallic Ni nanoparticles, which can expose more reaction sites. Importantly, the direct access to metallic Ni by thermal reduction of a small amount of urea boosts the experimental safety, economy, and maneuverability, which is more conducive to the industrial production of catalysts in large quantities. The experimental and mechanistic analysis showed that the construction of Ni/CeO2 heterojunctions induces electron redistribution at the interface, accelerating the dissociation of water and the binding of reaction intermediates, consequently achieving lower water dissociation energies and more thermoneutral ΔGH* value to accelerate HER proceeding. Notably, the elaborated Mott–Schottky Ni/CeO2 HJ-NSs electrocatalyst has exceptional HER activity due to the synergistic effect of Ni and CeO2, requiring an overpotential of only 72 mV to achieve 10 mA cm−2 in 1.0 M KOH electrolyte with a grandiloquent 50 h long-term electrocatalytic durability. This synthetic strategy provides some reference for the more economical and safe synthesis of other 2D Mott–Schottky heterostructure nanosheets.
2. Results and Discussion
Ni/CeO2 HJ-NSs are conveniently synthesized by a simple and scalable self-assembly and thermal reduction strategy, as shown in Figure 1. Briefly, aromatic organic ligands with polycarboxylic acid groups are widely used as connectors of metal ions in one-dimensional and even multidimensional structures for the construction of desirable structures and practical functions, especially Pyromellitic acid hydrate (PAH) with geometric centrosymmetry. The morphology of PAH with four potential binding sites belongs to the classical polycarboxylic acid O-donor ligands [28]. To begin with, the formation of the self-assembly Ce-based coordination polymer NSs through the solvothermal reaction serves as a self-sacrificing template and precursor to lay the foundation for the next annealing treatment with nickel acetate in air. Meanwhile, the steric effects generated by the conjugation of benzene ring and carboxyl group and the weakly acidic reaction environment provide the feasibility for the formation of 2D sheet structures [29,30]. As observed in the scanning electron microscope (SEM) image (Figure S1a, Supporting Information), the Ce-based coordination polymer NSs consists of the copious amounts of uniform 2D NSs arranged in random stacks with a disordered morphology. Further analysis from the cross-section image shows that the average thickness of the ultrathin potato chip-like NSs is about 18.5 nm, and the length and width are only a few micrometers (Figure S1b, Supporting Information). Subsequently, the entropy increase and entropy decrease of the reaction system under controlled annealing operation, the Ce-based coordination polymer precursors with 2D NSs structure, and nickel acetate further self-assemble to heterojunction NSs composed of relatively uniformly dispersed nanoparticles. Finally, the Ni2+ ions can be readily converted to nanoscale metal Ni while the CeO2 composition remains unchanged by the selective reduction of a small amount of urea. For comparison, CeO2 was fabricated under the same experimental conditions, except that Ni precursors were not involved.
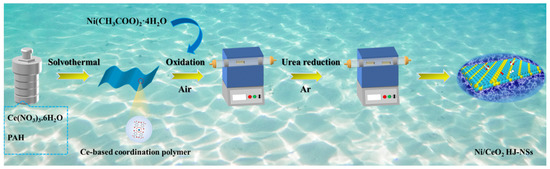
Figure 1.
Schematic representation of the synthesis process of Ni/CeO2 HJ-NSs.
After the annealing process, the X-ray powder diffraction (XRD) patterns can be observed that all the diffraction peaks are well indexed to CeO2 with fluorite-structured (Figure S2, Supporting Information), confirming that the Ce-based coordination polymer NSs precursors can be completely transformed into CeO2 crystals. With controlled annealing operations, the mixture of precursors and nickel acetate can be formed into NSs composed of more uniformly dispersed nanoparticles by further self-assembly. It is worth noting that the oodles of pores and oxygen vacancies (Ov) on the catalyst surface accompanied by the release of CO2 and H2O at high temperatures. (Detailed discussion on oxygen vacancies at XPS) The Ni/CeO2 HJ-NSs electrocatalyst is precisely prepared by selective reduction of a small amount of urea. As shown in Figure 2a. The diffraction peaks at 44.5°, 51.9°, and 76.4° can be well matched to the fcc-phased metal Ni (JCPDS no. 04-0850) (111), (200) and (200) crystal planes, respectively, and the rest of the diffraction peaks can be easily corresponded to the fluorite-structured of CeO2 (JCPDS no. 43-1002). SEM, a commonly used tool to characterize the morphology, can explain the samples formed after urea reduction in more detail. The Ni/CeO2 HJ-NSs catalyst is a glutinous rice potpourri-like 2D NSs morphology composed of nanoparticles with satisfied dispersion and ultrathin characteristics, as clearly seen in the low-magnification SEM (Figure 2b). Meanwhile, the NSs with similar average thickness as the precursors are characterized in the partially enlarged side view (inset of Figure 2b), demonstrating the stability of the Ce-based coordination polymers in high temperature calcination. Additionally, the magnified SEM image (Figure 2c) further shows that the 2D NSs are composed of many nanoparticles in close contact without massive agglomeration.

Figure 2.
Morphological characterization of the formed Ni/CeO2 HJ-NSs. (a) XRD pattern, (b,c) SEM images, with the inset in (b) showing SEM image of side view of Ni/CeO2 HJ-NSs. (d–f) HRTEM images, the red dotted box of the inset of (d) is an enlarged view of the interface in Ni/CeO2 HJ-NSs. (g,h) FFT images of orange dotted box (I zone) and light green dotted box (II zone) in (d).
We further employ transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) imaging to determine the microstructure and crystal structure of Ni/CeO2 HJ-NSs catalyst. TEM (Figure S2a,b, Supporting Information) unambiguously demonstrates that Ni/CeO2 hetero-nanoparticles with paired interconnections form 2D lamellar structures, the bulk seams formed by high temperature sintering are ubiquitous, which is consistent with the above SEM observation. Moreover, the particle size distribution (Figure S2c, Supporting Information) visualizes a large number of Ni/CeO2 nanoparticles with an average size of about 32.8 nm. Furthermore, as shown in the HR-TEM image in Figure 2d, the interplanar distance (Figure 2e,f) of well-defined lattice fringes of each pair of crystalline Ni/CeO2 hetero-nanoparticles (red dotted box at the bottom right corner) were precisely measured to be 0.203 and 0.312 nm can be easily matched to the (111) plane of the face-centered cubic (fcc) structure of Ni and the (111) plane of CeO2, respectively. The presence of a heterojunction interface between the Ni and CeO2 phases can be clearly observed, as shown by the cyan dashed line in the Figure 2d. It is worth mentioning that the Mott–Schottky heterojunction interface with built-in electric field composed of metallic Ni and n-type semiconductor CeO2 can accelerate the electron transfer and lower the energy barrier during the HER reaction. The corresponding fast Fourier transform (FFT) images (Figure 2g,h) in I and II regions also further confirm the presence of the (111) facets of CeO2 and Ni with heterojunctions. To further investigate the catalyst structure, we determined the nitrogen adsorption/desorption isotherm curves of Ni/CeO2 HJ-NSs and evaluated the corresponding pore size distribution, as shown in Figure S4 (Supporting Information). A typical V-shaped adsorption isotherm model can be seen in the figures. Meanwhile, the centered pore size distribution of 3.4 nm calculated by the Barret–Joyner–Halenda (BJH) model indicates that the Ni/CeO2 HJ-NSs are mainly composed of mesoporous, which are formed by the gas generation of the Ce-based coordination polymer and nickel acetate during the high temperature pyrolysis. The nanoporous feature facilitates the electrolyte penetration and migration into the catalyst interior, allowing more active sites to participate in the reaction and accelerating the rapid separation of products, thus improving the overall catalytic efficiency.
The inductively coupled plasma optical emission spectrometer (ICP-OES) accurately detects all metallic elements and some non-metallic elements contained in the catalysts and the corresponding ratio relationships, which allows further understanding of the catalysts qualitatively and quantitatively. As shown in Table S1, the atomic ratio of Ni, Ce of Ni/CeO2 HJ-NSs is almost 11:2, indicating that the molar ratio of Ni and CeO2 is about 11:2. The surface elemental composition and valence states of Ni/CeO2 HJ-NSs were further investigated by X-ray photoelectron spectroscopy (XPS). The full scan spectrum in Figure 3a again demonstrates the coexistence of Ni, Ce, and O in Ni/CeO2 HJ-NSs, which is consistent with the results of XRD and ICP-OES analysis. Figure 3b clearly presents the high-resolution photoelectron spectra of Ni 2p for Ni/CeO2 HJ-NSs. The fitted peaks with binding energies of 853.10 and 871.46 eV are attributed to the Ni0 of Ni/CeO2 HJ-NSs. The two peaks 2p3/2 (856.01 eV) and 2p1/2 (874.21 eV) of Ni2+ were obtained by deconvolution, which may be caused by the inevitable surface oxidation upon exposure to air. However, the oxidized state of Ni does not show up in the XRD pattern, which may be the reason for the small amount or the amorphous state. The remaining peaks at 860.53 binding energy is corresponding to satellite peak. Compared with pure Ni [31], the peaks of Ni/CeO2 HJ-NSs are significantly shifted toward the high binding energy (Figure S5a, Supporting Information), which again confirms that the built-in electric field formed between the Ni and CeO2 heterojunction interface promotes the electron transfer from Ni to CeO2.
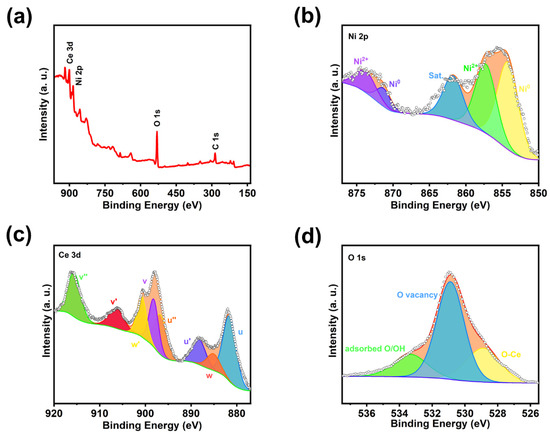
Figure 3.
Compositional analyses of the resultant Ni/CeO2 HJ-NSs. (a) XPS surveys, (b–d) high-resolution XPS spectra of (b) Ni 2p, (c) Ce 3d, and (d) O 1s.
The valence changes of Ce for Ni/CeO2 HJ-NSs are further analyzed by Ce 3d high-resolution spectrum, as displayed in Figure 3c. We label the Ce3+ peak as w and the 3d5/2 and 3d3/2 peaks of Ce4+ as u and v, respectively. The peaks named as u (881.58 eV), u’ (888.28 eV) and u’’ (896.88 eV) correspond to the 3d5/2 peaks of Ce4+. The peaks of v, v’ and v’’ at 898.18, 906.48 and 915.68 eV, respectively, assigned to the 3d3/2 peak positions of Ce4+ [32,33]. In addition, the remaining w and w’ peaks are attributed to Ce3+. These results reveal the coexistence of trivalent and tetravalent Ce in Ni/CeO2 HJ-NSs. In the environment of high temperature and chemical reduction, the lattice oxygen in metal oxides will be detached from the original junction position, leading to the absence of oxygen in the structure, thus forming oxygen vacancies (Ov). It is shown that the electrons formed during the oxidation of CeO2 are usually transferred to Ce atoms, which will trigger the conversion of Ce4+ to Ce3+. In other words, the formation of Ov in CeO2 is accompanied by a simultaneous increase in Ce3+ content [34]. Based on the peak area in XPS (Figure 3c and Figure S6 (Supporting Information)), the concentration of Ce3+ species in the overall Ce can be calculated by the following Equation (1):
where A(Ce3+) and A(Ce4+) represent the peak integration areas of Ce3+ and Ce4+ in XPS, respectively [35]. The Ni/CeO2-HJ NSs have a larger Ce3+ value of 23% with respect to pure CeO2 (16%), indicating the presence of partial electron transfer from Ni to CeO2 at the Ni/CeO2 HJ-NSs heterojunction surface. The conversion of Ce4+ to Ce3+ generates more oxygen vacancies, thus favoring a faster HER process. In addition, the obvious shift of the peaks of Ni/CeO2 HJ-NSs toward the lower binding energy (Figure S5b, Supporting Information) further verifies that CeO2 acts as an electron acceptor for the metallic Ni phase through the Ni/CeO2 interface [36]. Figure 3d shows the high-resolution XPS spectra of O1s of Ni/CeO2 HJ-NSs, where the Ni/CeO2 HJ-NSs pattern can be deconvoluted into a composition of three peaks, corresponding to the lattice oxygen in the Ce-O bond (528.88 eV), the oxygen vacancy (530.89 eV), and the surface adsorbed the hydroxyl group in water molecules or the adsorbed oxygen (533.33 eV) [37]. The oxygen vacancies have the largest peak area, indicating that the process of preparing Ni/CeO2 HJ-NSs heterostructures by the urea reduction strategy generates a large number of oxygen vacancies. It has been widely proposed that the production of oxygen vacancies can significantly tune the surface electronic structure of active sites and optimize the adsorption energies of reaction intermediates, thereby increasing the charge transfer rate and enhancing the intrinsic activity [38,39,40]. In addition, the inherent defect states induced in the energy band caused by oxygen vacancies and the overall negative shift of the energy band can make the conduction band downshift and cause a band gap reduction of CeO2, which endows it with the characteristics of a simple semiconductor and is more conducive to electron mobility, thus improving the electrical conductivity of the material. In addition, the defect states induced in the intrinsic energy band and the overall negative shift of the energy band caused by oxygen vacancies can trigger the downward shift of the conduction band and the reduction of the band gap of CeO2, endowing it with the characteristics of a degenerate semiconductor, which is more favorable for electron movement and thus improving the electrical conductivity of the material [41,42].
To evaluate the electrocatalytic performance of the prepared Ni/CeO2 HJ-NSs, the HER catalytic performance in 1.0 M KOH electrolyte at room temperature using a typical three-electrode system is systematically examined. The electrodes of Ni/CeO2 HJ-NSs catalysts loaded on carbon cloth (CC), graphite rod, and saturated calomel electrode (SCE) are used as working electrodes, counter electrode, and reference electrode, respectively. During the tests, potentials are referenced to the reversible hydrogen electrode (RHE) scale. In addition, unless otherwise stated, iR correction was applied to all initial data. For comparison purposes, CeO2, NiO/CeO2 and commercial Pt/C (20 wt%) are also fully examined under the same test conditions.
The LSV curves for all catalysts are clearly shown in Figure 4a, from which it is undemanding to observe that the commercial Pt/C catalyst has miracle performance with an overpotential of only 59 mV at 10 mA cm−2. Benefiting from the formed heterojunction interface, the Ni/CeO2 HJ-NSs catalyst with a 2D glutinous rice potpourri-like has lower overpotential and higher current density compared with other catalysts. In order to investigate more deeply the reaction kinetics of the Ni/CeO2 HJ-NSs and the main reaction process of HER, the linear part of the Tafel slope was obtained by the conversion of the LSV curve in HER. The mathematical expression of the conversion is: η = a + blgj, where j is the current density and b is the Tafel slope. The Tafel slope can be used to analyze the mechanism of HER, the rate-limiting step in HER can be judged from the value of the obtained Tafel slope, thus revealing the principle of reaction kinetics. In alkaline solutions, the reaction process of HER may undergo two reaction mechanisms, which are as follows [43]:
H2O + e− → = Hads+ OH− Volmer step (adsoption) slope ≈ 120 mVdec−1
Hads + H2O + e−→ = H2 + OH− Heyrovsky step (electrochemical desorption) slope ≈ 40 mVdec−1
Or
Hads + Hads → = H2 Tafel step (chemical desorption) slope ≈ 30 mVdec−1
The outstanding performance of Ni/CeO2 HJ-HSs catalysts can be more intuitively observed by plotting the Tafel slopes and the three-dimensional color difference map of overpotential at a current density of 10 mA cm−2 of all catalysts (Figure 4b). Undoubtedly, the commercial Pt/C catalysts have the lowest overpotentials and the smallest Tafel slopes. The Ni/CeO2 HJ-HSs catalysts have a smaller Tafel value of 65 mV dec−1 and an overpotential of only 72 mV at 10 mA cm−2 compared to NiO/CeO2 NSs (125 mV dec−1, 150 mV), indicating faster reaction kinetics. In addition, the value of Tafel slope also indicates that the HER of Ni/CeO2 HJ-HSs follows the Volmer-Heyrovsky mechanism and that the rate-limiting step of this reaction is the Heyrovsky reaction (i.e., the electrochemical desorption step). It was previously reported that metallic nickel has not been ideal as HER catalyst in alkaline solutions in industry due to its high overpotential (~200 mV) and large Tafel slope [16,44]. Therefore, the well-prepared Ni/CeO2 HJ-NSs catalysts effortlessly achieve optimal performance compared to single-component metallic nickel thanks to the synergistic effect with CeO2. More notably, the impressive performance of Ni/CeO2 HJ-NSs catalysts exceeds that of many previously reported non-precious metal-based HER catalysts, as listed in Table S2 (Supporting Information). Moreover, the value of current density, defined as the exchange current density (j0), reflects the intrinsic activity of the electrocatalyst in HER, by extrapolating the linear part of the Tafel slope to an overpotential of 0 V (Figure 4c). Undoubtedly as expected, Ni/CeO2 HJ-NSs catalysts exhibit the highest j0 value, further confirming their state-of-the-art intrinsic activity.
To further investigate the charge transfer kinetics of HER, electrochemical impedance spectra (EIS) were measured in the frequency range of 0.01 to 105 Hz. For the representation of the EIS, the Nyquist plot can more intuitively reflect the relationship between the imaginary and real parts of the impedance. Moreover, the charge transfer resistance (Rct) corresponding to the semicircle in the high-frequency region, obtained by fitting the equivalent circuit model, can further evaluate the charge transfer rate of all catalysts in the HER process. Not surprisingly, the “3D Rainbow Bridge” diagram in Figure 4d clearly shows that Ni/CeO2 HJ-NSs have a smaller Rct (6.2 Ω) compared to NiO/CeO2 NSs (9.6 Ω) and CeO2 NSs (13.3 Ω), reflecting the higher conductivity and the faster charge transfer kinetics between the electrode and the electrolyte. Electrochemical active surface area (ECSA) is also an important descriptor for the assessment of HER activity and. As a rule, the former and the latter have a positive correlation. It is well known that the double-layer capacitance (Cdl) is composed of a simple charge distribution of chemically inactive particles near the electrodes in the electrolyte. In previous studies, the ECSA is proportional to the Cdl. In view of this, the ECSA of different catalysts was estimated by measuring the cyclic voltammetry (CV) of different scan rates (20, 40, 60, 80, 100, 120 mV s−1) in the non-Faraday region to obtain the Cdl values (Figures S7 and S8a, Supporting Information). The Ni/CeO2 HJ-NSs have large Cdl (6.83 mF cm−2), which is significantly higher than that of NiO/CeO2 NSs (3.40 mF cm−2) and CeO2 NSs (1.87 mF cm−2). The ECSA (Figure S8b, Supporting Information) for Ni/CeO2 HJ-NSs, NiO/CeO2 and CeO2 are calculated as 85.38, 42.50 and 23.38 cm−2, respectively. A larger ECSA can enable more active sites to participate in HER, thus increasing the electrocatalytic reaction rate. Simultaneously, the current density in the LSV curves of HER was normalized by ECSA to exclude geometric effects allowing further analysis of the intrinsic activity of the different catalysts (Figure S8c, Supporting Information). To drive a jECSA of 0.4 mA cm−2, Ni/CeO2 HJ-NSs, NiO/CeO2 NSs, and CeO2 NSs require overpotentials of 203.4, 244.4, and 513 mV, respectively, indicating the Ni/CeO2 HJ-NSs catalysts have higher intrinsic HER catalytic activity, and in agreement with the LSV data. Likewise, the intrinsic activity of catalysts can also be investigated by measuring the turnover frequency (TOF) of different catalysts (Figure S9, Supporting Information). The trend between the overpotential and TOF can be clearly seen in Figure S9a (Supporting Information). At the same overpotential, Ni/CeO2 HJ-HSs exhibit superior HER performance with higher TOF than NiO/CeO2 NSs and CeO2 NSs. The TOF values of different catalysts at an overpotential of 0.1 V (Figure S9b, Supporting Information) can be observed more clearly. Meanwhile, the heterostructures composed of hetero-nanoparticles formed by introducing metal Ni in CeO2 greatly enhances the intrinsic activity of the catalysts compared with CeO2 catalysts alone.
It is worth noting that an eminent catalyst should not only have high activity, as the stability required for long-term operation is also an important factor. Among them, the corrosion resistance of the catalyst and the adhesion to the test electrode are the two main aspects of attention, where the higher of corrosion resistance can maintain the high activity and long-term electrocatalytic process in the electrolyte, while the stronger of adhesion can avoid the shedding phenomenon during the catalyst operation. The long-term stability test of Ni/CeO2 HJ-NSs catalysts was investigated by the chronopotentiometry in Figure 4e. Ni/CeO2 HJ-HSs were tested at a constant current density of 10 mA cm−2 for up to 50 h. It was clearly seen that no significant degradation of voltage and current density occurred, strongly demonstrating the outstanding electrochemical stability. In conclusion, the impressive HER performance of the rationally designed Ni/CeO2 HJ-NSs catalysts was corroborated by a series of tests above (Figure 4f).

Figure 4.
Electrochemical characterization of HER properties of Ni/CeO2 HJ-NSs in 1.0 M KOH solution. (a) LSV polarization curves, (b) Tafel slopes and overpotentials required to achieve 10 mA cm−2 of various catalysts. (c) Exchange current density curves. (d) EIS Nyquist plots. (e) Chronopotentiometric curve of Ni/CeO2 HJ-NSs. (f) Comparison of electrochemical performance of the prepared catalysts.
In addition, the reason for the high HER performance of the Ni/CeO2 HJ-NSs catalyst is further analyzed by the schematic diagram in Figure 5. The work function of metallic Ni is lower compared to that of the n-type semiconductor CeO2. Hence, electrons will spontaneously pass through the Mott–Schottky heterojunction interface until equilibrium of the work function is reached [45]. More electrons accumulated at the CeO2 interface will generate a local chemically reducing environment that facilitates the conversion of Ce4+ to Ce3+. More notably, the content of Ce3+ is positively correlated with the amount of oxygen vacancies (as discussed in the above XPS) [46]. High concentrations of oxygen vacancies are even more capable of promoting HER processes [47]. The change in oxygen vacancy concentration due to the strong interaction between Ni and CeO2, which alters the electronic properties of CeO2 as well as enhances the electron enrichment and overall electrical conductivity at the interface, further increases the electron transfer efficiency and improves the catalytic reaction process. Therefore, the high overpotential and large Tafel slope compared to single component metal Ni in alkaline solution accelerates the Volmer step in the HER process by electron coupling with the Ov-rich CeO2, conferring a faster reaction kinetic process. Based on the above discussion of the mechanism, the substantial increase in oxygen vacancy concentration caused by the Mott–Schottky structure supports the excellent alkaline HER performance of Ni/CeO2 HJ-NSs.
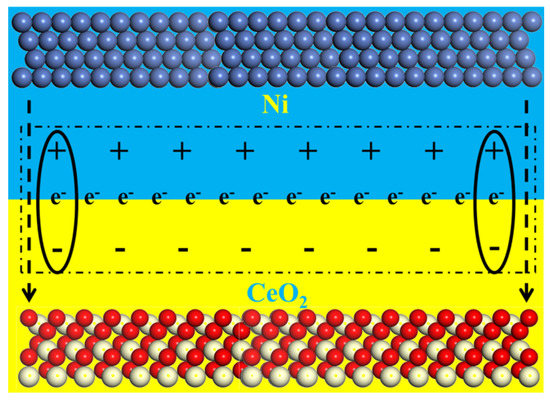
Figure 5.
Schematic diagram of the interfacial electronic structure of Ni/CeO2 HJ-NSs for HER in the alkaline solution.
3. Conclusions
In conclusion, we rationally developed an efficient glutinous rice potpourri-like Mott–Schottky 2D nanosheet electrocatalyst Ni/CeO2 HJ-HSs for HER via a straightforward self-assembly and urea reduction tactic. The experimental findings and mechanism studies corroborate that the formation of Ni/CeO2 heterojunctions induces the occurrence of the electron directional migration process, resulting in the formation of a built-in electric field at the interface and a localizing electron density, which significantly improves the overall electrical conductivity, leading to faster water dissociation and optimal binding energy of the reaction intermediates, ultimately achieving more excellent HER kinetics. As a result, the designed Ni/CeO2 HJ-HSs demonstrate marvelous HER catalytic performance in 1 M KOH electrolyte with an overpotential of only 72 mV at 10 mA cm−2, as well as a moderate Tafel slope of 65 mV dec−1 and a brilliant long time electrocatalytic durability after 50 h, laying a solid foundation for further in-depth research. This work provides a certain reference for the simpler, more economical, and safe synthesis of other 2D Mott–Schottky heterostructure nanosheets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15249443/s1, Figure S1. (a) SEM image of Ce-based coordination polymer. (b) Enlarged a cross-section image of light blue dotted box in (a). Figure S2. XRD pattern of CeO2 NSs. Figure S3. (a) TEM image, (b) enlarged TEM image, and (c) particle size distribution of Ni/CeO2. Figure S4. Nitrogen adsorption-desorption isotherms of Ni/CeO2 HJ-NSs, pore size distribution (inset of S4). Figure S5. Comparisons of the (a) Ni 2p and (b) Ce 3d XPS spectra for different catalysts. Figure S6. Deconvoluted Ce 3d XPS spectra for pure CeO2 catalysts. Figure S7. CV curves of different samples in the non-Faradaic region (1.10-1.20 V (vs. RHE)) obtained at different scanning rates. (a) Ni/CeO2 HJ-NSs, (b) NiO/CeO2 NSs, and (c) CeO2 NSs. Figure S8. (a) The Cdl, (b) corresponding ECSA, and (c) HER polarization curves with the current density normalized by ECSA of Ni/CeO2 HJ-NSs, NiO/CeO2 NSs and CeO2 NSs. Figure S9. (a) TOF plots for Ni/CeO2 HJ-NSs, NiO/CeO2 NSs and CeO2 NSs. (b) The TOF values at the overpotential of 0.1 V. Table S1. Elemental analysis of Ni, Ce (measured by ICP-OES) for CeO2 HJ-NSs catalyst. Table S2. Comparison of HER performance in alkaline environment between Ni/CeO2 HJ-NSs and previously reported representative catalysts. References [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.Z. and G.Z.; methodology, Y.Z.; validation, G.Z. and H.S.; data curation, H.S.; writing—original draft preparation, G.Z.; writing—review and editing, Y.Z.; supervision, Y.Z.; project administration, Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Fundamental Research Funds for the Central Universities (2682022ZTPY049, 2682020CX57).
Data Availability Statement
The data presented in this study are available in article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gao, K.R.; Wang, X.D.; Zheng, H.J.; Cao, J.Y.; Mi, L.R.; Huo, Q.H.; Yang, H.P.; Liu, J.H.; He, C.X. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Yang, X.B.; Wang, Q.; Cui, X.Q.; Zou, H.B.; Tong, X.L.; Yang, N.J. Facet-Selective hydrogen evolution on Rh2P electrocatalysts in pH-Universal media. Chem. Eng. J. 2022, 449, 137790. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Wu, P.; Gao, M.R.; Zhang, X.L.; Gao, F.Y.; Ju, H.X.; Wu, R.; Gao, Q.; You, R.; Huang, W.X.; et al. Doping-induced structural phase transition in cobalt diselenide enables enhanced hydrogen evolution catalysis. Nat. Commun. 2018, 9, 2533. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S.Z. The Hydrogen Evolution Reaction in Alkaline Solution: From Theory, Single Crystal Models, to Practical Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 7568–7579. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Mohajernia, S.; Schmuki, P.; Altomare, M. Site-selective Pt dewetting on WO3-coated TiO2 nanotube arrays: An electron transfer cascade-based H2 evolution photocatalyst. Appl. Catal. B 2018, 237, 198–205. [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Kim, M.G.; Hou, L.Q.; Liu, X.; Cho, J. Ru-incorporated oxygen-vacancy-enriched MoO2 electrocatalysts for hydrogen evolution reaction. Appl. Catal. B 2022, 307, 121204. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Z.; Wang, D.; Sun, X.; Yang, X. In Situ Electrochemically Activated CoMn-S@NiO/CC Nanosheets Array for Enhanced Hydrogen Evolution. ACS Catal. 2016, 6, 2797–2801. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liu, Q.; Yin, J.; Sun, D.; Zhang, M.; Xu, L.; Tang, Y.; Zhang, Y. Immobilization of Ni3Co Nanoparticles into N-Doped Carbon Nanotube/Nanofiber Integrated Hierarchically Branched Architectures toward Efficient Overall Water Splitting. Adv. Sci. 2020, 7, 1902371. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J.Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, S.Y.; Ma, L.X.; Guo, Y.J.; Sun, J.; Zhang, N.; Jiang, R.B. Water-Induced Formation of Ni2P-Ni12P5 Interfaces with Superior Electrocatalytic Activity toward Hydrogen Evolution Reaction. Small 2021, 17, 2006770. [Google Scholar] [CrossRef] [PubMed]
- You, H.H.; Wu, D.S.; Si, D.H.; Cao, M.N.; Sun, F.F.; Zhang, H.; Wang, H.M.; Liu, T.F.; Cao, R. Monolayer NiIr-Layered Double Hydroxide as a Long-Lived Efficient Oxygen Evolution Catalyst for Seawater Splitting. J. Am. Chem. Soc. 2022, 144, 9254–9263. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Yan, F.; Liu, L.; Zhu, C.; Li, B.; Chen, Y. Ni/MoC heteronanoparticles encapsulated within nitrogen-doped carbon nanotube arrays as highly efficient self-supported electrodes for overall water splitting. Chem. Eng. J. 2021, 406, 126815. [Google Scholar] [CrossRef]
- Janjua, M.B.I.; Le Roy, R.L. Electrocatalyst performance in industrial water electrolysers. Int. J. Hydrogen Energy 1985, 10, 11–19. [Google Scholar] [CrossRef]
- Fan, L.L.; Liu, P.F.; Yan, X.C.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.L.; Yao, X.D. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, H.Y.; Kang, J.L.; Ma, L.Y.; Chen, G.X.; Huang, Q.; Zhang, Z.J.; Liu, E.Z.; Lu, H.M.; Li, J.X.; et al. A freestanding nanoporous NiCoFeMoMn high-entropy alloy as an efficient electrocatalyst for rapid water splitting. Chem. Eng. J. 2022, 435, 134898. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Jia, Y.; Gao, G.P.; Yan, X.C.; Chen, N.; Chen, J.; Soo, M.T.; Wood, B.; Yang, D.J.; Du, A.J.; et al. Graphene Defects Trap Atomic Ni Species for Hydrogen and Oxygen Evolution Reactions. Chem 2018, 4, 285–297. [Google Scholar] [CrossRef]
- Zeng, H.B.; Chen, S.Q.; Jin, Y.Q.; Li, J.W.; Song, J.D.; Le, Z.C.; Liang, G.F.; Zhang, H.; Xie, F.Y.; Chen, J.; et al. Electron Density Modulation of Metallic MoO2 by Ni Doping to Produce Excellent Hydrogen Evolution and Oxidation Activities in Acid. ACS Energy Lett. 2020, 5, 1908–1915. [Google Scholar] [CrossRef]
- Feng, Y.; Guan, Y.; Zhou, E.; Zhang, X.; Wang, Y. Nanoscale Double-Heterojunctional Electrocatalyst for Hydrogen Evolution. Adv. Sci. 2022, 9, 2201339. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.; Song, S.; Ning, M.; Zhu, Q.; Zhou, J.; Gao, G.; Chen, Z.; Zhou, Q.; Xing, X.; et al. Efficient Alkaline Water/Seawater Hydrogen Evolution by a Nanorod-Nanoparticle-Structured Ni-MoN Catalyst with Fast Water-Dissociation Kinetics. Adv. Mater. 2022, 34, 2201774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yu, L.H.; Liu, W.X.; Dai, X.J.; Niu, X.X.; Fu, W.Q.; Shi, W.H.; Wu, F.F.; Cao, X.H. Structural advantages and enhancement strategies of heterostructure water-splitting electrocatalysts. Cell Rep. Phys. Sci. 2021, 2, 100443. [Google Scholar] [CrossRef]
- Wen, S.; Huang, J.; Li, T.; Chen, W.; Chen, G.; Zhang, Q.; Zhang, X.; Qian, Q.; Ostrikov, K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B 2022, 316, 121678. [Google Scholar] [CrossRef]
- Li, J.Y.; Xia, Z.M.; Xue, Q.Y.; Zhang, M.K.; Zhang, S.; Xiao, H.; Ma, Y.Y.; Qu, Y.Q. Insights into the Interfacial Lewis Acid-Base Pairs in CeO2-Loaded CoS2 Electrocatalysts for Alkaline Hydrogen Evolution. Small 2021, 17, 2103018. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, J.F.; Shi, Y.M.; Liu, D.; Zhang, B. Metallic WO2-Carbon Mesoporous Nanowires as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 6983–6986. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.M.; Jiao, L.F. Electronic Redistribution: Construction and Modulation of Interface Engineering on CoP for Enhancing Overall Water Splitting. Adv. Funct. Mater. 2020, 30, 1909618. [Google Scholar] [CrossRef]
- Cao, R.; Sun, D.F.; Liang, Y.C.; Hong, M.C.; Tatsumi, K.; Shi, Q. Syntheses and characterizations of three-dimensional channel-like polymeric lanthanide complexes constructed by 1,2,4,5-benzenetetracarboxylic acid. Inorg. Chem. 2002, 41, 2087–2094. [Google Scholar] [CrossRef]
- Li, Q.Y.; He, M.H.; Shen, Z.D.; Yang, G.W.; Yuan, Z.Y. pH Dependent structural diversity of lead compounds based on new flexible ligand 3-(2-pyridyl)-1-pyrazolyl acetic acid. Inorg. Chem. Commun. 2012, 20, 214–218. [Google Scholar] [CrossRef]
- Chi, Y.N.; Cui, F.Y.; Jia, A.R.; Ma, X.Y.; Hu, C.W. pH-Dependent syntheses of copper-quinoxaline-polyoxotungatate hybrids: Variable role of Keggin-type polyanion in different pH conditions. Crystengcomm 2012, 14, 3183–3188. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhou, J. Interactions of Ni Nanoparticles with Reducible CeO2(111) Thin Films. J. Phys. Chem. C 2012, 116, 9544–9549. [Google Scholar] [CrossRef]
- Tian, L.; Liu, H.; Zhang, B.; Liu, Y.; Lv, S.; Pang, L.; Li, J. Ni and CeO2 Nanoparticles Anchored on Cicada-Wing-like Nitrogen-Doped Porous Carbon as Bifunctional Catalysts for Water Splitting. ACS Appl. Nano Mater. 2022, 5, 1252–1262. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, J.; Sun, W.; Shen, Z. Fabrication of homogeneously Cu2+/La3+-doped CeO2 nanosheets and their application in CO oxidation. J. Mater. Chem. A 2017, 5, 9717–9722. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Ce3+-ion, Surface Oxygen Vacancy, and Visible Light-induced Photocatalytic Dye Degradation and Photocapacitive Performance of CeO2-Graphene Nanostructures. Sci. Rep. 2017, 7, 5928. [Google Scholar] [CrossRef] [PubMed]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS studies of damage induced by X-ray irradiation on CeO2 surfaces. Surf. Sci. 1990, 234, L253–L258. [Google Scholar] [CrossRef]
- Li, G.; Jang, H.; Liu, S.; Li, Z.; Kim, M.G.; Qin, Q.; Liu, X.; Cho, J. The synergistic effect of Hf-O-Ru bonds and oxygen vacancies in Ru/HfO2 for enhanced hydrogen evolution. Nat. Commun. 2022, 13, 1270. [Google Scholar] [CrossRef]
- Cai, J.; Ding, J.; Wei, D.; Xie, X.; Li, B.; Lu, S.; Zhang, J.; Liu, Y.; Cai, Q.; Zang, S. Coupling of Ru and O-Vacancy on 2D Mo-Based Electrocatalyst Via a Solid-Phase Interface Reaction Strategy for Hydrogen Evolution Reaction. Adv. Energy Mater. 2021, 11, 2100141. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, M.Y.; Yan, D.Y.; Mao, J.; Liu, H.; Hu, W.B.; Du, X.W.; Ling, T.; Qiao, S.Z. Engineering oxygen vacancy on NiO nanorod arrays for alkaline hydrogen evolution. Nano Energy 2018, 43, 103–109. [Google Scholar] [CrossRef]
- Yang, F.; Bao, X.; Li, P.; Wang, X.; Cheng, G.; Chen, S.; Luo, W. Boosting Hydrogen Oxidation Activity of Ni in Alkaline Media through Oxygen-Vacancy-Rich CeO2/Ni Heterostructures. Angew. Chem. Int. Ed. 2019, 58, 14179–14183. [Google Scholar] [CrossRef]
- Zheng, T.T.; Sang, W.; He, Z.H.; Wei, Q.S.; Chen, B.W.; Li, H.L.; Cao, C.; Huang, R.J.; Yan, X.P.; Pan, B.C.; et al. Conductive Tungsten Oxide Nanosheets for Highly Efficient Hydrogen Evolution. Nano Lett. 2017, 17, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Tang, F.W.; Wang, M.; Zhan, W.B.; Hu, H.X.; Li, Y.R.; Friend, R.H.; Song, X.Y. Femtosecond visualization of oxygen vacancies in metal oxides. Sci. Adv. 2020, 6, eaax9427. [Google Scholar] [CrossRef] [PubMed]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Norskov, J.K. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Su, H.; Yu, Q.-Y.; Zhang, B.; Wang, H.-H.; Li, X.-H.; Chen, J.-S. Janus Co/CoP Nanoparticles as Efficient Mott–Schottky Electrocatalysts for Overall Water Splitting in Wide pH Range. Adv. Energy Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Z.; Hao, L.; Xing, Z.P.; Dai, Y.; Xu, X.; Pan, S.Y.; Duan, Y.Q.; Zou, J.L. Nano Ce2O2S with Highly Enriched Oxygen-Deficient Ce3+ Sites Supported by N and S Dual-Doped Carbon as an Active Oxygen-Supply Catalyst for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 22518–22529. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Liu, H.; Liu, Y.; Zhao, F.W.; Li, J.Q.; He, X.M. Defective TiO2-graphene heterostructures enabling in-situ electrocatalyst evolution for lithium-sulfur batteries. J. Energy Chem. 2021, 62, 508–515. [Google Scholar] [CrossRef]
- Wen, Q.; Duan, J.; Wang, W.; Huang, D.; Liu, Y.; Shi, Y.; Fang, J.; Nie, A.; Li, H.; Zhai, T. Engineering a Local Free Water Enriched Microenvironment for Surpassing Platinum Hydrogen Evolution Activity. Angew. Chem. Int. Ed. 2022, 134, e202206077. [Google Scholar] [CrossRef]
- Yuan, S.; Xia, M.; Liu, Z.; Wang, K.; Xiang, L.; Huang, G.; Zhang, J.; Li, N. Dual synergistic effects between Co and Mo2C in Co/Mo2C heterostructure for electrocatalytic overall water splitting. Chem. Eng. J. 2022, 430, 132697. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Gao, M.-R.; Liu, S.; Luo, J.-L. Hierarchically assembling cobalt/nickel carbonate hydroxide on copper nitride nanowires for highly efficient water splitting. Appl. Catal. B 2021, 292, 120148. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, Z.; Liao, J.; Liang, H.; Wei, B.; Xu, X.; Xu, H.; Zheng, J.; Zhu, W.; Cavallo, L.; et al. Construction of hydroxide pn junction for water splitting electrocatalysis. Appl. Catal. B 2021, 292, 120160. [Google Scholar] [CrossRef]
- Zang, Z.; Wang, X.; Li, X.; Zhao, Q.; Li, L.; Yang, X.; Yu, X.; Zhang, X.; Lu, Z. Co9S8 Nanosheet Coupled Cu2S Nanorod Heterostructure as Efficient Catalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 9865–9874. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhang, L.; Bukhvalov, D.; Chen, Z.; Zou, Z.; Shang, L.; Yang, X.; Yan, D.; Han, F.; Zhang, T. Hierarchical ultrathin carbon encapsulating transition metal doped MoP electrocatalysts for efficient and pH-universal hydrogen evolution reaction. Nano Energy 2020, 70, 104445. [Google Scholar] [CrossRef]
- Wang, P.; Qin, R.; Ji, P.; Pu, Z.; Zhu, J.; Lin, C.; Zhao, Y.; Tang, H.; Li, W.; Mu, S. Synergistic Coupling of Ni Nanoparticles with Ni3C Nanosheets for Highly Efficient Overall Water Splitting. Small 2020, 16, 2001642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical Nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a Highly Efficient Electrocatalyst for Overall Water Splitting in a Wide pH Range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, M.; He, W.; Wang, H.; Jian, M.; Zhang, Y. Blue rose-inspired approach towards highly graphitic carbons for efficient electrocatalytic water splitting. Carbon 2019, 150, 21–26. [Google Scholar] [CrossRef]
- Han, W.; Chen, L.; Ma, B.; Wang, J.; Song, W.; Fan, X.; Li, Y.; Zhang, F.; Peng, W. Ultra-small Mo2C nanodots encapsulated in nitrogen-doped porous carbon for pH-universal hydrogen evolution: Insights into the synergistic enhancement of HER activity by nitrogen doping and structural defects. J. Mater. Chem. A 2019, 7, 4734–4743. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, K.; Liu, S.; Chen, X.; Cheng, Y.; Cheong, W.-C.; Chen, Z.; Zheng, L.; Zhang, J.; Li, X.; et al. Construction of CoP/NiCoP Nanotadpoles Heterojunction Interface for Wide pH Hydrogen Evolution Electrocatalysis and Supercapacitor. Adv. Energy Mater. 2019, 9, 1901213. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, A.; Jiao, Y.; Zheng, H.; Wang, X.; Xie, Y.; Wang, L.; Tian, C.; Fu, H. Two-Dimensional Porous Molybdenum Phosphide/Nitride Heterojunction Nanosheets for pH-Universal Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 6673–6681. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Liu, S.; Sun, K.; Cheng, Y.; Liu, M.; Wang, Z.; Li, X.; Zhang, J. Construction of multi-dimensional core/shell Ni/NiCoP nano-heterojunction for efficient electrocatalytic water splitting. Appl. Catal. B 2019, 259, 118039. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, A.-L.; Xiao, W.; Chao, D.; Zhang, X.; Tiep, N.H.; Chen, S.; Kang, J.; Wang, X.; Ding, J.; et al. In Situ Grown Epitaxial Heterojunction Exhibits High-Performance Electrocatalytic Water Splitting. Adv. Mater. 2018, 30, 1705516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Long, X.; Lin, H.; Zhou, D.; An, Y.; Yang, S. Enhancing Full Water-Splitting Performance of Transition Metal Bifunctional Electrocatalysts in Alkaline Solutions by Tailoring CeO2 –Transition Metal Oxides–Ni Nanointerfaces. ACS Energy Lett. 2018, 3, 290–296. [Google Scholar] [CrossRef]
- Kou, T.; Smart, T.; Yao, B.; Chen, I.; Thota, D.; Ping, Y.; Li, Y. Theoretical and Experimental Insight into the Effect of Nitrogen Doping on Hydrogen Evolution Activity of Ni3S2 in Alkaline Medium. Adv. Energy Mater. 2018, 8, 1703538. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).