Microscopic Analysis of Hydrogen Production from Methane Sono-Pyrolysis
Abstract
1. Introduction
2. Model
- Equation (1) (the modified Keller–Miksis equation [25]) describes the radial dynamics, R(t), of the bubble during its oscillation in compressible medium (water);
- Equations (3) and (4) provide the internal bubble pressure and temperature during oscillation;
- Equation (5) (the Hertz–Knudsen formula [26]) describes the mass flux, dm/dt, of water evaporation and condensation at the bubble–liquid interface;
- Equations (6)–(8) (heat dissipation by conduction [27]) describe the heat exchange dQ/dt inside and outside the bubble during oscillation;
- Equation (9) describes the temporal variation in the internal energy of a bubble;
- Equations (10)–(15) describe the change, with time, in quantities of H2O, CH4 and all other species ‘k’ within the bubble during oscillation.
3. Results and Discussions
3.1. Kinetics of Bubble in Presence of Methane
3.2. Model Efficiency
3.3. Effect of Methane Dose on the Bubble Chemistry and Dynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Af (Ar) | Pre-exponential factor of the forward (reverse) reaction, [(cm3 mol−1 s−1) for two-body reaction and (cm6 mol−2 s−1) for three-body reaction]. |
| bf (br) | Temperature exponent of the forward (reverse) reaction. |
| c | Speed of sound in the liquid medium, (m s−1). |
| Cp | Heat capacity concentration inside the bubble (J m−3 K−1) |
| Eaf (Ear) | Activation energy of the forward (reverse) reaction, (cal mol−1). |
| f | Frequency of ultrasonic wave, (Hz). |
| Ia | Acoustic intensity of ultrasonic irradiation, (W m−2). |
| kf (kr) | Forward (reverse) reaction constant, [(cm3 mol−1 s−1) for two-body reaction and (cm6 mol−2 s−1) for three-body reaction]. |
| MH2O | Molar mass of water (kg mol−1). |
| Evaporation-condensation rate of water (kg m-² s−1). | |
| n | Molar amount (mol). |
| p | Pressure inside a bubble, (Pa). |
| PA | Amplitude of the acoustic pressure, (Pa). |
| PB | Liquid pressure on the external side of the bubble wall, (Pa) |
| pmax | Maximum pressure inside a bubble (Pa). |
| Pv | Vapor pressure of water, (Pa). |
| p∞ | Ambient static pressure, (Pa). |
| Q | Energy transferred by heat exchange (J s−1) |
| R | Radius of the bubble, (m). |
| Rg | Ideal gas constant (J/mol K). |
| Rmax | Maximum radius of the bubble, (m). |
| R0 | Ambient bubble radius, (m). |
| t | Time, (s). |
| T | Temperature inside a bubble, (K). |
| Tmax | Maximum temperature inside a bubble, (K). |
| T∞ | Bulk liquid temperature, (K). |
| Production rate of the kth species (mol s−1 m−3) | |
| V | Volume of the bubble (m3) |
| x | Thermal diffusivity inside the bubble (m² s−1) |
| Greek letters | |
| α | Accommodation coefficient. |
| λΙ | Thermal conductivity of species i (W m−1 K−1). |
| λμιξ | Thermal conductivity of the mixture (W m−1 K−1). |
| µ | Dynamic viscosity (Pa s). |
| ργ | Density inside the bubble (kg m−3). |
| ρH2O | Density of water vapor inside the bubble (Kg m−3). |
| ρλ | Density of liquid water, (kg m−3). |
| ρσατ,H2O | Saturated vapor density (Kg m−3). |
| s | Surface tension of liquid water, (N m−1). |
| υki | Stoichiometric coefficient of the kth chemical species in the ith reaction |
References
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O. Correlations between the sonochemical production rate of hydrogen and the maximum temperature and pressure reached in acoustic bubbles. Arab. J. Sci. Eng. 2018, 43, 6109–6117. [Google Scholar] [CrossRef]
- Chibani, A.; Merouani, S.; Bougriou, C.; Hamadi, L. International Journal of Heat and Mass Transfer Heat and mass transfer during the storage of hydrogen in LaNi 5 -based metal hydride: 2D simulation results for a large scale, multi-pipes fixed-bed reactor. Int. J. Heat Mass Transf. 2019, 147, 118939. [Google Scholar] [CrossRef]
- Chibani, A.; Bougriou, C.; Merouani, S. Simulation of hydrogen absorption/desorption on metal hydride LaNi5-H2: Mass and heat transfer. Appl. Therm. Eng. 2018, 142, 110–117. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrog. Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Balat, M. Possible methods for hydrogen production. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 31, 39–50. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Islam, M.H.; Lamb, J.J.; Lien, K.M.; Burheim, O.S.; Hihn, J.-Y.; Pollet, B.G. Novel fuel production based on sonochemistry and sonoelectrochemistry. ECS Trans. 2019, 92, 1. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen Production from Waste Materials Bio-hydrogen production from waste materials. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O. The sonochemical approach for hydrogen production. In Sustainable Green Chemical Processes and Their Allied Applications. Nanotechnology in the Life Sciences; Inamuddin, Asiri, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–29. [Google Scholar]
- Islam, M.H.; Burheim, O.S.; Pollet, B.G.; Islam, H.; Burheim, O.S.; Pollet, B.G.; Islam, M.H.; Burheim, O.S.; Pollet, B.G. Sonochemical and Sonoelectrochemical Production of Hydrogen. Ultrason. Sonochem. 2018, 51, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G. Sonochemistry: Environmental science and engineering applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Tuziuti, T.; Yasui, K.; Sivakumar, M.; Iida, Y.; Miyoshi, N. Correlation between acoustic cavitation noise and yield enhancement of sonochemical reaction by particle addition. J. Phys. Chem. A 2005, 109, 4869–4872. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Nakamura, H.; Bandow, H.; Maeda, Y.; Nagata, Y. Sonolysis of organic liquid: Effect of vapour pressure and evaporation rate. Ultrason. Sonochem. 1999, 6, 203–209. [Google Scholar] [CrossRef]
- Buettner, J.; Gutierrez, M.; Henglein, a. Sonolysis of water-methanol mixtures. J. Phys. Chem. 1991, 95, 1528–1530. [Google Scholar] [CrossRef]
- Kerboua, K.; Hamdaoui, O.; Al-Zahrani, S. Sonochemical production of hydrogen: A numerical model applied to the recovery of aqueous methanol waste under Oxygen-Argon atmosphere. Environ. Prog. Sustain. Energy 2020, in press. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Methanol sono-pyrolysis for hydrogen recovery: Effect of methanol concentration under an argon atmosphere. Chem. Eng. J. 2021, 433, 133272. [Google Scholar] [CrossRef]
- Henglein, A. Sonolysis of carbon dioxide, nitrous oxide and methane in aqueous solution. Z. Naturforsch. B 1985, 40, 100–107. [Google Scholar] [CrossRef]
- Henglein, A. Chemical effects of continuous and pulsed ultrasound in aqueous solutions. Ultrason. Sonochem. 1995, 2, 115–121. [Google Scholar] [CrossRef]
- Hart, E.J.; Fischer, C.H.; Henglein, A. Sonolysis of hydrocarbons in aqueous solution. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1990, 36, 511–516. [Google Scholar] [CrossRef]
- Arsentev, S.D. Sonochemical Transformations of Methane and Ethylene in Aqueous Solutions under Conditions of Cavitation. Phys. Chem. Solut. 2020, 94, 1811–1815. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Effect of carbon tetrachloride (CCl4) sonochemistry on the size of active bubbles for the production of reactive oxygen and chlorine species in acoustic cavitation field. Chem. Eng. J. 2021, 426, 130251. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Carbon tetrachloride (CCl4) sonochemistry: A comprehensive mechanistic and kinetics analysis elucidating how CCl4 pyrolysis improves the sonolytic degradation of nonvolatile organic contaminants. Sep. Purif. Technol. 2021, 275, 118614. [Google Scholar] [CrossRef]
- Yasui, K. Effects of thermal conduction on bubble dynamics near the sonoluminescence threshold. J. Acoust. Soc. Am. 1995, 98, 2772–2782. [Google Scholar] [CrossRef]
- Sochard, S.; Wilhelm, A.M.; Delmas, H. Modelling of free radicals production in a collapsing gas-vapour bubble. Ultrason. Sonochem. 1997, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Toegel, R.; Lohse, D. Phase diagrams for sonoluminescing bubbles: A comparison between experiment and theory. J. Chem. Phys. 2003, 118, 1863–1875. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O. Computer simulation of chemical reactions occurring in collapsing acoustical bubble: Dependence of free radicals production on operational conditions. Res. Chem. Intermed. 2015, 41, 881–897. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Extreme conditions in a dissolving air nanobubble. Phys. Rev. E 2016, 94, 013106. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Liley, P.E.; Saxena, S. Thermal conductivity (Nonmetallic liquids and gases). Thermophys. Prop. Matter 1970, 3, 512. [Google Scholar]
- Yasui, K.; Yasui, K. Effect of volatile solutes on sonoluminescence. J. Chem. Phys. 2002, 2945, 2945. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Mechanism of the sonochemical production of hydrogen. Int. J. Hydrog. Energy 2015, 40, 4056–4064. [Google Scholar] [CrossRef]
- Yasui, K. Chemical reactions in a sonoluminescing bubble. J. Phys. Soc. Jpn. 1997, 66, 2911–2920. [Google Scholar] [CrossRef]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Alternative model of single-bubble sonoluminescence. Phys. Rev. E 1997, 56, 6750–6760. [Google Scholar] [CrossRef]
- Merouani, S.; Ferkous, H.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. New interpretation of the effects of argon-saturating gas toward sonochemical reactions. Ultrason. Sonochem. 2015, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Alghyamah, A. Insight into the impact of excluding mass transport, heat exchange and chemical reactions heat on the sonochemical bubble yield: Bubble size-dependency. Ultrason. Sonochem. 2021, 73, 105511. [Google Scholar] [CrossRef]
- Lide, D.R.; Haynes, W.M.M.; Baysinger, G.; Berger, L.I.; Kehiaian, H.V.; Roth, D.L.; Zwillinger, D.; Frenkel, M.; Goldberg, R.N. CRC Handbook of Chemistry and Physics, 90th ed.; Internet Version; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Alghyamah, A. A complete analysis of the effects of transfer phenomenons and reaction heats on sono-hydrogen production from reacting bubbles: Impact of ambient bubble size. Int. J. Hydrog. Energy 2021, 46, 18767–18779. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Ashokkumar, M. An alternative technique for determining the number density of acoustic cavitation bubbles in sonochemical reactors. Ultrason. Sonochem. 2022, 82, 105872. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Lee, J.; Kozuka, T.; Towata, A.; Iida, Y. The range of ambient radius for an active bubble in sonoluminescence and sonochemical reactions. J. Chem. Phys. 2008, 128, 184705. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O. The size of active bubbles for the production of hydrogen in sonochemical reaction field. Ultrason. Sonochem. 2016, 32, 320–327. [Google Scholar] [CrossRef]
- Jiang, Y.; Petrier, C.; Waite, T.D. Sonolysis of 4-chlorophenol in aqueous solution: Effects of substrate concentration, aqueous temperature and ultrasonic frequency. Ultrason. Sonochem. 2006, 13, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Beckett, M.A.; Hua, I. Elucidation of the 1,4-dioxane decomposition pathway at discrete ultrasonic frequencies. Environ. Sci. Technol. 2000, 34, 3944–3953. [Google Scholar] [CrossRef]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Lee, J.; Ashokkumar, M.; Kentish, S.; Grieser, F. Determination of the size distribution of sonoluminescence bubbles in a pulsed acoustic field. J. Am. Chem. Soc. 2005, 127, 16810–16811. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Theoretical investigation of the effect of ambient pressure on bubble sonochemistry: Special focus on hydrogen and reactive radicals production. Chem. Phys. 2021, 547, 111171. [Google Scholar] [CrossRef]
- Rassokhin, D.N.; Kovalev, G.V.; Bugaenko, L.T. Temperature Effect on the Sonolysis of Methanol-water Mixtures. J. Am. Chem. Soc. 1995, 1984, 344–347. [Google Scholar] [CrossRef]
- Kerboua, K.; Hamdaoui, O. Void fraction, number density of acoustic cavitation bubbles, and acoustic frequency: A numerical investigation. J. Acoust. Soc. Am. 2019, 146, 2240. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Ferkous, H.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. A method for predicting the number of active bubbles in sonochemical reactors. Ultrason. Sonochem. 2014, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S.; Chibani, A.; Hamdaoui, O.; Yasui, K.; Ashokkumar, M. Estimation of the number density of active cavitation bubbles in a sono-irradiated aqueous solution using a thermodynamic approach. Ultrasonics 2022, 126, 106824. [Google Scholar] [CrossRef]
- Gireesan, S.; Pandit, A.B. Modeling the effect of carbon-dioxide gas on cavitation. Ultrason. Sonochem. 2017, 34, 721–728. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Chapter 2—The energy forms and energy conversion. In Energy Aspects of Acoustic Cavitation and Sonochemistry; Hamdaoui, O., Kerboua, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 23–35. ISBN 978-0-323-91937-1. [Google Scholar]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Chapter 9—Energy Controlling Mechanisms: Relationship with Operational Conditions; Hamdaoui, O., Kerboua, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–155. ISBN 978-0-323-91937-1. [Google Scholar]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Computational engineering study of hydrogen production via ultrasonic cavitation in water. Int. J. Hydrog. Energy 2016, 41, 832–844. [Google Scholar] [CrossRef]
- Kerabchi, N.; Merouani, S.; Hamdaoui, O. Relationship between liquid depth and the acoustic generation of hydrogen: Design aspect for large cavitational reactors with special focus on the role of the wave attenuation. Int. J. Green Energy 2019, 16, 423–434. [Google Scholar] [CrossRef]
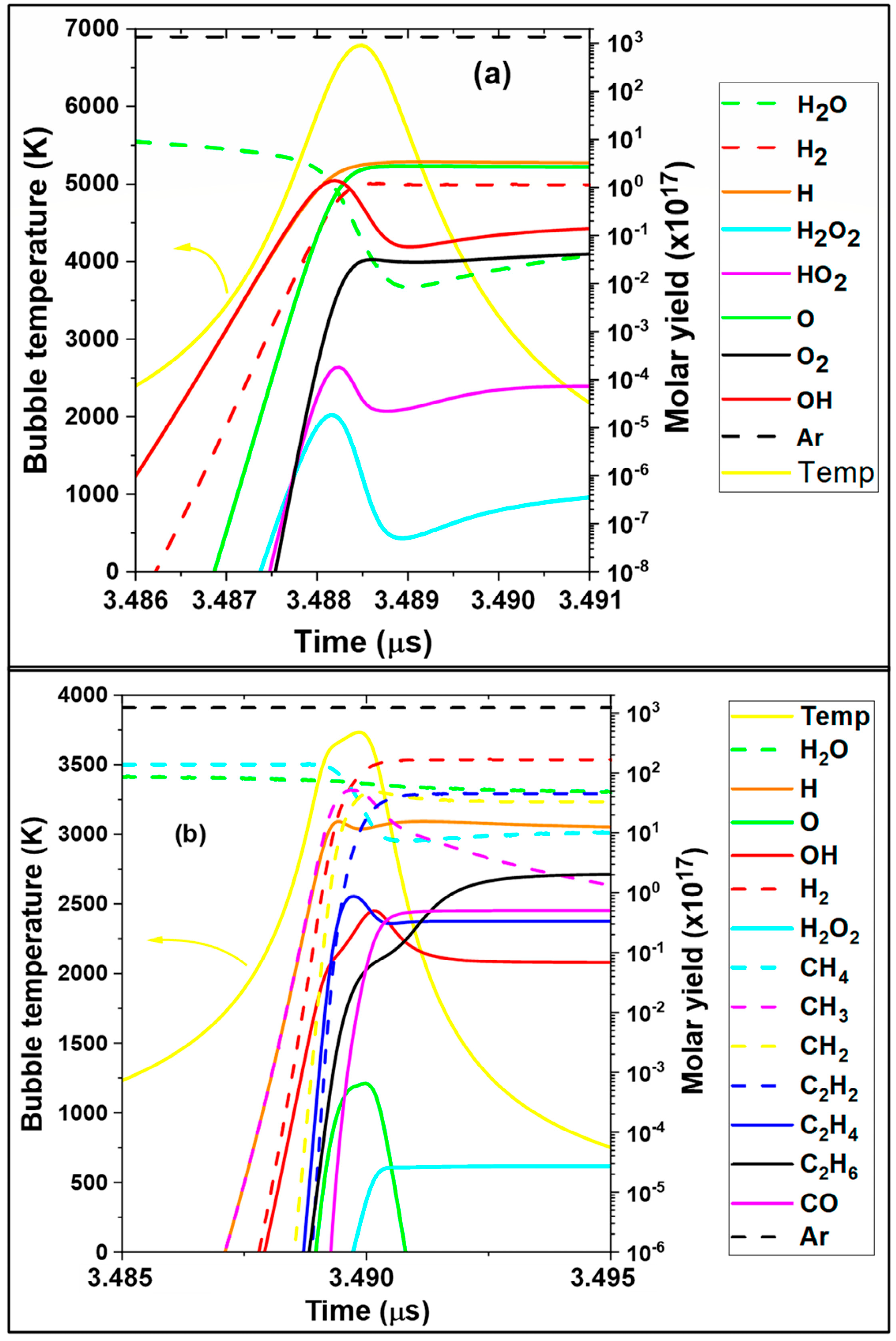
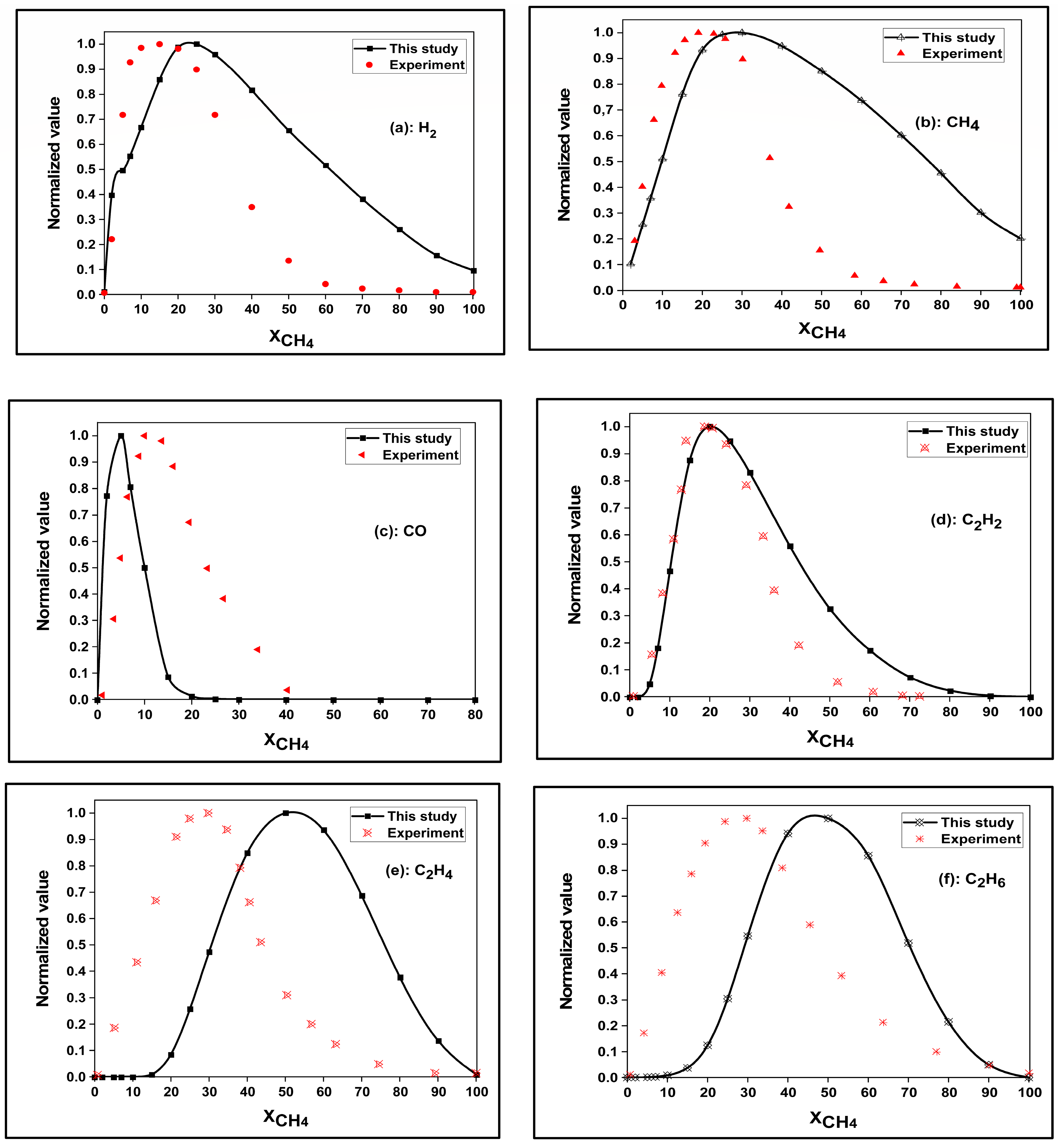
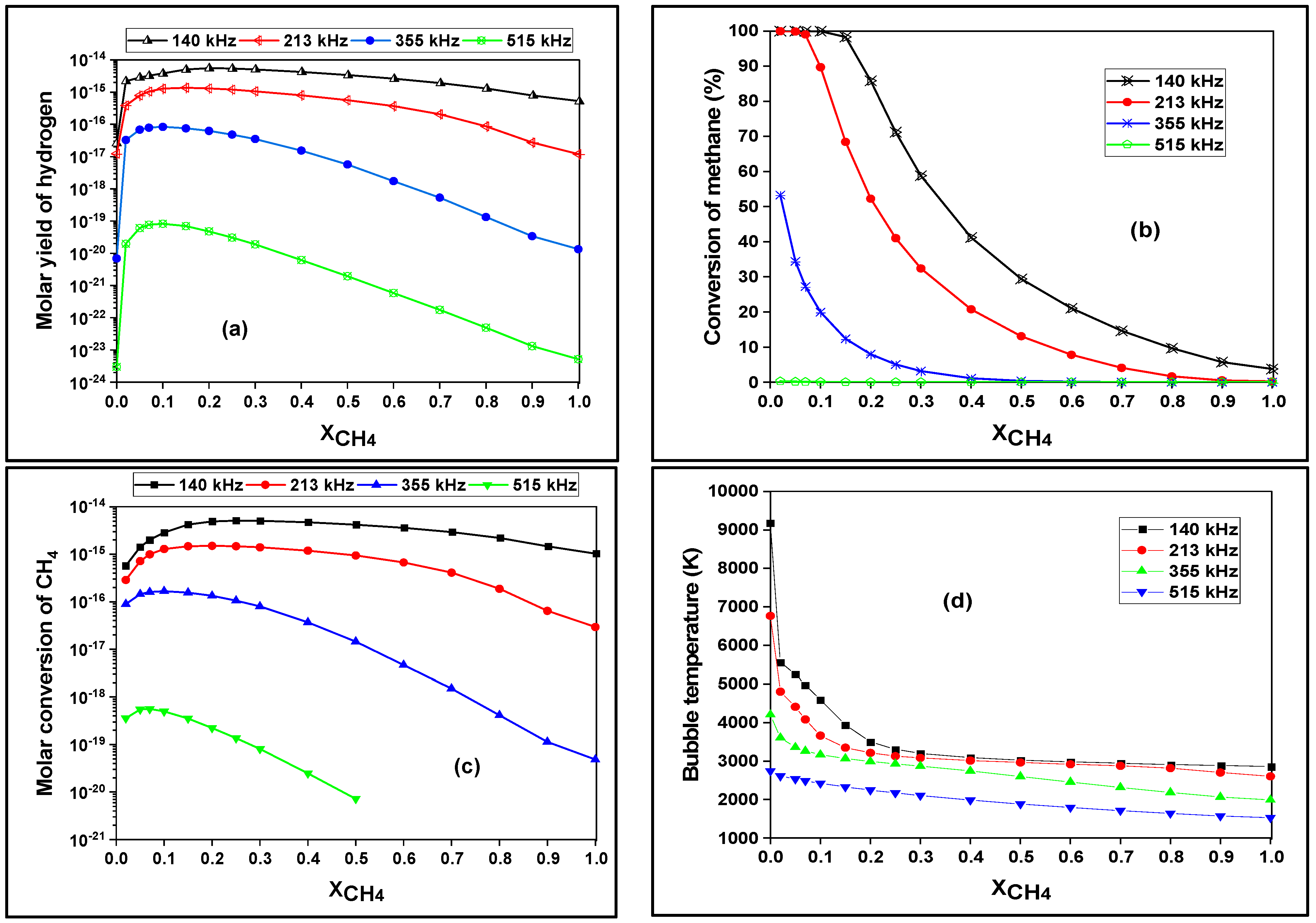
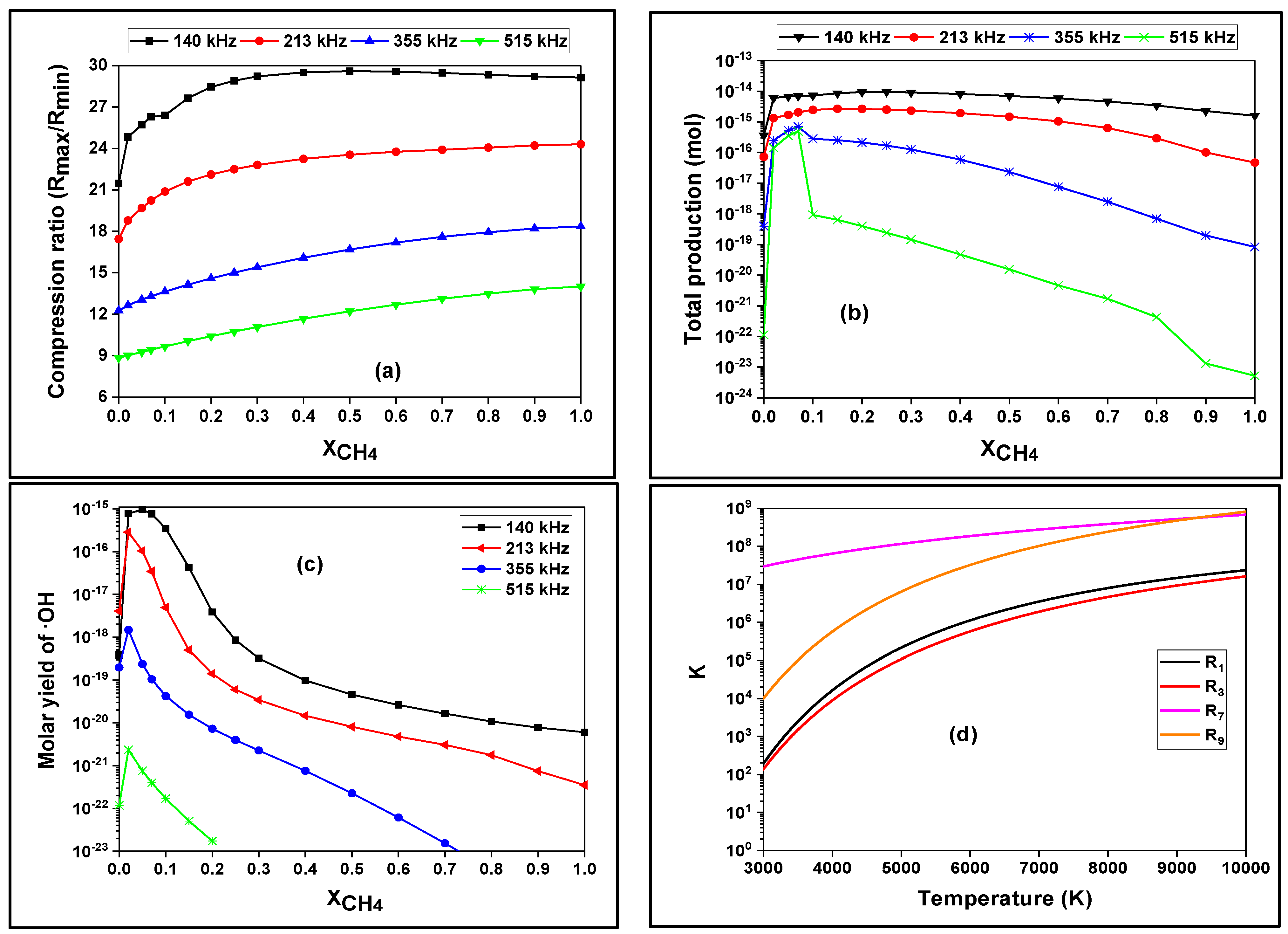
| |
| (1) | |
| |
| (2) | |
| |
| (3) | |
| (4) | |
| |
| (5) | |
| |
| (6) | |
| (7) | |
| (8) | |
| |
| (9) | |
| |
| |
| (10) | |
| |
| (11) | |
| where: | |
| (12) | |
| (13) | |
| (14) | |
| (15) |
| Reaction | A | n | Ea | ΔH | |
|---|---|---|---|---|---|
| 1 | H2O + M → H• + •OH + M | 1.912 × 107 | −1.83 | 28.35 | 508.82 |
| 2 | O2+M → O + O + M | 4.515 × 1011 | −0.64 | 28.44 | 505.4 |
| 3 | •OH + M → O + H•+ M | 9.88 × 1011 | −0.74 | 24.43 | 436.23 |
| 4 | H• + O2 → O + •OH | 1.915 × 108 | 0.0 | 3.93 | 69.17 |
| 5 | H• + O2 + M → HO2• + M | 1.475 | 0.6 | 0.0 | −204.80 |
| 6 | O + H2O → •OH + •OH | 2.97 | 2.02 | 3.21 | 72.59 |
| 7 | HO2• + H• → H2 + O2 | 1.66 × 107 | 0.0 | 1.97 × 10−1 | −239.67 |
| 8 | HO2• + H• → •OH + •OH | 7.079 × 107 | 0.0 | 7.06 × 10−2 | −162.26 |
| 9 | HO2• + O → •OH + O2 | 3.25 × 107 | 0.0 | 0.0 | −231.85 |
| 10 | HO2• + •OH → H2O + O2 | 2.89 × 107 | 0.0 | −1.19 × 10−1 | −304.44 |
| 11 | H2+M → H• + H• + M | 4.577 × 1013 | −1.4 | 24.98 | 444.47 |
| 12 | O + H2 → H• + •OH | 3.82 × 106 | 0.0 | 1.9 | 8.23 |
| 13 | •OH + H2 → H• + H2O | 2.16 × 102 | 1.52 | 8.25 × 10−1 | −64.35 |
| 14 | H2O2 + O2 → HO2• + HO2• | 4.634 × 1010 | −0.35 | 12.12 | 175.35 |
| 15 | H2O2 + M → •OH + •OH +M | 2.951 × 108 | 0.0 | 11.59 | 217.89 |
| 16 | H2O2+H• → H2O+•OH | 2.410 × 107 | 0.0 | 9.5 × 10−1 | −290.93 |
| 17 | H2O2 + H• → H2 + HO2• | 6.025 × 107 | 0.0 | 1.9 | −64.32 |
| 18 | H2O2 + O → •OH + HO2• | 9.550 | 2.0 | 9.5 × 10−1 | −56.08 |
| 19 | H2O2 + •OH → H2O + HO2• | 1.0 × 106 | 0.0 | 0.0 | −128.67 |
| 20 | H• + •OH + M → H2O + M | 2.2 × 1010 | −2.0 | 0.0 | −508.82 |
| 21 | O + O + M → O2 + M | 6.165 × 103 | −0.5 | 0.0 | −505.4 |
| 22 | O + H• + M → •OH + M | 4.714 × 106 | −1.0 | 0.0 | −436.23 |
| 23 | O + •OH → H• + O2 | 5.481 × 105 | 0.39 | −7.01 × 10−2 | −69.17 |
| 24 | HO2• +M → H• + O2 + M | 3.09 × 106 | 0.53 | 11.7 | 204.80 |
| 25 | •OH+•OH → O + H2O | 1.465 × 10−1 | 2.11 | −6.94 × 10−1 | −72.59 |
| 26 | H2 + O2 → HO2•+ H• | 3.164 × 106 | 0.35 | 13.3 | 239.67 |
| 27 | •OH + •OH → HO2• + H• | 2.027 × 104 | 0.72 | 8.8 | 162.26 |
| 28 | •OH+O2 → HO2• + O | 3.252 × 106 | 0.33 | 12.75 | 231.85 |
| 29 | H2O + O2 → HO2• + •OH | 5.861 × 107 | 0.24 | 16.53 | 304.44 |
| 30 | H• + H• + M → H2 + M | 1.146 × 108 | −1.68 | 1.96 × 10−1 | −444.47 |
| 31 | H• + •OH → O + H2 | 2.667 × 10−2 | 2.65 | 1.17 | −8.23 |
| 32 | H• + H2O → •OH + H2 | 2.298 × 103 | 1.40 | 4.38 | 64.35 |
| 33 | HO2• + HO2• → H2O2 + O2 | 4.2 × 108 | 0.0 | 2.87 | −175.35 |
| 34 | •OH + •OH + M → H2O2 + M | 1.0 × 102 | −0.37 | 0.0 | −217.89 |
| 35 | H2O + •OH → H2O2 + H• | 1.269 × 102 | 1.31 | 17.08 | 290.93 |
| 36 | H2 + HO2• → H2O2 + H• | 1.041 × 105 | 0.70 | 5.74 | 64.32 |
| 37 | •OH + HO2• → H2O2 + O | 8.66 × 10−3 | 2.68 | 4.45 | 56.08 |
| 38 | H2O + HO2• → H2O2 + •OH | 1.838 × 104 | 0.59 | 7.4 | 128.67 |
| Reaction | A | n | Ea | ΔH | |
|---|---|---|---|---|---|
| 1 | H2O+M → H• + •OH + M | 1.912 × 107 | −1.83 | 28.35 | 508.82 |
| 2 | O2 + M → O + O + M | 4.515 × 1011 | −0.64 | 28.44 | 505.4 |
| 3 | •OH + M → O + H• + M | 9.88 × 1011 | −0.74 | 24.43 | 436.23 |
| 4 | H• + O2 + M → HO2• + M | 1.475 | 0.6 | 0.0 | −204.80 |
| 5 | H2 + M → H• + H•+ M | 4.577 × 1013 | −1.4 | 24.98 | 444.47 |
| 6 | H2O2 + M → •OH + •OH + M | 2.951 × 108 | 0.0 | 11.59 | 217.89 |
| 7 | CH4 + •OH → CH3• + H2O | 0.19 | 2.4 | 8.81 | −57.69 |
| 8 | CH4 + H• → CH3• + H2 | 1.313 × 10−2 | 3.0 | 33.61 | 3.33 |
| 9 | CH4 + M → CH3• + H+ M | 1.04 × 1012 | 0.0 | 402.97 | 439.27 |
| 10 | CH3• + CH3• +M → C2H6 + M k0 k∞ | 1.27 × 1029 3.614 × 107 | −7.0 0 | 11.55 0.0 | −376.66 |
| 11 | CH3• + CH3• → C2H4 + H2 | 1.0 × 108 | 0.0 | 133.76 | −240.34 |
| 12 | C2H4+ M → C2H2 + H2 + M | 9.33 × 1010 | 0.0 | 38.8 | 175.74 |
| 13 | O + C2H2 → CH2• + CO | 4.086 × 102 | 1.5 | 7.07 | −197.51 |
| 14 | CH2• + O → CO + H2 | 4.818 × 107 | 0.0 | 0.0 | −750.09 |
| 15 | CO + O2 → CO2 + O | 2.529 × 106 | 0.0 | 199.44 | −33.83 |
| 16 | C2H2 + •OH → CO + CH3• | 4.84 × 10−10 | 4.0 | 8.39 | −229.51 |
| 17 | CH2• + CH2• → C2H2 + 2H• | 2.0 × 108 | 0.0 | 44.99 | −116.64 |
| 18 | CH3• + H• → CH2• + H2 | 2.0 × 108 | 0.0 | 63.1 | 26.02 |
| 19 | O + O + M → O2 + M | 6.165 × 103 | −0.5 | 0.0 | −505.4 |
| 20 | HO2• + M → H• + O2 + M | 3.09 × 106 | 0.53 | 11.7 | 204.80 |
| 21 | H• + H• + M → H2 + M | 1.146 × 108 | −1.68 | 1.96 × 10−1 | −444.47 |
| 22 | •OH + •OH + M → H2O2 +M | 1.0 × 102 | −0.37 | 0.0 | −217.89 |
| 23 | CH3• + H• + M → CH4 + M k0 k∞ | 1.42 × 1021 1.27 × 1010 | −4.8 −0.6 | 10.22 1.6 | −439.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehane, A.; Merouani, S. Microscopic Analysis of Hydrogen Production from Methane Sono-Pyrolysis. Energies 2023, 16, 443. https://doi.org/10.3390/en16010443
Dehane A, Merouani S. Microscopic Analysis of Hydrogen Production from Methane Sono-Pyrolysis. Energies. 2023; 16(1):443. https://doi.org/10.3390/en16010443
Chicago/Turabian StyleDehane, Aissa, and Slimane Merouani. 2023. "Microscopic Analysis of Hydrogen Production from Methane Sono-Pyrolysis" Energies 16, no. 1: 443. https://doi.org/10.3390/en16010443
APA StyleDehane, A., & Merouani, S. (2023). Microscopic Analysis of Hydrogen Production from Methane Sono-Pyrolysis. Energies, 16(1), 443. https://doi.org/10.3390/en16010443






