Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell
Abstract
:1. Introduction
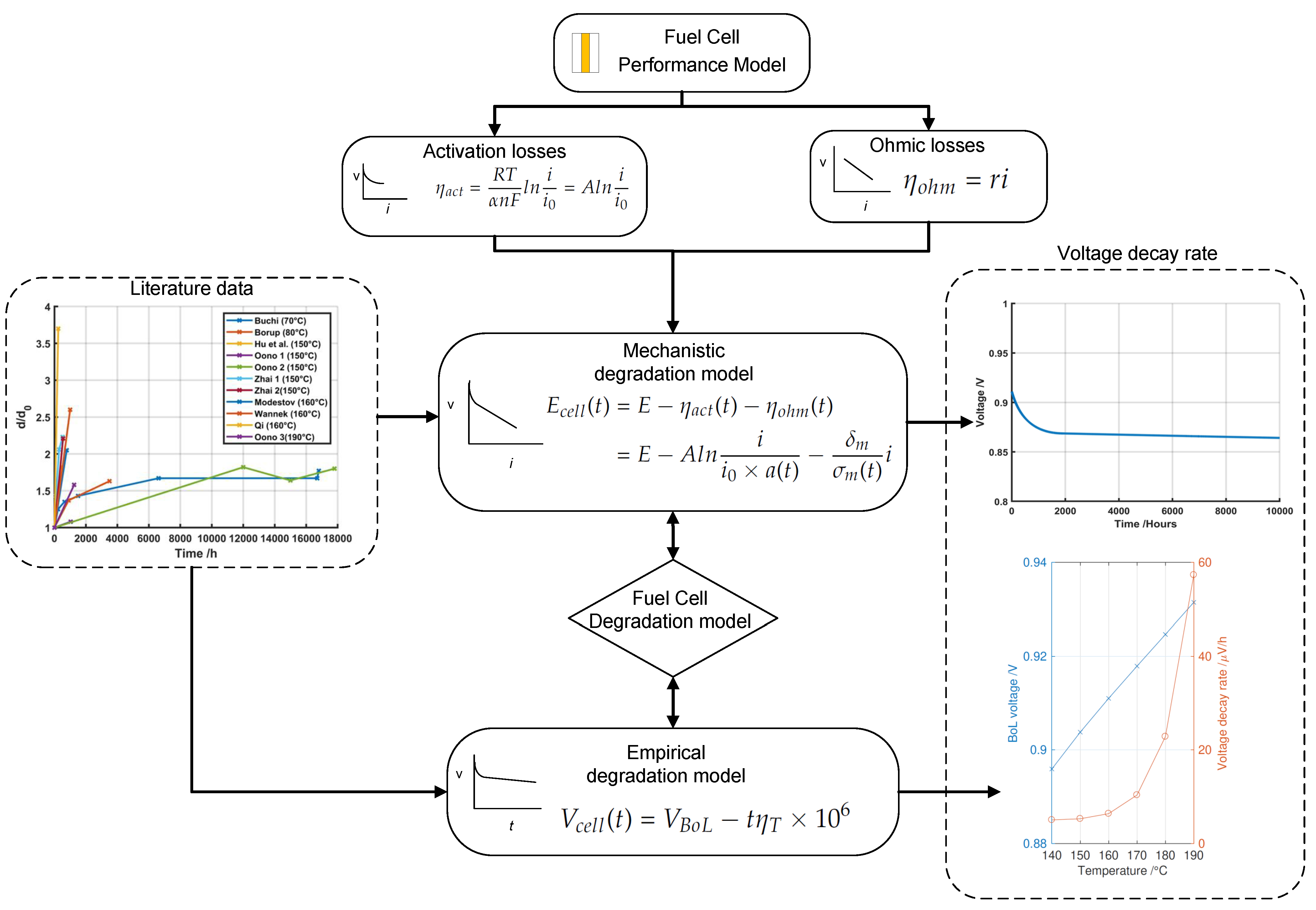
2. Modeling Approach
- There are no gradients, i.e., all parameters are considered uniform across the cell.
- All fluids are ideal and well mixed.
- The product water at the cathode is in vapor phase.
- The anode overpotential is neglected.
2.1. Fuel Cell Performance Model
2.1.1. Activation Losses
2.1.2. Ohmic Losses
2.2. Degradation Model
2.2.1. Mechanical–Chemical Degradation Model
Activation Losses
Ohmic Losses
2.2.2. Temperature Dependence of Voltage Degradation
3. Results and Discussion
3.1. Activation Losses
3.1.1. Particle Agglomeration
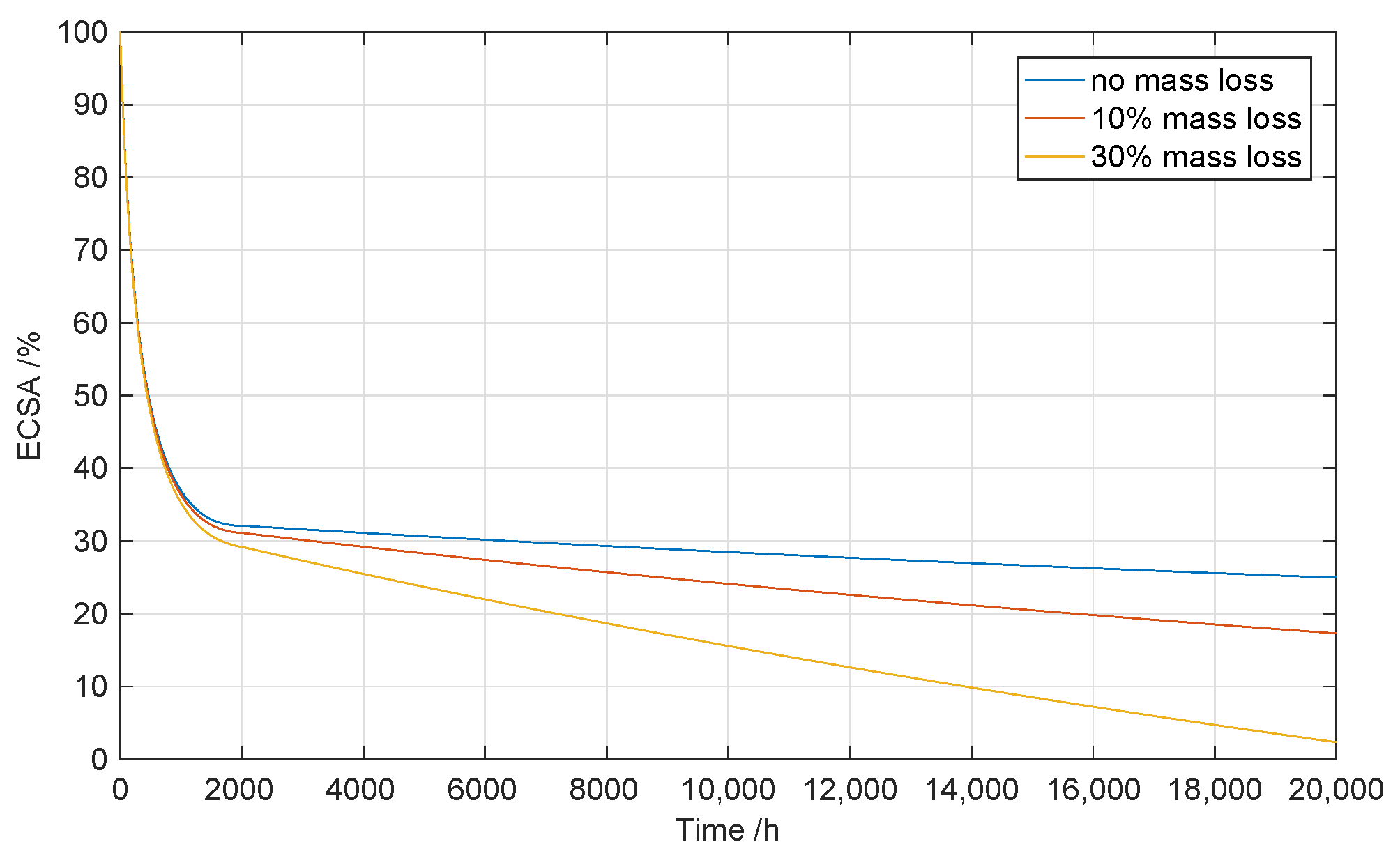
3.1.2. Loss of Active Catalyst Material
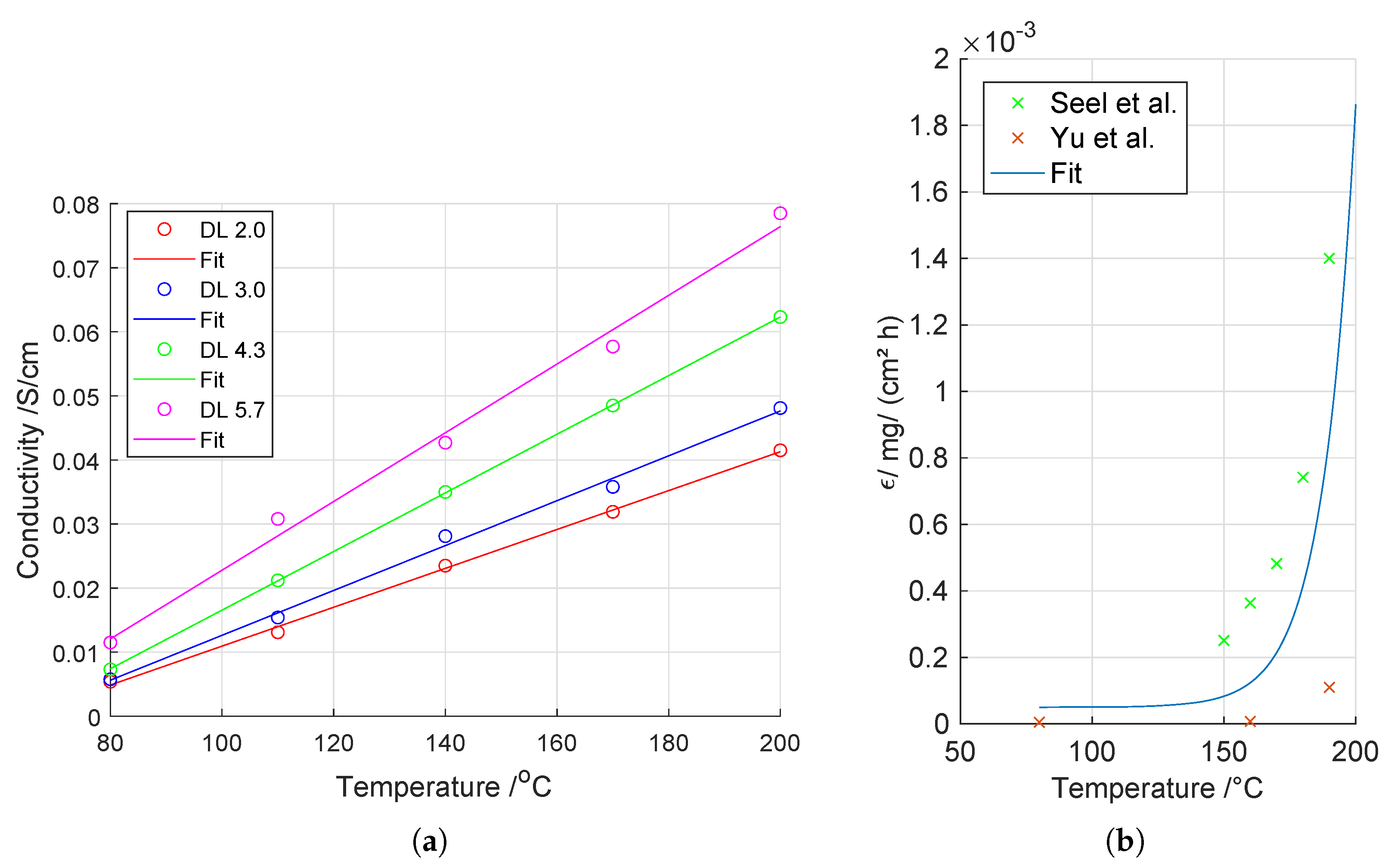
3.2. Ohmic Losses
3.3. Cell Voltage Degradation
3.4. Effect of Temperature on Voltage Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APU | Auxiliary power units |
| BOL | Beginning of life |
| CL | Catalyst layer |
| DL | Acid doping level |
| EOL | End of life |
| ECSA | Electrochemical active surface area |
| GDL | Gas diffusion layer |
| HT-PEMFC | High-temperature proton exchange membrane fuel cells |
| LT-PEMFC | Low-temperature proton exchange membrane fuel cells |
| OCV | Open circuit voltage |
| PA | Phosphoric acid |
| PBI | Polybenzimidazole |
| PtX | Power-to-X |
| RH | Relative humidity |
| CHP | Micro-combined heat and power generation |
| Greek Letters | |
| Charge transfer coefficient | |
| Density, | |
| Voltage decay rate, | |
| Conductivity, | |
| Evaporation rate of PA, | |
| Overpotential, | |
| Thickness, | |
| Gas stoichiometric ratio | |
| Symbols | |
| Degradation factor | |
| Reactants activities | |
| Products activities | |
| A | Tafel slope, |
| Geometric area of the MEA, | |
| Particle surface area, | |
| Coefficients for exchange current density | |
| d | Particle diameter, |
| Reversible cell voltage, | |
| F | Faraday constant, |
| i | Current density, |
| Exchange current density, | |
| Temperature coefficient | |
| Pt loading, | |
| Total mass of platinum, | |
| Phosphoric acid specific mass, | |
| M | Molecular weight, |
| n | Number of electrons |
| Q | Reaction quotient, |
| r | Particle radius (Equation (9)), |
| r | Membrane resistance, |
| Area specific resistance, | |
| R | Universal gas constant, |
| Specific platinum surface area, | |
| t | Time, |
| T | Temperature, |
| Utilization factor | |
| Reactants stoichiometric coefficient | |
| Products stoichiometric coefficient | |
| V | Particle volume, |
| Molar fraction of P2O5 |
References
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.R.; Friedrichs, J.; Dinkelacker, F. A Study on Electrofuels in Aviation. Energies 2018, 11, 392. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Kær, S.K. Thermodynamic analyses of a moderate-temperature process of carbon dioxide hydrogenation to methanol via reverse water-gas shift with in situ water removal. Ind. Eng. Chem. Res. 2019, 58, 10559–10569. [Google Scholar] [CrossRef]
- Araya, S.S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef] [Green Version]
- Martinho, D.L.; Araya, S.S.; Sahlin, S.L.; Liso, V.; Li, N.; Berg, T.L. Modeling a Hybrid Reformed Methanol Fuel Cell & Battery System for Telecom Backup Applications. Energies 2022, 15, 3218. [Google Scholar] [CrossRef]
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Loreti, G.; Facci, A.L.; Ubertini, S. High-Efficiency Combined Heat and Power through a High-Temperature Polymer Electrolyte Membrane Fuel Cell and Gas Turbine Hybrid System. Sustainability 2021, 13, 12515. [Google Scholar] [CrossRef]
- Automotive—Advent Technologies. Available online: https://www.advent.energy/automotive/ (accessed on 22 July 2022).
- Automotive—Blue World Technologies. Available online: https://www.blue.world/markets/automotive/ (accessed on 22 July 2022).
- Commercial collaboration between Tuco Marine and Blue World Technologies—Blue World Technologies. Available online: https://www.blue.world/commercial-collaboration-between-tuco-marine-and-blue-world-technologies/ (accessed on 22 July 2022).
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Wang, Z.; Sun, H.; Sun, G. Degradation study of high temperature proton exchange membrane fuel cell under start/stop and load cycling conditions. Int. J. Hydrogen Energy 2021, 46, 24353–24365. [Google Scholar] [CrossRef]
- Bandlamudi, V.; Bujlo, P.; Linkov, V.; Pasupathi, S. The Effect of Potential Cycling on High Temperature PEM Fuel Cell with Different Flow Field Designs. Fuel Cells 2019, 19, 231–243. [Google Scholar] [CrossRef]
- Leader, J.O.; Yue, Y.; Walluk, M.R.; Trabold, T.A. Voltage degradation of high-temperature PEM fuel cells operating at 200C under constant load and start-stop conditions. Int. J. Hydrogen Energy 2022, 47, 18820–18830. [Google Scholar] [CrossRef]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Inbody, M.A. PEM fuel cell electrocatalyst durability measurements. J. Power Sources 2006, 163, 76–81. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Zhai, Y.; Liu, G.; Yi, B. 500h Continuous aging life test on PBI/H3PO4 high-temperature PEMFC. Int. J. Hydrogen Energy 2006, 31, 1855–1862. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Liu, G.; Hu, J.; Yi, B. Degradation Study on MEA in H3PO4/PBI High-Temperature PEMFC Life Test. J. Electrochem. Soc. 2007, 154, B72–B76. [Google Scholar] [CrossRef]
- Büchi, F.; Inaba, M.; Schmidt, T. Polymer Electrolyte Fuel Cell Durability; Springer: New York, NY, USA, 2009; Volume 1, pp. 1829–1841. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Cleghorn, S.J.C.; Mayfield, D.K.; Moore, D.a.; Moore, J.C.; Rusch, G.; Sherman, T.W.; Sisofo, N.T.; Beuscher, U. A polymer electrolyte fuel cell life test: 3 years of continuous operation. J. Power Sources 2006, 158, 446–454. [Google Scholar] [CrossRef]
- Prokop, M.; Drakselova, M.; Bouzek, K. Review of the experimental study and prediction of Pt-based catalyst degradation during PEM fuel cell operation. Curr. Opin. Electrochem. 2020, 20, 20–27. [Google Scholar] [CrossRef]
- Hansen, T.W.; Delariva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or ostwald ripening? Accounts Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Yata, K.; Tamaguchi, T. Ostwald Ripening of Silver in Lead-Borosilicate Glass. Materials Science Forum 1993, 126–128, 643–646. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Liu, G. Progress of high temperature polybenzimidazole proton exchange membrane: A systematic review. J. Phys. Conf. Ser. 2021, 2076, 012032. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Long-term cell degradation mechanism in high-temperature proton exchange membrane fuel cells. J. Power Sources 2012, 210, 366–373. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Li, Q. High Temperature Proton Exchange Membranes for Fuel Cells. Ph.D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2005. [Google Scholar]
- Oono, Y.; Sounai, A.; Hori, M. Prolongation of lifetime of high temperature proton exchange membrane fuel cells. J. Power Sources 2013, 241, 87–93. [Google Scholar] [CrossRef]
- Kannan, A.; Kabza, A.; Scholta, J. Long term testing of start-stop cycles on high temperature PEM fuel cell stack. J. Power Sources 2015, 277, 312–316. [Google Scholar] [CrossRef]
- Schmidt, T.J. Durability and Degradation in High-Temperature Polymer Electrolyte Fuel Cells. ECS Trans. 2006, 1, 19–31. [Google Scholar] [CrossRef]
- Moçotéguy, P.; Ludwig, B.; Scholta, J.; Barrera, R.; Ginocchio, S. Long term testing in continuous mode of HT-PEMFC based H3PO4/PBI celtec-PMEAs for μ-CHP applications. Fuel Cells 2009, 9, 325–348. [Google Scholar] [CrossRef]
- Moçotéguy, P.; Ludwig, B.; Scholta, J.; Nedellec, Y.; Jones, D.J.; Rozière, J. Long-term testing in dynamic mode of HT-PEMFC H3PO4/PBI celtec-P based membrane electrode assemblies for micro-CHP applications. Fuel Cells 2010, 10, 299–311. [Google Scholar] [CrossRef]
- Moradi, M.; Moheb, A.; Javanbakht, M.; Hooshyari, K. Experimental study and modeling of proton conductivity of phosphoric acid doped PBI-Fe2TiO5 nanocomposite membranes for using in high temperature proton exchange membrane fuel cell (HT-PEMFC). Int. J. Hydrogen Energy 2016, 41, 2896–2910. [Google Scholar] [CrossRef]
- BASF. Celtec MEAs; Technical Report; BASF: Ludwigshafen, Germany, 2008. [Google Scholar]
- Galbiati, S.; Baricci, A.; Casalegno, A.; Marchesi, R. Degradation in phosphoric acid doped polymer fuel cells: A 6000 h parametric investigation. Int. J. Hydrogen Energy 2013, 38, 6469–6480. [Google Scholar] [CrossRef]
- Simon Araya, S.; Juhl Andreasen, S.; Venstrup Nielsen, H.; Knudsen Kær, S. Investigating the effects of methanol-water vapor mixture on a PBI-based high temperature PEM fuel cell. Int. J. Hydrogen Energy 2012, 37, 18231–18242. [Google Scholar] [CrossRef]
- Modestov, A.; Tarasevich, M.; Filimonov, V.; Zagudaeva, N. Degradation of high temperature MEA with PBI-H3PO4 membrane in a life test. Electrochim. Acta 2009, 54, 7121–7127. [Google Scholar] [CrossRef]
- Oono, Y.; Fukuda, T.; Sounai, A.; Hori, M. Influence of operating temperature on cell performance and endurance of high temperature proton exchange membrane fuel cells. J. Power Sources 2010, 195, 1007–1014. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Durability and Reliability in High-Temperature Reformed Hydrogen PEFCs. ECS Trans. 2006, 3, 861–869. [Google Scholar] [CrossRef]
- Wannek, C.; Konradi, I.; Mergel, J.; Lehnert, W. Redistribution of phosphoric acid in membrane electrode assemblies for high-temperature polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2009, 34, 9479–9485. [Google Scholar] [CrossRef]
- Wannek, C.; Kohnen, B.; Oetjen, H.F.; Lippert, H.; Mergel, J. Durability of ABPBI-based MEAs for high temperature PEMFCs at different operating conditions. Fuel Cells 2008, 8, 87–95. [Google Scholar] [CrossRef]
- Vang, J.R. HTPEM Fuel Cell Impedance: Mechanistic Modelling and Experimental Characterisation. Ph.D. Thesis, Department of Energy Technology, Aalborg University, Aalborg, Denmark, 2014. [Google Scholar]
- O’Hayre, R.; Cha, S.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Sahlin, S.L.; Araya, S.S.; Andreasen, S.J.; Kær, S.K. Electrochemical Impedance Spectroscopy (EIS) Characterization of Reformate-operated High Temperature PEM Fuel Cell Stack. Int. J. Power Energy Res. 2017, 1, 20–40. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, H.; Jin, S.; Choi, S.M.; Kim, H.J.; Seo, M.H.; Kim, W.B. A Review on Recent Progress in the Aspect of Stability of Oxygen Reduction Electrocatalysts for Proton-Exchange Membrane Fuel Cell: Quantum Mechanics and Experimental Approaches. Energy Technol. 2019, 7, 1900312. [Google Scholar] [CrossRef]
- Chugh, S.; Chaudhari, C.; Sonkar, K.; Sharma, A.; Kapur, G.; Ramakumar, S. Experimental and modelling studies of low temperature PEMFC performance. Int. J. Hydrogen Energy 2020, 45, 8866–8874. [Google Scholar] [CrossRef]
- de Beer, C.; Barendse, P.; Pillay, P.; Rengaswamy, R.; Bullecks, B. Derivation of an equivalent electrical circuit model for degradation mechanisms in high temperature pem fuel cells in performance estimation. In Proceedings of the 2014 IEEE Energy Conversion Congress and Exposition (ECCE), Pittsburgh, PA, USA, 14–18 September 2014; pp. 4583–4590. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.; Kim, J.; Sohn, Y.J. One-dimensional modeling and analysis for performance degradation of high temperature proton exchange membrane fuel cell using PA doped PBI membrane. Solid State Ionics 2014, 262, 319–323. [Google Scholar] [CrossRef]
- Qi, Z.; Buelte, S. Effect of open circuit voltage on performance and degradation of high temperature PBI-H3PO4 fuel cells. J. Power Sources 2006, 161, 1126–1132. [Google Scholar] [CrossRef]
- Lim, I.S.; Park, J.Y.; Kang, D.G.; Choi, S.H.; Kang, B.; Kim, M.S. Numerical study for in-plane gradient effects of cathode gas diffusion layer on PEMFC under low humidity condition. Int. J. Hydrogen Energy 2020, 45, 19745–19760. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/Acid Complexes as High-Temperature Membranes. In Fuel Cells II, Advances in Polymer Science; Scherer, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 216, pp. 63–124. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Zhai, Y.; Liu, G.; Hu, J.; Yi, B. Performance degradation studies on PBI/H3PO4 high temperature PEMFC and one-dimensional numerical analysis. Electrochim. Acta 2006, 52, 394–401. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Xing, D.; Shao, Z.G. The stability of Pt/C catalyst in H3PO4/PBI PEMFC during high temperature life test. J. Power Sources 2007, 164, 126–133. [Google Scholar] [CrossRef]
- Bett, J.; Kinoshita, K.; Stonehart, P. Crystallite growth of platinum dispersed on graphitized carbon black. J. Catal. 1974, 35, 307–316. [Google Scholar] [CrossRef]
- Seel, D.C.; Benicewicz, B.C.; Xiao, L.; Schmidt, T.J. High-Temperature Polybenzimidazole-Based Membranes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 5–6, pp. 1–13. [Google Scholar]
- Yu, S.; Xiao, L.; Benicewicz, B.C. Durability Studies of PBI-based High Temperature PEMFCs. Fuel Cells 2008, 8, 165–174. [Google Scholar] [CrossRef]
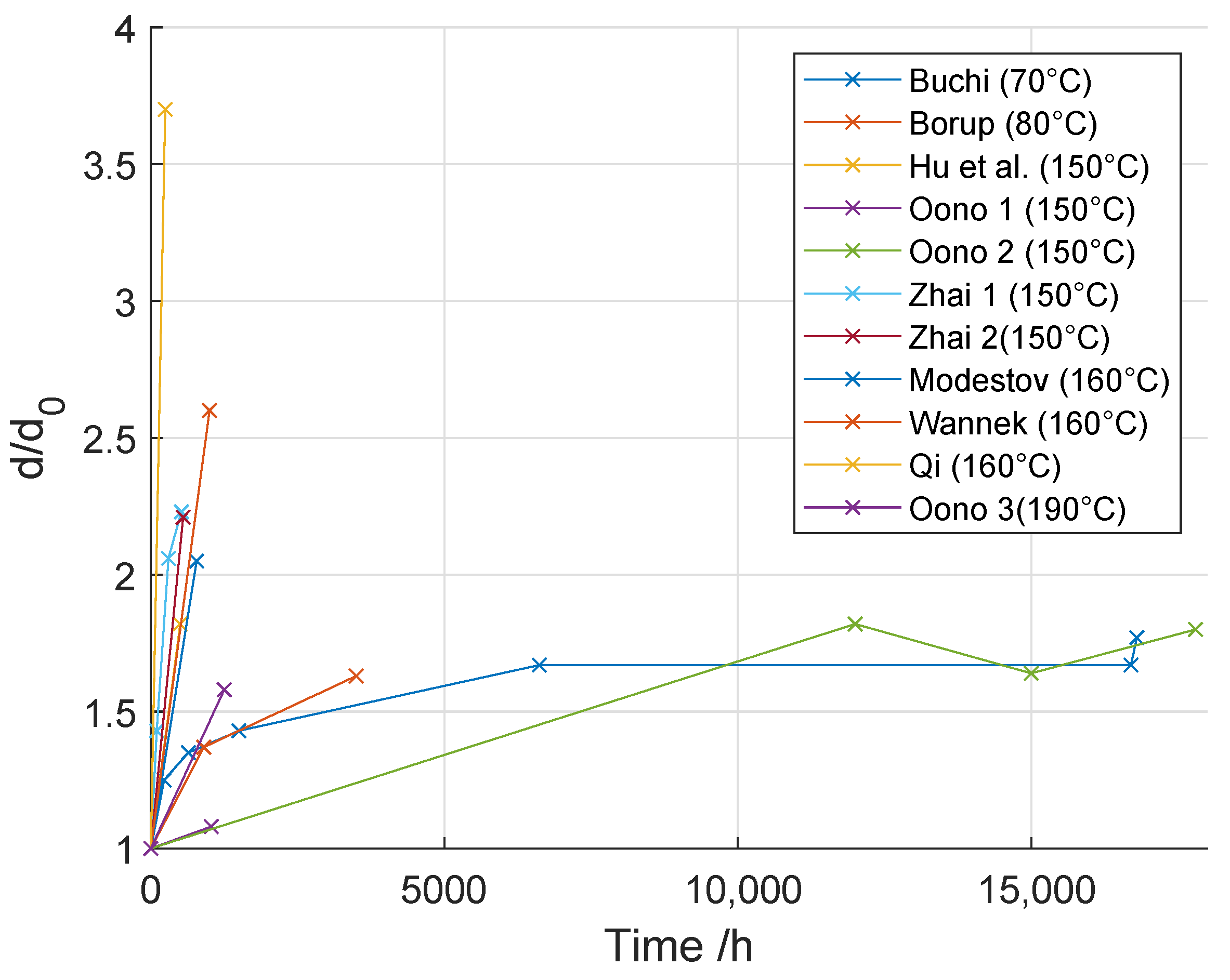





| Reference | Investigated Parameter | Voltage Decay Rate | T | i | / | Cycling/Steady State | Fuel | Duration | MEA |
|---|---|---|---|---|---|---|---|---|---|
| (μV/h) | (°C) | (A/cm2) | (h) or (Number of Cycles) | ||||||
| BASF [35] | t | 6 | std | std | std | ss | H2 | ||
| cycle | 47 | std | std | n/a | 2 h on/2 h off | H2 | 200 cyc | P1000 | |
| cycle | 35 | std | std | n/a | 2 h on/2 h off | H2 | 200 cyc | P1100W | |
| Galbiati et al. [36] | t | 8.6 | std | std | std | ss | H2 | 950 h | P2100 |
| T | 19 | 180 | std | std | ss | H2 | 400 h | P2100 | |
| i | 4.5 | std | 0.4 | std | ss | H2 | 800 h | P2100 | |
| 8.5 | std | std | 1.2/4 | ss | H2 | 800 h | P2100 | ||
| Hu et al. [16] | t | 150 | 150 | 0.64 | n/a | ss | H2 | 500 h | own |
| Simon Araya et al. [37] | t | 5 | std | 0.22 | 1.2/4 | ss | H2 | 123 h | Celtec-P |
| fuel impurities | 900 | std | 0.22 | 1.2/4 | ss | 5% CH3OH/H2O | 400 h | Celtec-P | |
| fuel impurities | 3400 | std | 0.22 | 1.2/4 | ss | 8% CH3OH/H2O | 200h | Celtec-P | |
| Modestov et al. [38] | t | 25 | std | std | 1.3/1.8 | ss | H2 | 780 h | own |
| Moçotéguy et al. [32] | t | 41 | std | 0.4 | 1.4/2 | ss | reformate | 1105 h | P1000 |
| Moçotéguy et al. [33] | t | 35 | std | std | std | ss | reformate | total: ∼650 h | P1000 |
| cycling | 139/117 | std | 0.2/0.4 | std | 6 h 0.2/6 h 0.4 | reformate | P1000 | ||
| cycling | 54/26 | std | 0.2/0.4 | std | 12 h 0.2/12 h 0.4 | reformate | P1000 | ||
| cycling | 67 | std | std | std | 12 h on/12 h off | reformate | P1000 | ||
| Oono et al. [39] | T | 3.6 | 150 | std | 3.7/3.7 | ss | H2 | >16,000 h | own |
| T | 13 | 170 | std | 3.7/3.7 | ss | H2 | 6400 h | own | |
| T | 59 | 190 | std | 3.7/3.7 | ss | H2 | 1220 h | own | |
| Oono et al. [26,29] | t | 3 (t < 14,000 h); 9 (t > 14,000 h) | 150 | std | 3.7/3.7 | ss | H2 | 17,860 h | FuMA-Tech |
| Schmidt and Baurmeister [40] | t | 20 | 180 | std | std | ss | H2 + H2O | ∼200 h | P1000 |
| fuel impurities | 20 | 180 | std | std | ss | reformate | 200 h | P1000 | |
| fuel impurities | 20 | 180 | std | std | ss | reformate + Sulfur | 3200 h | P1000 | |
| Schmidt and Baurmeister [19] | t | 5 | std | std | std | ss | H2 | 6.000 h | P1000 |
| cycle | 11 | std | std | std | 12 h on/12 h off | H2 | 240 cyc | P1000 | |
| Kannan et al. [30] | t | 13.25 | 140 | 0.25 | 1.25/2.5 | ss | reformate | >1600 h | P1100W |
| i/cycling | 11 | 120–180 | 0.03 | 1.25/2.5 | cyc | reformate | 4160 h, 1562 cyc | P1100W | |
| i/cycling | 26 | 120–180 | 0.25 | 1.25/2.5 | cyc | reformate | P1100W | ||
| i/cycling | 133 | 120–180 | OCV | 1.25/2.5 | cyc | reformate | P1100W | ||
| Wannek et al. [41] | t | std | std | 2/2 | ss | H2 | 1000 h | custom FuMA-Tech | |
| Wannek et al. [42] | t | 25 | std | st | 2/2 | ss | H2 | >1000 h | FuMA-Tech |
| t | 20 | std | std | 2/2 | ss | H2 | order of hundreds | FuMA-Tech | |
| i/cycling | 180 | std | std | 2/2 | load variation | H2 | FuMA-Tech | ||
| cycling | 120 | std | std | 2/2 | off 1/d | H2 | FuMA-Tech |
| −0.005812 | |
| 0.0001675 | |
| −0.007636 | |
| 6.518 × 10 | |
| 0.0005617 | |
| 0.9958 |
| Parameter | Value | Unit | |
|---|---|---|---|
| Charge transfer coefficient | 0.5 | ||
| Cell area | 45 | ||
| Membrane thickness | 1 × 10 | m | |
| DL | Acid doping level | 5 | PRU |
| I | Cell current | 9 | A |
| L | Initial PBI loading | 6 | |
| L | Initial platinum loading | 0.75 | |
| m | Loss of active Pt | 0 | mg/( × h) |
| p | Pressure (pan = pcat) | 1 | atm |
| u | Utilization | 1 | |
| W | PA concentration | 0.95 | |
| Anode feed | 100% H2 | ||
| Cathode feed | 21% O2 (air) | ||
| i | Current density | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Frensch, S.; Liso, V.; Li, N.; Sahlin, S.L.; Cinti, G.; Simon Araya, S. Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell. Energies 2022, 15, 5651. https://doi.org/10.3390/en15155651
Zhou M, Frensch S, Liso V, Li N, Sahlin SL, Cinti G, Simon Araya S. Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell. Energies. 2022; 15(15):5651. https://doi.org/10.3390/en15155651
Chicago/Turabian StyleZhou, Mengfan, Steffen Frensch, Vincenzo Liso, Na Li, Simon Lennart Sahlin, Giovanni Cinti, and Samuel Simon Araya. 2022. "Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell" Energies 15, no. 15: 5651. https://doi.org/10.3390/en15155651
APA StyleZhou, M., Frensch, S., Liso, V., Li, N., Sahlin, S. L., Cinti, G., & Simon Araya, S. (2022). Modeling the Performance Degradation of a High-Temperature PEM Fuel Cell. Energies, 15(15), 5651. https://doi.org/10.3390/en15155651










