Research Summary and Literature Review on Modelling and Simulation of Heat Pumps for Simultaneous Heating and Cooling for Buildings
Abstract
:1. Introduction
2. Thermodynamic Aspects and Optimization Methods
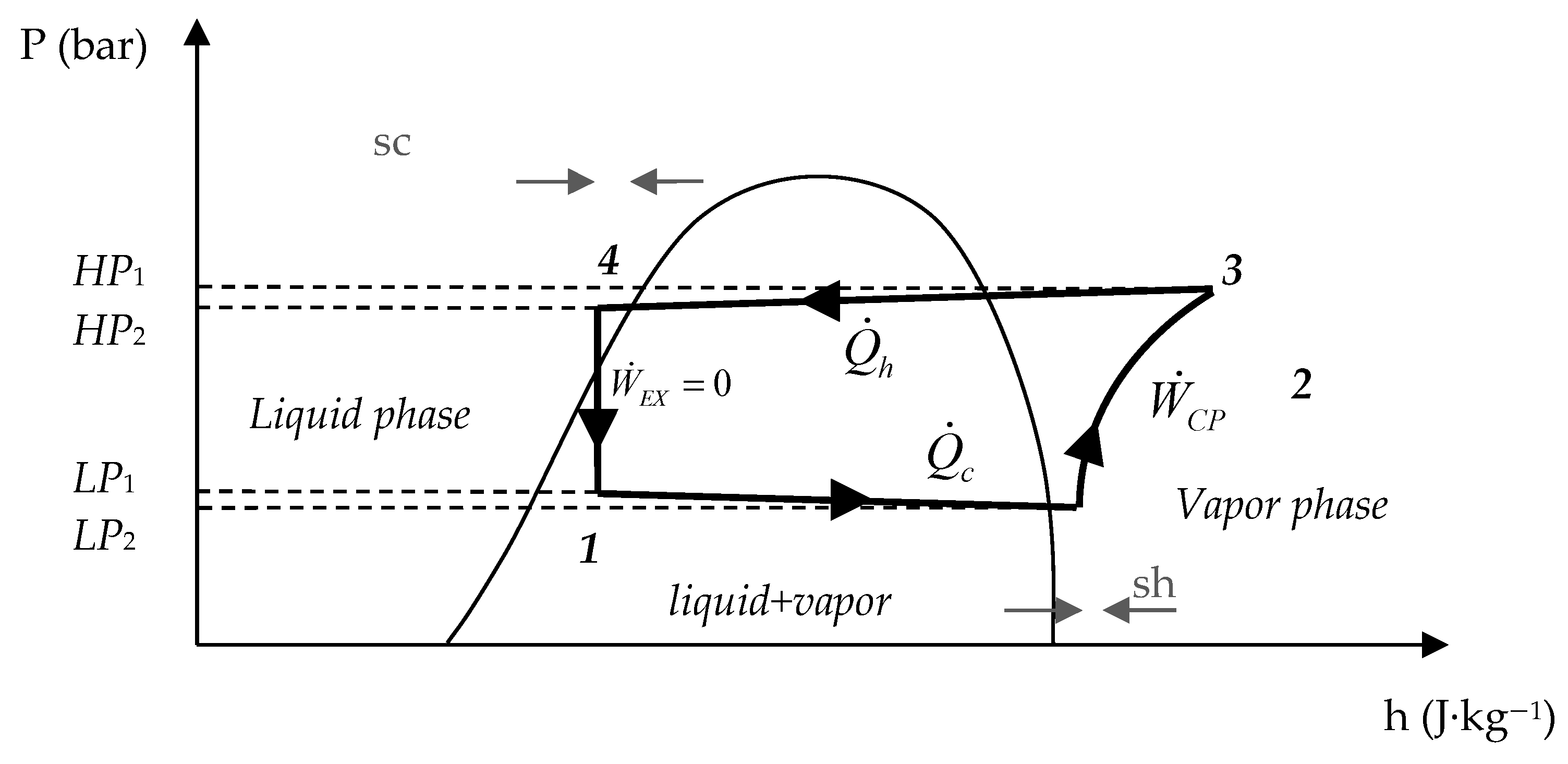
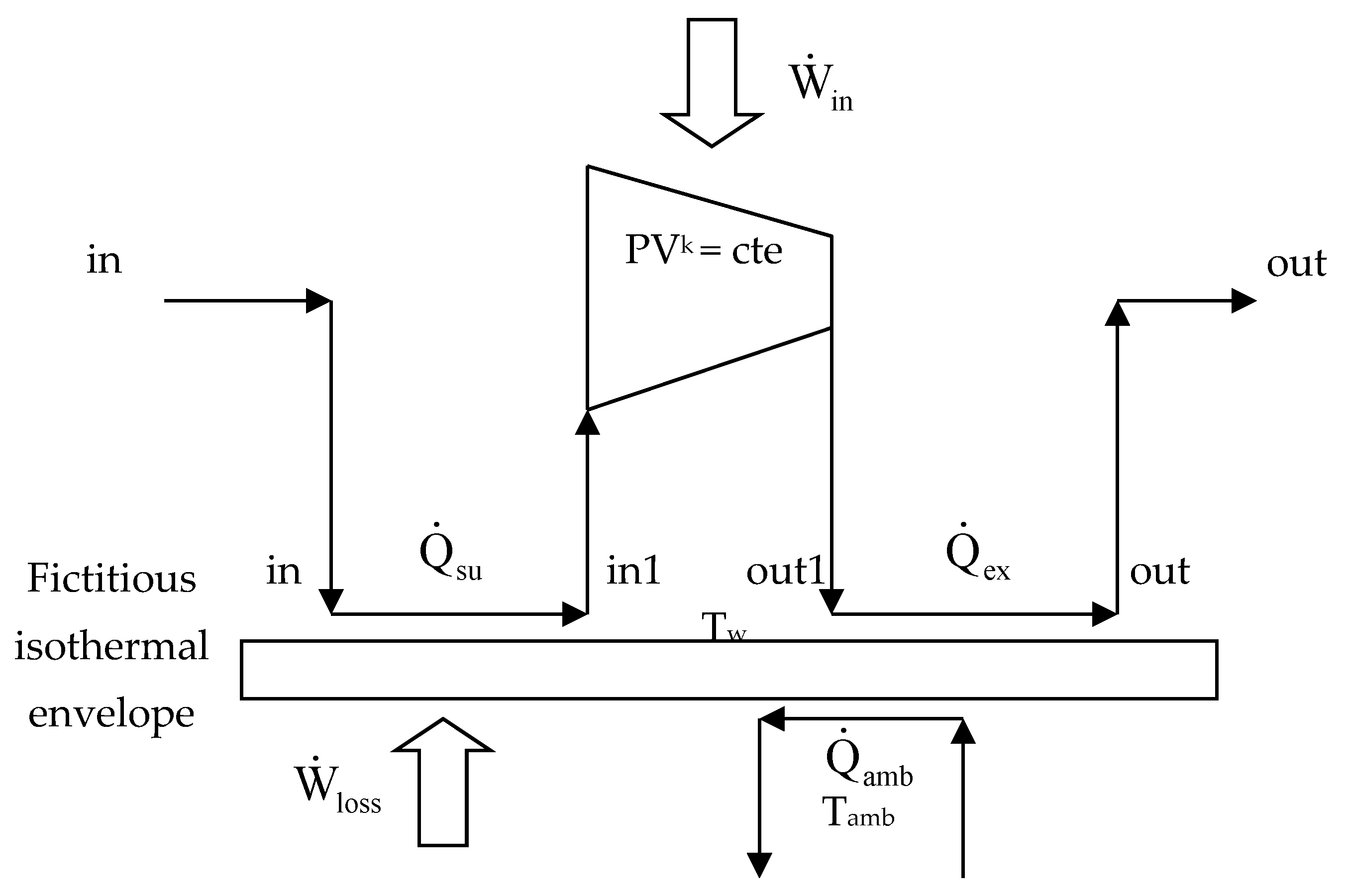
2.1. Energy Analysis
2.2. Exergy Analysis
2.3. Complementary Methods
3. Refrigerants
3.1. Safety Issues
- IEC 60335-2-24: Particular requirements for refrigerating appliances, ice-cream appliances and icemakers.
- IEC 60335-2-34: Particular requirements for motor-compressors.
- IEC 60335-2-89: Particular requirements for commercial refrigerating appliances and icemakers with an incorporated or remote refrigerant unit or motor-compressor.
- IEC 60335-2-40: Particular requirements for electrical heat pumps, air-conditioners and dehumidifiers.
3.2. Environmental Issues
- GWP is assessed over a 100-year horizon (kgCO2);
- m is the mass of fluid (kg);
- L is the annual leakage rate (commonly 2 to 5%/year);
- n is the system’s lifetime (commonly 15 to 20 years);
- α is the recovery rate at end of life (commonly 75 to 85%);
- E is the annual electric energy consumption (kWh/year);
- and β is the emission factor representing the CO2 content per kWh consumed (kgCO2/kWh).
3.3. Principal Families of Refrigerants
- The hundreds digit is the number of atoms of carbon in the molecule minus 1.
- The tens digit is the number of hydrogen atoms plus one.
- The units’ digit directly indicates the number of fluorine atoms.
- 4, it is a zeotropic or quasi-azeotropic mixture with a temperature shift at constant pressure between the bubble temperature and the dew temperature greater than 1 K. For example, R404A is a mixture of HFC134a, HFC125 and HFC143a, R407C is a mixture of HFC32, HFC125 and HFC134a and R410A, widely used in heat pumps, is a mixture of HFC32 and HFC125.
- 5, these are azeotropic mixtures (boiling at fixed temperature and composition). For example, R502 is a mixture of HCFC22 and CFC115. R513A is a mixture of HFO1234yf and HFC134a.
- 6, it is an organic compound other than CFCs, HCFCs and HFCs. For example, isobutene is referenced to R600a. Propane, C3H8 or R290, follows the classic CFC nomenclature.
- 7, it is an inorganic compound followed by the value of the molar mass. For example, R717 is the benchmark for ammonia, 17 being the molar mass of the ammonia molecule, R718 and R744 are water and carbon dioxide, respectively, used as refrigerants.
3.3.1. Chlorofluorocarbons (CFC) and Hydrochlorofluorocarbons (HCFC)
3.3.2. Hydrofluorocarbons (HFC)
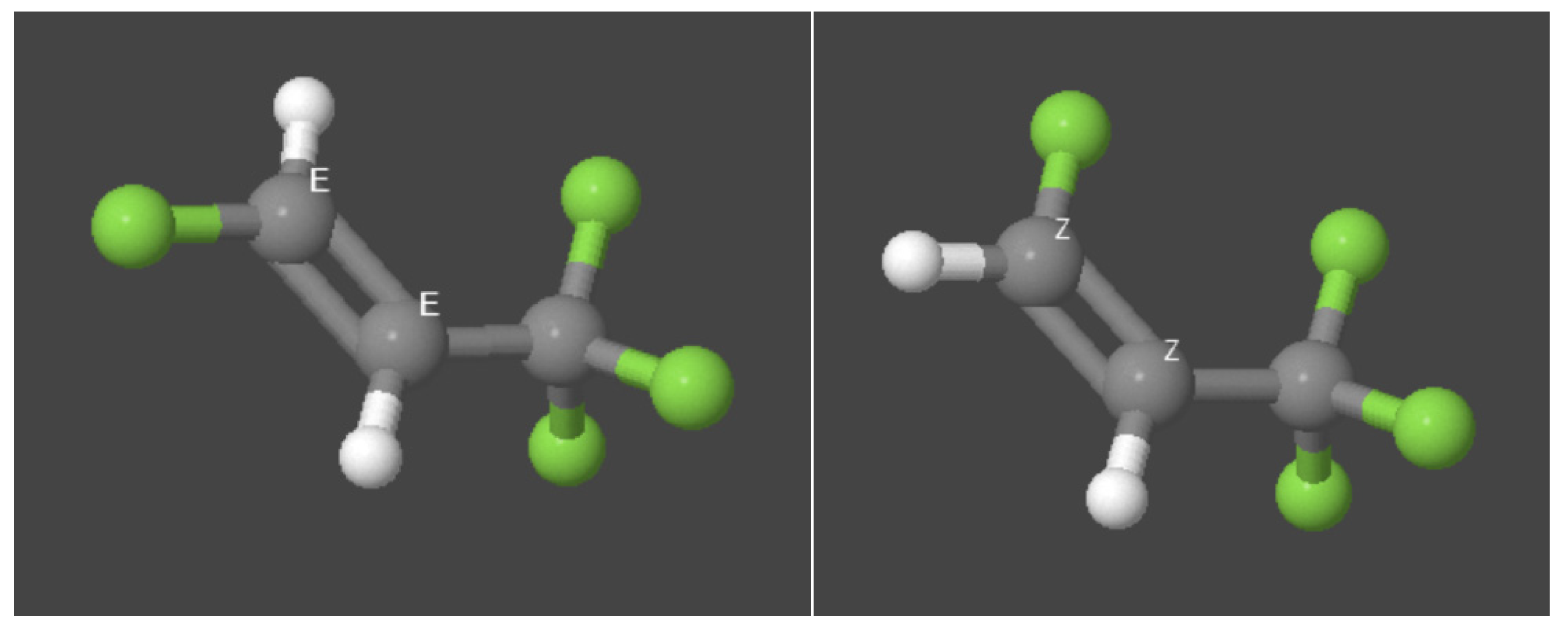
3.3.3. Hydrofluoroolefins (HFO)
3.3.4. Ammonia (R717, NH3)
3.3.5. Hydrocarbons (HC)
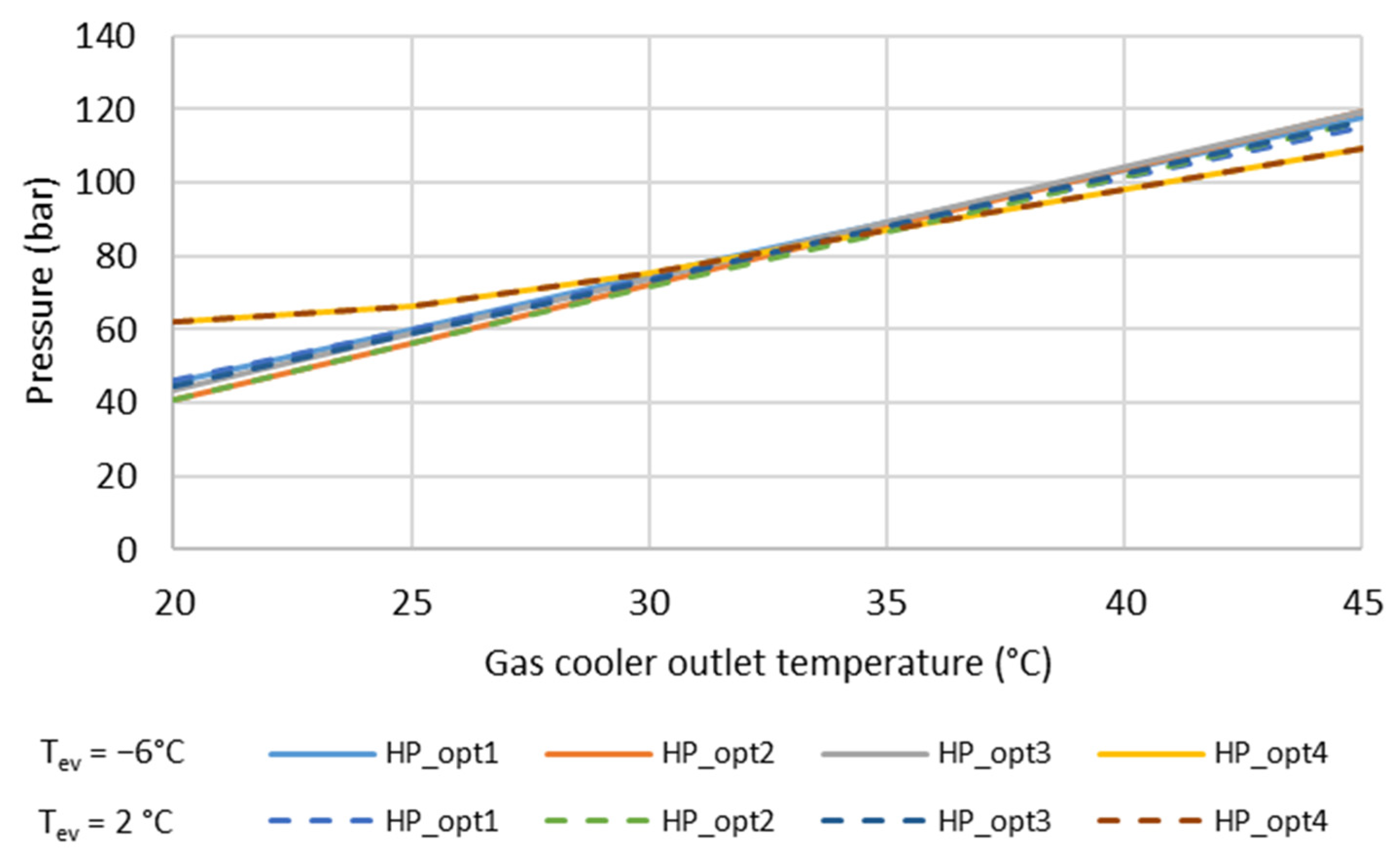
3.3.6. Carbon Dioxide (R744, CO2)
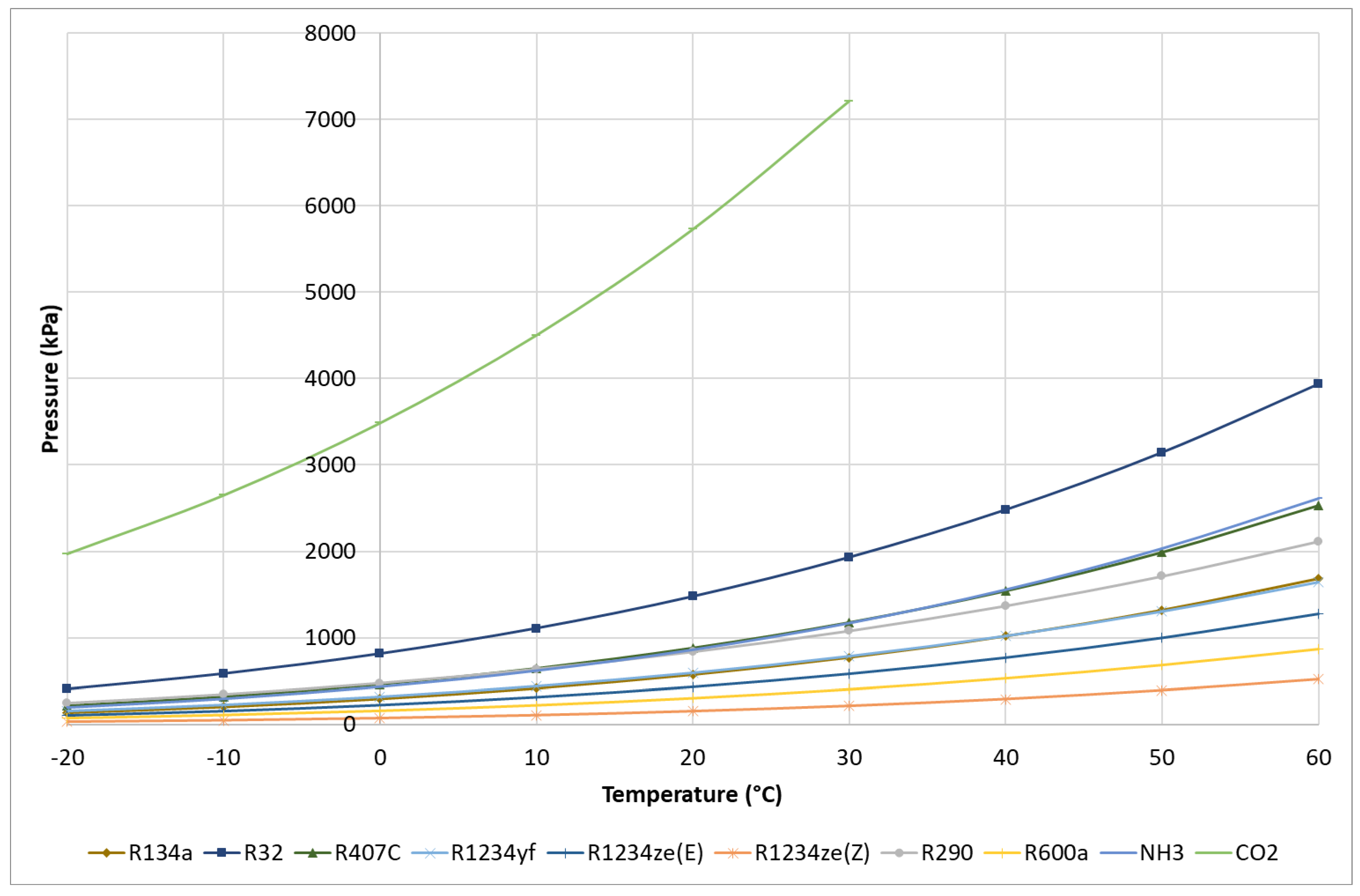
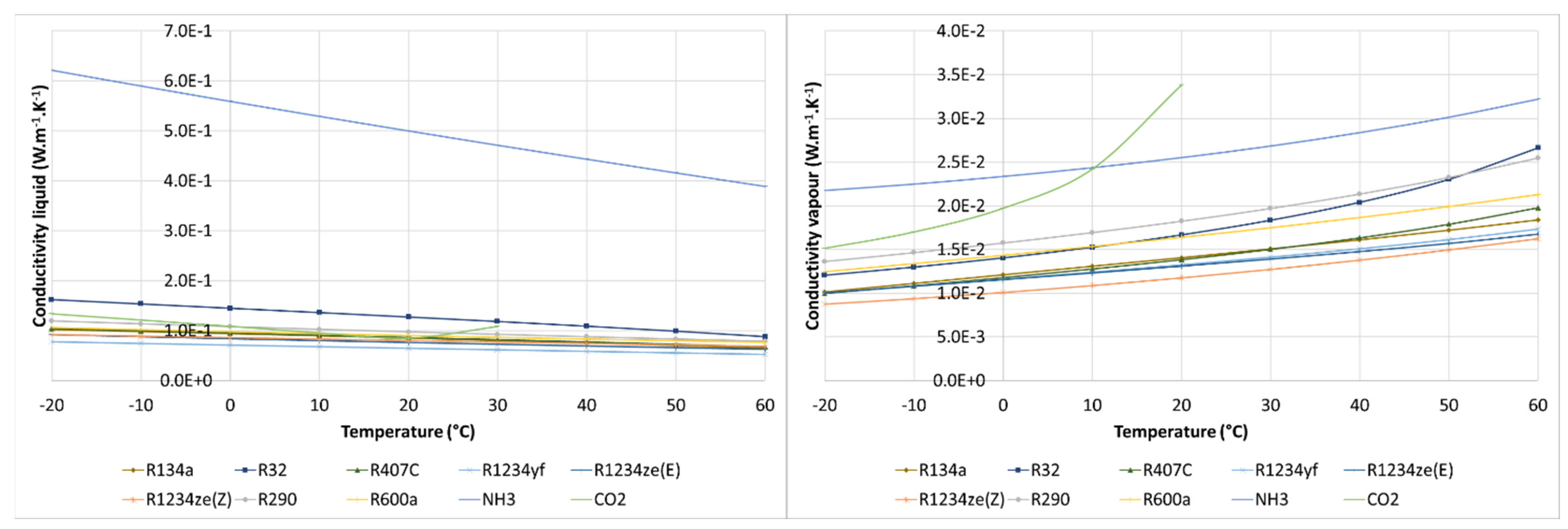
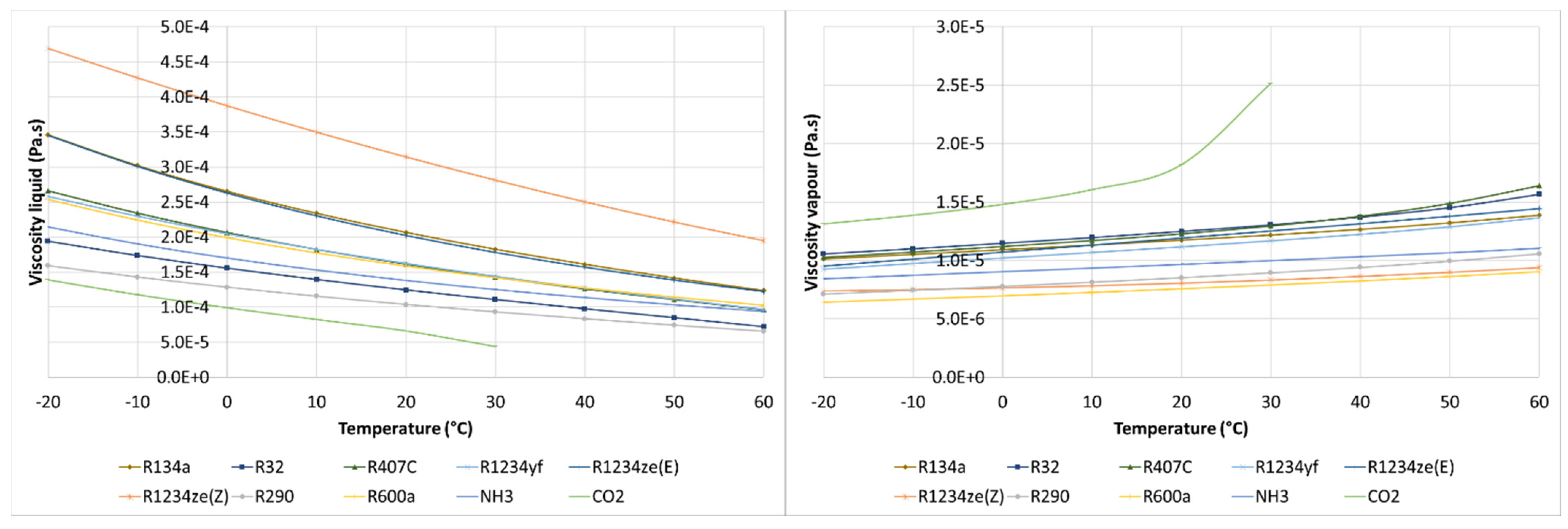
3.4. Comparison of Thermophysical Properties
- CFC R12;
- HCFC R22;
- HFCs R134a and R32;
- mixture of HFCs R407C;
- HFOs R1234yf, R1234ze(E) and R1234ze(Z);
- HCs R290 and R600a;
- NH3;
- CO2.
3.5. Comparison of Refrigerants through Cycle Performance
- Superheating is equal to 5 K.
- A non-useful superheating in the suction line is equal to 1 K.
- Subcooling is equal to 2 K.
- Pressure drops inside heat exchangers are neglected.
- Isentropic efficiency is equal to 0.7.
3.6. Discussion on Refrigerants
4. Applications of Heat Pumps for Simultaneous Heating and Cooling
- low and high temperature ambient heating;
- ambient cooling or air conditioning (with humidity control);
- domestic hot water production;
- seawater or brackish water desalination;
- commercial refrigeration;
- industrial refrigeration;
- industrial processes.
- the outside air;
- the air extracted by mechanical ventilation;
- groundwater from water tables;
- surface water;
- a building water loop as an internal balancing network;
- wastewater;
- condensed water from cooling towers;
- the ground by a water loop in a geothermal well or by a direct expansion of refrigerant fluid;
- solar collectors;
- heat rejected by industrial processes.
4.1. Simultaneous Heating and Cooling in Buildings
- Hotel: 135 L/day and per person at 60 °C [56].
- Residential: 40 L/day and per person at 60 °C [56].
- Offices: 5 L/day and per person at 60 °C [57].
- Shop: 10 L/day and per person at 45 °C [56].
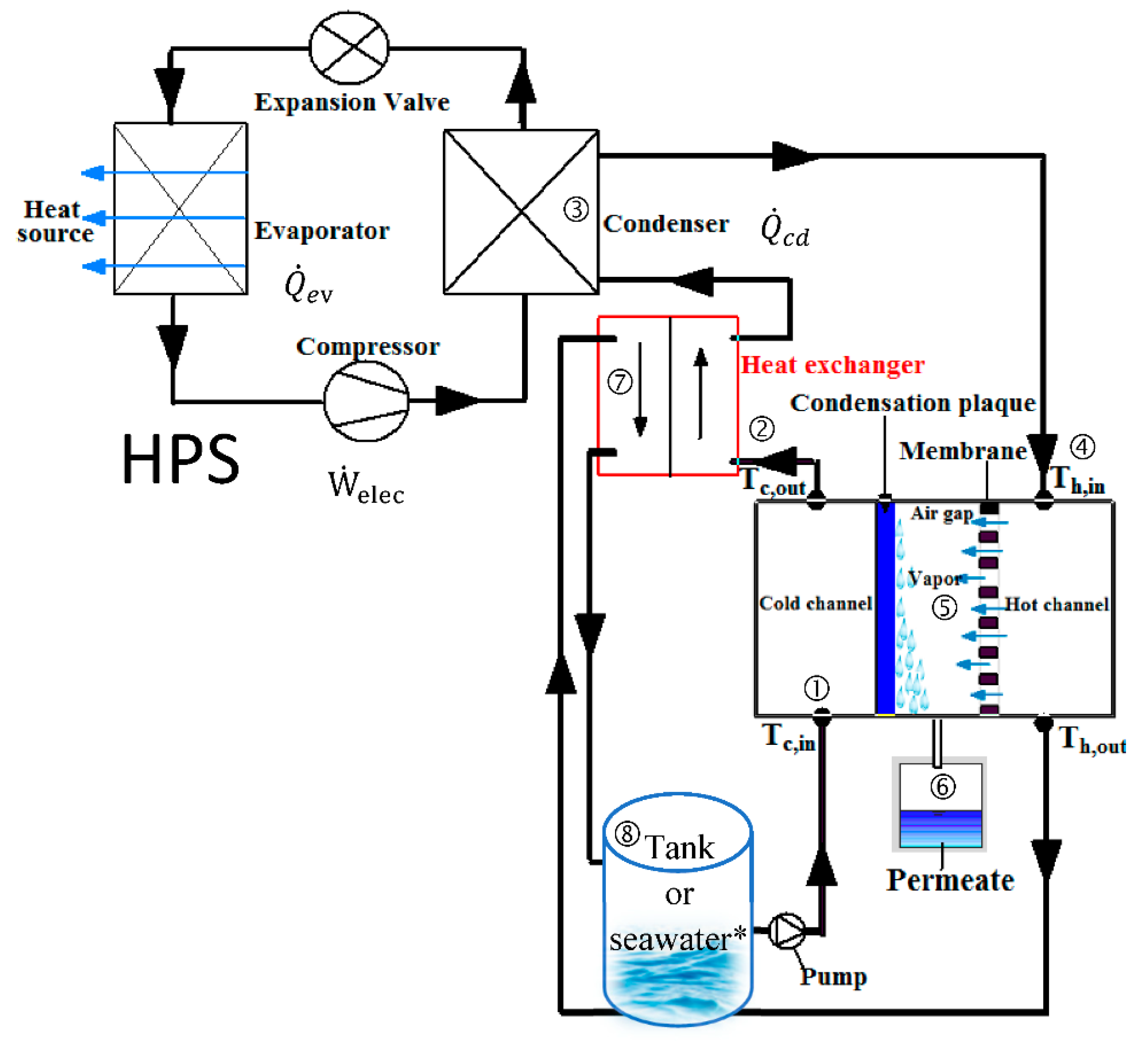
4.2. Simultaneous Cooling and Desalination
4.3. Discussion on Simultaneous Heating and Cooling Demands
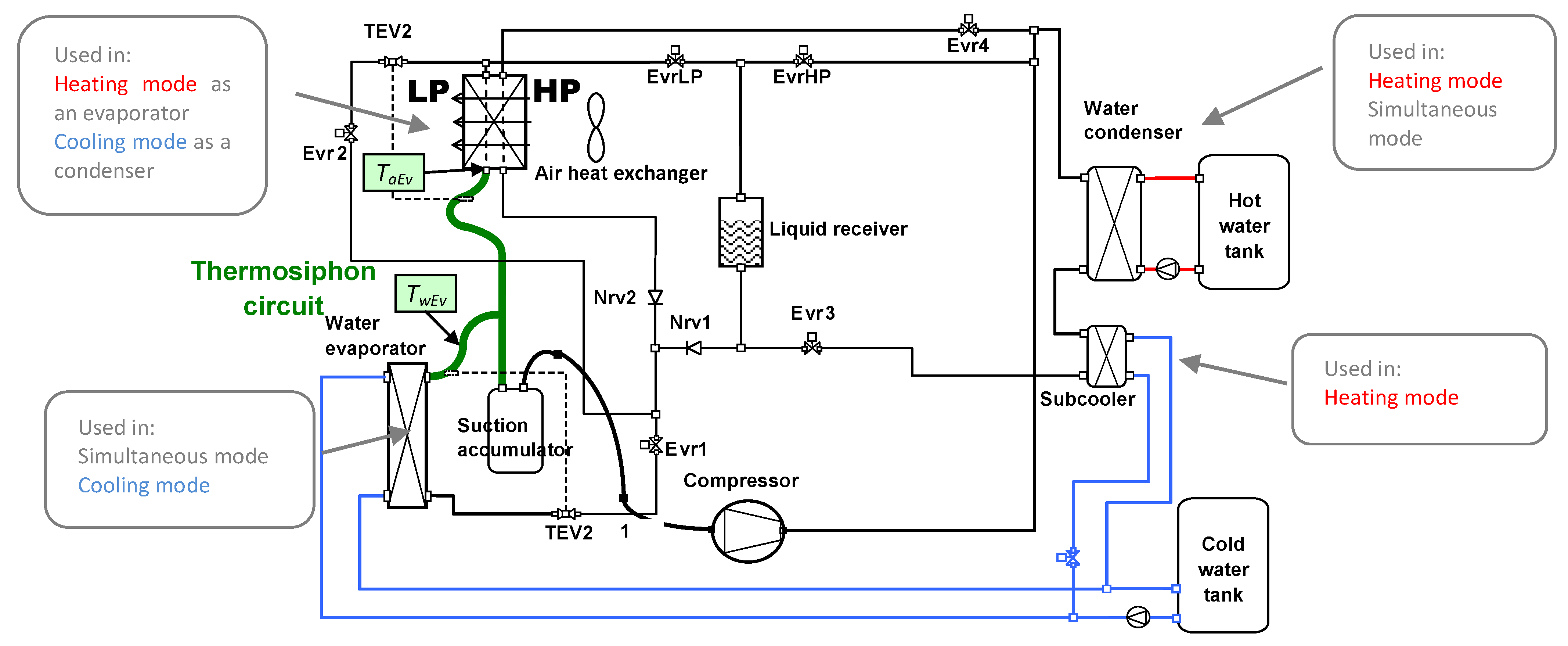
5. Literature Review of Simultaneous Heating and Cooling Heat Pump Systems
5.1. Patents
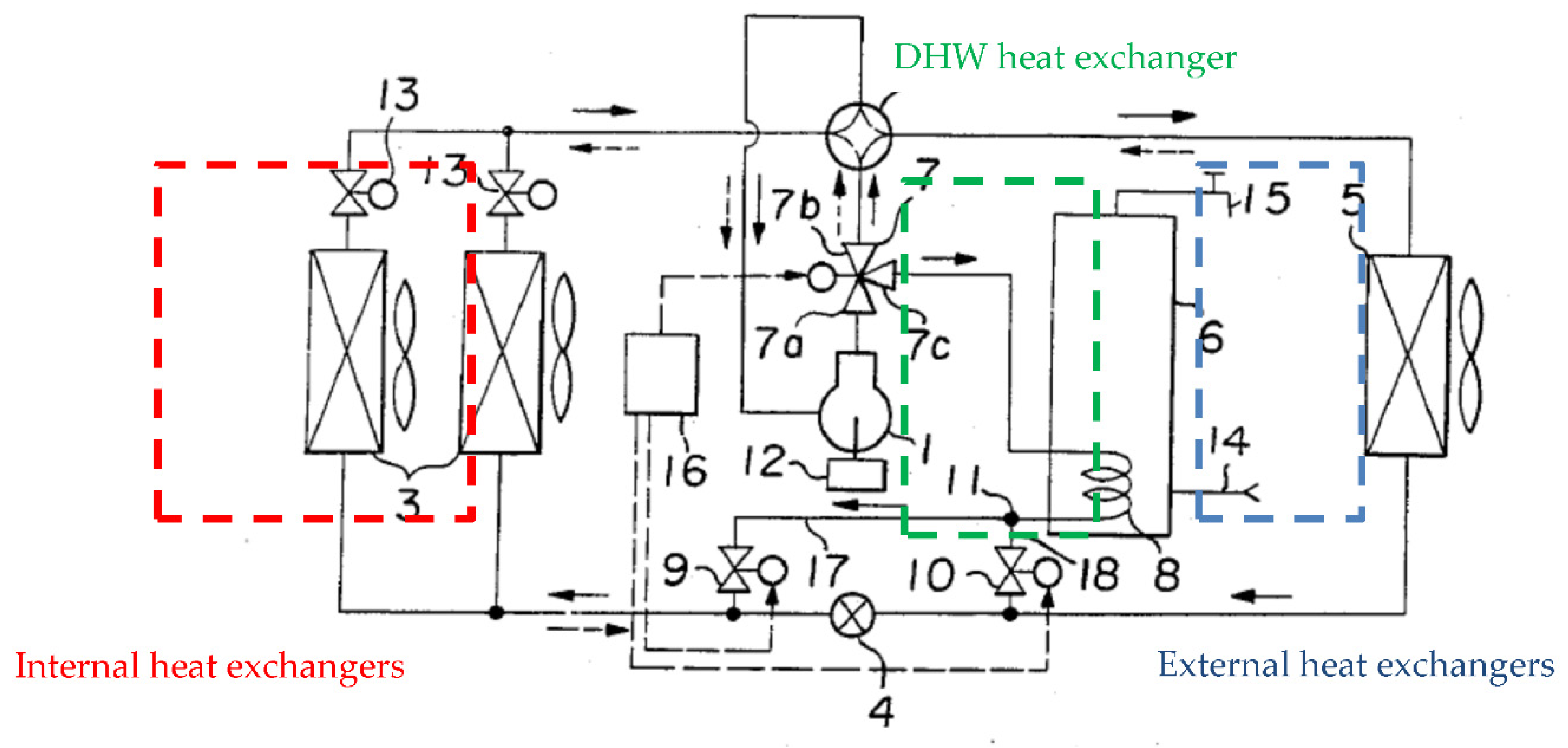
- Heating only mode (condensation in the internal exchangers and evaporation in the external air exchanger).
- Heating and DHW production mode (condensation in the internal exchangers as well as the coil and evaporation in the (external air exchanger).
- Cooling only mode (condensation in the external air exchanger and evaporation in the internal exchangers).
- Cooling and DHW production mode (condensation in the coil and evaporation in the internal exchangers).
5.2. Scientific Articles
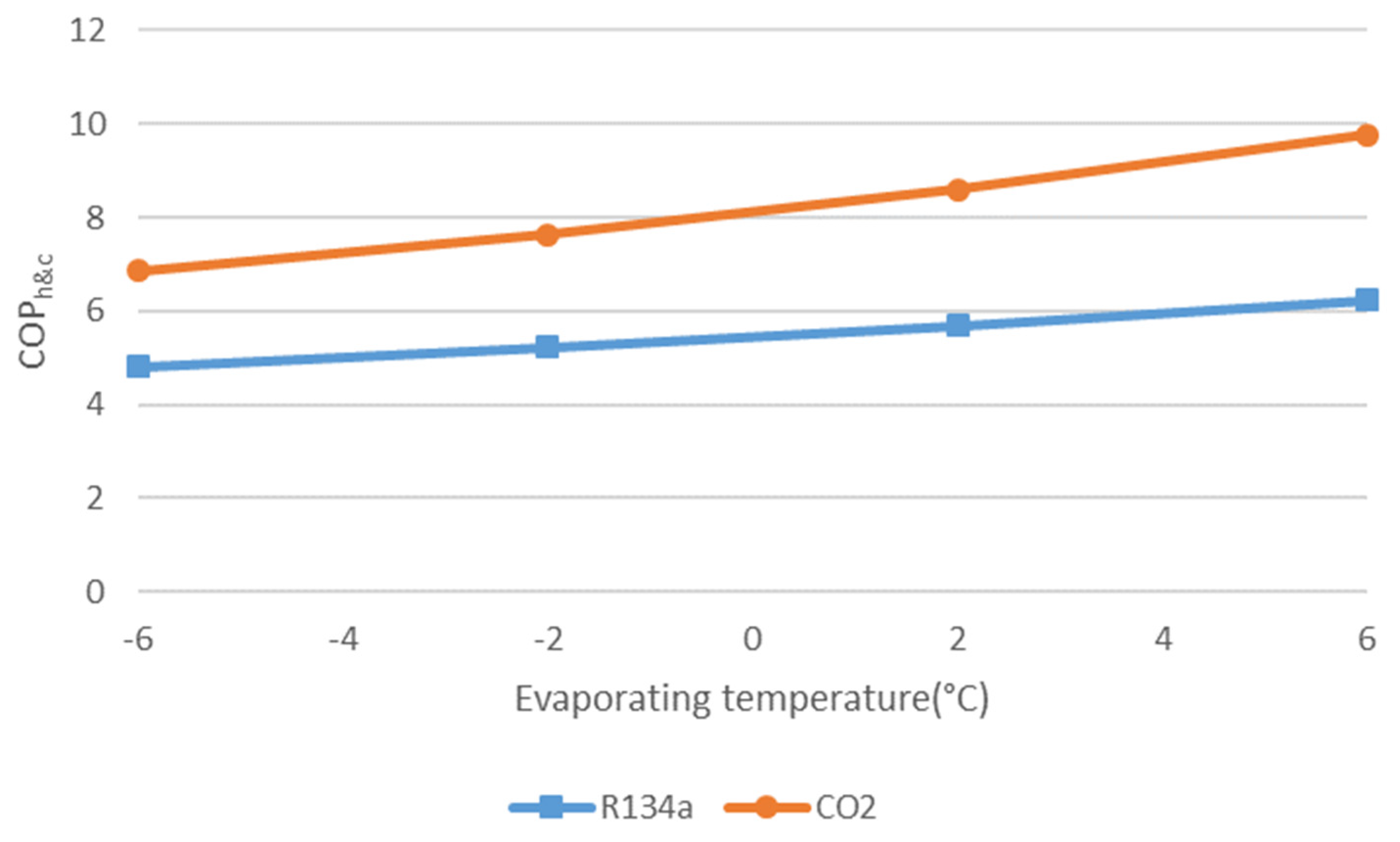
- A HPS improves the seasonal coefficient of performance compared to a reversible heat pump. The improvement can reach up to 20% depending on the study case.
- Seasonal coefficient of performance and exergy efficiency are better when simultaneous demands are high. The exergy efficiency is very sensitive to this aspect.
- CO2 exhibits better performance improvements than R407C when switching from a reversible heat pump to an HPS.
- The propane used in a HPS gives better energy and environmental performance than HFC R407C and HFO R1234yf.
- The environmental impact assessed in terms of TEWI is reduced by the use of a HPS compared to a reversible heat pump.
- The GOR (gained output ratio) described below;
- The PR (performance ratio) in kg of freshwater produced per MJ of energy consumed;
- The ExR (exergy ratio) in kg of freshwater produced per MJ of exergy consumed;
- Specific energy consumption (or SEC for specific energy consumption) in kWh/m3;
- For membrane systems, the production of fresh water in l·m−2·h−1, sometimes called LMH relating to the membrane surface.
- Entry of seawater into the cold channel to cool the condensation plate.
- Preheating by the heat of the brine, provided by an internal exchanger.
- Heats to the set temperature thanks to the condensation of the refrigerant from the HPS.
- Entering the hot channel.
- Evaporation of pure water at the membrane surface, vapor transfer then condensation on the cold plate.
- Collecting of the permeate.
- Reuse the brine for preheating in the internal exchanger.
- Discharge of the brine into a tank or directly into the sea depending on the application (symbol * in the figure).
6. Conclusions
Funding
Conflicts of Interest
Nomenclature
| Abbreviations | |
| CFC | chlorofluorocarbon |
| DHW | domestic hot water |
| ECBCS | Energy Conservation in Building Community Systems |
| EES | Engineering Equation Solver |
| HC | hydrocarbon |
| HCFC | hydrochlorofluorocarbon |
| HFC | hydrofluorocarbon |
| HFO | hydrofluoroolefin |
| HPS | heat pump for simultaneous heating and cooling |
| IEA | International Energy Agency |
| MD | membrane distillation |
| PE | primary energy |
| RE | environmental regulation |
| RT | thermal regulation |
| TFA | trifluoroacetic acid |
| TRNSYS | Transient System Simulation Software |
| Latin letters | |
| c | relative clearance volume (-) |
| COP | coefficient of performance (-) |
| Cp | specific heat (J·kg−1·K−1) |
| E | energy consumption (kWh) |
| Ex | exergy (J) |
| ex | specific exergy (J·kg−1) |
| exergy rate (W) | |
| ExR | exergy ratio (kg·MJ−1) |
| g | gravitational acceleration (m·s−2) |
| GOR | gained output ratio (-) |
| GWP | Global Warming Potential (kgCO2 equivalent over a 100-year horizon) |
| h | specific enthalpy (kJ·kg−1) |
| HP | high pressure (Pa), (bar) |
| k | polytropic coefficient (-) |
| L | annual leakage rate (%·an−1) |
| LEL | lower explosive limit (%) |
| LP | low pressure (Pa), (bar) |
| m | mass (kg) |
| mass flow rate (kg·s−1) | |
| n | lifetime (years), number of moles (mol) |
| ODP | ozone depletion potential (-) |
| P | pressure (Pa) |
| PR | performance ratio (kg·MJ−1) |
| Q | heat amount (J) |
| thermal capacity (W) | |
| S | entropy (J·K−1) |
| s | specific entropy (J·kg−1·K−1) |
| SCOP | seasonal COP (-) |
| SEC | specific energy consumption (kWh·m−3) |
| T | temperature (K), (°C) |
| t | time (s) |
| TEWI | total equivalent warming impact (kgCO2) |
| tt | total time (s) |
| U | internal energy (J) |
| u | velocity (m·s−1) |
| UEL | upper explosive limit (%) |
| V | volume (m3) |
| v | specific volume (m3·kg−1) |
| Vswept | swept volume (m3·s−1) |
| mechanical power (W) | |
| z | altitude (m) |
| Greek letters | |
| α | recovery rate (%) |
| β | greenhouse gas emission ratio (kgCO2·kWh−1) |
| Δ | discrepancy |
| η | efficiency (-) |
| μi0 | chemical potential (J·mol−1) |
| ρ | density (kg·m−3) |
| Subscripts | |
| 0 | reference state |
| amb | ambient |
| aux | auxiliaries |
| c | cooling |
| cw | cold water |
| CD | condenser |
| cd | condensation |
| ch | chemical |
| CP | compressor |
| crit | critical |
| D | destructed |
| eco | economical |
| elec | electric |
| EV | evaporator |
| ev | evaporation |
| EX | expansion valve |
| ex | exergetic |
| gc | gas cooler |
| h | heating |
| h&c | heating and cooling |
| hw | hot water |
| in | internal, inlet |
| is | isentropic |
| kin | kinetic |
| meca | mechanical |
| opt | optimal |
| out | outlet |
| ph | physical |
| pot | potential |
| pro | process |
| so | source |
| th | thermal |
| vap | vaporization |
| vol | volumetric |
| w | water |
| wm | without movement |
References
- Agence de l’Environnement et de la Maîtrise de l’Energie. Climat, Air et Energie—Chiffres-Clés; ADEME: Montrouge, France, 2015. [Google Scholar]
- Pardiñas, Á.Á.; Jokiel, M.; Schlemminger, C.; Selvnes, H.; Hafner, A. Modeling of a CO2-Based Integrated Refrigeration System for Supermarkets. Energies 2021, 14, 6926. [Google Scholar] [CrossRef]
- Byrne, P. Advances in air-source heat pump water heaters. In Novel Concepts for Energy-Efficient Water Heating Systems. Theoretical Analysis and Experimental Investigation; Barbin, D.F., Silveira, V., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013; Chapter 4; pp. 93–122. [Google Scholar]
- Byrne, P.; Ghoubali, R.; Miriel, J. Scroll compressor modelling for heat pumps using hydrocarbons as refrigerants. Int. J. Refrig. 2014, 41, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I.; Cengel, Y.A. Energy, entropy and exergy concepts and their roles in thermal engineering. Entropy 2001, 3, 116–149. [Google Scholar] [CrossRef]
- Torío, H.; Angelotti, A.; Schmidt, D. Exergy analysis of renewable energy-based climatisation systems for buildings: A critical view. Energy Build. 2009, 41, 248–271. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Exergy Analysis of Heating, Refrigerating and Air Conditioning: Methods and Applications. In Exergy and Its Ties to the Environment, Economics, and Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 1; pp. 1–42. [Google Scholar]
- Ahamed, J.U.; Saidur, R.; Masjuki, H.H. A review on exergy analysis of vapor compression refrigeration system. Renew. Sustain. Energy Rev. 2011, 15, 1593–1600. [Google Scholar] [CrossRef]
- Diaby, A.T.; Byrne, P.; Loulergue, P.; Balannec, B.; Szymczyk, A.; Mare, T.; Sow, O. Design study of the coupling of an air gap membrane distillation unit to an air conditioner. Desalination 2017, 420, 308–317. [Google Scholar] [CrossRef]
- Byrne, P.; Ghoubali, R. Exergy analysis of heat pumps for simultaneous heating and cooling. Appl. Therm. Eng. 2019, 149, 414–424. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G. Advanced exergetic evaluation of refrigeration machines using different working fluids. Energy 2009, 34, 2248–2258. [Google Scholar] [CrossRef]
- Byrne, P.; Diaby, A.T.; Ghoubali, R.; Maré, T.; Sow, O. Fluides frigorigènes naturels et fluides a faible GWP pour petites pompes à chaleur. In Proceedings of the Colloque Interuniversitaire Franco-Québécois, CIFQ 2017, Saint-Lô, France, 22–24 May 2017. [Google Scholar]
- ANSI/ASHRAE Standard 34; Designation and Safety Classification of Refrigerants. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2004.
- AFNOR, NF EN 378-1; Systèmes de réfrigération et pompes à chaleur—Exigences de sécurité et d’environnement—Partie 1: Exigences de base, définitions, classification et critères de choix. Afnor EDITIONS: Saint-Denis, France, 2012.
- Directive 2014/68/UE; du Parlement Européen et du Conseil du 15 mai 2014 relative à l’Harmonisation des léGislations des États Membres Concernant la Mise à Disposition sur le Marché des éQuipements Sous Pression. European Union: Brussels, Belgium, 2014.
- IEC 60335; Household and Similar Electrical Appliances—Safety. International Electrotechnical Commission: Geneva, Switzerland, 2002.
- Granryd, E. Hydrocarbons as refrigerants—An overview. Int. J. Refrig. 2001, 24, 15–24. [Google Scholar] [CrossRef]
- Palm, B. Hydrocarbons as refrigerants in small heat pump and refrigeration systems—A review. Int. J. Refrig. 2008, 31, 552–563. [Google Scholar] [CrossRef]
- Corberan, J.M.; Segurado, J.; Colbourne, D.; Gonzalvez, J. Review of standards for the use of hydrocarbon refrigerants in A/C, heat pump and refrigeration equipement. Int. J. Refrig. 2008, 31, 748–756. [Google Scholar] [CrossRef]
- Guilpart, J. Evaluation des risques liés aux fluides frigorigènes naturels. Tech. L’Ingénieur 1999, BE9750, 19. [Google Scholar] [CrossRef]
- Claret, J.C. Retour D’expérience: L’ammoniac et La Réfrigeration; SEI/BARPIED00389; Ministère de L’environnement, Service de L’environnement Industriel: Paris, France, 1995.
- Islam, M.A.; Srinivasan, K.; Thu, K.; Saha, B.B. Assessment of total equivalent warming impact (TEWI) of supermarket refrigeration systems. Int. J. Hydrogen Energy 2017, 42, 26973–26983. [Google Scholar] [CrossRef]
- Davies, T.W.; Caretta, O. A low carbon, low TEWI refrigeration system design. Appl. Therm. Eng. 2004, 24, 1119–1128. [Google Scholar] [CrossRef]
- Cavallini, A. Working fluids for mechanical refrigeration. Invited paper at the 19th International Congress of Refrigeration, The Hague, August 1995. Int. J. Refrig. 1996, 19, 485–496. [Google Scholar] [CrossRef]
- Llopis, R.; Calleja-Anta, D.; Maiorino, A.; Nebot-Andrés, L.; Sánchez, D.; Cabello, R. TEWI analysis of a stand-alone refrigeration system using low-GWP fluids with leakage ratio consideration. Int. J. Refrig. 2020, 118, 279–289. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) No 517/2014: On Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006; Official Journal of European Union: Brussels, Belgium, 2014. [Google Scholar]
- UNEP. The Kigali Amendment to the Montreal Protocol: HFC Phase-down; Ozone Action Fact Sheet United Nations Environment Program (UNEP): Nairobi, Kenya, 2016; pp. 1–7. [Google Scholar]
- Réseau Action Climat France. Available online: http://www.rac-f.org (accessed on 28 March 2022).
- Navarro-Esbri, J.; Mendoza-Miranda, J.M.; Mota-Babiloni, A.; Barragan-Cervera, A.; Belman-Flores, J.M. Experimental analysis of R1234yf as a drop-in replacement for R134a in a vapor compression system. Int. J. Refrig. 2013, 36, 870–880. [Google Scholar] [CrossRef]
- Zilio, C.; Brown, J.S.; Schiochet, G.; Cavallini, A. The refrigerant R1234yf in air conditioning systems. Energy 2011, 36, 6110–6120. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, D. A brief performance comparison of R1234yf and R134a in a bench tester for automobile applications. Appl. Therm. Eng. 2012, 35, 240–242. [Google Scholar] [CrossRef]
- Henne, S.; Shallcross, D.E.; Reimann, S.; Xiao, P.; Brunner, D.; O’Doherty, S.; Buchmann, B. Future emissions and atmospheric fate of HFC-1234yf from mobile air conditioners, in Europe. Environ. Sci. Technol. 2012, 46, 1650–1658. [Google Scholar] [CrossRef]
- Fukuda, S.; Kondou, C.; Takata, N.; Koyama, S. Low GWP refrigerants R1234ze(E) and R1234ze(Z)for high temperature heat pumps. Int. J. Refrig. 2014, 40, 161–173. [Google Scholar] [CrossRef]
- Calm, J.M. Emissions and environmental impacts from air-conditioning and refrigeration systems. Int. J. Refrig. 2002, 25, 293–305. [Google Scholar] [CrossRef]
- Duminil, M. Histoire et Evolution des Fluides Frigorigènes des Systèmes Frigorifiques à Compression. Rev. GÉNÉRale Du Froid Du Cond. d’Air 2008, 1080, 45–55. [Google Scholar]
- Chang, Y.S.; Kim, M.S.; Ro, S.T. Performance and heat transfer characteristics of hydrocarbon refrigerants in a heat pump system. Int. J. Refrig. 2000, 23, 232–242. [Google Scholar] [CrossRef]
- Liao, S.M.; Zhao, T.S.; Jakobsen, A. A correlation of optimal pressures in transcritical carbon dioxide cycles. Appl. Therm. Eng. 2000, 20, 831–841. [Google Scholar] [CrossRef]
- Byrne, P.; Miriel, J.; Lénat, Y. Design and simulation of a heat pump for simultaneous heating and cooling using HFC or CO2 as a working fluid. Int. J. Refrig. 2009, 32, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, H.; Cai, S.W.; Shao, L.L.; Zhang, C.L. Minimizing COP loss from optimal high pressure correlation for transcritical CO2 cycle. Appl. Therm. Eng. 2015, 89, 656–662. [Google Scholar] [CrossRef]
- Diaby, A.T.; Byrne, P.; Mare, T. Simulation of heat pumps for simultaneous heating and cooling using CO2. Int. J. Refrig. 2019, 106, 616–627. [Google Scholar] [CrossRef]
- Qi, P.C.; He, Y.L.; Wang, X.L.; Meng, X.Z. Experimental investigation of the optimal heat rejection pressure for a transcritical CO2 heat pump water heater. Appl. Therm. Eng. 2013, 56, 120–125. [Google Scholar] [CrossRef]
- Lorentzen, G. Revival of Carbon Dioxide as a Refrigerant. Int. J. Refrig. 1994, 17, 292–301. [Google Scholar] [CrossRef]
- Neksa, P. CO2 Heat Pump Systems. Int. J. Refrig. 2002, 25, 421–427. [Google Scholar] [CrossRef]
- Kusakari, K. The spread situation and the future view of the CO2 refrigerant heat pump water heater in Japan. In Proceedings of the 7th IIR Gustav Lorentzen Conference on Natural Working Fluids, Trondheim, Norway, 29–31 May 2006. [Google Scholar]
- Stene, J. Residential CO2 heat pump system for combined space heating and hot water heating. Int. J. Refrig. 2005, 28, 1259–1265. [Google Scholar] [CrossRef] [Green Version]
- Fournaison, L. Evolution des Fluides Frigoporteurs. Rev. Générale Du Froid Du Cond. d’Air 2008, 1080, 45–55. [Google Scholar]
- AFNOR, NF EN 14511-3; Climatiseurs, Groupes Refroidisseurs de Liquide et Pompes à Chaleur Avec Compresseur Entraîné par Moteur éLectrique Pour le Chauffage et la Réfrigération. Partie 3: Méthodes D’Essai. Afnor EDITIONS: Saint-Denis, France, 2004.
- AFNOR, NF EN 16147; Pompes à Chaleur Avec Compresseur Entraîné Par Moteur Électrique—Essais, Détermination Des Performances et Exigences Pour le Marquage Des Appareils Pour eau Chaude Sanitaire. Afnor EDITIONS: Saint-Denis, France, 2017.
- Klein, S. Engineering Equation Solver, Version 10.200-3D, ©1992–2017; F-Chart Software: Madison, WI, USA, 2017. [Google Scholar]
- Technical University of Denmark, Department of Mechanical Engineering. Coolpack, A Collection of Simulation Tools for Refrigeration (1997–2001); Technical University of Denmark: Lyngby, Denmark, 2001. [Google Scholar]
- Stabat, P. Analysis of Heating and Cooling Demands in the Purpose of Assessing the Reversibility and Heat Recovery Potentials, IEA EBC Annex 48 Project Report R1. 2011. Available online: https://www.iea-ebc.org/Data/publications/EBC_Annex_48_Final_Report_R1.pdf (accessed on 28 March 2022).
- Bertagnolio, S.; Stabat, P. Review of Heat Recovery and Heat Pumping Solutions, IEA EBC Annex 48 Project Report R2. 2011. Available online: https://www.iea-ebc.org/Data/publications/EBC_Annex_48_Final_Report_R2.pdf (accessed on 28 March 2022).
- Ghoubali, R.; Byrne, P.; Miriel, J.; Bazantay, F. Simulation study of heat pumps for simultaneous heating and cooling coupled to buildings. Energy Build. 2014, 72, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Byrne, P.; Miriel, J.; Lénat, Y. Modelling and simulation of a heat pump for simultaneous heating and cooling. Build.Simul. Int. J. 2012, 5, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Klein, S. TRNSYS 17: A Transient System Simulation Program, Solar Energy Laboratory; University of Wisconsin: Madison, WI, USA, 2010; Available online: http://sel.me.wisc.edu/TRNSYS (accessed on 28 March 2022).
- Recknagel, H.; Sprenger, E.; Schramek, E.R. Recknagel, Manuel Pratique du Génie Climatique: Chauffage et Production d’eau Chaude Sanitaire; PYC Edition: Paris, France, 1996; p. 222. [Google Scholar]
- AICVF. Association des Ingénieurs en Climatique, Ventilation et Froid. Volume 6, Bâtiments Non réSidentiels; AICVF: Paris, France, 2000. [Google Scholar]
- Byrne, P.; Fournaison, L.; Delahaye, A.; Ait Oumeziane, Y.; Serres, L.; Loulergue, P.; Szymczyk, A.; Mugnier, D.; Malaval, J.-L.; Bourdais, R.; et al. A review on the coupling of cooling, desalination and solar photovoltaic systems. Renew. Sustain. Energy Rev. 2015, 47, 703–717. [Google Scholar] [CrossRef]
- Hunt, J.D.; de Assis Brasil Weber, N.; Zakeri, B.; Diaby, A.T.; Byrne, P.; Leal Filho, W.; Smith Schneider, P. Deep seawater cooling and desalination: Combining seawater air conditioning and desalination. Sustain. Cities Soc. 2021, 74, 103257. [Google Scholar] [CrossRef]
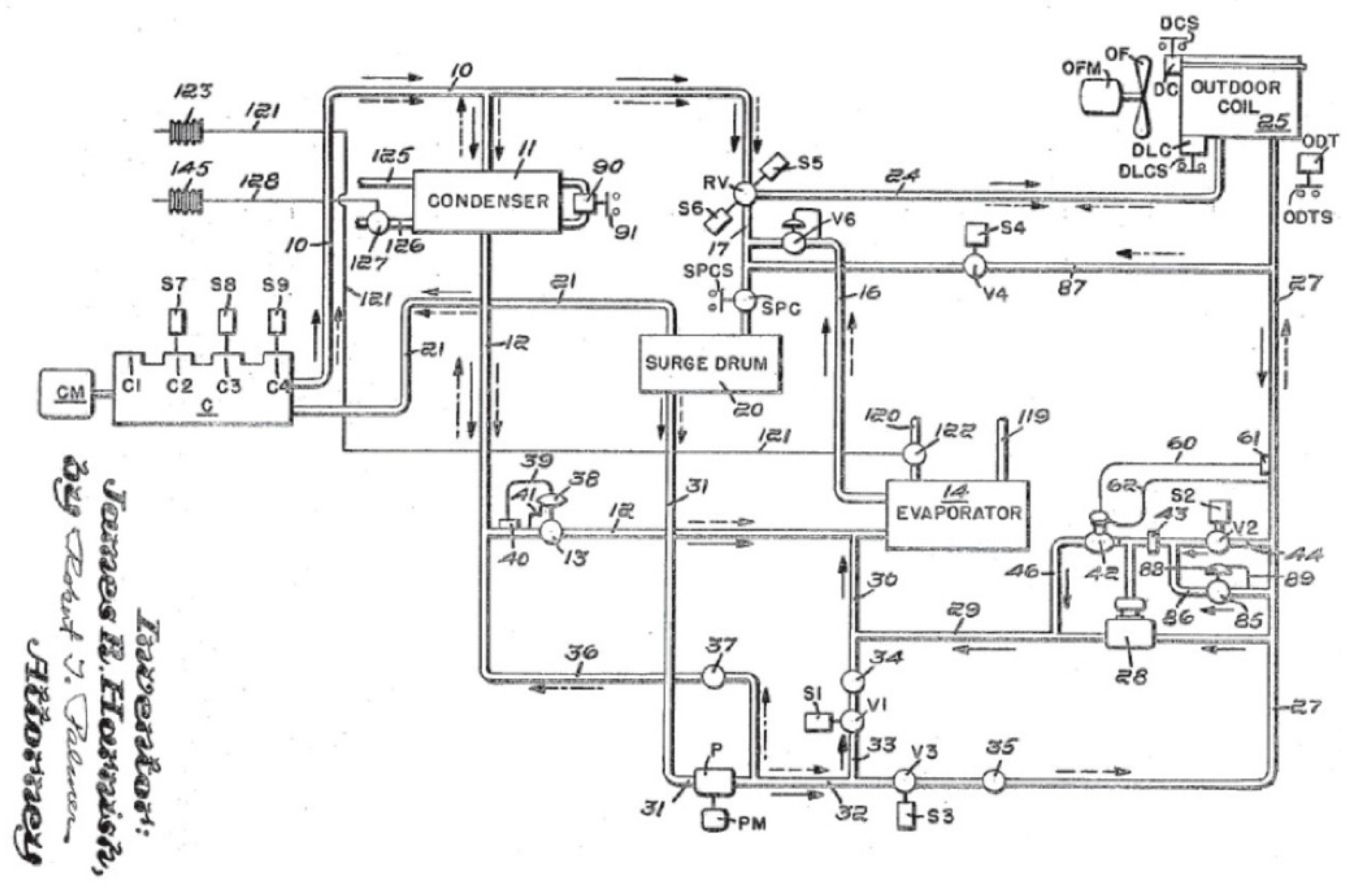
- Zimmerman, W.G. Heating and Cooling Air Conditioning System. U.S. Patent 2,581,744, 8 January 1952. [Google Scholar]
- Harnish, J.R.; Westinghouse Electric Corporation. Heat Pumps for Simultaneous Cooling and Heating. U.S. Patent 3,264,839, 9 August 1966. [Google Scholar]
- Yamazaki, K.; Otsubo, M.; Okuma, K.; Mitsubishi Electric Corp. Room-Warming/Cooling and Hot-Water Supplying Heat-Pump Apparatus. U.S. Patent 4,592,206, 3 June 1986. [Google Scholar]
- Matsuura, T.; Union Kogyo Kabushiki Kaisha. Apparatus and Method for Heating and Cooling with a Refrigerant. U.S. Patent 5,211,023, 18 May 1993. [Google Scholar]
- Jungwirth, C.A. Apparatus for Simultaneous Heating Cooling and Humidity Removal. U.S. Patent 6,751,972, 22 June 2004. [Google Scholar]
- Leballais, H.; CLIMATIK Sarl. Heating and Refrigerating System for e.g., Room of Building, Has Auxiliary Exchanger Connected to Loop and Operating in Condensing Mode in Place of Condenser or in Evaporating Mode in Place of Evaporator. F.R. Patent 2,886,388, 26 October 2007. [Google Scholar]
- Moreau, C. Mobile Confort Holding. Multi-Energy Thermodynamic Device with Simultaneous Production of Hot Water, Warm Water, Cold Water and Electricity. E.P. Patent 2,085,721, 5 August 2009. [Google Scholar]
- Ghoubali, R.; Bazantay, F.; Méar, L.; Miriel, J.; Byrne, P. Refrigerating Circuit, Facility Comprising such a Circuit and Corresponding Method. Patent WO2015/015104A1, 5 February 2015. [Google Scholar]
- Marnet, P.; Mantran, B.; Mallet, J.A.; Rybaka, R. La Centrale Thermodynamique de la Maison de l’ORTF, Application à la Climatisation; Annales de l’Institut Technique du Bâtiment et des Travaux Publics: Paris, France, 1964; p. 204. [Google Scholar]
- Le Goff, P. Exergetic, economic or ecologic optimisations of heat-cold-pumps. Entropie 1999, 220–221, 6–11. [Google Scholar]
- Lecrivain, E.; Laroche, G.; Vallot, A. La production simultanée d’eau glacée et d’eau chaude à 95 °C par une thermofrigopompe d’une laiterie. Int. J. Refrig. 1982, 5, 221–225. [Google Scholar] [CrossRef]
- Ghosh, V.S.; Devotta, S.; Patwardhan, S. The economics of heat pump systems for simultaneous heating and cooling. Heat Recovery Syst. 1987, 7, 159–166. [Google Scholar] [CrossRef]
- Naveteur, J.; Bruss, C. Chauffage et Rafraîchissement de la CAF de Lyon: Une Réussite Exemplaire! Rev. GÉNÉRale Du Froid Du Cond. d’Air 2007, 1079, 23–26. [Google Scholar]
- CFP. La «Thermofrigopompe», une technique économe… et peu émettrice de CO2. Chaud Froid Plomb. 2001, 632, 67–71. [Google Scholar]
- CFP. Un immeuble parisien climatisé par une thermofrigopompe sur nappe phréatique. Chaud Froid Plomb. 2003, 660, 62–68. [Google Scholar]
- Wesser, S.; Haëntjens, H. Systèmes à eau: Rendements optimisés grâce à la nappe. Clim Prat. 2006, 76, 49–53. [Google Scholar]
- MAD. Climatisation d’Infogrames par Thermofrigopompes. MAD l’Outil. Froid 2002, 47, 32–37. [Google Scholar]
- Bouttefroy, P. Thermofrigopompe chez IBM Corbeil. Rev. GénéRale Du Froid 1990, 6, 19–23. [Google Scholar]
- White, S.D.; Cleland, D.J.; Cotter, S.D.; Stephenson, R.A.; Kallu, R.D.S.; Fleming, A.K. A heat pump for simultaneous refrigeration and water heating. In IPENZ Transactions 1997; Engineers New Zealand: Wellington, New Zealand, 1997; Volume 24, No. 1/EMCh. [Google Scholar]
- Sappa, F. Chaud et Froid à l’Infirmerie protestante. Clim Prat. 2003, 52, 30–32. [Google Scholar]
- Haëntjens, H. Système à eau: Ayphassorho Met l’Exergy en Musique. Clim Prat. 2006, 76, 55–58. [Google Scholar]
- Gong, G.; Zeng, W.; Wang, L.; Wu, C. A new heat recovery technique for air-conditioning/heat-pump system. Appl. Therm. Eng. 2008, 28, 2360–2370. [Google Scholar] [CrossRef]
- Gong, G.; Chen, F.; Su, H.; Zhou, J. Thermodynamic simulation of condensation heat recovery characteristics of a single stage centrifugal chiller in a hotel. Appl. Energy 2012, 91, 326–333. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Gopal, M.R. Optimization of a transcritical CO2 heat pump cycle for simultaneous cooling and heating applications. Int. J. Refrig. 2004, 27, 830–838. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Gopal, M.R. Simulation of a transcritical CO2 heat pump cycle for simultaneous cooling and heating applications. Int. J. Refrig. 2006, 29, 735–743. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Gopal, M.R. Performance of a Transcritical CO2 heat pump for simultaneous water cooling and Heating. Int. J. Mech. Mechatron. Eng. 2010, 4, 571–577. [Google Scholar]
- Fatouh, M.; Elgendy, E. Experimental investigation of a vapor compression heat pump used for cooling and heating applications. Energy 2011, 36, 2788–2795. [Google Scholar] [CrossRef]
- Agrawal, N.; Bhattacharyya, S. Experimental investigations on adiabatic capillary tube in a transcritical CO2 heat pump system for simultaneous water cooling and heating. Int. J. Refrig. 2011, 34, 476–483. [Google Scholar] [CrossRef]
- Liu, X.; Ni, L.; Lau, S.; Li, H. Performance analysis of a multi-functional heat pump system in heating mode. Appl. Therm. Eng. 2013, 51, 698–710. [Google Scholar] [CrossRef]
- Liu, X.; Lau, S.K.; Li, H. Optimization and analysis of a multi-functional heat pump system with air source and gray water source in heating mode. Energy Build. 2014, 69, 1–13. [Google Scholar] [CrossRef]
- Kang, H.; Joo, Y.; Chung, H.; Kim, Y.; Choi, J. Experimental study on the performance of a simultaneous heating and cooling multi-heat pump with the variation of operation mode. Int. J. Refrig. 2009, 32, 1452–1459. [Google Scholar] [CrossRef]
- Joo, Y.; Kang, H.; Ahn, J.H.; Lee, M.; Kim, Y. Performance characteristics of a simultaneous cooling and heating multi-heat pump at partial load conditions. Int. J. Refrig. 2011, 34, 893–901. [Google Scholar] [CrossRef]
- Jung, H.W.; Kang, H.; Chung, H.; Ahn, J.H.; Kim, Y. Performance optimization of a cascade multi-functional heat pump in various operation modes. Int. J. Refrig. 2014, 42, 57–68. [Google Scholar] [CrossRef]
- Boahen, S.; Anka, S.K.; Lee, K.H.; Choi, J.M. Performance characteristics of a cascade multi-functional heat pump in the winter season. Energy Build. 2021, 253, 111511. [Google Scholar] [CrossRef]
- Fricker, J.; Zoughaib, A. Simultaneous heating and cooling production devices composed by reverse cycle systems under variable loads. Int. J. Refrig. 2015, 55, 1–16. [Google Scholar] [CrossRef]
- Liu, Y.; Groll, E.A.; Yazawa, K.; Kurtulus, O. Theoretical analysis of energy-saving performance and economics of CO2 and NH3 heat pumps with simultaneous cooling and heating applications in food processing. Int. J. Refrig. 2016, 65, 129–141. [Google Scholar] [CrossRef]
- Liu, Y.; Groll, E.A.; Yazawa, K.; Kurtulus, O. Energy-saving performance and economics of CO2 and NH3 heat pumps with simultaneous cooling and heating applications in food processing: Case studies. Int. J. Refrig. 2017, 73, 111–124. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Jiang, Y. Novel beverage heating and fast-cooling processes separately using an absorption chiller and using electric heat pumps. Int. J. Refrig. 2018, 94, 87–101. [Google Scholar] [CrossRef]
- Shin, D.U.; Ryu, S.R.; Kim, K.W. Simultaneous heating and cooling system with thermal storage tanks considering energy efficiency and operation method of the system. Energy Build. 2019, 205, 109518. [Google Scholar] [CrossRef]
- Deymi-Dashtebayaz, M.; Namanlo, S.V.; Arabkoohsar, A. Simultaneous use of air-side and water-side economizers with the air source heat pump in a data center for cooling and heating production. Appl. Therm. Eng. 2019, 161, 114133. [Google Scholar] [CrossRef]
- Best, R.; Eisa, M.A.R.; Holland, F.A. Thermodynamic design data for absorption heat pump systems operating on ammonia-water—Part III. Simultaneous cooling and heating. Heat Recovery Syst. CHP 1987, 7, 187–194. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, R.; Wang, Y.; You, S.; Zhang, H.; Xia, J.; Wei, S. Mathematical modeling and performance analysis of an integrated solar heating and cooling system driven by parabolic trough collector and double-effect absorption chiller. Energy Build. 2019, 202, 109400. [Google Scholar] [CrossRef]
- Wang, J.; Belusko, M.; Liu, M.; Semsarilar, H.; Liddle, R.; Alemu, A.; Evans, M.; Zhao, C.; Hudson, J.; Bruno, F. A comprehensive study on a novel transcritical CO2 heat pump for simultaneous space heating and cooling—Concepts and initial performance. Energy Convers. Manag. 2021, 243, 114397. [Google Scholar] [CrossRef]
- Artuso, P.; Tosato, G.; Rossetti, A.; Marinetti, S.; Hafner, A.; Banasiak, K.; Minetto, S. Dynamic Modelling and Validation of an Air-to-Water Reversible R744 Heat Pump for High Energy Demand Buildings. Energies 2021, 14, 8238. [Google Scholar] [CrossRef]
- Shin, D.-U.; Jeong, C.-H. Energy Savings of Simultaneous Heating and Cooling System According to Indoor Set Temperature Changes in the Comfort Range. Energies 2021, 14, 7691. [Google Scholar] [CrossRef]
- Byrne, P.; Miriel, J.; Lénat, Y. Experimental study of a heat pump for simultaneous heating and cooling—Part 1: Basic concepts and performance verification. Appl. Energy 2011, 88, 1841–1847. [Google Scholar] [CrossRef] [Green Version]
- Byrne, P.; Miriel, J.; Lénat, Y. Experimental study of an air-source heat pump for simultaneous heating and cooling—Part 2: Dynamic behaviour and two-phase thermosiphon defrosting technique. Appl. Energy 2011, 88, 3072–3078. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, M.U.; Foslie, S.S.; Moen, O.M.; Bantle, M.; Eikevik, T.M. Integrated high temperature heat pumps and thermal storage tanks for combined heating and cooling in the industry. Appl. Therm. Eng. 2021, 189, 116731. [Google Scholar] [CrossRef]
- Al-Sayyab, A.K.S.; Mota-Babiloni, A.; Navarro-Esbrí, J. Novel compound waste heat-solar driven ejector-compression heat pump for simultaneous cooling and heating using environmentally friendly refrigerants. Energy Convers. Manag. 2021, 228, 113703. [Google Scholar] [CrossRef]
- Al-Sayyab, A.K.S.; Navarro-Esbrí, J.; Mota-Babiloni, A. Energy, exergy, and environmental (3E) analysis of a compound ejector-heat pump with low GWP refrigerants for simultaneous data center cooling and district heating. Int. J. Refrig. 2022, 133, 61–72. [Google Scholar] [CrossRef]
- Calise, F.; Cappiello, F.L.; d’Accadia, M.D.; Petrakopoulou, F.; Vicidomini, M. A solar-driven 5th generation district heating and cooling network with ground-source heat pumps: A thermo-economic analysis. Sustain. Cities Soc. 2022, 76, 103438. [Google Scholar] [CrossRef]
- Quirosa, G.; Torres, M.; Solter, V.M.; Chacartegui, R. Energetic and economic analysis of decoupled strategy for heating and cooling production with CO2 booster heat pumps for ultra-low temperature district network. J. Build. Eng. 2022, 45, 103538. [Google Scholar] [CrossRef]
- Kong, R.; Deethayat, T.; Asanakham, A.; Kiatsiriroat, T. Performance and economic evaluation of a photovoltaic/thermal (PV/T)-cascade heat pump for combined cooling, heat and power in tropical climate area. J. Energy Storage 2020, 30, 101507. [Google Scholar] [CrossRef]
- Amin, Z.M.; Hawlader, M.N.A. Analysis of solar desalination system using heat pump. Renew. Energy 2015, 74, 116–123. [Google Scholar] [CrossRef]
- Attia, A.A.A. New proposed system for freeze water desalination using auto reversed R-22 vapor compression heat pump. Desalination 2010, 254, 179–184. [Google Scholar] [CrossRef]
- Koschikowski, J.; Wieghaus, M.; Rommel, M. Solar thermal-driven desalination plants based on membrane distillation. Desalination 2003, 156, 295–304. [Google Scholar] [CrossRef]
- Diaby, A.T.; Byrne, P.; Loulergue, P.; Sow, O.; Maré, T. Experimental Study of a Heat Pump for Simultaneous Cooling and Desalination by Membrane Distillation. Membranes 2021, 11, 725. [Google Scholar] [CrossRef]
- Bindels, M.; Nelemans, B. Theoretical analysis of heat pump assisted air gap membrane distillation. Desalination 2021, 518, 115282. [Google Scholar] [CrossRef]
- Bindels, M.; Nelemans, B. Cost reduction of heat pump assisted membrane distillation by using variable electricity prices. Desalination 2022, 530, 115669. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, F.; Guo, J.; Liu, J.; Zhai, H.; Duan, Z. Performance analysis of a novel combined cooling, heating and power system based on carbon dioxide energy storage. Energy Convers. Manag. 2019, 188, 151–161. [Google Scholar] [CrossRef]
- Mohammadi, K.; Powell, K. Thermodynamic and economic analysis of different cogeneration and trigeneration systems based on carbon dioxide vapor compression refrigeration systems. Appl. Therm. Eng. 2020, 164, 114503. [Google Scholar] [CrossRef]
- Byrne, P.; Lalanne, P. Parametric Study of a Long-Duration Energy Storage Using Pumped-Hydro and Carbon Dioxide Transcritical Cycles. Energies 2021, 14, 4401. [Google Scholar] [CrossRef]






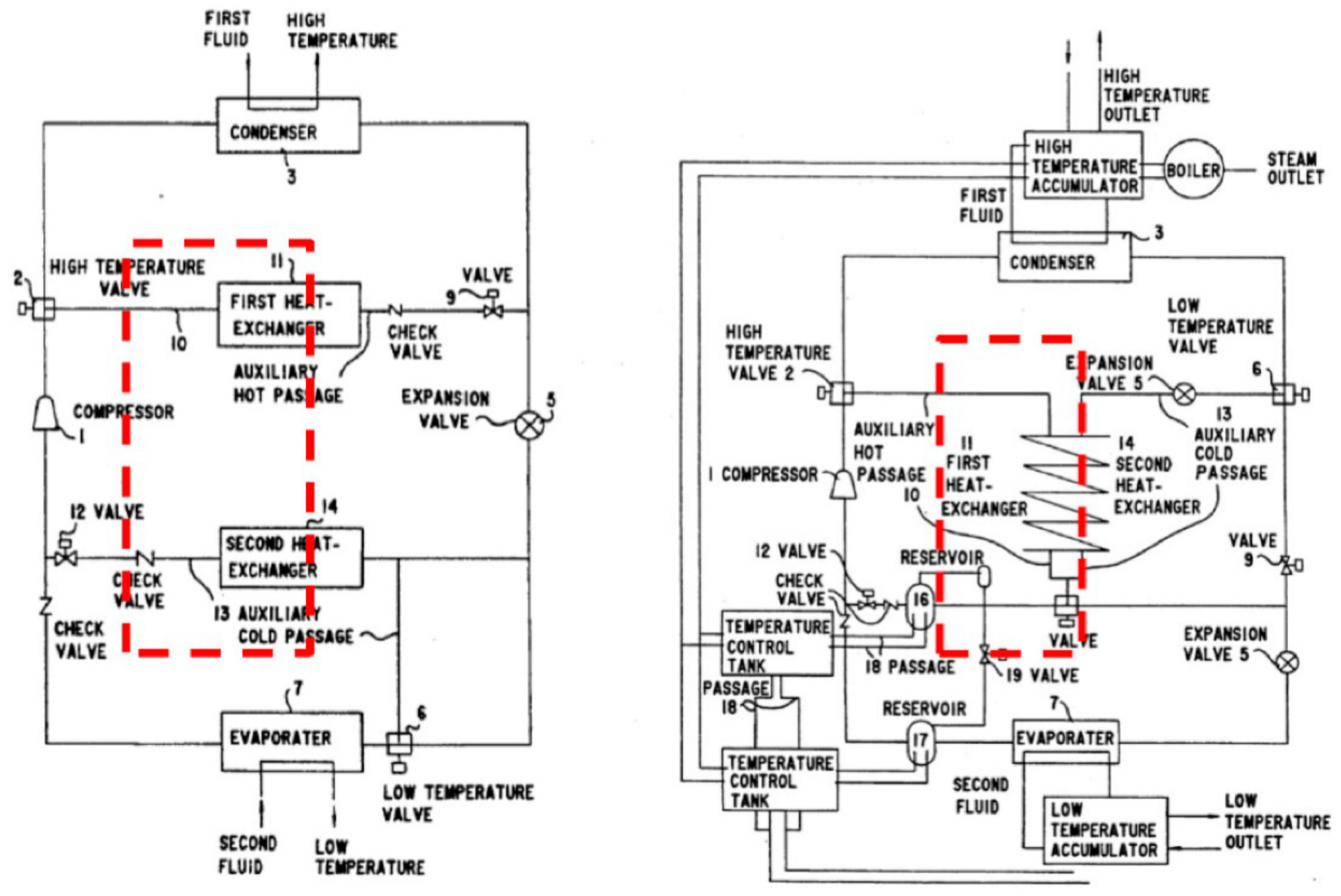
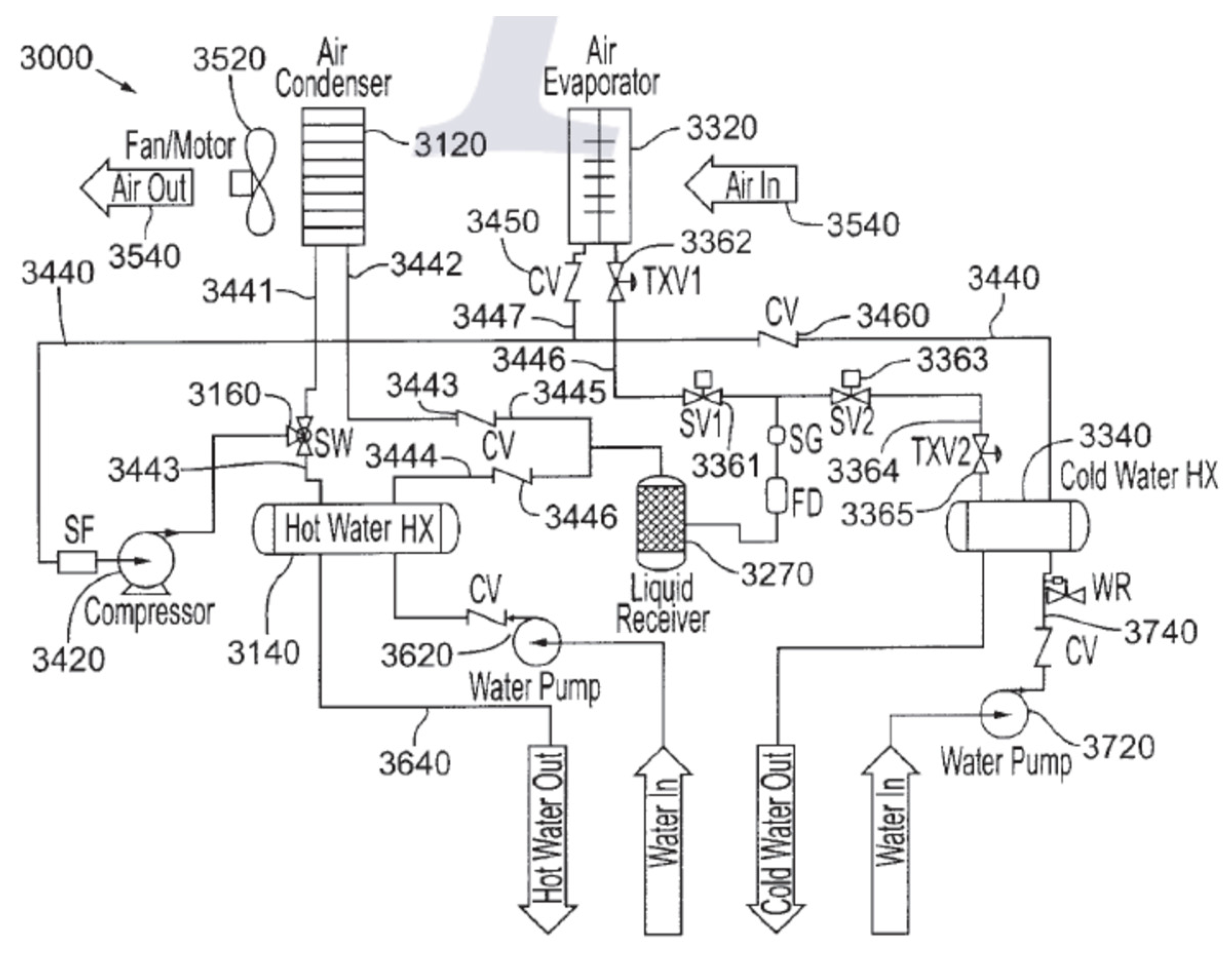
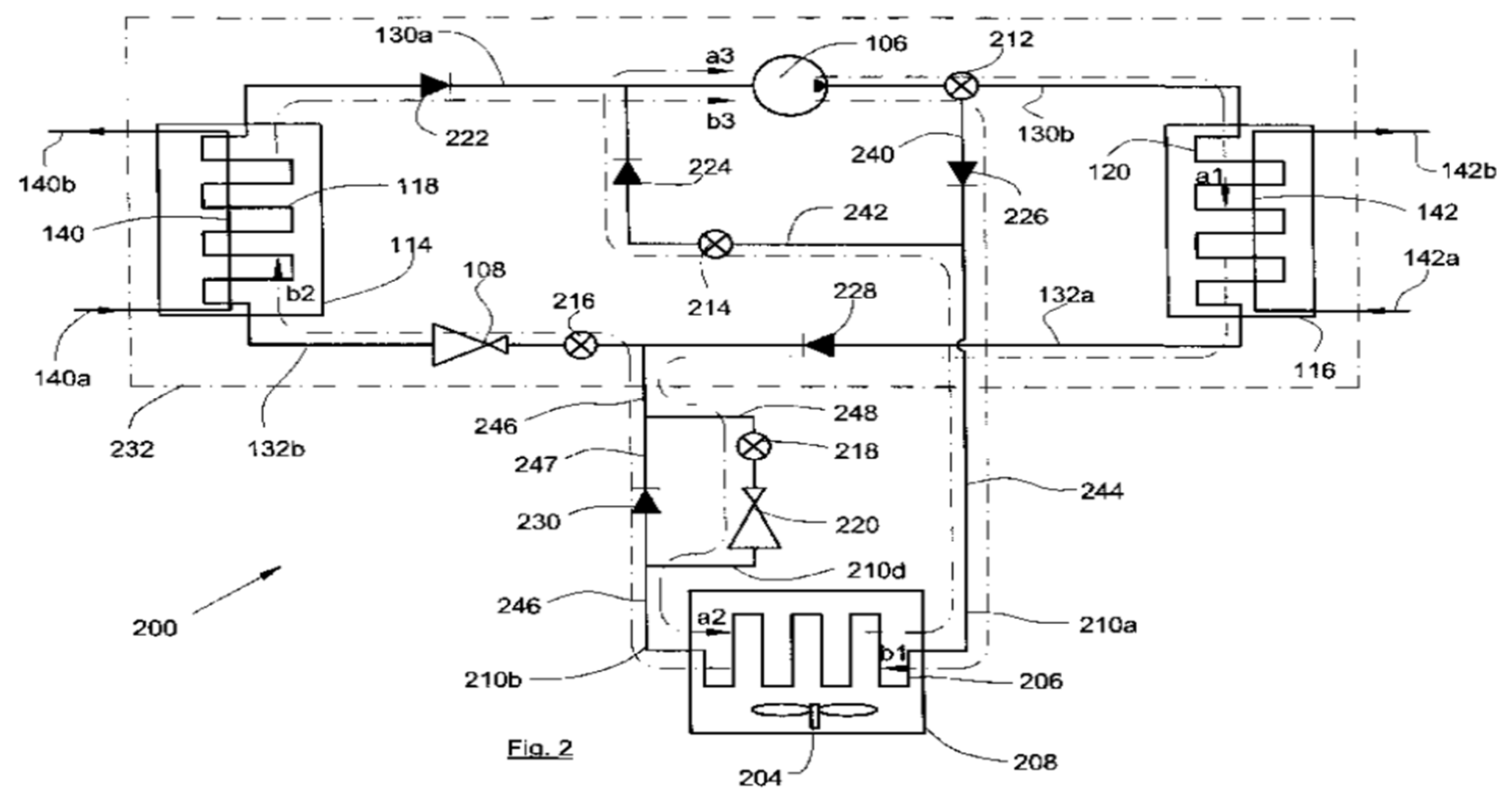
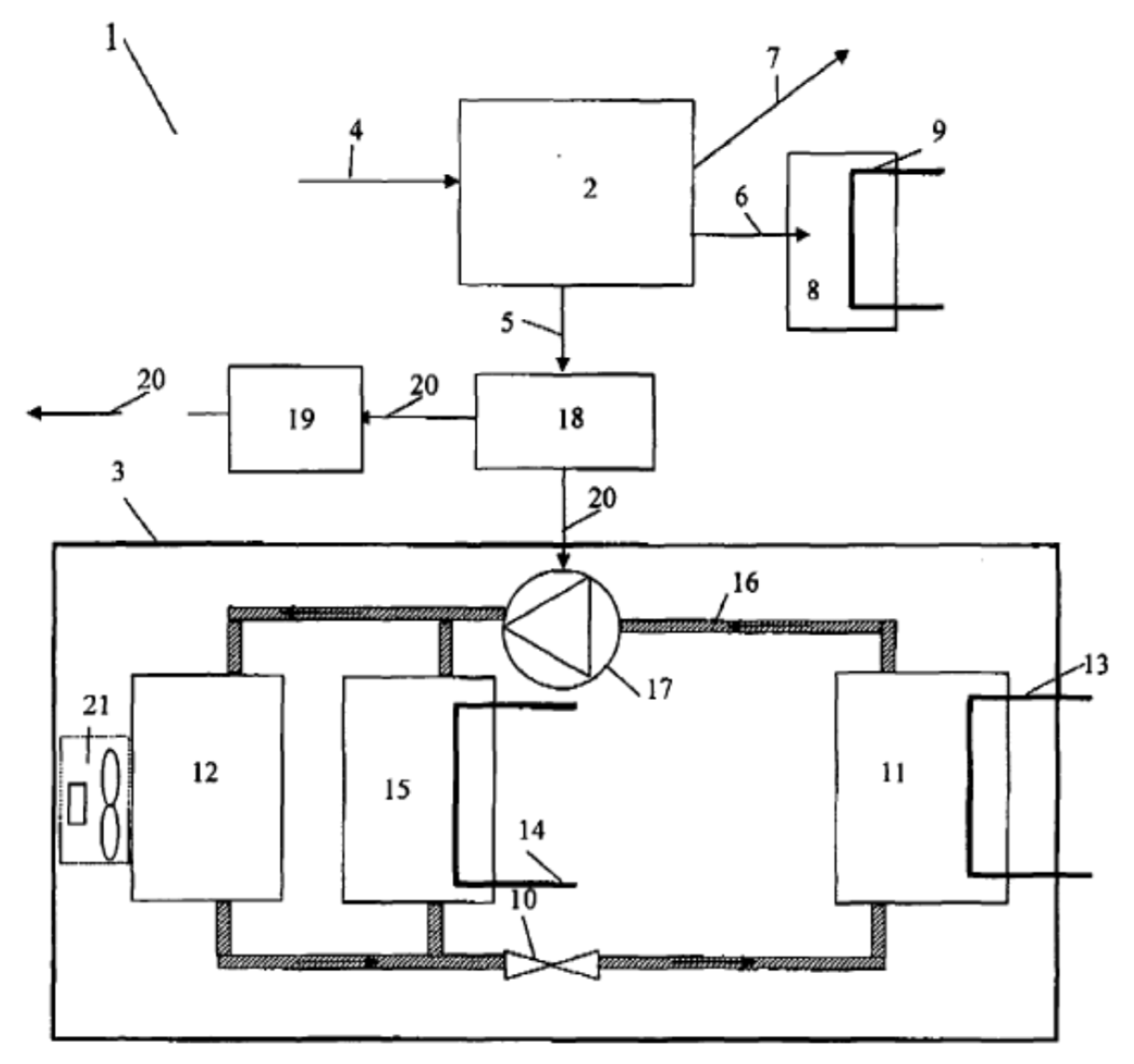

| Refrigerant | Hydrocarbon |
|---|---|
| Safety group (EN 378/1-2012/ASHRAE 34-2010) | A3 |
| Pressure Equipment Directive (2014/68/EU) [15] | 1 |
| A—General occupation (residential, hotels, schools, etc.) | <1.5 kg |
| B—Supervised occupation (offices, laboratories, manufacturing premises, etc.) | 1 kg below ground level and 2.5 kg above ground level |
| C—Occupancy only with restricted access (refineries, cold stores, etc.) | 1 kg below ground level and 10 kg above ground level |
| Fluid | R134a (1,1,1,2-Tetrafluoroethane) | R32 (Difluoromethane) |
|---|---|---|
| Chemical formula | C2H2F4 | CH2F2 |
| GWP100years (kgCO2) | 1430 | 650 |
| ODP | 0 | 0 |
| Potential risks | Climate change | Climate change Flammability A2L |
| Fluid | R1234yf (2,3,3,3-Tetrafluoroprop-1-ene) | R1234ze(E) (Trans-1,3,3,3-Tétrafluoroprop-1-ene) | R1234ze(Z) (Cis-1,3,3,3-Tétrafluoroprop-1-ene) |
|---|---|---|---|
| Chemical formula | CH2=CFCF3 | CFH=CH-CF3 | CFH=CH-CF3 |
| GWP100years (kgCO2) | 4 | 7 | <10 |
| ODP | 0 | 0 | 0 |
| Potential risks | Slight flammability Water acidification | Slight flammability Water acidification | Slight flammability Water acidification |
| Fluid | R717 (Ammoniac) |
|---|---|
| Chemical formula | NH3 |
| GWP100years (kgCO2) | 0 |
| ODP | 0 |
| Potential risks | High toxicity Corrosion of copper Slight flammability |
| Refrigerant | Application Domain | Temperature Levels | Equivalent Synthetized Fluid |
|---|---|---|---|
| R600a (isobutene) | Domestic refrigeration | High and middle temperature | R12, R134a |
| R290 (propane) | Commercial, industrial, freezers, air conditioning, heat pump | High, middle and low temperature | R22, R404A, R407C, R507A |
| R1270 (propene) | industrial refrigeration, air conditioning, heat pumps, tertiary, industrial | High, middle and low temperature | R22, R404A, R407C, R507A |
| Fluid | R600a (Isobutene) | R290 (Propane) | R1270 (Propene) |
|---|---|---|---|
| Chemical formula | CH3CH2CH3 | C4H10 | C3H6 |
| GWP100yeas (kgCO2) | 3 | 3 | 2 |
| ODP | 0 | 0 | 0 |
| Potential risks | High flammability | ||
| LEL % (v/v) | 2.2 | 1.8 | 2 |
| UEL % (v/v) | 10 | 9.8 | 11.2 |
| Auto-ignition temperature | 470 °C | 460 °C | 485 °C |
| Fluid | R744 (Carbon Dioxide) |
|---|---|
| Chemical formula | CO2 |
| GWP100years (kgCO2) | 1 |
| ODP | 0 |
| Potential risks | High pressure |
| Refrigerant | Molar Mass (g/mol) | Critical Temperature (°C) | Critical Pressure (bar) | Specific Volume at Tdew = 0 °C (m3/kg) | Latent Heat at Tdew = 0 °C (kJ/kg) | Latent Heat at Tdew = 50 °C (kJ/kg) |
|---|---|---|---|---|---|---|
| R12 | 120.90 | 112.00 | 41.14 | 0.05542 | 151.5 | 121.5 |
| R22 | 86.47 | 96.10 | 49.89 | 0.04708 | 205.0 | 154.1 |
| R134a | 102.00 | 101.00 | 40.59 | 0.06925 | 198.6 | 151.8 |
| R32 | 52.02 | 78.10 | 57.84 | 0.04526 | 315.3 | 209.6 |
| R407C | 86.20 | 86.20 | 46.32 | 0.05087 | 218.2 | 157.0 |
| R1234yf | 114.00 | 94.70 | 33.82 | 0.05653 | 163.3 | 122.2 |
| R1234ze(E) | 114.00 | 109.40 | 36.32 | 0.08389 | 184.2 | 145.1 |
| R1234ze(Z) | 114.00 | 150.10 | 35.33 | 0.27900 | 220.5 | 189.0 |
| R290 | 44.10 | 96.70 | 42.47 | 0.09651 | 374.7 | 284.3 |
| R600a | 58.12 | 134.70 | 36.40 | 0.23530 | 355.0 | 298.5 |
| NH3 | 17.03 | 132.30 | 113.30 | 0.28920 | 1262.0 | 1050.0 |
| CO2 | 44.01 | 30.98 | 73.77 | 0.01024 | 230.9 | supercritical state |
| Mode | Tin,air (°C) | Tout,air (°C) | Tin,cw (°C) | Tout,cw (°C) | Tin,hw (°C) | Tout,hw (°C) | Tev (°C) | Tcd (°C) |
|---|---|---|---|---|---|---|---|---|
| (1) heating only | 7 | −1 | - | - | 30 | 35 | −6 | 40 |
| (2) cooling only | 27 | 35 | 12 | 7 | - | - | 2 | 40 |
| (3) DHW only | 7 | −1 | - | - | 15 | 55 | −6 | 60 |
| (4) simultaneous heating and cooling | - | - | 12 | 7 | 30 | 35 | 2 | 40 |
| (5) simultaneous DHW and cooling | - | - | 12 | 7 | 15 | 55 | 2 | 60 |
| Building Type | Surface (m²) | Number of Thermal Zones | Gains | |||
|---|---|---|---|---|---|---|
| Occupation | Lighting (W/m²) | Number of Electrical Devices (P = 230 W) | ||||
| Number of Persons | Hours | |||||
| (a) Hotel | 1440 | 30 | Bedrooms: 45 Welcome zone: 3 | 20 h–7 h 8 h–18 h | 10 | Welcome zone: 2 |
| (b) Residential | 675 | 15 | 24 | 6 h–9 h 18 h–24 h | 5 | 64 |
| (c) Offices | 792 | 12 | 123 | 8 h–20 h | 10 | 141 |
| (d) Shop | 1467 | 5 | 134 | 8 h–21 h | 10 | 9 |
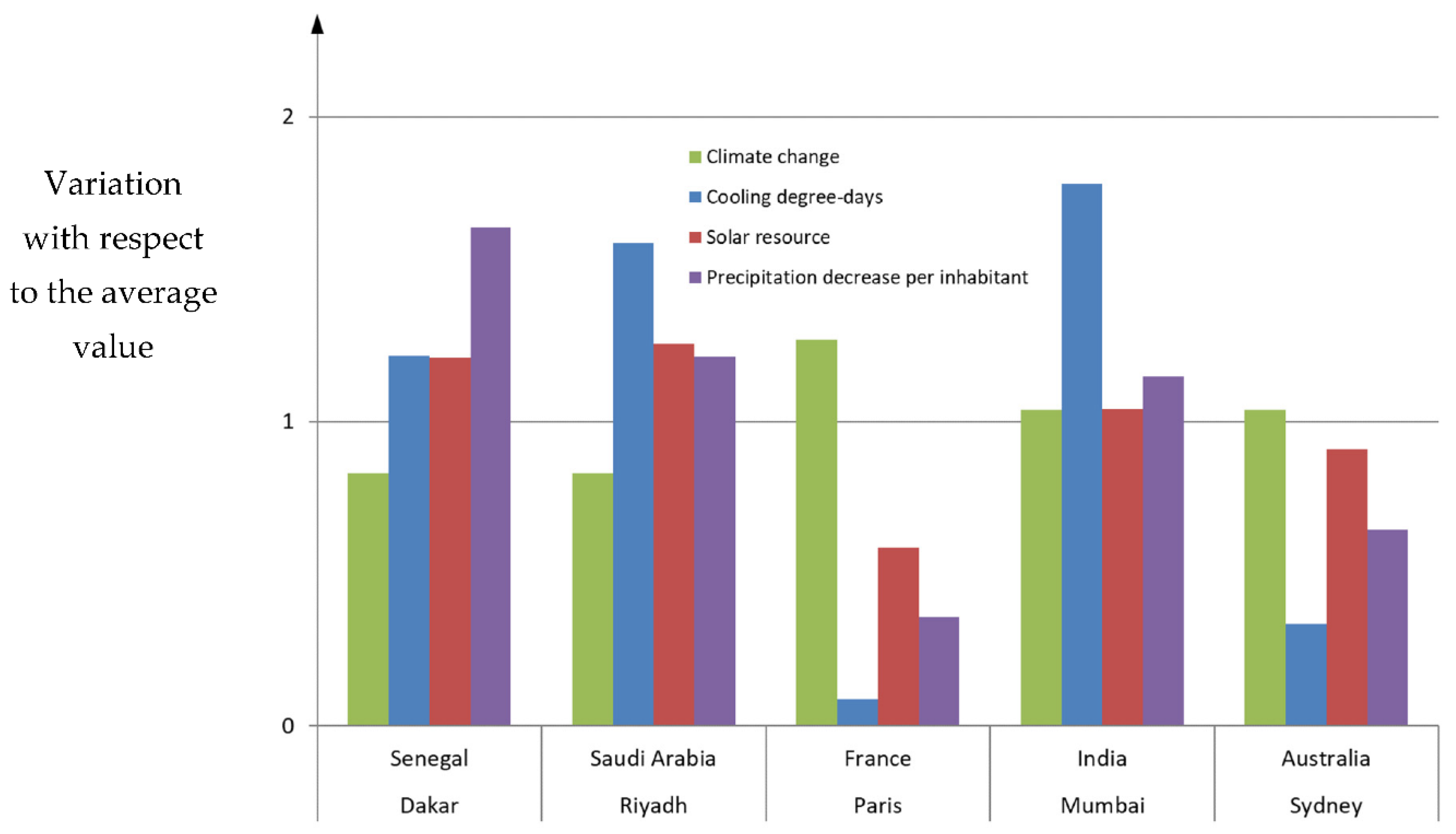
| Climate | Hotel | Residential | Offices | Shop | Shop with 730 kWh Commercial Cooling | Shop with 104 kWh Commercial Cooling |
|---|---|---|---|---|---|---|
| Oceanic | 18.97% | 28.00% | 28.17% | 10.17% | 7.64% | 26.82% |
| Continental | 15.31% * | 22.50% | 22.57% | 6.86% | 5.15% | 18.09% |
| Mediterranean | 20.47% | 30.52% | 24.37% | 10.26% | 7.71% | 27.06% |
| Article | Building | Site | Fluid | Performance Index | Comments | |
|---|---|---|---|---|---|---|
| [38] International Journal of Refrigeration | Hotel | Paris | R407C | SCOP = 3.57 | +16.7% | Compared to a reversible heat pump |
| CO2 | SCOP = 3.26 | +20.7% | ||||
| R407C | = 26.40% | +11.0% | ||||
| CO2 | = 27.48% | +49.5% | ||||
| R407C | TEWI = 113,112 kgCO2 | −12.0% | ||||
| CO2 | TEWI = 95,359 kgCO2 | −27.4% | ||||
| [54] Building Simulation | Hotel | Rennes | R407C | SCOP = 4.34 | +13.0% | Compared to a reversible heat pump |
| R407C | = 36% | +16.1% | ||||
| Marseille | R407C | SCOP = 5.33 | +6.2% | |||
| R407C | = 33% | +6.5% | ||||
| Brussels | R407C | SCOP = 3.61 | +18.7% | |||
| R407C | = 36% | +56.5% | ||||
| [53] Energy and Buildings | Low-energy residential building | Rennes | R407C | SCOP = 2.84 | Part of consumptions is covered by an auxiliary electric heater. HPS power is 4 times lower than the one in [92] | |
| R290 | SCOP = 3.21 | |||||
| R1234yf | SCOP = 3.05 | |||||
| R407C | TEWI = 26,362 kgCO2 | |||||
| R290 | TEWI = 12,709 kgCO2 | |||||
| R1234yf | TEWI = 13,741 kgCO2 | |||||
| Fluid | COP (-) | GOR (-) | SEC (kWh/m3) | ηeco (%) | ηpro (%) |
|---|---|---|---|---|---|
| R1234yf | 2.8 | 4.2 | 312.4 | 24.8 | 30.1 |
| R290 | 3.2 | 4.5 | 278.5 | 28.7 | 35.9 |
| R744 | 2.7 | 9.5 | 68.1 | 4.4 | 13.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrne, P. Research Summary and Literature Review on Modelling and Simulation of Heat Pumps for Simultaneous Heating and Cooling for Buildings. Energies 2022, 15, 3529. https://doi.org/10.3390/en15103529
Byrne P. Research Summary and Literature Review on Modelling and Simulation of Heat Pumps for Simultaneous Heating and Cooling for Buildings. Energies. 2022; 15(10):3529. https://doi.org/10.3390/en15103529
Chicago/Turabian StyleByrne, Paul. 2022. "Research Summary and Literature Review on Modelling and Simulation of Heat Pumps for Simultaneous Heating and Cooling for Buildings" Energies 15, no. 10: 3529. https://doi.org/10.3390/en15103529
APA StyleByrne, P. (2022). Research Summary and Literature Review on Modelling and Simulation of Heat Pumps for Simultaneous Heating and Cooling for Buildings. Energies, 15(10), 3529. https://doi.org/10.3390/en15103529






