Application of a Polyacrylate Latex to a Lithium Iron Phosphate Cathode as a Binder Material
Abstract
1. Introduction
2. Materials and Methods
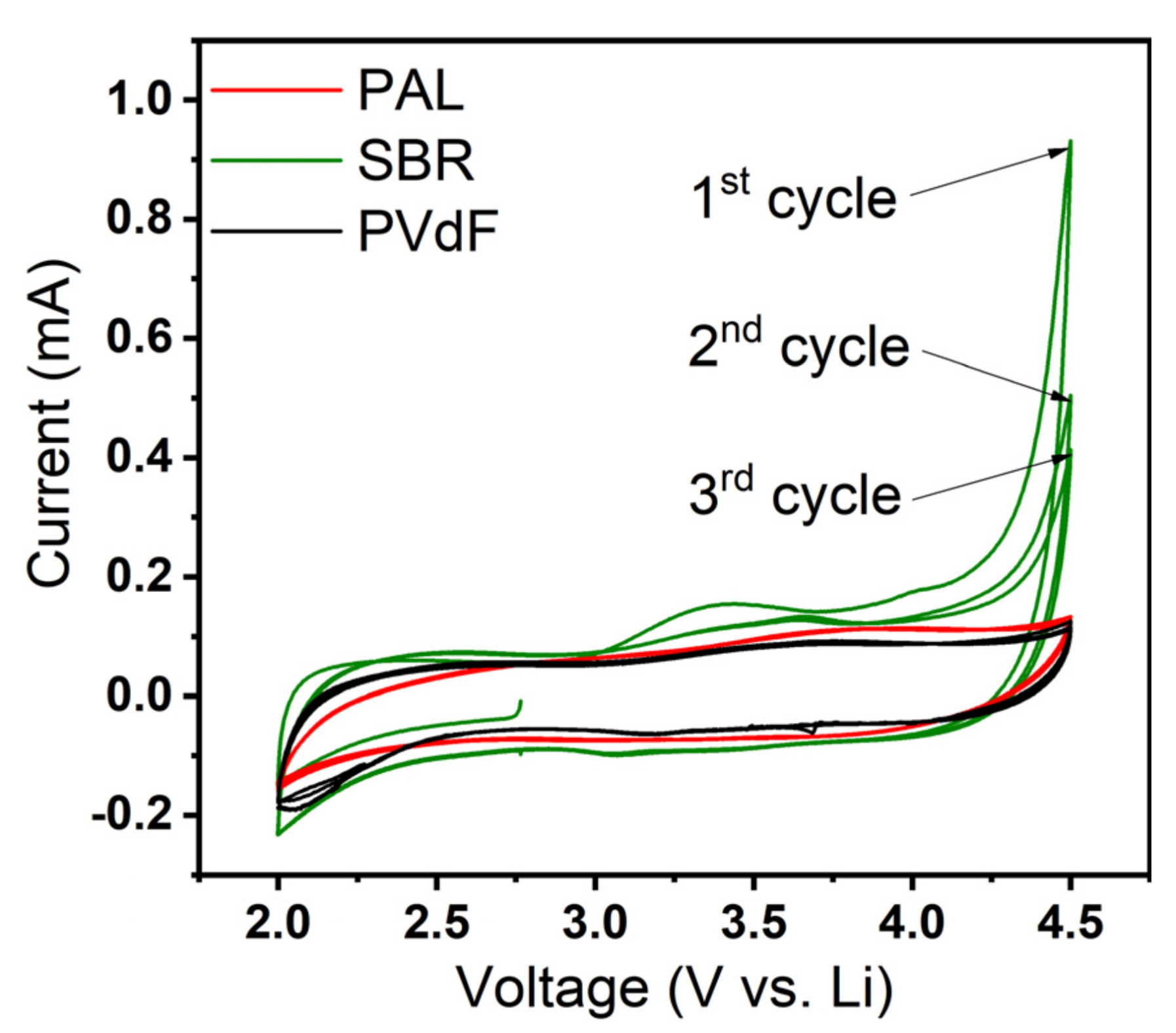
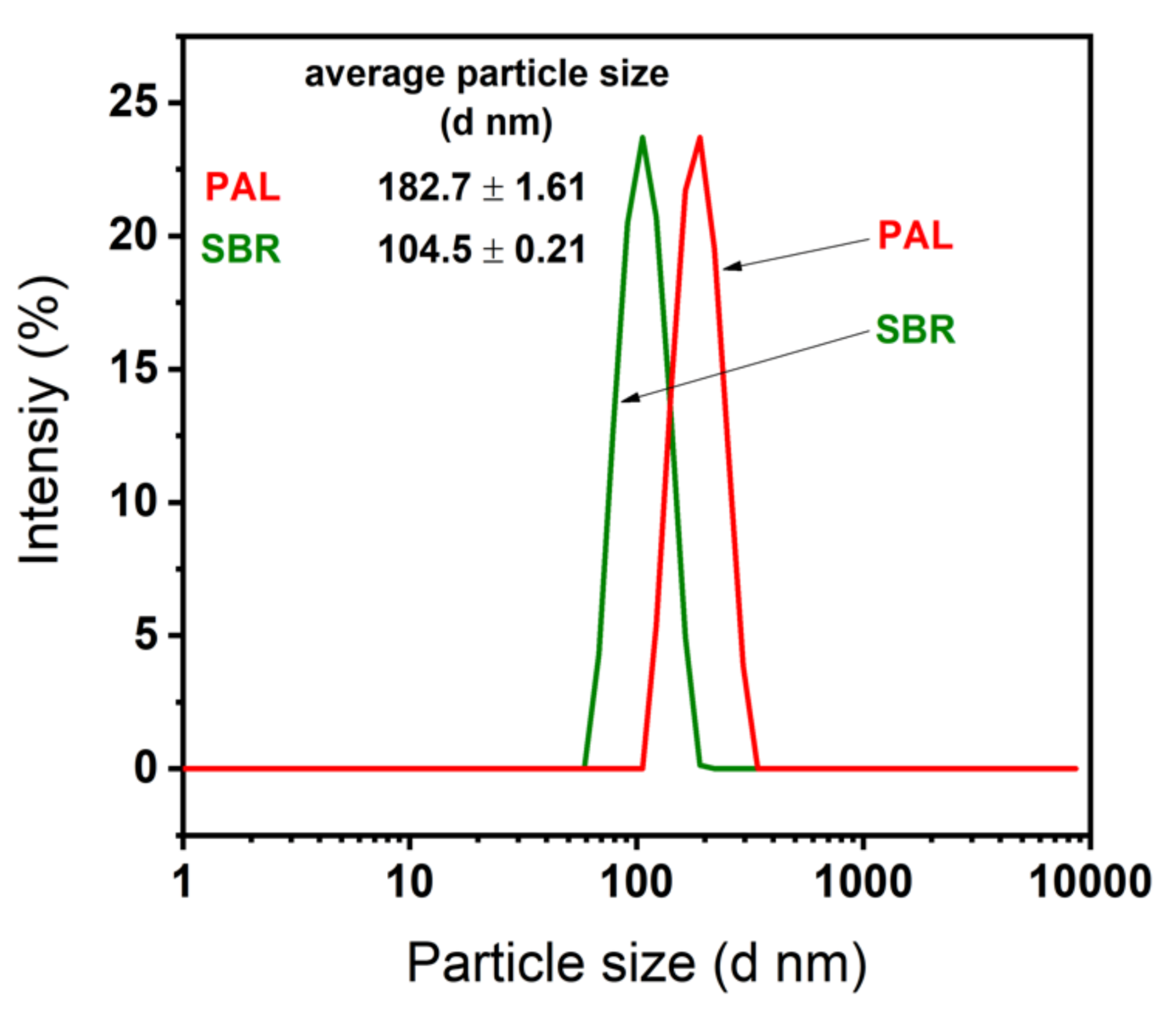
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Zhong, H.; Wang, J.; Zhang, L. Investigation on Xanthan Gum as Novel Water Soluble Binder for LiFePO4 Cathode in Lithium-Ion Batteries. J. Alloys Compd. 2017, 714, 409–418. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A Review on Lithium-Ion Battery Ageing Mechanisms and Estimations for Automotive Applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the Sustainability of Lithium Ion Battery Industry—A Review and Perspective. Energy Storage Mater. 2020. [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-Ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent Advances and Historical Developments of High Voltage Lithium Cobalt Oxide Materials for Rechargeable Li-Ion Batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Wan, J.; Zhu, J.; Xiang, Y.; Zhong, G.; Liu, X.; Li, Y.; Zhang, K.H.L.; Hong, C.; Zheng, J.; Wang, K.; et al. Revealing the Correlation between Structure Evolution and Electrochemical Performance of High-Voltage Lithium Cobalt Oxide. J. Energy Chem. 2021, 54, 786–794. [Google Scholar] [CrossRef]
- Matsuda, T.; Ando, K.; Myojin, M.; Matsumoto, M.; Sanada, T.; Takao, N.; Imai, H.; Imamura, D. Investigation of the Influence of Temperature on the Degradation Mechanism of Commercial Nickel Manganese Cobalt Oxide-Type Lithium-Ion Cells during Long-Term Cycle Tests. J. Energy Storage 2019, 21, 665–671. [Google Scholar] [CrossRef]
- Schmitt, J.; Maheshwari, A.; Heck, M.; Lux, S.; Vetter, M. Impedance Change and Capacity Fade of Lithium Nickel Manganese Cobalt Oxide-Based Batteries during Calendar Aging. J. Power Sources 2017, 353, 183–194. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Kim, S.; Kim, Y. Fluorination of Free Lithium Residues on the Surface of Lithium Nickel Cobalt Aluminum Oxide Cathode Materials for Lithium Ion Batteries. Mater. Des. 2016, 100, 175–179. [Google Scholar] [CrossRef]
- Wong, D.; Shrestha, B.; Wetz, D.A.; Heinzel, J.M. Impact of High Rate Discharge on the Aging of Lithium Nickel Cobalt Aluminum Oxide Batteries. J. Power Sources 2015, 280, 363–372. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A Brief Review on Hydrometallurgical Technologies for Recycling Spent Lithium-Ion Batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Manthiram, A. A Reflection on Lithium-Ion Battery Cathode Chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Shin, H.C.; Cho, W.I.; Jang, H. Electrochemical Properties of the Carbon-Coated LiFePO4 as a Cathode Material for Lithium-Ion Secondary Batteries. J. Power Sources 2006, 159, 1383–1388. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, Z.; Feng, H.; Li, J. Sucrose-Assisted Loading of LiFePO4 Nanoparticles on Graphene for High-Performance Lithium-Ion Battery Cathodes. Chem. Eur. J. 2013, 19, 5631–5636. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, J. Enhanced Electrochemical Performance of LiFePO4 Originating from the Synergistic Effect of ZnO and C Co-Modification. Nanomaterials 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Zhang, J.; Liu, Y.; Zhang, W.; Chen, Y.; Wang, C. Direct Regeneration of Spent LiFePO4 Cathode Material by a Green and Efficient One-Step Hydrothermal Method. ACS Sustain. Chem. Eng. 2020, 8, 17622–17628. [Google Scholar] [CrossRef]
- Chand, P.; Kumar, S.; Singh, V.; Singh, N.P. Investigation of the Structural and Electrical Behavior of LiFePO4 as Cathode Material for Energy Storage Application. Mater. Today Proc. 2020, 32, 483–486. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High Mass Loading Additive-Free LiFePO4 Cathodes with 500 Μm Thickness for High Areal Capacity Li-Ion Batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Elmarakbi, A.; Metawa, H.; Shenashen, M.A. Multifaceted Geometric 3D Mesopolytope Cathodes and Its Directional Transport Gates for Superscalable LIB Models. Appl. Mater. Today 2020, 19, 100590. [Google Scholar] [CrossRef]
- Nam, Y.J.; Cho, S.-J.; Oh, D.Y.; Lim, J.-M.; Kim, S.Y.; Song, J.H.; Lee, Y.-G.; Lee, S.-Y.; Jung, Y.S. Bendable and Thin Sulfide Solid Electrolyte Film: A New Electrolyte Opportunity for Free-Standing and Stackable High-Energy All-Solid-State Lithium-Ion Batteries. Nano Lett. 2015, 15, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shkrob, I.A.; Zhang, S.; Zhang, L.; Zhang, J.; Li, Y.; Liao, C.; Zhang, Z.; Lu, W.; Zhang, L. The Existence of Optimal Molecular Weight for Poly(Acrylic Acid) Binders in Silicon/Graphite Composite Anode for Lithium-Ion Batteries. J. Power Sources 2018, 378, 671–676. [Google Scholar] [CrossRef]
- Qi, Y.; Nguyen, M.H.T.; Oh, E.-S. Enhancement of the Lithium Titanium Oxide Anode Performance by the Copolymerization of Conductive Polypyrrole with Poly(Acrylonitrile/Butyl Acrylate) Binder. J. Appl. Electrochem. 2020, 50, 431–438. [Google Scholar] [CrossRef]
- Ren, W.-F.; Le, J.-B.; Li, J.-T.; Hu, Y.-Y.; Pan, S.-Y.; Deng, L.; Zhou, Y.; Huang, L.; Sun, S.-G. Improving the Electrochemical Property of Silicon Anodes through Hydrogen-Bonding Cross-Linked Thiourea-Based Polymeric Binders. ACS Appl. Mater. Interfaces 2020. [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Influence of the PVdF Binder on the Stability of LiCoO2 Electrodes. Electrochem. Commun. 2005, 7, 1298–1304. [Google Scholar] [CrossRef]
- Han, B.; Piernas-Muñoz, M.J.; Dogan, F.; Kubal, J.; Trask, S.E.; Bloom, I.D.; Vaughey, J.T.; Key, B. Probing the Reaction between PVDF and LiPAA vs Li7Si3: Investigation of Binder Stability for Si Anodes. J. Electrochem. Soc. 2019, 166, A2396–A2402. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Hou, Y.; Li, P.; Sun, H.; Niu, Q.J. Fabrication of a Superhydrophilic PVDF Membrane with Excellent Chemical and Mechanical Stability for Highly Efficient Emulsion Separation. Sep. Purif. Technol. 2020, 251, 117408. [Google Scholar] [CrossRef]
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and Economic Analysis of Solvent-Based Lithium-Ion Electrode Drying with Water and NMP. Dry. Technol. 2018, 36, 234–244. [Google Scholar] [CrossRef]
- Lee, S.; Kim, E.-Y.; Lee, H.; Oh, E.-S. Effects of Polymeric Binders on Electrochemical Performances of Spinel Lithium Manganese Oxide Cathodes in Lithium Ion Batteries. J. Power Sources 2014, 269, 418–423. [Google Scholar] [CrossRef]
- Prosini, P.P.; Carewska, M.; Cento, C.; Masci, A. Poly Vinyl Acetate Used as a Binder for the Fabrication of a LiFePO4-Based Composite Cathode for Lithium-Ion Batteries. Electrochimica Acta 2014, 150, 129–135. [Google Scholar] [CrossRef]
- Soeda, K.; Yamagata, M.; Ishikawa, M. Alginic Acid as a New Aqueous Slurry-Based Binder for Cathode Materials of LIB. ECS Trans. 2015, 64, 13. [Google Scholar] [CrossRef]
- Isozumi, H.; Horiba, T.; Kubota, K.; Hida, K.; Matsuyama, T.; Yasuno, S.; Komaba, S. Application of Modified Styrene-Butadiene-Rubber-Based Latex Binder to High-Voltage Operating LiCoO2 Composite Electrodes for Lithium-Ion Batteries. J. Power Sources 2020, 468, 228332. [Google Scholar] [CrossRef]
- Qiu, L.; Shao, Z.; Wang, D.; Wang, W.; Wang, F.; Wang, J. Enhanced Electrochemical Properties of LiFePO4 (LFP) Cathode Using the Carboxymethyl Cellulose Lithium (CMC-Li) as Novel Binder in Lithium-Ion Battery. Carbohydr. Polym. 2014, 111, 588–591. [Google Scholar] [CrossRef]
- Bigoni, F.; De Giorgio, F.; Soavi, F.; Arbizzani, C. Sodium Alginate: A Water-Processable Binder in High-Voltage Cathode Formulations. J. Electrochem. Soc. 2017, 164, A6171–A6177. [Google Scholar] [CrossRef]
- Chong, J.; Xun, S.; Zheng, H.; Song, X.; Liu, G.; Ridgway, P.; Wang, J.Q.; Battaglia, V.S. A Comparative Study of Polyacrylic Acid and Poly(Vinylidene Difluoride) Binders for Spherical Natural Graphite/LiFePO4 Electrodes and Cells. J. Power Sources 2011, 196, 7707–7714. [Google Scholar] [CrossRef]
- Aslan, M.; Weingarth, D.; Jäckel, N.; Atchison, J.S.; Grobelsek, I.; Presser, V. Polyvinylpyrrolidone as Binder for Castable Supercapacitor Electrodes with High Electrochemical Performance in Organic Electrolytes. J. Power Sources 2014, 266, 374–383. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of Styrene Butadiene Rubber and Sodium Methyl Cellulose as Binder for Negative Electrodes in Lithium-Ion Batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, X.; Zhang, D.; Qiu, H.; Fu, Q.; Na, H.; Guo, Z.; Du, F.; Chen, G.; Wei, Y. Water Soluble Styrene Butadiene Rubber and Sodium Carboxyl Methyl Cellulose Binder for ZnFe2O4 Anode Electrodes in Lithium Ion Batteries. J. Power Sources 2015, 285, 227–234. [Google Scholar] [CrossRef]
- Gordon, R.; Orias, R.; Willenbacher, N. Effect of Carboxymethyl Cellulose on the Flow Behavior of Lithium-Ion Battery Anode Slurries and the Electrical as Well as Mechanical Properties of Corresponding Dry Layers. J. Mater. Sci. 2020, 55, 15867–15881. [Google Scholar] [CrossRef]
- Versaci, D.; Nasi, R.; Zubair, U.; Amici, J.; Sgroi, M.; Dumitrescu, M.A.; Francia, C.; Bodoardo, S.; Penazzi, N. New Eco-Friendly Low-Cost Binders for Li-Ion Anodes. J. Solid State Electrochem. 2017, 21, 3429–3435. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kinoshita, Y.; Misaki, K.; Matsuyama, T.; Komaba, S. Electrochemical Properties of LiCoO 2 Electrodes with Latex Binders on High-Voltage Exposure. J. Electrochem. Soc. 2015, 162, A538–A544. [Google Scholar] [CrossRef]
- Komaba, S.; Okushi, K.; Ozeki, T.; Yui, H.; Katayama, Y.; Miura, T.; Saito, T.; Groult, H. Polyacrylate Modifier for Graphite Anode of Lithium-Ion Batteries. Electrochem. Solid State Lett. 2009, 12, A107. [Google Scholar] [CrossRef]
- Han, Z.-J.; Yabuuchi, N.; Shimomura, K.; Murase, M.; Yui, H.; Komaba, S. High-Capacity Si–Graphite Composite Electrodes with a Self-Formed Porous Structure by a Partially Neutralized Polyacrylate for Li-Ion Batteries. Energy Environ. Sci. 2012, 5, 9014–9020. [Google Scholar] [CrossRef]
- Qi, Y.; Nguyen, M.H.T.; Oh, E.-S. Effect of Conductive Polypyrrole in Poly(Acrylonitrile-Co-Butyl Acrylate) Water–Based Binder on the Performance of Electrochemical Double-Layer Capacitors. J. Solid State Electrochem. 2020. [CrossRef]
- Nguyen, M.H.T.; Oh, E.-S. Application of a New Acrylonitrile/Butylacrylate Water-Based Binder for Negative Electrodes of Lithium-Ion Batteries. Electrochem. Commun. 2013, 35, 45–48. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Electrochemical Impedance Study on the Low Temperature of Li-Ion Batteries. Electrochimica Acta 2004, 49, 1057–1061. [Google Scholar] [CrossRef]



| Electrode | Composite Thickness (μm) | Composite Surface Resistivity (Ohm cm2) | Interface Resistance (Ohm cm2) | Composite Volume Resistivity (Ohm cm) |
|---|---|---|---|---|
| PAL | 16.4 | 1.19 × 10−2 | 2.93 | 7.27 |
| SBR | 16.9 | 1.85 × 10−2 | 1.85 | 7.59 |
| PVdF | 18.5 | 2.75 × 10−2 | 8.91 | 12.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Qi, Y.; Oh, E.-S. Application of a Polyacrylate Latex to a Lithium Iron Phosphate Cathode as a Binder Material. Energies 2021, 14, 1902. https://doi.org/10.3390/en14071902
Tian M, Qi Y, Oh E-S. Application of a Polyacrylate Latex to a Lithium Iron Phosphate Cathode as a Binder Material. Energies. 2021; 14(7):1902. https://doi.org/10.3390/en14071902
Chicago/Turabian StyleTian, Mi, Yanchunxiao Qi, and Eun-Suok Oh. 2021. "Application of a Polyacrylate Latex to a Lithium Iron Phosphate Cathode as a Binder Material" Energies 14, no. 7: 1902. https://doi.org/10.3390/en14071902
APA StyleTian, M., Qi, Y., & Oh, E.-S. (2021). Application of a Polyacrylate Latex to a Lithium Iron Phosphate Cathode as a Binder Material. Energies, 14(7), 1902. https://doi.org/10.3390/en14071902






