Small-Scale Biodiesel Production Plants—An Overview
Abstract
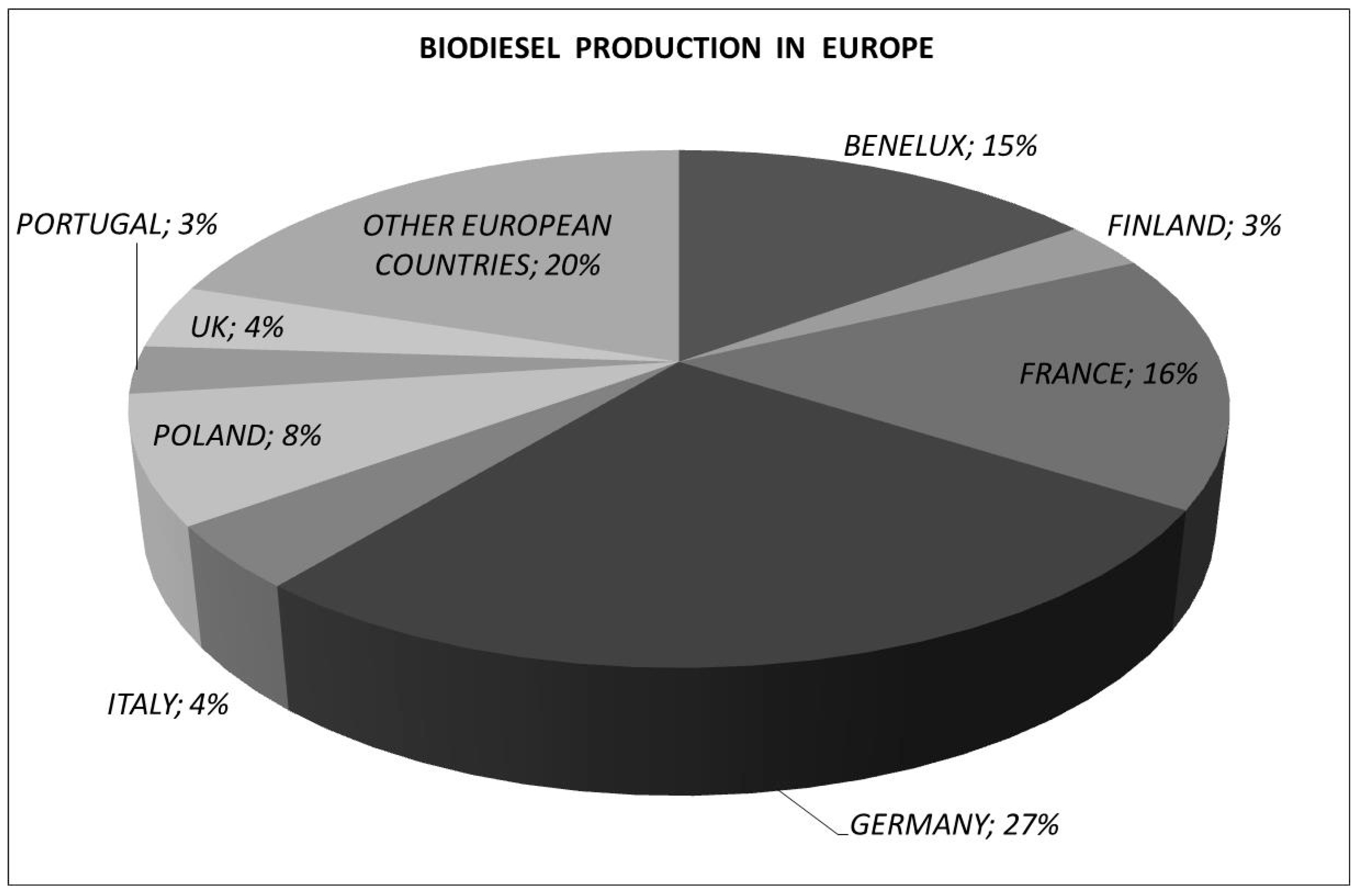
1. Introduction
2. Oils Used in Small-Scale Biodiesel Production
2.1. Own-Produced Oils
2.2. Purchased Oils
2.3. Recovered Oils from Wastes
3. Small-Scale Biodiesel Plants
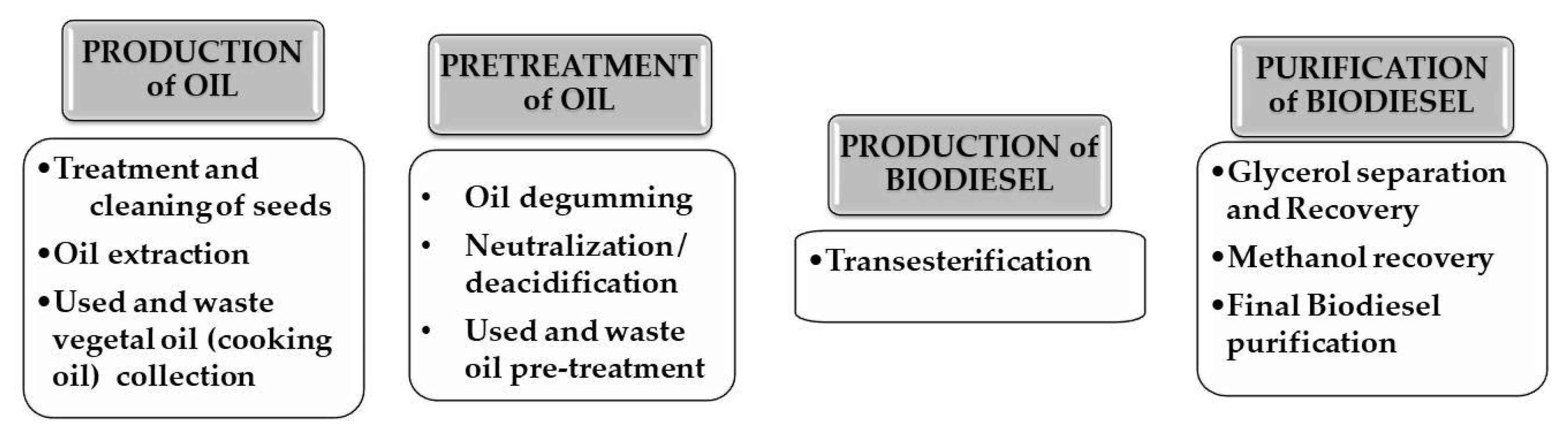
3.1. Small-Plant Units
3.1.1. Pre-Treatment of Waste Oils
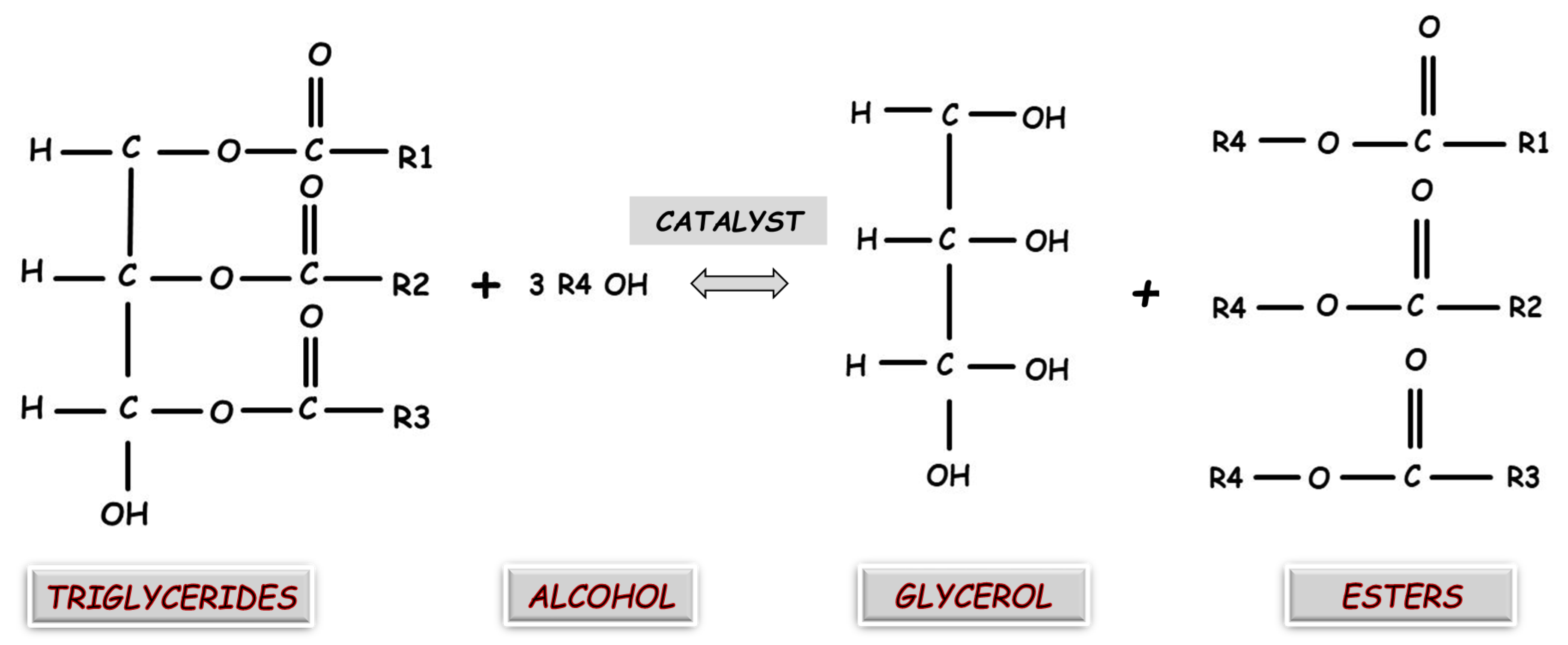
3.1.2. Reactor
3.1.3. Separation of Biodiesel from By-Products
3.1.4. Purification
3.2. Material Selection and Chemical Compatibility
- -
- Metals: aluminum, steel, stainless steel;
- -
- Elastomers: Viton, fluorosilicone, HiFluor, fluorocarbon, Chemraz, Teflon;
- -
- Polymers: polypropylene, polyethylene, high-density polyethylene, fluorinated materials, nylon.
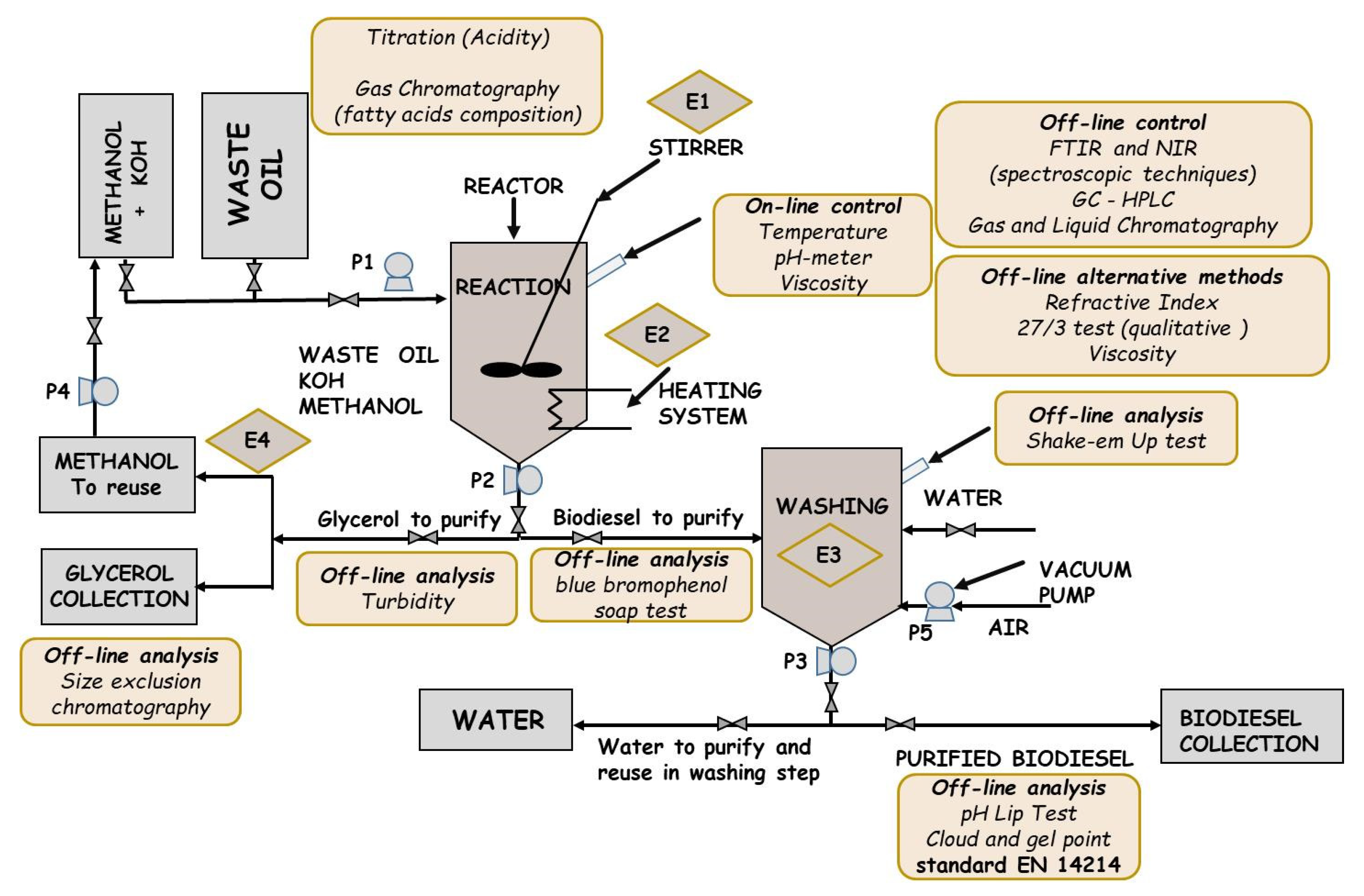
3.3. Analysis Methods in Small Plants
3.3.1. Analysis in Pre-Treatment
3.3.2. Analysis during Reaction and before Product Separation
3.3.3. Analysis before Biodiesel Washing
3.3.4. Analysis during Biodiesel Water Washing
3.3.5. Analysis on Biodiesel
3.4. Energy Consumption
3.4.1. Energy Storage
3.4.2. Energy Indicators for Small-Scale Plants
3.5. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.; Polasky, S.; Nelson, E.; Tilman, D.; Huo, H.; Ludwig, L.; Neumann, J.; Zheng, H.; Bonta, D. Climate Change and Health Costs of Air Emissions from Biofuels and Gasoline. Proc. Natl. Acad. Sci. USA 2009, 106, 2077–2082. [Google Scholar] [CrossRef]
- European Committee for Standardization. Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods; European Committee for Standardization: Brussels, Belgium, 2008. [Google Scholar]
- European Biomass Industry Association. Available online: https://www.eubia.org/ (accessed on 28 February 2021).
- SRS Plus. Available online: www.biodiesel-intl.com/standards_e/standards.htm (accessed on 28 February 2021).
- EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1410967635606&uri=CELEX:02009L0028-20130701 (accessed on 28 February 2021).
- British Standard. Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods; British Standard: London, UK, 2010. [Google Scholar]
- Farm Advisory System. Available online: https://ec.europa.eu/info/food-farming-fisheries/key-policies/common-agricultural-policy/income-support/cross-compliance/fas_en (accessed on 28 February 2021).
- Calabrò, V.; Ricca, E.; De Paola, M.G.; Curcio, S.; Iorio, G. Kinetics of Enzymatic Trans-Esterification of Glycerides for Biodiesel Production. Bioprocess Biosyst. Eng. 2010, 33, 701–710. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Transesterification of Vegetable Oil to Biodiesel Fuel Using Alkaline Catalyst. Fuel 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Miao, X.; Li, R.; Yao, H. Effective Acid-Catalyzed Transesterification for Biodiesel Production. Energy Convers. Manag. 2009, 50, 2680–2684. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Naccarato, S.; Albo, L.; De Paola, M.G.; Chakraborty, S.; Curcio, S.; Calabrò, V. Enzymatic Transesterification of Waste Vegetable Oil to Produce Biodiesel. Ecotoxicol. Environ. Saf. 2015, 121, 229–235. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Albo, L.; Policicchio, M.F.; Chakraborty, S.; Calabro, V. Comparative Analysis of Immobilized Biocatalyst: Study of Process Variables in Trans-Esterification Reaction. 3 Biotech 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Schumacher, J.; Gustafson, C. Economics of Small-Scale Biodiesel Production. Available online: https://farm-energy.extension.org/economics-of-small-scale-biodiesel-production/ (accessed on 28 February 2021).
- National Fire Protection Association. Guide to Fire Hazard Properties of Flammable Liquids, Gases and Volatile Solids; National Fire Protection Association: Quincy, MA, USA, 1994. [Google Scholar]
- National Fire Protection Association. Flammable and Combustible Liquids Code—Volume 30; National Fire Protection Association: Quincy, MA, USA, 2008. [Google Scholar]
- Methanol Source Request. Available online: www.methanol.org (accessed on 28 February 2021).
- You, Y.-D.; Shie, J.-L.; Chang, C.-Y.; Huang, S.-H.; Pai, C.-Y.; Yu, Y.-H.; Chang, C.H. Economic Cost Analysis of Biodiesel Production: Case in Soybean Oil. Energy Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
- Panvini, A. Biodiesel—Produzione, Utilizzo, Valutazioni Economiche Ed Ambientali. Available online: https://www.cti2000.it/Bionett/BioNett_Biodiesel_ITA.pdf (accessed on 28 February 2021).
- Learn More About Small Scale Biodiesel Production. Available online: http://www.springboardbiodiesel.com/small-scale-biodiesel-production (accessed on 28 February 2021).
- Sarantopoulos, I.; Che, F.; Tsoutsos, T.; Bakirtzoglou, V.; Azangue, W.; Bienvenue, D.; Ndipen, F.M. An Evaluation of a Small-Scale Biodiesel Production Technology: Case Study of Mango’ o Village, Center Province, Cameroon. Phys. Chem. Earth 2009, 34, 55–58. [Google Scholar] [CrossRef]
- Al-attab, K.; Wahas, A.; Almoqry, N.; Alqubati, S. Biodiesel Production from Waste Cooking Oil in Yemen: A Techno-Economic Investigation. Biofuels 2016. [Google Scholar] [CrossRef]
- Kumaran, P.; Mazlini, N.; Hussein, I.; Nazrain, M.; Khairul, M. Technical Feasibility Studies for Langkawi WCO (Waste Cooking Oil) Derived-Biodiesel. Energy 2011, 36, 1386–1393. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.D.; Chen, H.; Zhang, X.; Mondol, J.; Shah, N.; Hewitt, N.J. Performance Analysis of Biofuel Fired Trigeneration Systems with Energy Storage for Remote Households. Appl. Energy 2017, 186, 530–538. [Google Scholar] [CrossRef]
- Canakci, M. The Potential of Restaurant Waste Lipids as Biodiesel Feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Transportation, Air Pollution, and Climate Change. Available online: https://www.epa.gov/transportation-air-pollution-and-climate-change (accessed on 28 February 2021).
- Used and Waste Oil and Grease for Biodiesel. Available online: https://farm-energy.extension.org/used-and-waste-oil-and-grease-for-biodiesel/ (accessed on 28 February 2021).
- What Is Biodiesel? Available online: https://biodieseleducation.org/Education/WhatIsBiodiesel.html (accessed on 21 February 2021).
- Wiltsee, G. Urban Waste Grease Resource Assessment; National Renewable Energy Lab.: Golden, CO, USA, 1998.
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.N.; Bayer, I.S.; Athanassiou, A. Bioplastics from Vegetable Waste: Via an Eco-Friendly Water-Based Process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Kolet, M.; Zerbib, D.; Nakonechny, F.; Nisnevitch, M. Production of Biodiesel from Brown Grease. Catalysts 2020, 10, 1189. [Google Scholar] [CrossRef]
- Spiller, R.; Knoshaug, E.P.; Nagle, N.; Dong, T.; Milbrandt, A.; Clippinger, J.; Peterson, D.; VanWychen, S.; Panczak, B.; Pienkos, P.T. Upgrading Brown Grease for the Production of Biofuel Intermediates. Bioresour. Technol. Rep. 2020, 9, 100344. [Google Scholar] [CrossRef]
- Pinotti, L.M.; Benevides, L.C.; Lira, T.S.; de Oliveira, J.P.; Cassini, S.T.A. Biodiesel Production from Oily Residues Containing High Free Fatty Acids. Waste Biomass Valorization 2018, 9, 293–299. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Antunes, P.W.P.; Santos, A.R.; Mordente, T.Z.; Pinotti, L.M.; Cassini, S.T.A. Transesterification of Sanitation Waste for Biodiesel Production. Waste Biomass Valorization 2017, 8, 463–471. [Google Scholar] [CrossRef]
- Huang, D.L.; Wang, Z.M.; Zhu, S.N. Optimization of Biodiesel Production from Acid Oil via Acid Catalysis. Int. Conf. Electr. Control Eng. ICECE Proc. 2011, 25, 1654–1657. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Antunes, P.W.P.; Mordente, T.Z.; Santos, A.R.; Pinotti, L.M.; Cassini, S.T.A. Biodiesel Production from Scum of Grease Traps and Sludge from Septic Tanks. Clean Technol. Environ. Policy 2017, 19, 1231–1237. [Google Scholar] [CrossRef]
- Waickman, Z. Biodiesel Labs; Loyola University of Chicago, Institute of Environmental Sustainability, Biodiesel Program: Chicago, IL, USA, 2017. [Google Scholar]
- Paladino, O.; Neviani, M. A Closed Loop Biowaste to Biofuel Integrated Process Fed with Waste Frying Oil, Organic Waste and Algal Biomass: Feasibility at Pilot Scale. Renew. Energy 2018, 124, 61–74. [Google Scholar] [CrossRef]
- Iglesias, L.; Laca, A.; Herrero, M.; Díaz, M. A Life Cycle Assessment Comparison between Centralized and Decentralized Biodiesel Production from Raw Sun Fl Ower Oil and Waste Cooking Oils. J. Clean. Prod. 2012, 37, 162–171. [Google Scholar] [CrossRef]
- Foroutan, R.; Esmaeili, H.; Ghyasi, F.F. Optimization of Biodiesel Production from Waste Cooking Oil by Alkaline Catalysts. J. Food Technol. Food Chem. 2018, 1, 1–7. [Google Scholar]
- Fallon, W.; Kirkpatrick, N.; Mahon, S.; Bonnell, R. The Design and Construction of a Small-Scale Biodiesel Plant; Bellairs Research Institute: Holetown, Barbados, 2005. [Google Scholar]
- Prakoso, T.; Reksowardojo, I.K.; Soerawidjaja, T.H. Small Scale Biodiesel Production Plant by Utilizing Two Tanks System. In Proceedings of the International Workshop on Automotive Technology, Engine and Alternative Fuels, Ho Chi Minh City, Vietnam, December 2008. [Google Scholar]
- Canakci, M.; Van Gerpen, J. Biodiesel Production from Oils and Fats with High Free Fatty Acids. Trans. Am. Soc. Agric. Eng. 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Van Gerpen, J.; Shanks, B.; Pruszko, R.; Clements, D.; Knothe, G. Biodiesel Production Technology; National Renewable Energy Lab.: Golden, CO, USA, 2004.
- Mićić, R.; Tomić, M.; Martinović, F.; Kiss, F.; Simikić, M.; Aleksic, A. Reduction of Free Fatty Acids in Waste Oil for Biodiesel Production by Glycerolysis: Investigation and Optimization of Process Parameters. Green Process. Synth. 2019, 8, 15–23. [Google Scholar] [CrossRef]
- Abomohra, A.E.F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of Fat, Oil and Grease (FOG) for Biodiesel Production: A Critical Review on the Recent Progress and Future Perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Hassan, Z.; Shaharuddin, H.; Wan Asma, I.; Siti Nur Ridhwah, M.R. Production and Applications of Biodiesel from High Acid Value Feedstocks. J. Mech. Eng. 2018, 5, 10–15. [Google Scholar]
- Clements, D. Preatreatment of High Free Fatty Acid Feedstocks. Biodiesel TechNotes 2018, 33, 78c–78i. [Google Scholar]
- Demirbas, A. Comparison of Transesterification Methods for Production of Biodiesel from Vegetable Oils and Fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- De Boer, K. Optimised Small Scale Reactor Technology, a New Approach for the Australian Biodiesel Industry; Murdoch University: Perth, Australia, 2010. [Google Scholar]
- Blinová, L.; Fiala, J.; Balog, K. Biodiesel Production from Waste Cooking Oil in Laboratory Scale. Appl. Mech. Mater. 2014, 448–453, 1656–1659. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonzalez, J.F.; Rodriguez-Reinares, A. Biodiesel from Used Frying Oil. Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Olaoye, J.O.; Adegite, J.O.; Salami, H.A. Development of a Laboratory Scale Biodiesel Batch Reactor. Int. Res. J. Eng. Technol. 2017, 4, 1698–1704. [Google Scholar]
- Bello, E.I.; Daniyan, I.A.; Akinola, A.O.; Ogedengbe, I.T. Development of a Biodiesel Processor. Res. J. Eng. Appl. Sci. 2013, 2, 182–186. [Google Scholar]
- Daniyan, I.A.; Adeodu, A.O.; Dada, O.M.; Aribidara, A.A. Design of a Small Scale Bio-Diesel Processor. J. Emerg. Trends Eng. Appl. Sci. 2013, 4, 576–580. [Google Scholar]
- Abbaszaadeh, A.; Ghobadian, B.; Najafi, G.; Motevali, A. Design, Fabrication and Evaluation of a Novel Biodiesel Processor System. Int. J. Renew. Energy Technol. Res. 2013, 2, 249–255. [Google Scholar]
- Mullenix, D.K. Optimization and Economics of Small-Scale, On-Farm Biodiesel Production Using Oilseed Crops and Waste Vegetable Oil; Auburn University: Auburn, AL, USA, 2011. [Google Scholar]
- Agnew, R.; Chai, M.; Lu, M.; Dendramis, N. Making Biodiesel from Recycled Cooking Oil Generated in Campus Dining Facilities. Sustainability 2009, 2, 303–307. [Google Scholar] [CrossRef]
- Ouanji, F.; Kacimi, M.; Ziyad, M.; Puleo, F.; Liotta, L.F. Production of Biodiesel at Small-Scale (10 L) for Local Power Generation. Int. J. Hydrog. Energy 2016, 42, 8914–8921. [Google Scholar] [CrossRef]
- Rahmat, B.; Setiasih, I.S.; Kastaman, R. Biodiesel Reactor Design with Glycerol Separation to Increase Biodiesel Production Yield. Makara Seri Teknol. 2013, 17, 11–16. [Google Scholar] [CrossRef]
- Anilkumar, G.; Raju, J.; Thomas, M. Design and Fabrication of Automatic Biodiesel Production Equipment. Int. J. Appl. Eng. Res. 2019, 14, 77–80. [Google Scholar]
- Shahare, P.; Harode, A.; Ingale, A.; Godbole, E.; Lilhare, A.; Deshmukh, R. Fabrication of Small Scale Biodiesel Plant. Int. J. Innov. Res. Technol. 2018, 5, 7–10. [Google Scholar]
- Ramesh, D.; Samapathrajan, A.; Venkatachalam, P. Production of Biodiesel from Jatropha Curcas Oil by Using Pilot Biodiesel Plant. Jatropha Online J. 2006, 18, 1–6. [Google Scholar]
- Oliveira, L.S.; Brasil, A.N.; Nunes, D.L. Design and Operation of a Mobile Biodiesel Production Unit. Chem. Biol. Environ. Eng. 2010. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Sanli, H. Biodiesel Production from Vegetable Oil and Waste Animal Fats in a Pilot Plant. Waste Manag. 2014, 34, 2146–2154. [Google Scholar] [CrossRef]
- Ko, M.J.; Park, J.J.; Hong, S.Y. Continuous Biodiesel Production Using in Situ Glycerol Separation by Membrane Bioreactor System. Bioprocess Biosyst. Eng. 2012, 35, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wall, J. Comparison of Methods for the Purification of Biodiesel; College of Graduate Studies—University of Idaho: Moscow, ID, USA, 2009. [Google Scholar]
- Ortega, M.A.C.; Alpírez, G.M.; Eliezer, A.; González, C.G.; Román, J.C.; Pérez Pelayo, L.J.; Ayala Bautista, J.R. Materials Technological Challenges for the Biodiesel Industry Development in Mexico. Mater. Process. Energy Commun. Curr. Res. Technol. Dev. 2013, 279–288. [Google Scholar]
- Coronado, M.; Montero, G.; García, C.; Schorr, M.; Valdez, B.; Eliezer, A. Equipment, Materials, and Corrosion in the Biodiesel Industry. Mater. Perform. 2020, 58, 34–38. [Google Scholar]
- Shrestha, D. Biodiesel TechNotes; National Biodiesel Education Program, University of Idaho: Moscow, ID, USA, 2017. [Google Scholar]
- Alleman, T.L.; Christensen, E.D.; Fioroni, G.; Moriarty, K. Biodiesel Handling and Use Guide, 5th ed.; National Renewable Energy Lab.: Cincinnati, OH, USA, 2016.
- Nedambale, N.; Ndlovu, N.; Ntombela, T.; Low, M.; Harding, K.G.; Matambo, T. Alternative Testing Methods to Determine the Quality of Biodiesel. Chem. Technol. 2014, 16. [Google Scholar]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by Transesterification of Rapeseed Oil Using Ultrasound: A Kinetic Study of Base-Catalysed Reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef]
- Martins, M.I.; Pires, R.F.; Alves, M.J.; Hori, C.E.; Reis, M.H.M.; Cardoso, V.L. Transesterification of Soybean Oil for Biodiesel Production Using Hydrotalcite as Basic Catalyst. Chem. Eng. Trans. 2013, 32, 817–822. [Google Scholar]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR Analysis for Quantification of Fatty Acid Methyl Esters in Biodiesel Produced by Microwave-Assisted Transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964–969. [Google Scholar] [CrossRef]
- Yuan, T.; Akochi-Koble, E.; Pinchuk, D.; van de Voort, F.R. FTIR On-Line Monitoring of Biodiesel Transesterification. Int. J. Renew. Energy Biofuels 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Felizardo, P.; Baptista, P.; Uva, M.S.; Menezes, J.C.; Neiva Correia, M.J. Monitoring Biodiesel Fuel Quality by near Infrared Spectroscopy. J. Near. Infrared Spectrosc. 2007, 15, 97–105. [Google Scholar] [CrossRef]
- De Lima, S.M.; Silva, B.F.A.; Pontes, D.V.; Pereira, C.F.; Stragevitch, L.; Pimentel, M.F. In-Line Monitoring of the Transesterification Reactions for Biodiesel Production Using NIR Spectroscopy. Fuel 2014, 115, 46–53. [Google Scholar] [CrossRef]
- Zawadzki, A.; Shrestha, D.; He, B. Use of a Spectrophotometer for Biodiesel Quality Sensing. In 2005 ASAE Annual Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005. [Google Scholar]
- Figueiredo, M.K.K.; Silva, C.E.R.; Alvarenga, A.V.; Costa-Félix, R.P.B. Monitoring Biodiesel Reactions of Soybean Oil and Sunflower Oil Using Ultrasonic Parameters. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2013. [Google Scholar]
- Tubino, M.; Rocha Junior, J.G.; Bauerfeldt, G.F. Biodiesel Synthesis with Alkaline Catalysts: A New Refractometric Monitoring and Kinetic Study. Fuel 2014, 125, 164–172. [Google Scholar] [CrossRef]
- Zabala, S.; Arzamendi, G.; Reyero, I.; Gandía, L.M. Monitoring of the Methanolysis Reaction for Biodiesel Production by Off-Line and on-Line Refractive Index and Speed of Sound Measurements. Fuel 2020, 121, 157–164. [Google Scholar] [CrossRef]
- Ellis, N.; Guan, F.; Chen, T.; Poon, C. Monitoring Biodiesel Production (Transesterification) Using in Situ Viscometer. Chem. Eng. J. 2008, 138, 200–206. [Google Scholar] [CrossRef]
- Testing for Small-Scale Biodiesel Quality. Available online: http://www.springboardbiodiesel.com/testing-small-scale-biodiesel-quality (accessed on 28 February 2021).
- Da Tech, R. The 3–27 Conversion Test. Available online: https://www.make-biodiesel.org/Quality-Testing/the-3-27-conversion-test.html (accessed on 28 February 2021).
- Da Tech, R. The Biodiesel PHLip Test. Available online: https://www.make-biodiesel.org/Quality-Testing/the-biodiesel-phlip-test.html (accessed on 28 February 2021).
- Energia Nel Settore Trasporti. Available online: https://www.gse.it/documenti_site/Documenti GSE/Rapporti statistici/Energia nei Trasporti 2017.pdf (accessed on 28 February 2021).
- ILSAP Relazioni Annuali 2017–2019, Dati Monitoraggio Emissioni. Available online: https://www.regione.calabria.it/website/portaltemplates/view/view.cfm?7725&7725 (accessed on 28 February 2021).
- Ayoola, A.A.; Oresegun, O.R.; Oladimeji, T.E.; Eneghalu, C. Energy Analysis of Biodiesel Production from Waste Groundnut Oil. Int. J. Res. Eng. Appl. Sci. 2019, 6, 202–208. [Google Scholar]
- Yari, N.; Mostafaei, M.; Naderloo, L.; Safieddin Ardebili, S.M. Energy Indicators for Microwave-Assisted Biodiesel Production from Waste Fish Oil. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–12. [Google Scholar] [CrossRef]
- Palanisamy, K.; Idlan, M.K.; Saifudin, N. Preliminary Evaluation of the Effectiveness of Moisture Removal and Energy Usage in Pretreatment Module of Waste Cooking Oil for Biodiesel Production. IOP Conf. Ser. Earth Environ. Sci. 2013, 16. [Google Scholar] [CrossRef]
- Mohammadshirazi, A.; Akram, A.; Rafiee, S.; Bagheri Kalhor, E. Energy and Cost Analyses of Biodiesel Production from Waste Cooking Oil. Renew. Sustain. Energy Rev. 2014, 33, 44–49. [Google Scholar] [CrossRef]
- Skarlis, S.; Kondili, E.; Kaldellis, J.K. Small-Scale Biodiesel Production Economics: A Case Study Focus on Crete Island. J. Clean. Prod. 2012, 20, 20–26. [Google Scholar] [CrossRef]
- Haase, S.; Craig, B. An Economic Analysis of Small-Scale Biodiesel Production: Implementation of Ethyl Ester Production in a Job Shop Setting. J. Undergrad. Res. 2004, 4, 7. [Google Scholar]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A Review on the Prospects of Sustainable Biodiesel Production: A Global Scenario with an Emphasis on Waste-Oil Biodiesel Utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]




| Biodiesel Properties | Unit | Lower–Upper Limit | Test-Method |
|---|---|---|---|
| Fatty Acid Methyl Esters (FAME) | % (m/m) * | 96.5 | EN 14103 |
| Density a 15 °C | kg/m3 | 860–900 | EN ISO 3675 EN 12185 |
| Viscosity at 40 °C | mm2/s | 3.5–5 | EN ISO 3104 |
| Acidity | mg KOH/g | max 0.5 | EN 14104 |
| Flash point | °C | 101 | EN ISO 3679 |
| Copper strip corrosion (at 50 °C, 3 h) | grad corrosion | Classe 1 | EN ISO 2160 |
| Stability to oxidation, 110 °C | hr | 6 | EN 14112 |
| Cetane number | - | min 51 | EN ISO 5165 |
| Iodine value | g iodine/100 g | max 120 | EN 14111 |
| Methanol | % (m/m) | 0.2 | EN 14110 |
| Monoglycerides | % (m/m) | max 0.8 | EN 14105 |
| Diglycerides | % (m/m) | max 0.2 | EN 14105 |
| Triglycerides | % (m/m) | max 0.2 | EN 14105 |
| Free glycerol | % (m/m) | max 0.02 | EN 14105 EN 14106 |
| Total glycerol | % (m/m) | max 0.25 | EN 14105 |
| Linoleic acid methyl ester | % (m/m) | max 12 | EN 14103 |
| Polyunsaturated methyl esters (more than 4 double bonds) | % (m/m) | max 1 | EN 15779 |
| Alkaline content (Na + K) | mg/kg | max 5 | EN 14108 EN 14109 EN 14538 |
| Alkaline earth metals (Ca,Mg) | mg/kg | max 5 | EN 14538 |
| Sulfur content | mg/kg | max 10 | EN ISO 20846 EN ISO 20884 |
| Phosphorus content | mg/kg | max 4 | EN 14107 |
| Carbon residue | % (mass) | max 0.3 | EN ISO 10370 |
| Sulfur ash | % (mass) | max 0.02 | ISO 3987 |
| Water content | mg/kg | max 500 | EN ISO 12937 |
| Total contamination | mg/kg | max 24 | EN 12662 |
| Sustainable Double Counting Biodiesel | Available Power MW | |
|---|---|---|
| Min | Max | |
| Biodiesel from used cooking oils in Italy (UCO) | 104 | 119 |
| Biodiesel from animal oils and fats in Italy | 361 | 409 |
| Biodiesel from processed used vegetable oils in Italy | 686 | 779 |
| Biodiesel from other agro-industrial waste | 9 | 10 |
| Ayoola et al. [88] | Yari et al. [89] | |
|---|---|---|
| Input energy (MJ/L) | 124.31 | 48.839 |
| Output energy (MJ/L) | 89.47 | 50.866 |
| Energy use efficiency | 0.72 | 1.041 |
| Net energy (MJ/L) | −35.04 | 2.027 |
| Energy productivity (kg/MJ) | 0.08 | 0.018 |
| Yield % | 92 | 92.44 |
| Items | Unit | Values |
|---|---|---|
| Specific energy available on an annual basis | kWh/kg per anno | 10.28–11.67 |
| Energy available on the market (annual data) | MJ/kg | 37–42 |
| Selling costs per unit of available energy | Euro/kWh | 0.081–0.092 |
| Capital cost (plant) (equal to 16.9% of the production cost and 12.23% of the revenue) per unit of available energy | Euro/kWh | 0.010–0.011 |
| O&M production costs (equal to 72% of the sales cost) per unit of available energy | Euro/kWh | 0.059–0.067 |
| Raw material costs (equal to 56.75% of the production cost) per unit of available energy this cost is included in the O&M | Euro/kWh | 0.033–0.038 |
| Capacity | Reactants | Start-Up Cost | Cost of Production | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Materials and Equipment | Assembly Labor Cost | Other Costs | Reactants | Labor Cost of Production | Electricity | Other Costs | Total | |||
| 10,000 t/year | Methanol Basic catalyst Vegetable oils | 2,080,000 € | - | 1,920,000 € 1 | 6,550,000 €/year | 300,000 €/year | 60,000 €/year | 810,000 €/year 2 | 780 €/t | [92] |
| 150 L/batch | Ethanol + methanol KOH Waste cooking oils | 1200$ | 610$ (61 h) | - | 31 $/batch | 28.2 $/batch (2.82 h/batch) | 1.99 $/batch (0.077 $/KWh) | - | 1.63 $/gallon | [93] |
| 208 L/batch | Methanol Waste vegetable oils | - | - | - | 1498 $/year | 1450 $/year | 27 $/year | 4314 $/year | 2.21 $/gallon | [56] |
| 600 L/day 165 t/year | Methanol NaOH Waste cooking oils | 48,800$ | - | 23,256$ 3 | 0.47 $/L | 1483.7 $/month | - | 490.7 $/month 4 + 0.08 $/L 5 | 0.67 $/L | [21] |
| 10 t/day | Methanol Sulfuric acid + KOH Waste chicken fat | - | - | - | 10,233 €/day | - | 273 €/day | - | 1.40 €/L | [64] |
| Methanol Sulfuric acid + KOH Waste fleshing oil | - | - | - | 9778 €/day | - | 219 €/day | - | 1.13 €/L | ||
| 90 t/day | Methanol Sulfuric acid + KOH Waste cooking oil | 4,049,000$ | - | 8,321,000$ 6 | 56,076 $/day | 94 $/day | 138 $/day | 339 $/day 7 | 0.519 $/L | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paola, M.G.; Mazza, I.; Paletta, R.; Lopresto, C.G.; Calabrò, V. Small-Scale Biodiesel Production Plants—An Overview. Energies 2021, 14, 1901. https://doi.org/10.3390/en14071901
De Paola MG, Mazza I, Paletta R, Lopresto CG, Calabrò V. Small-Scale Biodiesel Production Plants—An Overview. Energies. 2021; 14(7):1901. https://doi.org/10.3390/en14071901
Chicago/Turabian StyleDe Paola, Maria Gabriela, Ivan Mazza, Rosy Paletta, Catia Giovanna Lopresto, and Vincenza Calabrò. 2021. "Small-Scale Biodiesel Production Plants—An Overview" Energies 14, no. 7: 1901. https://doi.org/10.3390/en14071901
APA StyleDe Paola, M. G., Mazza, I., Paletta, R., Lopresto, C. G., & Calabrò, V. (2021). Small-Scale Biodiesel Production Plants—An Overview. Energies, 14(7), 1901. https://doi.org/10.3390/en14071901








