Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Material Fabrication
2.2. Material Characterization
2.3. Electrochemical Measurements
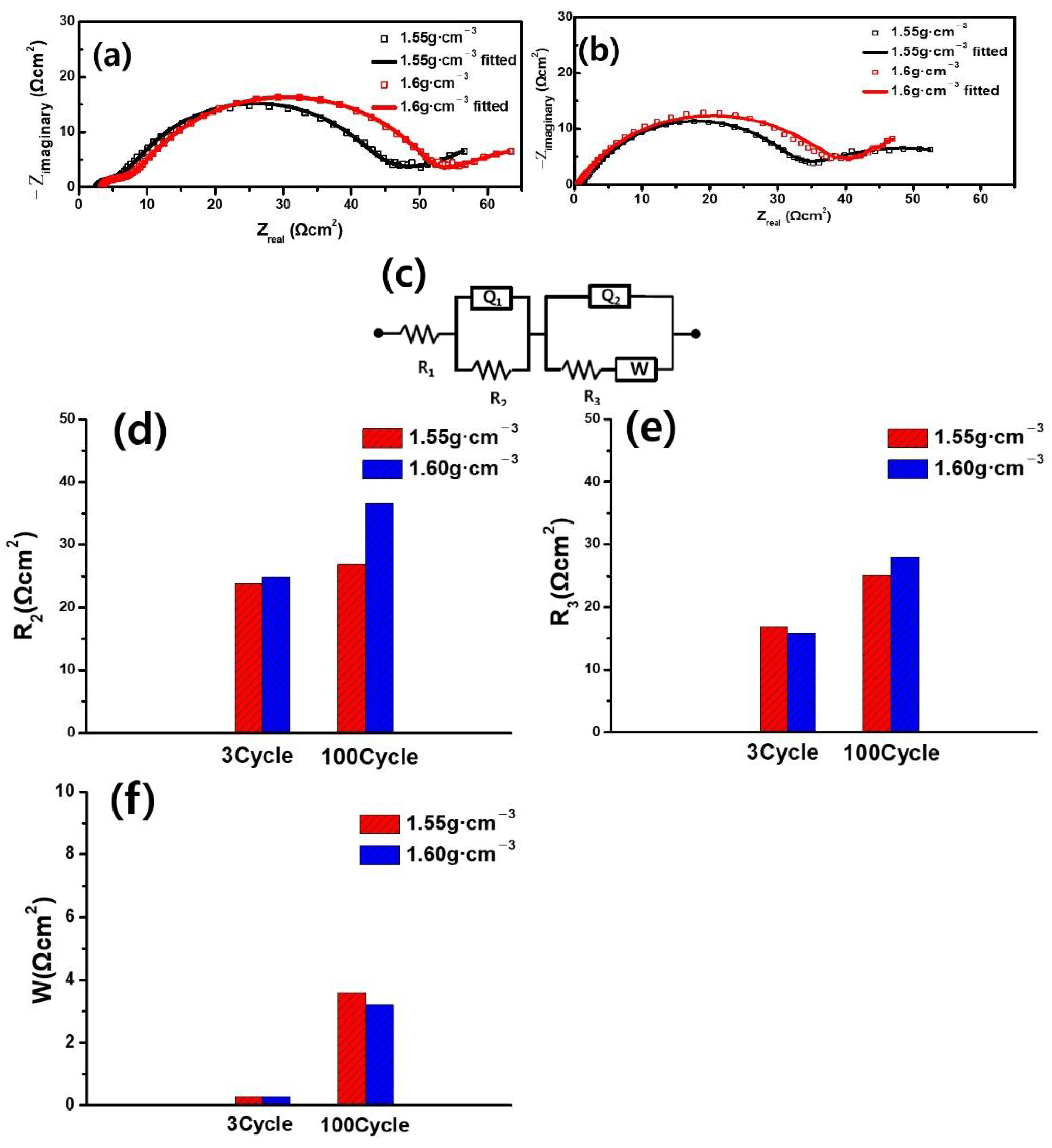
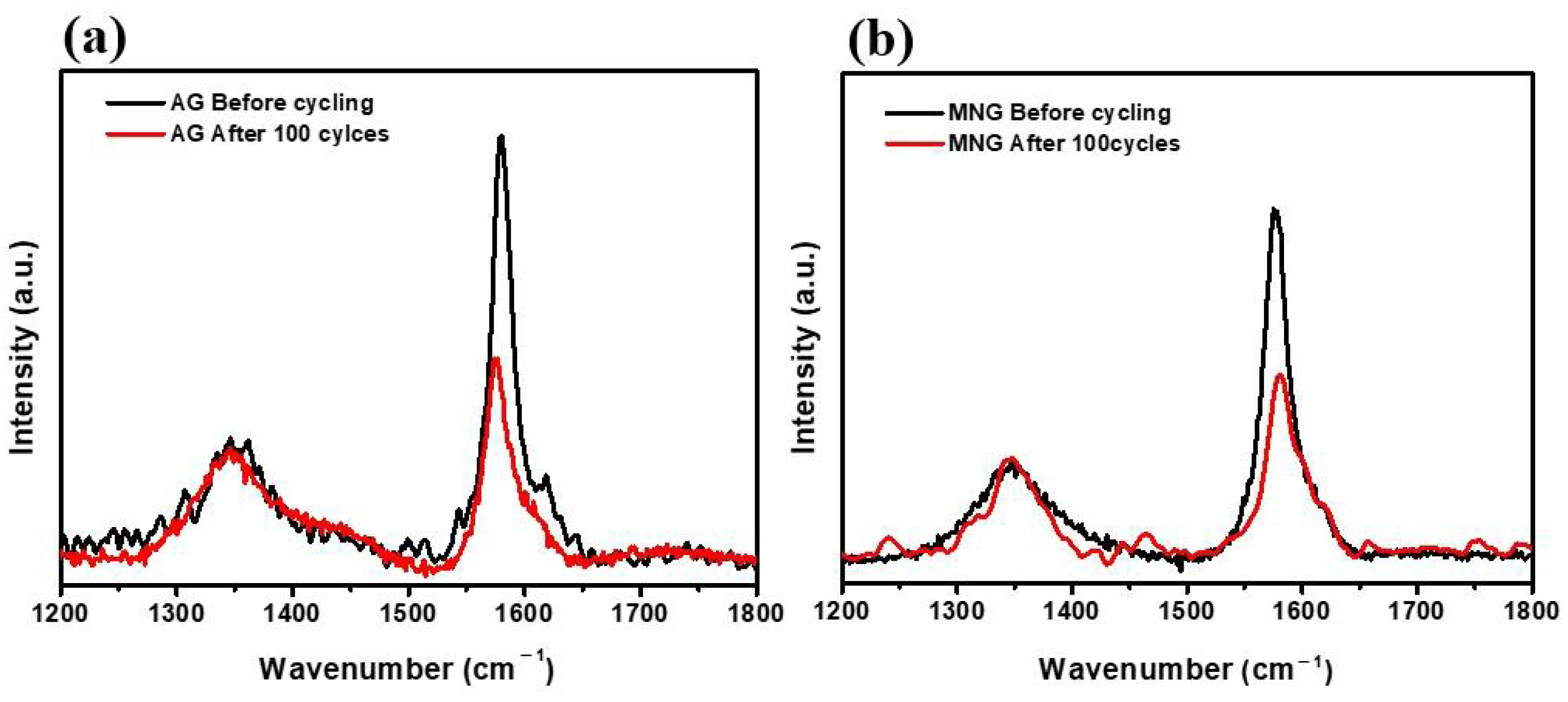
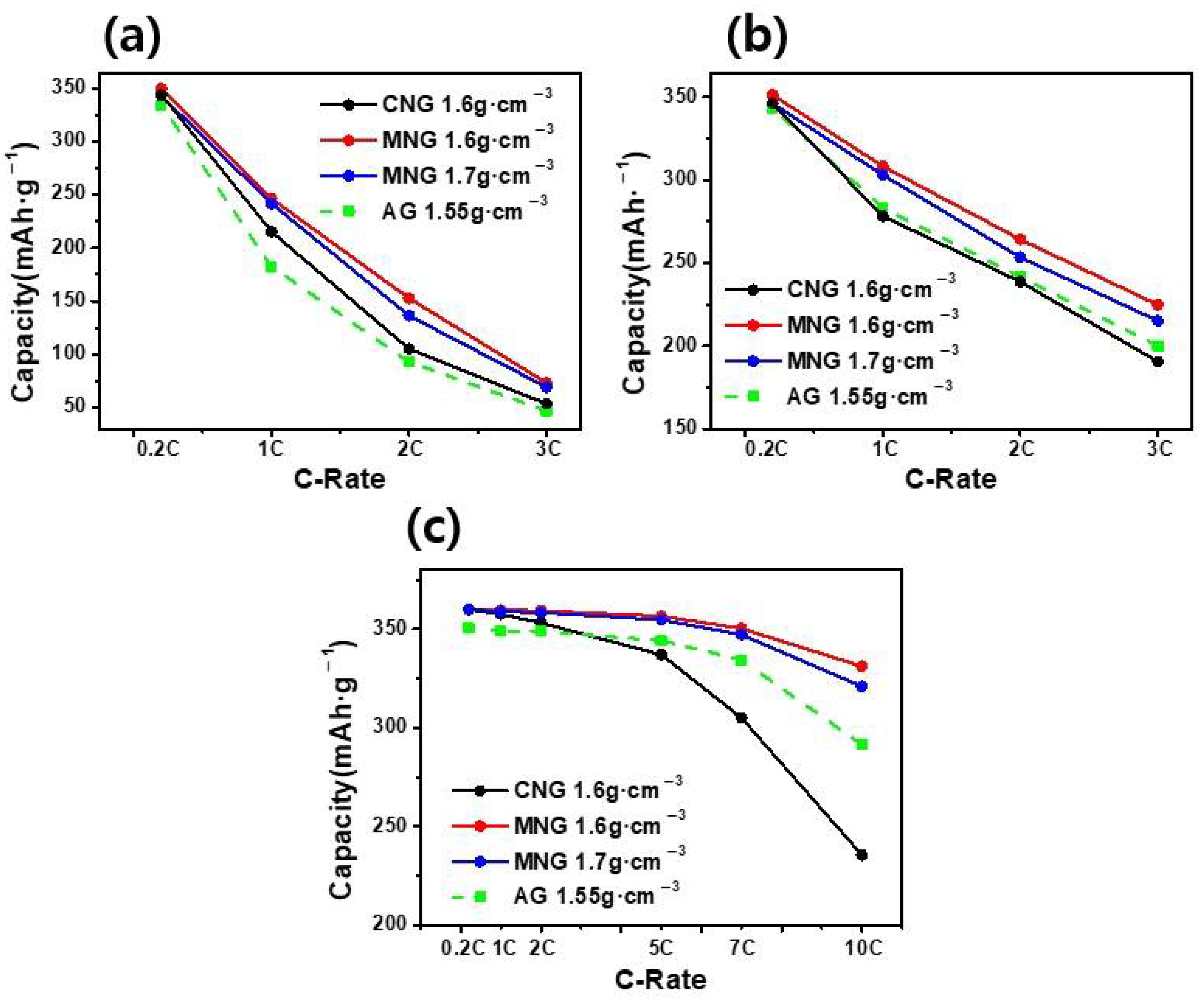
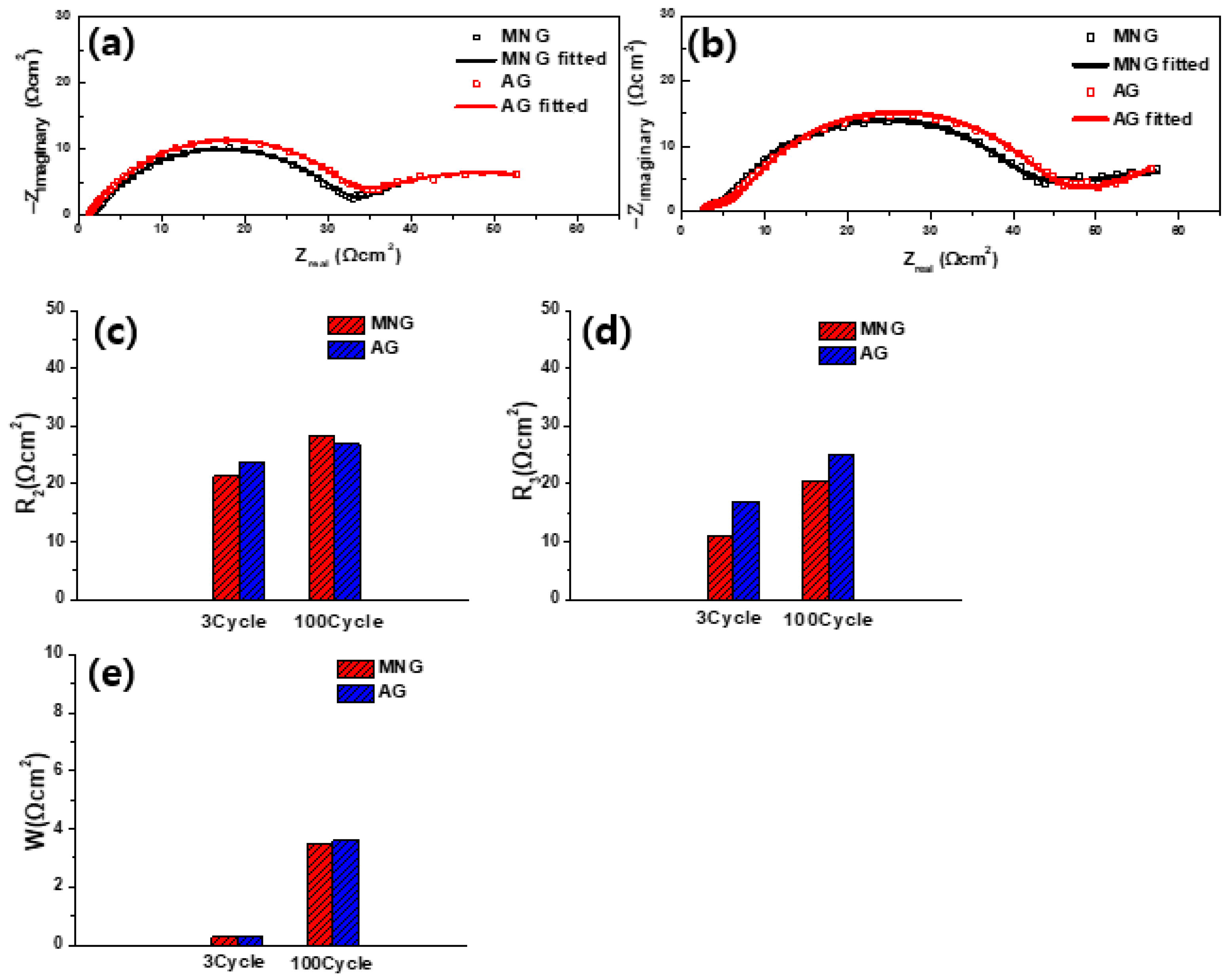
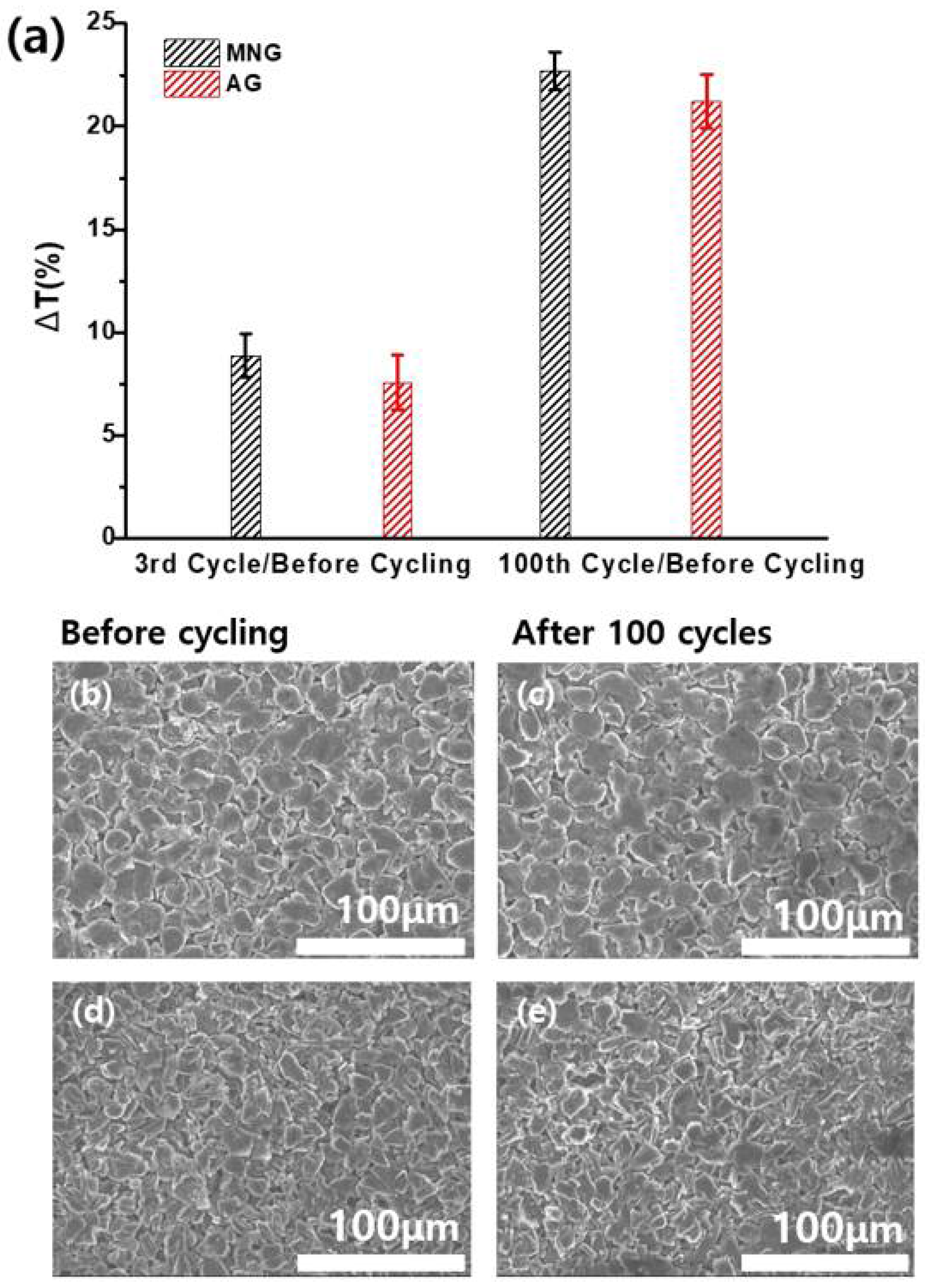
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endo, M.; Kim, C.; Nishimura, K.; Fujino, T.; Miyashita, K. Recent development of carbon materials for Li ion batteries. Carbon 2000, 38, 183–197. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A. Cycling performance of low-cost lithium ion batteries with natural graphite and LiFePO. J. Power Sources 2003, 119–121, 955–958. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K. Spherical Carbon-Coated Natural Graphite as a Lithium-Ion Battery-Anode Material. Angew. Chem. Int. Ed. 2003, 42, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Lee, T.-W.; Shin, M.-S.; Lim, S.-H.; Lee, S.-M. Modification for Improving the Electrochemical Performance of Spherically-Shaped Natural Graphite as Anode Material for Lithium-Ion batteries. J. Electrochem. Soc. 2016, 163, A3078–A3086. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Hara, Y.; Adachi, Y. Effect of Carbon Coating on Electrochemical Performance of Treated Natural Graphite as Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2000, 147, 1245–1250. [Google Scholar] [CrossRef]
- Glazier, S.L.; Li, J.; Louli, A.J.; Allen, J.P.; Dahn, J.R. An Analysis of Artificial and Natural Graphite in Lithium Ion Pouch Cells Using Ultra-High Precision Coulometry, Isothermal Microcalorimetry, Gas Evolution, Long Term Cycling and Pressure Measurements. J. Electrochem. Soc. 2017, 164, A3545–A3555. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Abe, T.; Ogumi, Z. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere Electronic supplementary information (ESI). Colour versions of Figs. 6, 8. J. Mater. Chem. 2004, 14, 1754–1758. Available online: http://www.rsc.org/suppdata/jm/b3/b316702j/ (accessed on 12 February 2021). [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K. Spherical Carbon-Coated Natural Graphite as a Lithium-Ion Battery-Anode Material. Angew. Chem. 2003, 115, 4335–4338. [Google Scholar] [CrossRef]
- Zaghib, K.; Song, X.; Guerfi, A.; Kostecki, R.; Kinoshita, K. Effect of particle morphology on lithium intercalation rates in natural graphite. J. Power Sources 2003, 124, 505–512. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, F.; Liu, C.; Tan, J.; Cheng, H.M. New Insight into Solid Electrolyte Interphase with Use of a Focused Ion Beam. J. Phys. Chem. B. 2005, 109, 22205–22211. [Google Scholar] [CrossRef] [PubMed]
- Striebel, K.A.; Sierra, A.; Shim, J.; Wang, C.-W.; Sastry, A.M. The Effect of Compression on Natural Graphite Anode Performance and Matrix Conductivity. J. Power Sources 2004, 134, 241–251. [Google Scholar] [CrossRef]
- Wang, C.-W.; Yi, Y.-B.; Sastry, A.M.; Shim, J.; Striebel, K.A. Particle Compression and Conductivity in Li-Ion Anodes with Graphite Additives. J. Electrochem. Soc. 2004, 151, 1489–1498. [Google Scholar] [CrossRef]
- Kuribayashi, I.; Yokoyama, M.; Yamashita, M. Battery characteristics with various carbonaceous materials. J. Power Sources 1995, 54, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Carbon-coated natural graphite prepared by thermal vapor decomposition process, a candidate anode material for lithium-ion battery. J. Power Sources 2001, 93, 123–129. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.; Oh, S.M. Surface modification of graphite by coke coating for reduction of initial irreversible capacity in lithium secondary batteries. J. Power Sources 2001, 94, 68–73. [Google Scholar] [CrossRef]
- Zhang, H.L.; Liu, S.H.; Li, F.; Bai, S.; Liu, C.; Tan, J.; Cheng, H.M. Electrochemical performance of pyrolytic carbon-coated natural graphite spheres. Carbon 2006, 44, 2212–2218. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, F.; Liu, C.; Cheng, H.M. Poly(vinyl chloride) (PVC) Coated Idea Revisited: Influence of Carbonization Procedures on PVC-Coated Natural Graphite as Anode Materials for Lithium Ion Batteries. J. Phys. Chem. C. 2008, 112, 7767–7772. [Google Scholar] [CrossRef]
- Wu, Y.S.; Wang, Y.H.; Lee, Y.H. Performance enhancement of spherical natural graphite by phenol resin in lithium ion batteries. J. Alloys Compd. 2006, 426, 218–222. [Google Scholar] [CrossRef]
- Wang, H.; Umeno, T.; Mizuma, K.; Yoshio, M. Highly conductive bridges between graphite spheres to improve the cycle performance of a graphite anode in lithium-ion batteries. J. Power Sources 2008, 175, 886–890. [Google Scholar] [CrossRef]
- Park, Y.S.; Oh, E.S.; Lee, S.M. Effect of polymeric binder type on the thermal stability and tolerance to roll-pressing of spherical natural graphite anodes for Li-ion batteries. J. Power Sources 2014, 248, 1191–1196. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries—A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Van Schalkwijk, W.; Scrosati, B. Advances in Lithium-Ion Batteries; Springer Publishing Company: New York, NY, USA, 2002; pp. 47–68. [Google Scholar]
- Agubra, V.; Fergus, J. Lithium Ion Battery Anode Aging Mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Baek, J.-K.; Lee, S.-M.; Park, H.-K.; Lee, K.-Y.; Kim, M.-H. Effect of carbon coating on elevated temperature performance of graphite as lithium-ion battery anode material. J. Power Sources 2004, 128, 61–66. [Google Scholar] [CrossRef]
- Barsoukov, E.; Kim, J.H.; Yoon, C.H.; Lee, H. Effect of Low-Temperature Conditions on Passive Layer Growth on Li Intercalation Materials: In Situ Impedance Study. J. Electrochem. Soc. 1998, 145, 2711–2717. [Google Scholar] [CrossRef]
- Piao, T.; Park, S.M.; Doh, C.H.; Moon, S.I. Intercalation of Lithium Ions into Graphite Electrodes Studied by AC Impedance Measurements. J. Electrochem. Soc. 1999, 146, 2794–2798. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, M.S.; Xu, K.; Allen, J.; Jow, T.R. Understanding Solid Electrolyte Interface Film Formation on Graphite Electrodes. Electrochem. Solid-state Lett. 2001, 4, A206–A208. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A474–A481. [Google Scholar] [CrossRef]
- Malifarge, S.; Delobel, B.; Delacourt, C. Experimental and Modeling Analysis of Graphite Electrodes with Various Thicknesses and Porosities for High-Energy-Density Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1275–A1287. [Google Scholar] [CrossRef]
- Habte, B.T.; Jiang, F. Effect of microstructure morphology on Li-ion battery graphite anode performance: Electrochemical impedance spectroscopy modeling and analysis. Solid State Ionics 2018, 314, 81–91. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, H. Dissecting anode swelling in commercial lithium-ion batteries. J. Power Sources 2012, 218, 52–55. [Google Scholar] [CrossRef]
- Mickelson, L.; Castro, H.; Switzer, E.; Friesen, C. Bulk Stress Evolution during Intercalation of Lithium in Graphite. J. Electrochem. Soc. 2014, 161, A2121–A2127. [Google Scholar] [CrossRef]
- Ohzuku, T.; Matoba, N.; Sawai, K. Direct evidence on anomalous expansion of graphite-negative electrodes on first charge by dilatometry. J. Power Sources 2001, 97–98, 73–77. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.M.; Ahn, S. Battery dimensional changes occurring during charge/discharge cycles-thin rectangular lithium ion and polymer cells. J. Power Sources 2003, 119–121, 833–837. [Google Scholar] [CrossRef]
- Harris, S.J.; Deshpande, R.D.; Qi, Y.; Dutta, I.; Cheng, Y.T. Mesopores inside electrode particles can change the Li-ion transport mechanism and diffusion-induced stress. J. Mater. Res. 2010, 25, 1433–1440. [Google Scholar] [CrossRef]
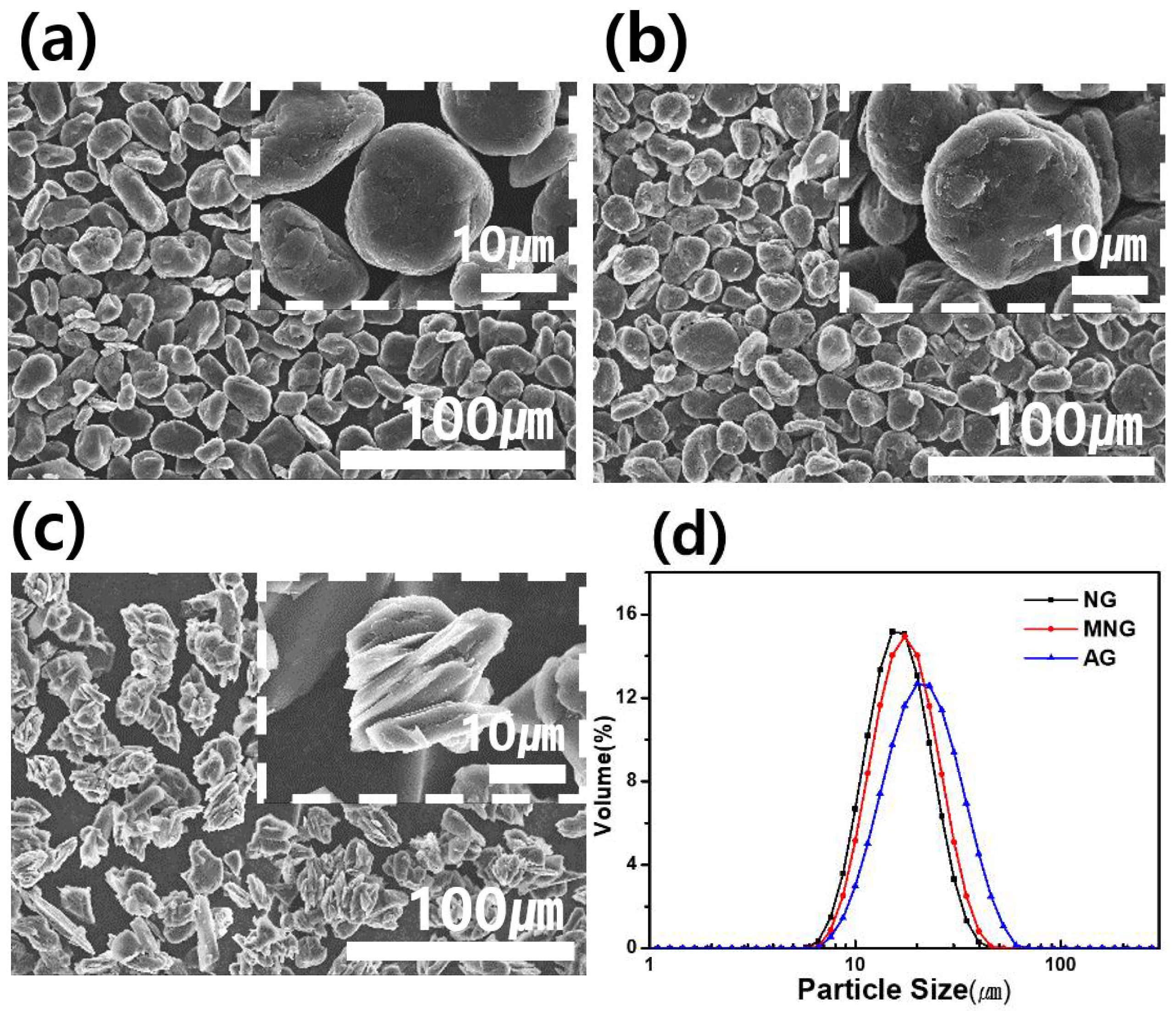




| Sample | MNG | AG | ||
|---|---|---|---|---|
| Tap density (g∙cm−3) | 1.07 | 0.85 | ||
| Electrode density (g∙cm−3) | 1.6 | 1.7 | 1.55 | 1.6 |
| Resistivity (μΩ∙cm) | 5.11 × 106 | 4.5 × 106 | 7.5 × 106 | 6.4 × 106 |
| Sample | Electrode Density (g∙cm−3) | Initial Capacity (mAh∙g−1) | Coulombic Efficiency (%) |
|---|---|---|---|
| MNG | 1.6 | 360.3 | 93 |
| 1.7 | 360.2 | 92.8 | |
| AG | 1.55 | 350.5 | 92.5 |
| 1.6 | 350.3 | 92.4 |
| Sample | AG | MNG | ||
|---|---|---|---|---|
| Electrode density(g·cm−3) | 1.55 | 1.6 | 1.6 | 1.7 |
| 3-cycle electrode | 2.00 × | 1.02 × | 7.89 × | 6.57 × |
| 100-cycle electrode | 1.8 × | 3.70 × | 1.91 × | 2.39 × |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-J.; Woo, S.-W.; Lee, Y.-J.; Kim, J.-Y.; Lee, S.-M. Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries. Energies 2021, 14, 1946. https://doi.org/10.3390/en14071946
Kwon H-J, Woo S-W, Lee Y-J, Kim J-Y, Lee S-M. Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries. Energies. 2021; 14(7):1946. https://doi.org/10.3390/en14071946
Chicago/Turabian StyleKwon, Hae-Jun, Sang-Wook Woo, Yong-Ju Lee, Je-Young Kim, and Sung-Man Lee. 2021. "Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries" Energies 14, no. 7: 1946. https://doi.org/10.3390/en14071946
APA StyleKwon, H.-J., Woo, S.-W., Lee, Y.-J., Kim, J.-Y., & Lee, S.-M. (2021). Achieving High-Performance Spherical Natural Graphite Anode through a Modified Carbon Coating for Lithium-Ion Batteries. Energies, 14(7), 1946. https://doi.org/10.3390/en14071946






