Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash
Abstract
1. Introduction
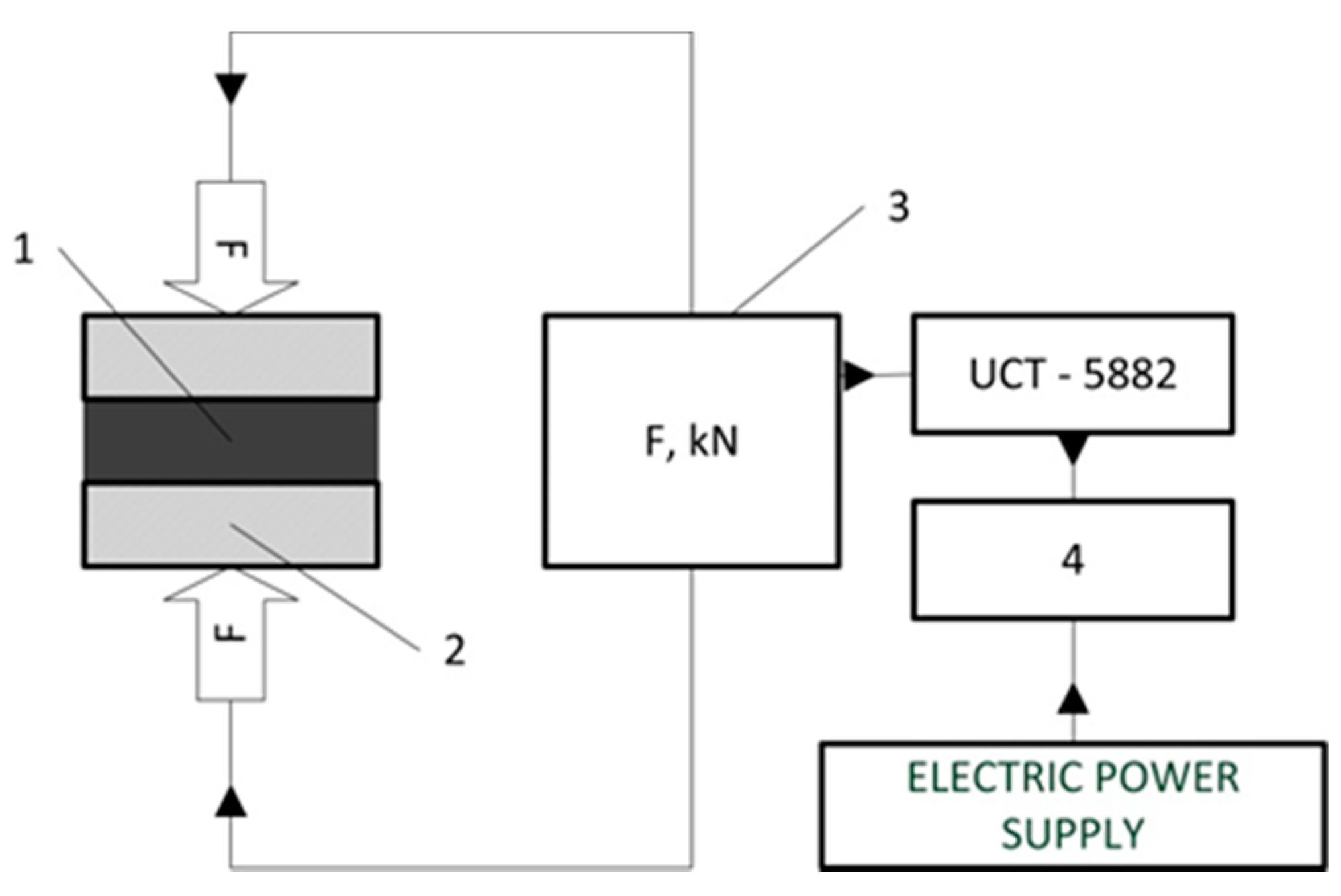
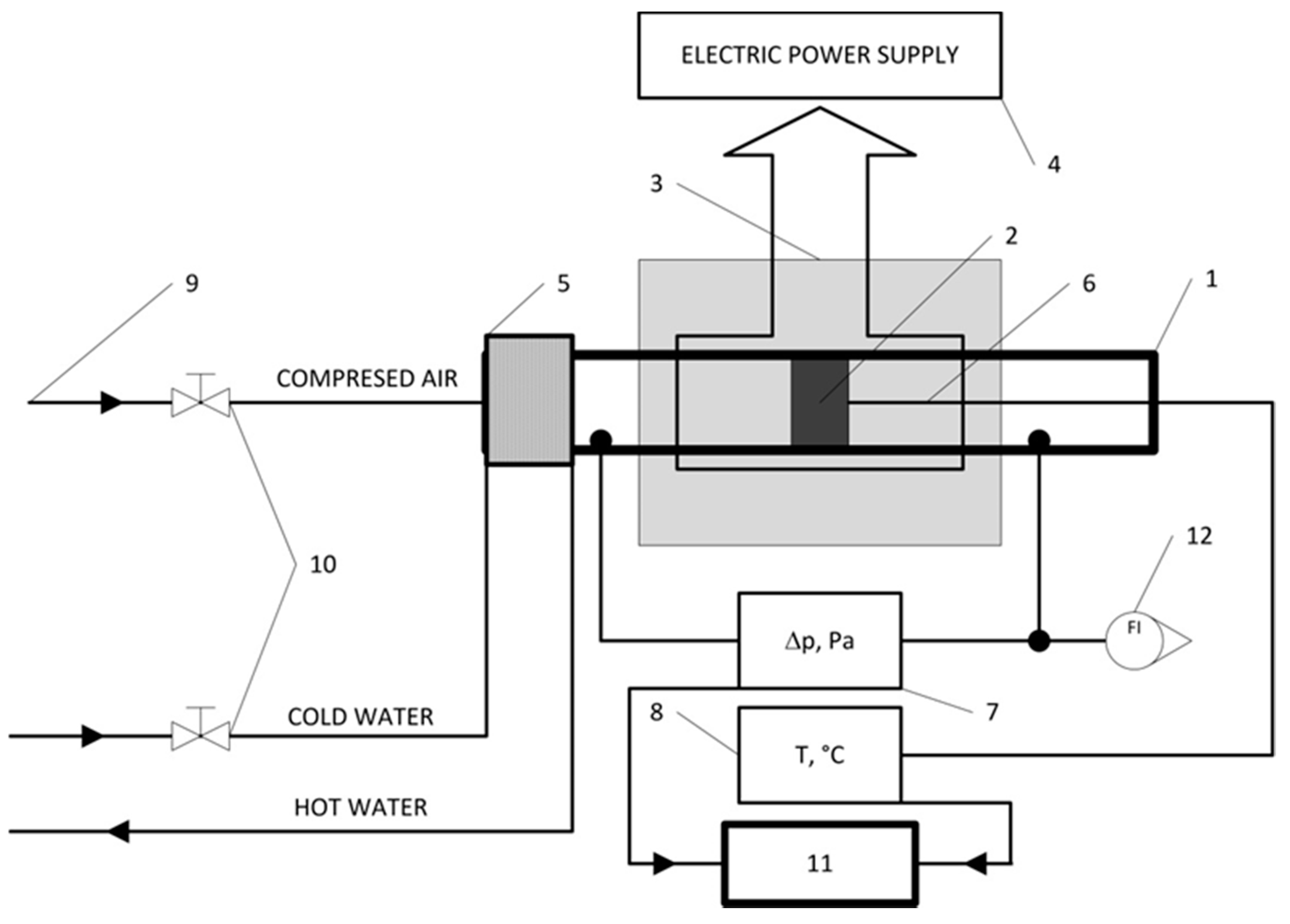
2. Materials and Methods
3. Results and Discussion
3.1. Samples Analysis
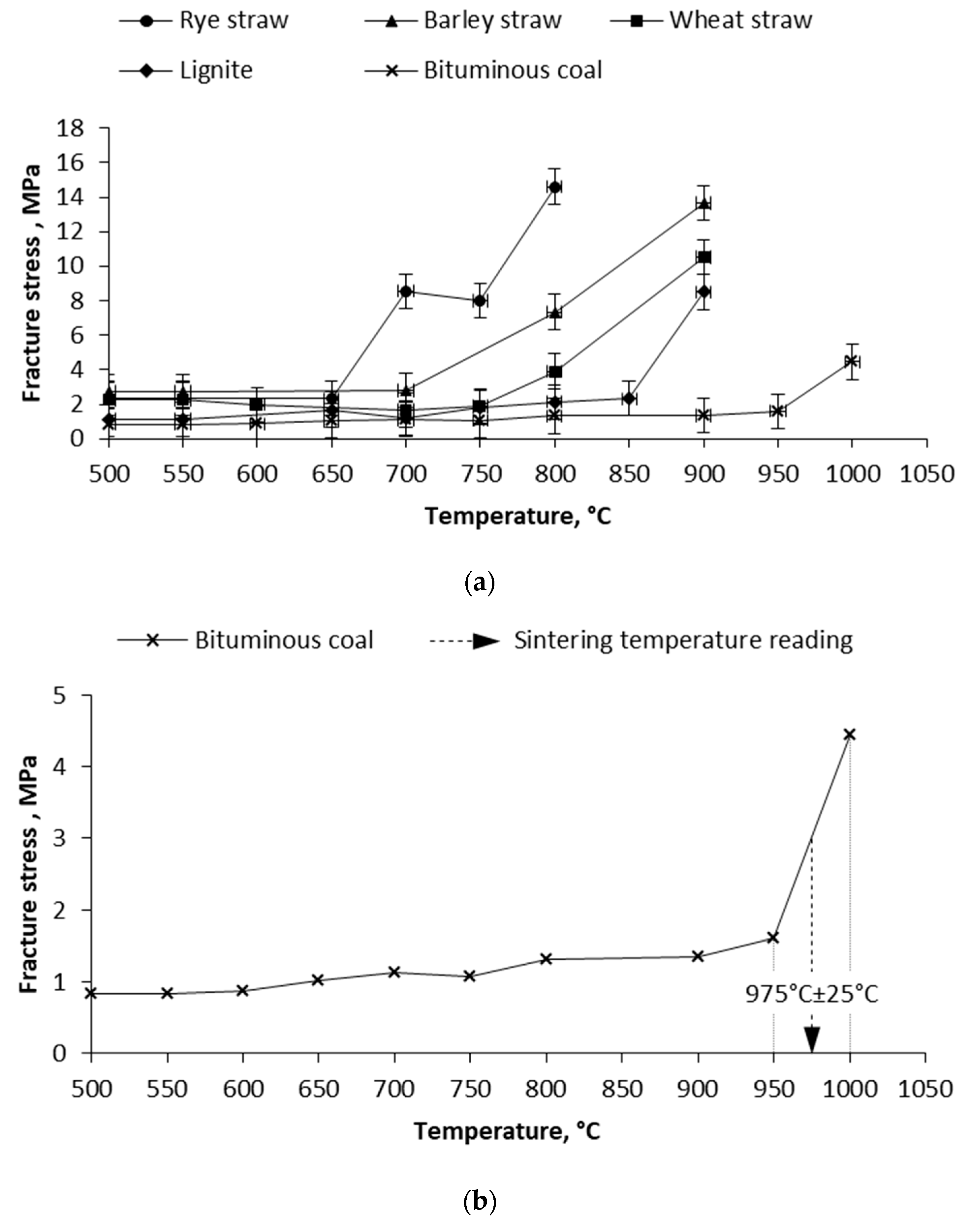
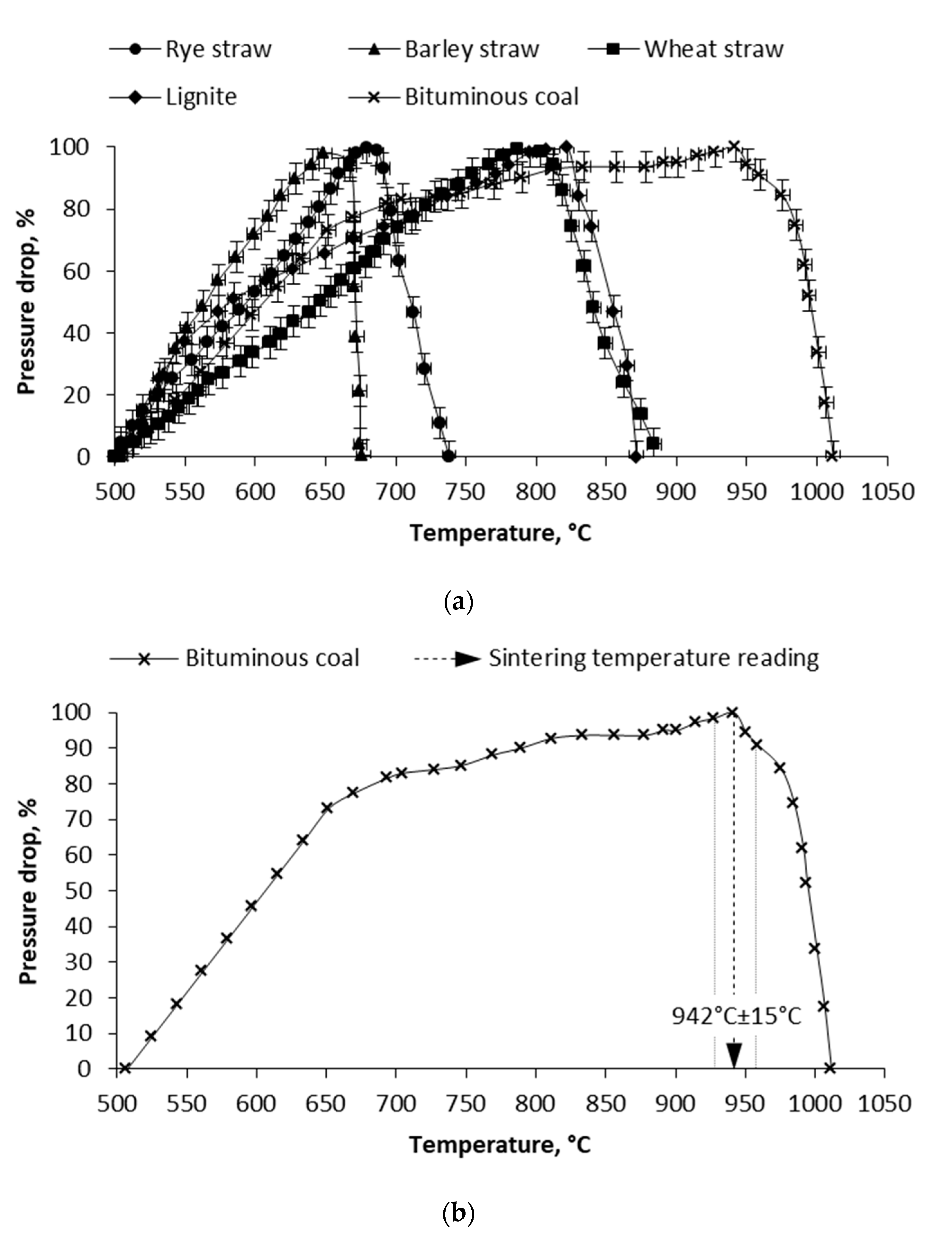
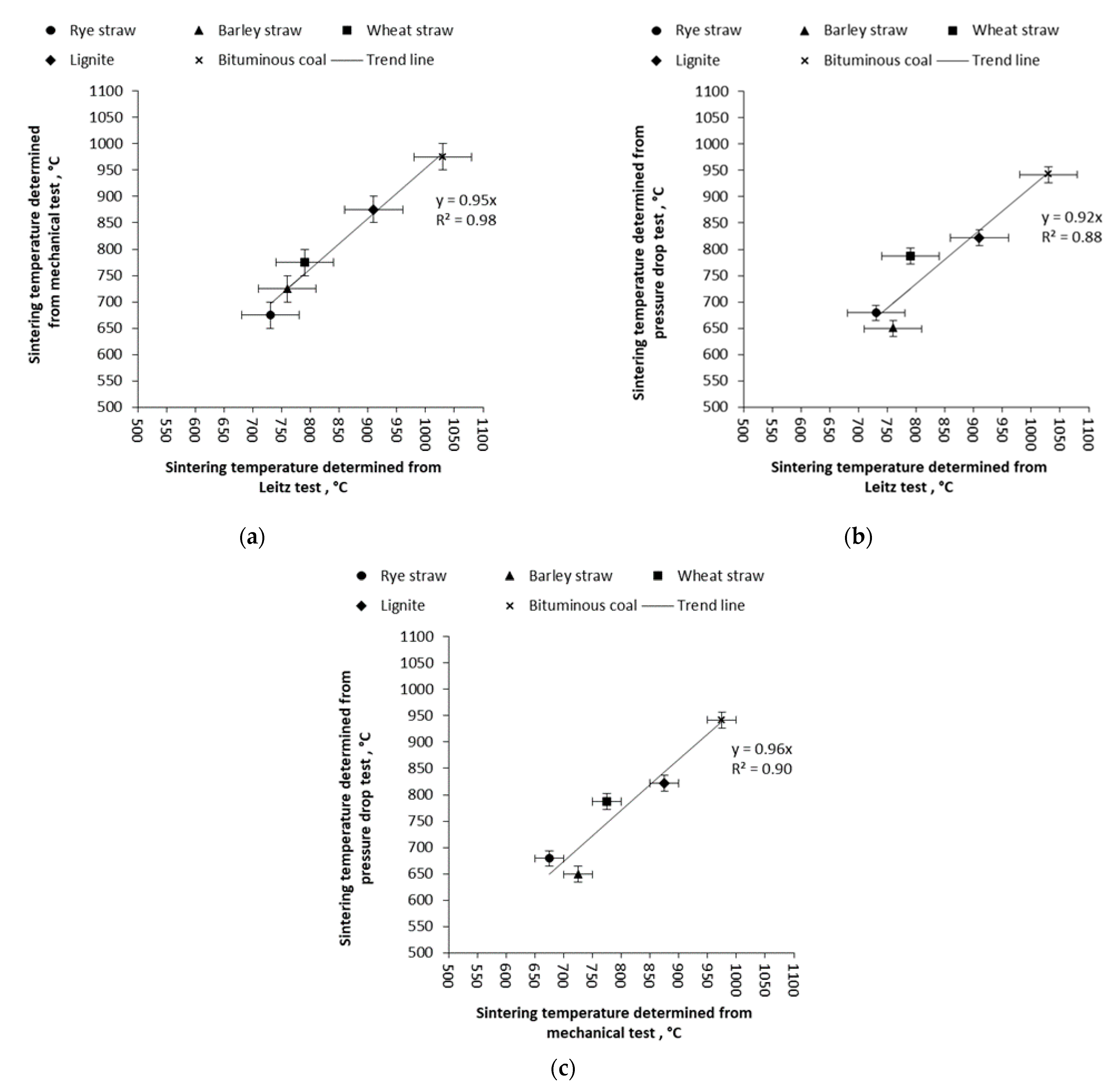
3.2. Mechanical Test and Pressure Drop Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tchapda, A.H.; Pisupati, S.V. A Review of Thermal Co-Conversion of Coal and Biomass/Waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behavior of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Nunes, L.; Matias, J.; Catalão, J. Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takeda, S.; Tsuru, T. Influence of minerał and chemical composition of coal ashes on their fusibility. Fuel Process. Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Elliott, L.K.; Wall, T.F.; Moghtaderi, B. Measurement of the Sintering Kinetics of Coal Ash. Energy Fuels 2000, 14, 994–1001. [Google Scholar] [CrossRef]
- Wall, T.; Creelman, R.; Gupta, R.; Gupta, S.; Coin, C.; Lowe, A. Coal ash fusion temperatures—New characterization techniques, and implications for slagging and fouling. Prog. Energy Combust. Sci. 1998, 24, 345–353. [Google Scholar] [CrossRef]
- Anthony, E.J.; Jia, L. Agglomeration and strength development of deposits in CFBC boilers firing high-sulfur fuels. Fuel 2000, 79, 1933–1942. [Google Scholar] [CrossRef]
- Jing, N.; Wang, Q.; Luo, Z.; Cen, K. Effect of different reaction atmospheres on the sintering temperature of Jincheng coal ash under pressurized conditions. Fuel 2011, 90, 2645–2651. [Google Scholar] [CrossRef]
- Shi, W.; Bai, J.; Kong, L.; Li, H.; Bai, Z.; Vassilev, S.V.; Li, W. An overview of the coal ash transition process from solid to slag. Fuel 2021, 287, 119537. [Google Scholar] [CrossRef]
- Bryers, R.W. Fireside slagging, fouling and high-temperature corrosion of heat-transfer surface due to impurities in stem rising fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Raask, E. Mineral Impurities in Coal Combustion: Behavior, Problems and Remedial Measures; Hemisphere Publishing Corporation: Washington, DC, USA, 1985; Chapter 10; pp. 137–160. [Google Scholar]
- Luan, C.; You, C.; Zhang, D. Composition and sintering characteristics of ashes from co–firing of coal and biomass in a la-boratory-scale drop tube furnace. Energy 2014, 69, 562–570. [Google Scholar] [CrossRef]
- Polish Standard PN-ISO 540:2001 Solid Fuels—Determination of Ash Fusibility at High Temperature Using the Pipe Method. Available online: https://sklep.pkn.pl/pn-iso-540-2001p.html (accessed on 19 February 2021).
- Du, S.; Yang, H.; Qian, K.; Wang, X.; Chen, H. Fusion and transformation properties of the inorganic components in biomass ash. Fuel 2014, 117, 1281–1287. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Meng, A.; Li, L.; Li, G. Study on ash fusion temperature using original and simulated biomass ashes. Fuel Process. Technol. 2013, 107, 107–112. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Gądek, W.; Pronobis, M.; Kalisz, S. Investigation of ash deposition in PF boiler during combustion of torrefied biomass. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Krakow, Poland, 14–17 November 2017; Volume 214, p. 012080. [Google Scholar]
- Magdziarz, A.; Wilk, M.; Gajek, M.; Nowak-Woźny, D.; Kopia, A.; Kalemba-Rec, I.; Koziński, J. Properties of ash genarated during sewage sludge combustion: A multifaceded analysis. Energy 2016, 113, 85–94. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass. Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Pronobis, M. The influence of biomass co-combustion on boiler fouling and efficiency. Fuel 2006, 85, 474–480. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Bryant, G.W.; Elliott, L.K.; Skrifvars, B.J.; Hupa, M.; Wall, T.F. Experimental Options for Determining the Temperature for the Onset of Sintering of Coal Ash. Energy Fuels 2000, 14, 227–233. [Google Scholar] [CrossRef]
- Jung, B.; Schobert, H.H. Viscous sintering of coal ashes. 1. Relationships of sinter point and sinter strength to particle size and composition. Energy Fuels 1991, 5, 555–561. [Google Scholar] [CrossRef]
- Jung, B.; Schobert, H.H. Viscous sintering of coal ashes. 2. Sintering behavior at short residence times in a drop tube furnace. Energy Fuels 1992, 6, 59–68. [Google Scholar] [CrossRef]
- Moroń, W.; Rybak, W. Strength method for determining the ash sintering temperature. In Proceedings of the Scientific and Technical Conference: Energy 2002, Wroclaw, Poland, 6–8 November 2002; pp. 503–508. [Google Scholar]
- Febrero, L.; Granada, E.; Perez, C.; Patiño, D.; Arce, E.; Arce-Fariña, M.E. Characterisation and comparison of biomass ashes with different thermal histories using TG-DSC. J. Therm. Anal. Calorim. 2014, 118, 669–680. [Google Scholar] [CrossRef]
- Qin, Y.-H.; Feng, M.-M.; Zhao, Z.-B.; Vassilev, S.V.; Feng, J.; Vassileva, C.G.; Li, W.-Y. Effect of biomass ash addition on coal ash fusion process under CO2 atmosphere. Fuel 2018, 231, 417–426. [Google Scholar] [CrossRef]
- Płaza, P.; Moroń, W.; Król, K.; Ferens, W. Sintering of ashes from coal and biofuels. Syst. J. Transdiscipl. Syst. Sci. 2006, 11, 525–532. [Google Scholar]
- Płaza, P.; Hercog, J.; Hrycaj, G.; Król, K.; Rybak, W. Predicting Ash Deposit Formation During Co-Firig of Coal with Biomass, Success and Visions for Bioenergy. In Thermal Processing of Biomass for Bioenergy, Biofuels and Bioproducts; Bridgwater, A.V., Ed.; European Commission: Salzburg, Austria, 2007; p. 10. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behavior of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 393–449. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Liu, F.; Yu, X.; Zeng, X.; Han, J.; Yu, G.; Xu, M. Impact of Oxy-fuel Combustion on Ash Properties and Sintering Strength Development. Energy Fuels 2018, 32, 4315–4322. [Google Scholar] [CrossRef]
- ISO. Polish Standard PN-EN ISO 18134-1:2015-11 Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 1: Total Moisture—Reference Method (ISO 18134-1:2015); ISO: London, UK, 2015. [Google Scholar]
- ISO. Polish Standard PN-EN ISO 18122:2016-01 Solid Biofuels—Determination of Ash Content (ISO 18122:2015); ISO: London, UK, 2015. [Google Scholar]
- ISO. Polish Standard PN-ISO 1928:2020-05 Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value; ISO: London, UK, 2020. [Google Scholar]
- ISO. Polish Standard PN-EN ISO 18123:2016-01 Solid Biofuels—Determination of the Content of Volatile Matter (ISO 18123:2015); ISO: London, UK, 2015. [Google Scholar]
- ISO. Polish Standard PN-G-04502:2014-11 Hard and Brown Coals—Sampling and Preparation of Samples for the Laboratory Tests—Primary Methods; ISO: London, UK, 2014. [Google Scholar]
- Abuelnuor, A.A.A.; Wahid, M.A.; Hosseini, S.E.; Saat, A.; Saqr, K.M.; Sait, H.H.; Osman, M. Characteristics of biomass in flameless combustion: A review. Renew. Sustain. Energy Rev. 2014, 33, 363–370. [Google Scholar] [CrossRef]
- Król, K.; Iskra, K.; Ferens, W.; Miodoński, J.M. Testing properties of sewage sludge for energy use. Environ. Prot. Eng. 2019, 45, 61–73. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Schimpke, R.; Klinger, M.; Krzack, S.; Meyer, B. Determination of the initial ash sintering temperature by cold compression strength tests with regard to mineral transitions. Fuel 2017, 194, 157–165. [Google Scholar] [CrossRef]
| Samples | Proximate Analysis | Ultimate Analysis | Heating Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | A | VM | FC | FR | C | H | N | S | O | Cl | HHV | |
| wt.% | wt.% | wt.% | wt.% | – | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | MJ/kg | |
| Wheat straw | 5.7 | 3.7 | 69.3 | 21.3 | 0.31 | 45.4 | 5.9 | 0.6 | 0.08 | 38.4 | 0.21 | 17.3 |
| Barley straw | 8.5 | 8.2 | 62.7 | 20.6 | 0.33 | 47.8 | 5.7 | 0.4 | 0.09 | 28.9 | 0.45 | 16.1 |
| Rye straw | 6.3 | 3.6 | 68.7 | 21.4 | 0.31 | 46.5 | 6.1 | 0.6 | 0.08 | 36.4 | 0.42 | 16.8 |
| Bituminous coal | 2.0 | 17.3 | 26.9 | 53.8 | 2.00 | 65.4 | 3.7 | 1.3 | 1.2 | 8.6 | 0.51 | 27.8 |
| Lignite coal | 10.7 | 19.5 | 41.6 | 28.2 | 0.68 | 43.5 | 4.9 | 0.7 | 2.6 | 17.2 | 0.88 | 16.6 |
| Samples | Wheat Straw | Barley Straw | Rye Straw | Bituminous Coal | Lignite Coal |
|---|---|---|---|---|---|
| Oxides | wt.% | wt.% | wt.% | wt.% | wt.% |
| SiO2 | 65.8 | 67.5 | 30.7 | 51.4 | 29.1 |
| Al2O3 | 0.9 | 0.2 | 0.3 | 25.5 | 17.6 |
| Fe2O3 | 0.5 | 0.2 | 0.3 | 6.6 | 11.3 |
| CaO | 8.0 | 4.9 | 12.8 | 4.6 | 3.0 |
| MgO | 2.0 | 1.5 | 5.6 | 3.3 | 2.9 |
| Na2O | 0.4 | 0.4 | 0.1 | 1.0 | 4.5 |
| K2O | 18.6 | 21.0 | 38.3 | 2.6 | 1.5 |
| P2O5 | 2.5 | 2.7 | 6.4 | 0.5 | 0.1 |
| TiO2 | – | – | – | 1.1 | 2.1 |
| SO3 | 1.2 | 1.5 | 5.6 | 3.2 | 27.8 |
| Mn3O4 | – | – | – | 0.1 | 0.1 |
| BaO | – | – | – | 0.2 | – |
| SrO | – | – | – | 0.1 | – |
| Parameter/Symbol | Value | Fuel | Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Extremely High | Wheat Straw | Barley Straw | Rye Straw | Bituminous Coal | Lignite Coal | |||
| Silica content in ash | SiO2 | <20 | 20–25 | >25 | 65.82 | 67.46 | 30.73 | 51.35 | 29.12 | wt.% | |
| Chlorine content in fuel | Cld | <0.2 | 0.2–0.3 | 0.3–0.5 | >0.5 | 0.22 | 0.49 | 0.45 | 0.52 | 0.99 | wt.% |
| Basic to acidic compounds ratio | B/A | <0.5 | 0.5–1.0 | 1.0–1.75 | 0.48 | 0.45 | 2.05 | 0.24 | 0.48 | – | |
| Bed agglomeration index | BAI | <0.15 | 0.03 | 0.01 | 0.01 | 1.83 | 1.89 | – | |||
| Babcock index | Rs | <0.6 | 0.6–2.0 | 2–2.6 | >2.6 | 0.04 | 0.04 | 0.17 | 0.29 | 1.39 | – |
| Fouling index | Fu | 0.6–2.0 | 2.0–40 | >40 | 9.15 | 9.75 | 78.63 | 0.86 | 2.86 | – | |
| Fouling factor | Rf | <0.2 | 0.2–0.5 | 0.5–1.0 | >1.0 | 6.10 | 6.49 | 51.91 | 0.64 | 2.61 | – |
| Slag viscosity index | Sr | >72 | 65–72 | <65 | 86.19 | 91.04 | 62.20 | 77.96 | 62.86 | – | |
| Initial deformation temperature | IDT | >1100 | 900–1100 | <900 | 790 | 760 | 730 | 1030 | 910 | °C | |
| Sample | Sintering Temperature Determined According to the Method: | ||
|---|---|---|---|
| Leitz, DT | Mechanical test | Pressure drop test | |
| Wheat straw | 790 °C ± 50 °C | 775 °C ± 25 °C | 787 °C ± 15 °C |
| Barley straw | 760 °C ± 50 °C | 725 °C ± 25 °C | 649 °C ± 15 °C |
| Rye straw | 730 °C ± 50 °C | 675 °C ± 25 °C | 679 °C ± 15 °C |
| Bituminous coal | 1030 °C ± 50 °C | 975 °C ± 25 °C | 942 °C ± 15 °C |
| Lignite coal | 910 °C ± 50 °C | 875 °C ± 25 °C | 822 °C ± 15 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, K.; Nowak-Woźny, D. Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash. Energies 2021, 14, 1126. https://doi.org/10.3390/en14041126
Król K, Nowak-Woźny D. Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash. Energies. 2021; 14(4):1126. https://doi.org/10.3390/en14041126
Chicago/Turabian StyleKról, Karol, and Dorota Nowak-Woźny. 2021. "Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash" Energies 14, no. 4: 1126. https://doi.org/10.3390/en14041126
APA StyleKról, K., & Nowak-Woźny, D. (2021). Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash. Energies, 14(4), 1126. https://doi.org/10.3390/en14041126







