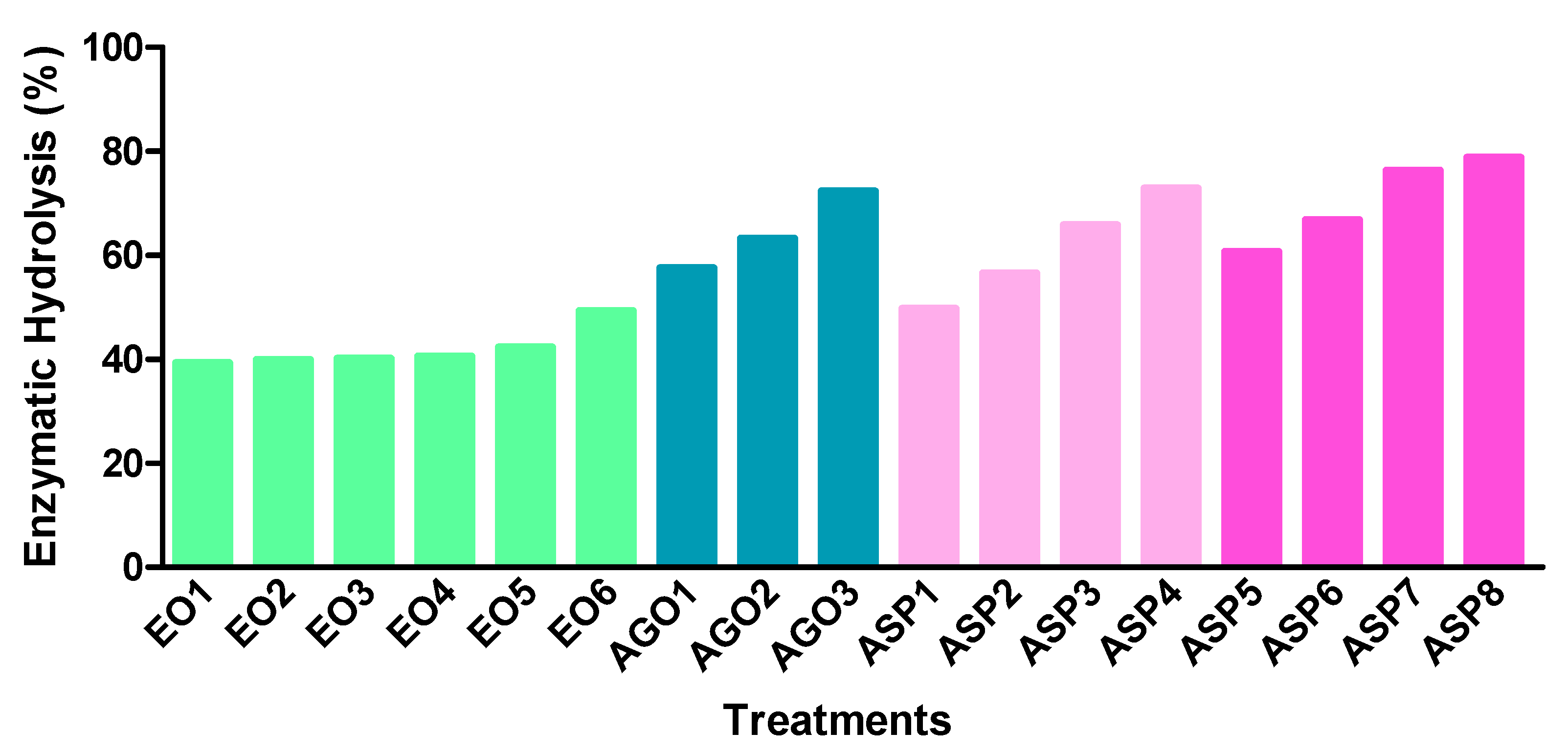Abstract
Residues of Cistus ladanifer obtained after commercial steam distillation for essential oil production were evaluated to produce cellulose enriched solids and added-value lignin-derived compounds. The delignification of extracted (CLRext) and extracted and hydrothermally pretreated biomass (CLRtreat) was studied using two organosolv processes, ethanol/water mixtures (EO), and alkali-catalyzed glycerol (AGO), and by an alkali (sodium hydroxide) process (ASP) under different reaction conditions. The phenolic composition of soluble lignin was determined by capillary zone electrophoresis and by Py-GC/MS, which was also used to establish the monomeric composition of both the delignified solids and isolated lignin. The enzymatic saccharification of the delignified solids was also evaluated. The ASP (4% NaOH, 2 h) lead to both the highest delignification and enzymatic saccharification (87% and 79%, respectively). A delignification of 76% and enzymatic hydrolysis yields of 72% were obtained for AGO (4% NaOH) while EO processes led to lower delignification (maximum lignin removal 29%). The residual lignin in the delignified solids were enriched in G- and H-units, with S-units being preferentially removed. The main phenolics present in the ASP and AGO liquors were vanillic acid and epicatechin, while gallic acid was the main phenolic in the EO liquors. The results showed that C. ladanifer residues can be a biomass source for the production of lignin-derivatives and glucan-rich solids to be further used in bioconversion processes.
1. Introduction
Lignin is an important source of compounds with functional activities and emerging applications in the cosmetic, pharmaceutical, and food industries. Compared to cellulose, the upgrading of lignin has deserved much less attention due to its recalcitrance, as well as its chemical and structural complexity. Although lignin is rich in phenolic and aliphatic hydroxyl groups, which are functional groups of interest for chemical modifications and reactions, it is often used only as filler or additive since separation and fragmentation processes are some of the hindrances for its utilization for chemicals production [1]. However, there has been a renewed interest in the study of lignin extraction processes to obtain added-value products [2]. These products can be grouped into three categories: biofuels, macromolecules, and monomeric aromatic compounds [3]. The lignins can be incorporated into polymeric materials, including conducting polymers, polyurethanes, and thermoplastic sealants [4]. They can also be used for the production of phenolic resins, binders, and adhesives [3,5]. However, the interest in the production of monomeric phenolic compounds, such as vanillin, ferulic acid, coumaric acid, p-hydroxybenzoic acid, vanillic acid, syringic acid, syringaldehyde, and p-hydroxybenzaldehyde, for example, is having increased interest. In fact, the development of new technologies for the production of lignin-based chemicals can lead to much higher value market opportunities than petrochemicals [6]. The market potential for high added-value products such as vanillin, phenol, BTX (benzene, toluene, and xylene), and carbon fibers, for example, is over $130 billion, and in 2020 this potential is expected to reach $208 billion [7].
The lignin source, as well as the extraction processes used to break down the lignin macromolecule into fragments of lower molecular mass, have an important influence on the physicochemical properties of the resulting products [8]. The most used delignification processes involve the action of alkalis that although mainly reacting with lignin, also affect the hemicelluloses. The most used agents are alkali metal hydroxides (sodium, potassium, or calcium) and one of the most known examples is the Kraft process (which uses sodium sulfide and sodium hydroxide) used in pulp and paper production [9,10]. It has the advantage that it may be performed at low temperatures, although the quality of lignin is rather low. These processes produce, at present, the highest volume of lignin but are slightly specific and require significant operations of wastewater treatment.
Organosolv processes are based on the utilization of aqueous mixtures of organic solvents such as acetone, ethanol, methanol, glycerol, formic acid, or acetic acid [8,11,12]. These are interesting delignification alternatives since they are able to obtain lignin with low ash content, higher purity (due to lower content of carbohydrates), and, in general, with low molecular weight and higher hydrophobicity [13]. Besides, the organosolv treatments lead to a liquid phase containing both hemicellulose and lignin-derived products that are free from sulfur [14]. Organosolv processes can also be used in combination with other catalysts, including acids, producing lignin dissolution and hemicellulose hydrolysis [12,15]. The overall economy of these processes should include the optimization of biomass fractionation, product recovery, and solvent recycling [15], although low boiling point solvents such as ethanol and acetone have the advantage of easy recovery by distillation and are themselves biorefinery products [16]. In contrast, high boiling point alcohols, such as ethylene glycol and glycerol, require higher energy consumption for their recovery [17] i.e., involving two distillation steps [18]. In a similar way, organic acids, such as formic acid and acetic acid, that are commonly recovered by evaporation, also need high energy inputs [12,19].
Besides the recovery of lignin for conversion into valuable products, these treatments may also produce cellulose-rich solids with enhanced enzymatic hydrolysis rate and yield [20] due to changes caused in the physicochemical properties of the pretreated solids. These include the increase of porosity, and of the surface area of the substrate, and the decrease of cell wall thickness, cellulose crystallinity, and degree of polymerization, as well as a decreased lignin and hemicellulose content [21].
The biomass used in this study was Cistus ladanifer, commonly known as rock-rose, is a native species from the Iberian Peninsula and one of the most important natural shrubs in the Mediterranean basin [22,23], occupying an area of c.a. 2 million hectares in the south/southwest of the Iberian Peninsula [24]. This plant is covered with a sticky exudate of a fragrant resin, known as labdanum, which in turn is valued in perfumery because of its high content of compounds from the Ambery’s olfactive family (amber odor) [25]. Cistus ladanifer is also used as feedstock for essential oil production, obtained by hydrodistillation or steam distillation, which can be sold at an attractive price (over 200 €/L) [25]. Furthermore, Cistus species have been employed in traditional medicine, since labdanum also contains phytochemicals with antioxidant, antibacterial, antifungal, and anticancer properties [26]. In general, the harvest of Cistus has been considered an important socio-economic activity for some rural communities [27].
The remained residues obtained after distillation or labdanum extraction present a favorable chemical composition to be used as a biorefinery feedstock [23]. The combination of extraction, hydrothermal processing (autohydrolysis), and delignification treatments are important processing routes for the full use of this biomass. Thus, the solids remaining from previous extraction and optimized hydrothermal processing for hemicellulose fractionation were subjected to delignification treatments using two mild organosolv processes: ethanol/water mixtures (EO) and alkali-catalyzed glycerol (AGO). For comparative purposes, an aqueous sodium hydroxide process (ASP) was tested and the processes were also applied to the extracted-only biomass.
This work aims to study the operational conditions for the delignification of extracted (CLRext) and extracted and hydrothermally pretreated solids (CLRtreat) of C. ladanifer residues using different delignification processes to produce cellulose-rich solids and maximize the recovery of the lignin-derived phenolic compounds in the liquid fraction. Lignins and remaining solids were characterized and the influence of the extraction methods on their chemical properties was evaluated.
2. Materials and Methods
2.1. Raw Material
The samples used in this work were (i) CLRext: Cistus ladanifer residues (CLR) obtained after essential oil distillation (kindly provided by Quinta Essência, Lda, Portel, Portugal), followed by extraction with ethanol and water; and (ii) CLRtreat: obtained by pre-treating CLRext by an autohydrolysis (hydrothermal) process under previously optimized conditions [22]. The CLRtreat sample was produced in sufficient amount and carefully homogenized to be used for the delignification studies. The average content of structural components and ash of the CLRext used in this work was 29% glucan, 17% xylan, 4% arabinan, 3% acetyl groups, 30% Klason lignin, and 3.30% ash. The CLRtreat biomass contained 35% glucan, 9% xylan, 1.4 % acetyl groups, 46% lignin, and 4.30% ash.
2.2. Delignification Treatments
2.2.1. Aqueous Sodium Hydroxide Process (ASP)
CLRext and CLRtreat were used as feedstock for an aqueous sodium hydroxide process (ASP). The treatment was carried out in an autoclave at 130 °C for 60 or 120 min. Each treatment used 10 g of solids (dry weight) and sodium hydroxide (NaOH) solutions at 2% or 4 % (w/v) with a solid:liquid ratio of 1:10 (w/w). At the end of the reaction, the flasks were cooled down to room temperature and the liquid and solid fractions were separated by filtration (Quantitative Filter-Lab nr 1235 filter paper). The liquor was stored at 4 °C for further analysis and the solid phase was washed with 1 L of hot distilled water (80 °C), dried for 48 h at 45 °C, and kept for another 48 h at room temperature before milling and storage until analysis.
2.2.2. Alkali-Catalyzed Glycerol Organosolv (AGO)
Alkali-catalyzed glycerol organosolv (AGO) was performed similarly to ASP. The delignification was carried out at 130 °C for 60 min and each treatment used CLRtreat and 50:50 water/glycerol (w/w) solution containing 2, 4, or 8 g of NaOH as a catalyst, in a solid: liquid ratio of 1:10 (w/w). After separation, the solid phases were washed with 1% NaOH solution (w/v) followed by 2 L of hot distilled water (80 °C) to remove possible adsorbed lignin.
2.2.3. Ethanol Organosolv Process (EO)
The ethanol organosolv process (EO) used CLRtreat solids as feedstock. The process was performed in a 600 mL stainless-steel reactor (Parr Instruments Co, Moline, IL, USA) using 50:50 ethanol/water mixtures and a 6:1 liquid-to-solid ratio (w/w). The temperature, agitation, and pressure were controlled by a Parr PID controller (model 4842). The reaction was carried out at non-isothermal conditions in a temperature range of 170 to 220 °C. Upon reaching the desired temperature, the reactor was cooled down by water circulating through a serpentine coil and introduced in a cold-water bath. The solid and liquid phases were separated by filtration and washed with 1 L ethanol/water solution and dried as described in Section 2.2.1.
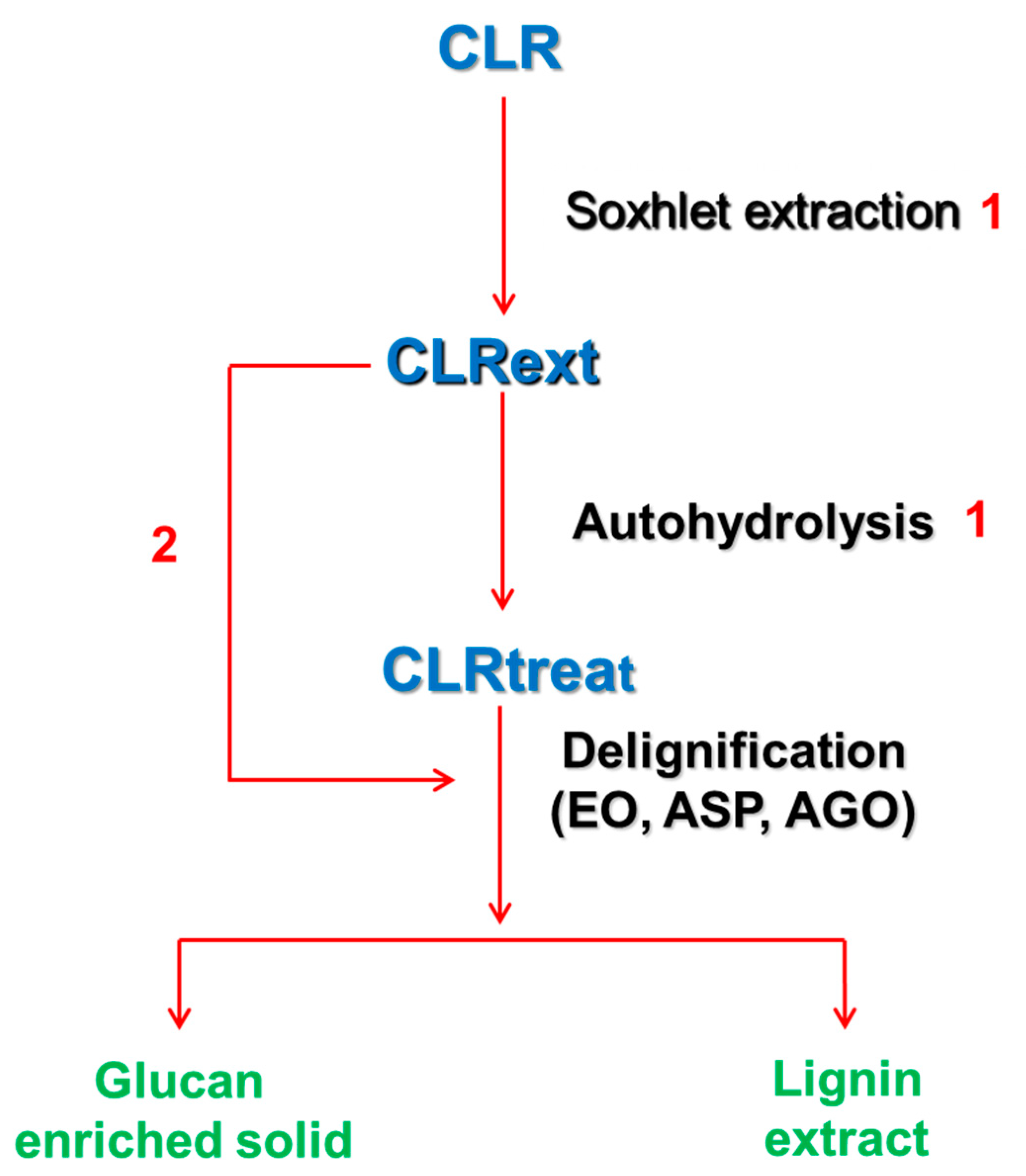
The diagram of sequential fractionation methods (extraction, autohydrolysis, and delignification) applied to this biomass is illustrated in Scheme 1.
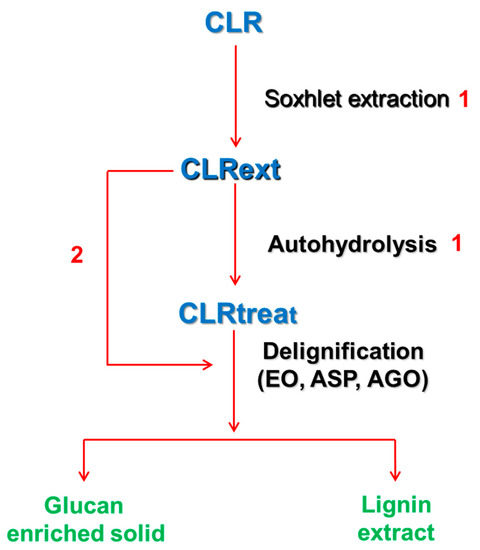
Scheme 1.
Sequential fractionation methods (extraction, autohydrolysis, and delignification) applied to the C. ladanifer biomass and the relevant samples used in this work. CLR—C. ladanifer residues obtained after commercial hydrodistillation. CLRext—Ethanol and water extracted CLR. CLRtreat—Extracted and hydrothermally treated CLRext. Sohxlet extraction and autohydrolysis conditions (1) are described in Alves-Ferreira et al. [22]. Process 2 was only applied to aqueous sodium hydroxide delignification.
2.3. Lignin Precipitation
Lignin was separated from the ASP and AGO liquors by acid precipitation, adding 72% H2SO4 to reach pH 2. The lignins precipitated from the ASP and AGO liquors were filtered, washed with hot distilled water (60 °C), and dried at 45 °C for 24 h. For the precipitation of the EO lignin, the liquor was diluted with distilled water (3:1), centrifuged at 5000 rpm for 10 min, and dried at 45 °C for 24 h.
2.4. Analytical Methods
Quantification of Carbohydrates and Lignin
The solids remaining after each delignification process were subjected to quantitative acid hydrolysis (QAH) according to the conditions used in previous work [23]. The liquors resulting from QAH were analyzed for glucose, xylose, arabinose, and acetic acid by HPLC (Agilent, Waldbronn, Germany) as described by Moniz et al. [28]. The solid residue from QAH was corrected for ash and considered as Klason lignin. The moisture content was determined by oven-drying at 100 °C to constant weight and the ash content was determined at 550 °C using NREL/TP-510-42622 protocol [29].
2.5. Chemical Characterization of Lignin and Lignin-Derived Compounds
2.5.1. Phenolic Profile by Capillary Zone Electrophoresis (CZE)
The lignin-derived products contained in the delignification liquors were analyzed by CZE (Agilent System, Waldbronn, Germany), equipped with a diode-array detector (DAD), and interfaced with a ChemStation data software under the operating conditions previously reported in Alves-Ferreira et al. [22]. Detection was performed at 200 and 375 nm, and phenolic compounds were identified by electrophoretic comparisons (migration times and UV spectra) using authentic standards.
Total phenolic compounds were determined by the Folin–Ciocalteu colorimetric method [30] using a microplate spectrophotometer (Thermo Scientific, Waltham, MA USA) as detailed elsewhere [22] and expressed as g of gallic acid equivalents per liter of liquor (gGAE/L).
2.5.2. Pyrolysis Experiments
Py-GC/MS was applied to study and compare the modifications on delignified solids and in the lignin isolated from the liquors. The solids analyzed were those corresponding to the conditions with the highest delignification obtained for each treatment (EO5, AGO3, ASP8) and the CLRtreat, for comparison purposes. The isolated lignin samples from the liquors correspond to precipitated EO5, AGO3, ASP7, and ASP8 liquors. The samples were pyrolyzed at 550 °C for 10 s in a 5150 CDS apparatus (CDS Analytical, Oxford, USA) linked to an Agilent GC 7890B coupled to a mass detector system 5977B using electron impact mode (EI at 70 eV) according to the operating conditions described before [22]. The pyrolysis products were identified by comparison with computer libraries (Wiley, NIST2014) and with the literature [31,32]. The percentage of each compound was calculated based on the total area of the chromatogram. The percentage of hydroxyphenyl (H), guaiacyl (G), and syringyl (S) lignin-derived products were separately summed. The S/G ratio and the S:G:H relation were calculated.
2.6. Enzymatic Hydrolysis
The enzymatic digestibility of the cellulose (measured as glucan) remaining in the delignified solids was evaluated using reference protocols (LAP-NREL/TP-510-42629) [33]. The dried solid fraction from the delignification process was digested in 5 mL of sodium citrate 0.1 M pH 4.8 buffer solution, 100 μL solution of sodium azide (2% w/v) and then supplemented with 60 FPU/g cellulose of Celluclast 1.5 L and 64 pNPGU/g cellulose of Novozyme 188 (Novozymes, Bagsvaerd, Denmark). The volume was adjusted with distilled water to 10 mL considering that the biomass has a density of 1. Incubation was carried out 150 rpm in an orbital incubator at 50 °C, for 72 h, in triplicate. [22]. Hydrolysates were analyzed by HPLC as described above. Final sugar concentrations were corrected for substrate and enzyme blanks. The enzymatic digestibility was determined by the ratio of digested cellulose to the initial cellulose loaded.
Filter Paper Activity (Celluclast 1.5 L) and β-glucosidase activity (Novozyme 188) were determined according to the procedures of Ghose [34] and Berghem et al. [35], respectively.
3. Results and Discussion
3.1. Chemical Composition of the Delignified Solids and Delignification Yield
The experimental conditions of the delignification processes and the chemical composition of the remaining solids, delignification yield, and solid yield obtained after treatment are shown in Table 1.

Table 1.
Experimental conditions for the delignification of C. ladanifer residues before (CLRext) and after hydrothermal treatment (CLRtreat) using three delignification processes-ethanol organosolv (EO), alkali-catalyzed glycerol (AGO), and aqueous sodium hydroxide process (ASP)-and results regarding delignification yield (% of the initial oven-dried material), solid yield (% of the delignified solid), contents of Klason lignin, glucan, and xylan (% of the delignified solid).
The ethanol organosolv process solubilized less lignin when compared to the other delignification methods, resulting in a solid residue still with high lignin content. The delignification obtained ranged from 13% to 28.9%, increasing with temperature increase up to 210 °C but dropping at 220 °C (21.6%). Wildschut et al. [36] also observed that delignification tends to increase with the increase of reaction temperature. The results obtained here are lower than those reported for ethanol-water delignification of other feedstocks, for example: palm fronds (43%), using 80% ethanol at 200 °C [11]; hydrothermally pretreated sugarcane bagasse followed by organosolv pretreatment (62%), using 50% ethanol at 190 °C, in a glycerin bath for 150 min [37]. It is also important to note that in general a catalyst, e.g., sulphuric acid, is also added. This was the case of the experiments carried out by Wildschut et al. and Chen et al. [36,38] where 30 mM of sulphuric acid was used for the delignification of wheat straw with 60% ethanol at 190 °C. The hemicellulosic fraction was only slightly affected by EO, except at 220 °C, where a major decrease was observed (corresponding to solubilization around 50%). Between 170 °C and 210 °C, there was slight solubilization of the xylan from 13% to 28%, while glucan mainly remained in the solid phase (maximum solubilization 7%). Since the ethanol organosolv treatment was not very effective for lignin removal, it was not possible to obtain solids with high glucan content (it ranged from 35.2% to 44.3%).
To try to improve the former results, sulphuric acid (50 mM) was also tested as a catalyst at 220 °C in the organosolv process. However, even under these conditions, the performance of the ethanol organosolv process for lignin solubilization was not improved but xylan removal increased to reach 78% of solubilization. Ethanol organosolv was also performed under isothermal conditions, but the lignin yields were even less satisfactory than those using non-isothermal conditions (data not shown).
The alkali-catalyzed glycerol organosolv using NaOH as catalyst (1%, 2%, and 4% (w/w)), for 60 min, at a mild temperature of 130 °C exhibited a greater delignification degree in comparison to the ethanol organosolv assays. The highest lignin removal was obtained for 4% NaOH concentration (AGO3), reaching a delignification yield of 76.3% and resulting in a solid containing 67.7% glucan, 4.4% xylan, and 18.4% residual lignin. AGO1 and AGO2 produced a delignification yield of 37.4% and 69.1%, respectively. The maximum delignification yield reached out for AGO was higher than the obtained by Sun et al. [39] for wheat straw, using 70% industrial glycerol at 220 °C for 3 h, and lower than that shown by Hundt et al. [40] for beech wood, using 97% glycerol and 8% KOH as a catalyst, at 190 °C, for 15 min. Novo et al. [41] reported delignification yields between 21–82% for sugarcane bagasse, using 80% glycerol under different conditions of time and temperature. The solid yield decreased with the NaOH concentration, achieving 73.4%, 61.0%, and 60.6 % for AGO1, AGO2, and AGO3, respectively. The amount of xylan solubilized also increased with the NaOH concentration (maximum solubilization 68%), while acetyl groups were completely removed from the solid residue at all the conditions. Glucan was almost not affected by AGO: solubilization of 12% occurred for AGO1 (using 2% NaOH) and there was not any degradation of glucan for the other conditions. Other studies with glycerol organosolv extraction methods also demonstrated high preservation of cellulose in the substrate [39,42].
For comparative purposes, an aqueous sodium hydroxide process (ASP) was also tested with two NaOH concentrations (2% and 4% (w/v)) and two-time periods (1 and 2 h) after reaching a temperature of 130 °C. This process was also applied to extracted biomass (CLRext) and hydrothermally treated biomass (CLRtreat). ASP showed an important influence in the delignification of CLRext and CLRtreat, being the most efficient method tested in this work for lignin extraction. The delignification corresponded to percentages from 68% to 78% for CLRext and from 73% to 87% for CLRtreat. Therefore, CLRtreat allowed the maximum lignin removal ca. 11.5% superior to the maximum removal obtained with CLRext. The results of delignification obtained for CLRext were similar taking into account the different reaction times (ASP1 vs. ASP2 and ASP3 vs. ASP4). For the CLRtreat, the delignification degree only improved 3.3% with the increase of reaction time (ASP5 vs. ASP6 and ASP7 vs. ASP8). These results suggest that NaOH concentration may influence lignin removal, while the reaction time seems not to have an important effect. Studies done by Wu et al. [43] demonstrated that the increase of the alkali concentration is important to improve the delignification degree of wheat straw. The hydrolytic capacity of NaOH is well known since it cleaves the ether and ester linkages in the lignin-carbohydrate complexes, and the ester and carbon-to-carbon bonds in lignin [44]. Different concentrations of NaOH solutions (0.3–10%) under temperatures ranging from 70 °C to 160 °C and 1–3 h reaction times led from moderate to high delignification yields (56–97%) in different lignocellulosic biomass [45,46,47].
Contrarily to other treatments, ASP had a slightly higher effect in glucan solubilization than the organosolv processes. This was mainly evident for the untreated biomass (about 14.9–18.2% for CLRtreat vs. 24.3–30.2% for CLRext). For CLRext glucan degradation increased with alkali load. However, this effect was not observed for CLRtreat. This is an advantage as CLRtreat is a feedstock that is the by-product of C. ladanifer distillation (CLR), of CLR extraction (CLRext), and of CLRext hydrothermal treatments to produced oligosaccharides and it is still very interesting to produce cellulose-enriched solids and lignin extracts. The alkali treatment also had an important effect on the xylan fraction, with solubilization of approximately 50% and 71% for CLRext (ASP4) and CLRtreat (ASP8), respectively. Similar to what occurred in AGO, acetyl groups were also fully solubilized at all conditions (data not shown). Arabinan was completely removed from CLRtreat by hydrothermal pretreatment, but CLRext still contained 4% arabinan. Almost complete solubilization of arabinan from CLRext was achieved in any of the conditions (92.5–94% of solubilization) (data not are shown). The ASP process displayed the lowest solid yields among all the treatments (from 39.7% to 49.3%) due to the higher lignin and carbohydrate hydrolysis.
According to these results, EO was the less effective delignification process, while the highest delignification and glucan yields were observed in the ASP followed by AGO, suggesting the efficiency of the alkali process for delignification of C. ladanifer to obtain a feedstock suitable for obtaining glucose solutions and lignin-derived phenolics.
The pH of the liquors from the AGO and ASP was 11.8 and 12.9, respectively, with higher pH values for the higher sodium hydroxide concentrations (Table 1). Ethanol organosolv liquors exhibited similar values (4.1–4.4), with a slight pH decrease with increasing temperature. The values obtained are lower than reported by Wildschut et al. [36] in ethanol organosolv liquors (4.2 to 5.9).
3.2. Enzymatic Hydrolysis
The results of the enzymatic digestibility of the cellulose remaining in solid fractions after delignification are presented in Figure 1. The extraction of lignin using chemicals, besides the rupture of lignin, leads to a swelling of the biomass, as well as to the increase in internal surface area and better access of hydrolytic enzymes [48].
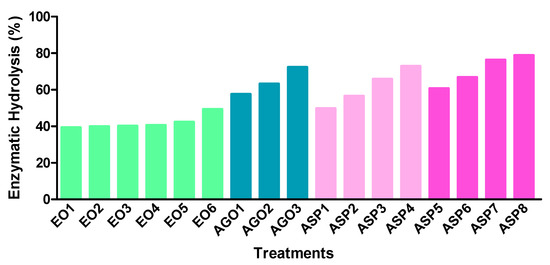
Figure 1.
Results of the cellulose enzymatic hydrolysis (% of hydrolyzed cellulose from delignified solid): ethanol organosolv (EO), alkali-catalyzed glycerol (AGO), and aqueous sodium hydroxide process (ASP).
EO treatment induced an increase in digestibility with the increase of reaction temperature. Organosolv performed at high temperatures may cause a higher rupture of the cell wall structure [38] and therefore better enzymatic access. However, the glucose yields obtained with EO solids were only about 39.3% to 49.3%. These results are in agreement with the lower delignification of CLRtreat obtained with these processes and contrast with the previously reported by Wildschut et al. [36]. These authors obtained a maximum enzymatic digestibility of 86% and 89% for wheat straw using aqueous ethanol without and with catalyst, respectively. Jang et al. [49] also reported a digestibility of over 80% for ethanol organosolv of Liriodendron tulipifera.
The results obtained for AGO and ASP solids showed a significant increase in the hydrolysis compared to the EO. Thus, values between 57.5–72.3% of cellulose digestibility were observed for AGO processed solids. These results are lower than those obtained for wheat straw (wet substrate) using crude and industrial glycerol [39,50]. Additionally, beechwood alkali-catalyzed glycerol delignification [40] and sugarcane glycerol/ sulphuric acid delignification [51] produced higher saccharification yields. CLRext (ASP1 to ASP4) showed enzymatic hydrolysis yields from 49.7% to 72.9% which are slightly below those obtained for CLRtreat (from 60.7% to 78.8%). Huang et al. [45] achieved 81% enzymatic hydrolysis of a sample pretreated by 10% NaOH at 160 °C and Wang et al. [47] indicated a hydrolysis efficiency of 79.2% for Sophora flavescens residues pretreated with 1.2% NaOH at 120 °C, for 2 h. Cellulose digestibility is increased with the removal of barriers such as lignin, xylan, and acetyl groups [52,53] and this could be observed in this work.
Therefore, the results showed that ASP and AGO are quite more effective than EO for fractionation of cellulose and lignin from C. ladanifer distillery residues as well as for the subsequent enzymatic hydrolysis of cellulose.
3.3. Phenolic Composition of Lignin Liquors
The liquid fractions resulting from delignification were characterized for total phenolics concentration (expressed as gallic acid equivalents) and by CZE for their phenolic profile. In general, the phenolic compounds yield increased with the delignification yield. However, this was not always observed when analyzing the samples individually. Ethanol extraction yielded lower total phenolic content (0.9–1.4 g/L), while AGO and ASP processes resulted in phenolic concentrations between 6.2 and 10.8 g/L (data not showed). Concentrations from 4.8 to 6 g/L of total phenolics were found for organosolv liquors from rice straw [13]. Phenolic compounds have shown interesting bioactivities, in particular, strong antioxidant potential. Thus, the antioxidant activity of lignins has suggested new applications of this polymer in cosmetics and pharmaceuticals industries [54].
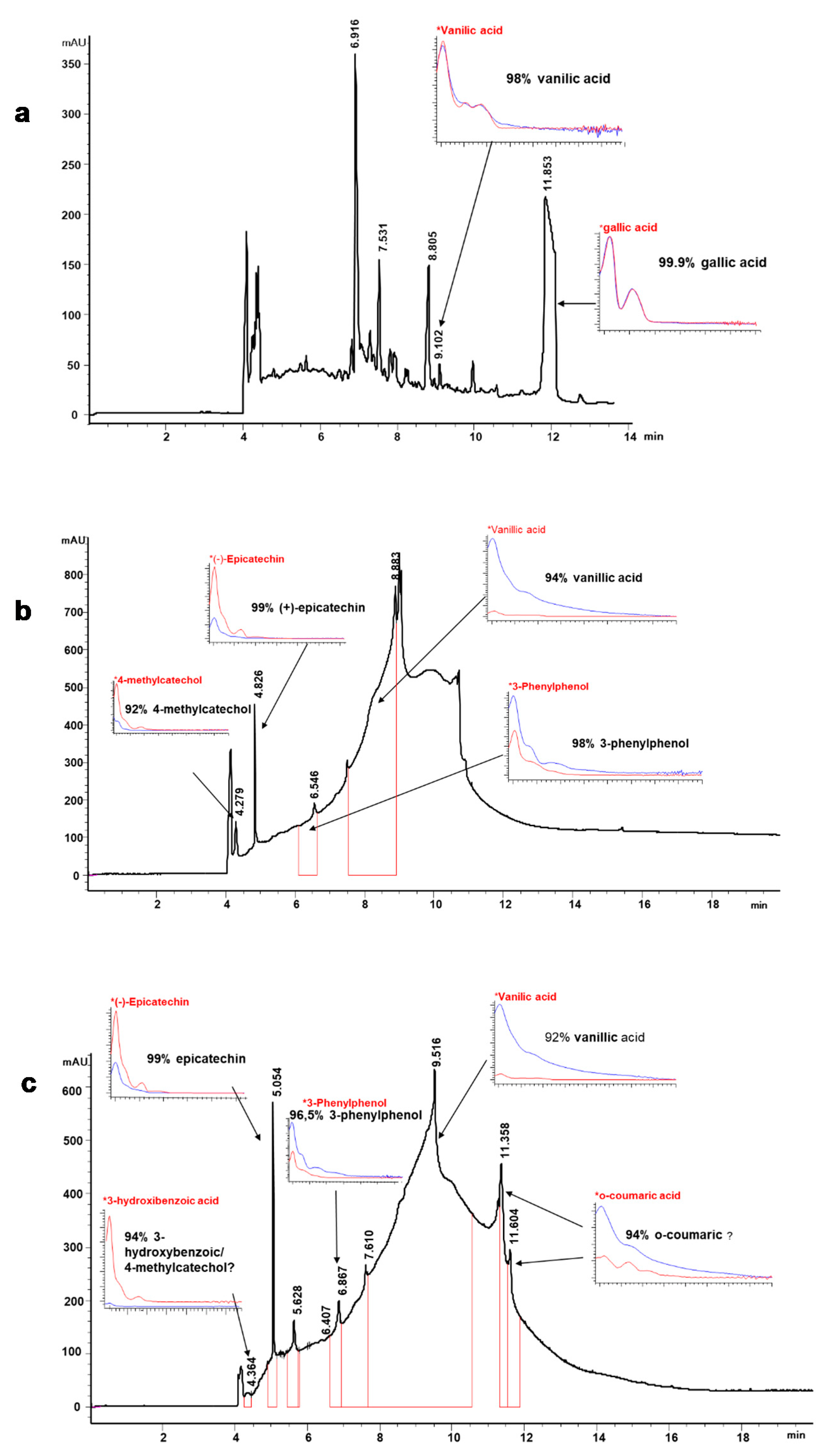
Figure 2 shows an example of the phenolic profile obtained by CZE where the lignin-derived non-volatile substances of EO6, AGO3, and ASP8 samples are shown. The electropherograms show the complexity of the liquor matrix, especially for AGO3 and ASP8, which were very viscous and with great content of lignin. Gallic acid and vanillic acid are the phenolic acids identified in the ethanol liquor (Figure 2a).
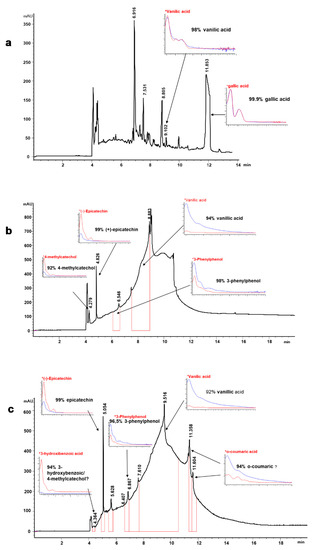
Figure 2.
Electropherogram (200 nm) showing the phenolic profile of the lignin products obtained after different delignification processes of treated C. ladanifer residues (CLRtreat). (a) ethanol organosolv lignin (EO6 sample); (b) alkali-catalyzed glycerol organosolv lignin (AGO3 sample); (c) aqueous sodium hydroxide lignin (ASP8 sample). The % matching was obtained by comparison with authentic standards (*) run under the same conditions as the samples. See text for CZE conditions.
These compounds were previously found in C. ladanifer extracts [22,55,56,57]. Benzoic aldehydes such as vanillin and syringaldehyde could also be identified in the same sample at 375 nm (data not shown). Vanillic acid was identified in all the samples, and especially in AGO3 and ASP8, this compound showed the most prominent peaks (Figure 2b,c). Vanillin or, its oxidized form, vanillic acid, are known to exhibit potential bio-activities as antibacterial [58], inhibitory effects on initiation of hepatocarcinogenesis [59], protective effect on cisplatin-induced renal injury [60]. Additionally, vanillin is widely used as a flavoring agent in food products and aromatic additives, and its global market is expected to reach USD 724.5 million by 2025 [61]. The compounds 3-phenyl phenol and epicatechin were found in AGO3 and ASP8 with very good matching (Figure 2b,c). Several studies reported that (−)-epicatechin, a flavan-3-ol, may contribute to the prevention of cardiovascular diseases and metabolic disorders, and demonstrated blood pressure-lowering capacity [62]. Other studies indicated pharmacological benefits of epicatechin such as inhibitory effects against human breast cancer cells [63], insulin-like activity [64], or parasitic activity [65].
Other compounds such as coumaric acid, 3-hydroxybenzoic acid, and 4-methylcatechol were detected in ASP8 and the latter was identified also in AGO3. However, due to the matrix complexity and overlapping peaks, these compounds were not identified with a high degree of certainty. Moniz et al. [13] also found coumaric acid in rice organosolv liquor. The biological activity of these phenolic compounds has been demonstrated in numerous studies: 4-methylcatechol as an anti-melanoma agent [66]; coumaric acid as a strong antimicrobial [67] and antioxidant [68].
Therefore, the potential bioactivity of lignins supports their promising application in the pharmaceutical, cosmetic, and food industries.
3.4. Characterisation of Delignified Solids and Isolated Lignins by Py-GC/MS Pyrolysis
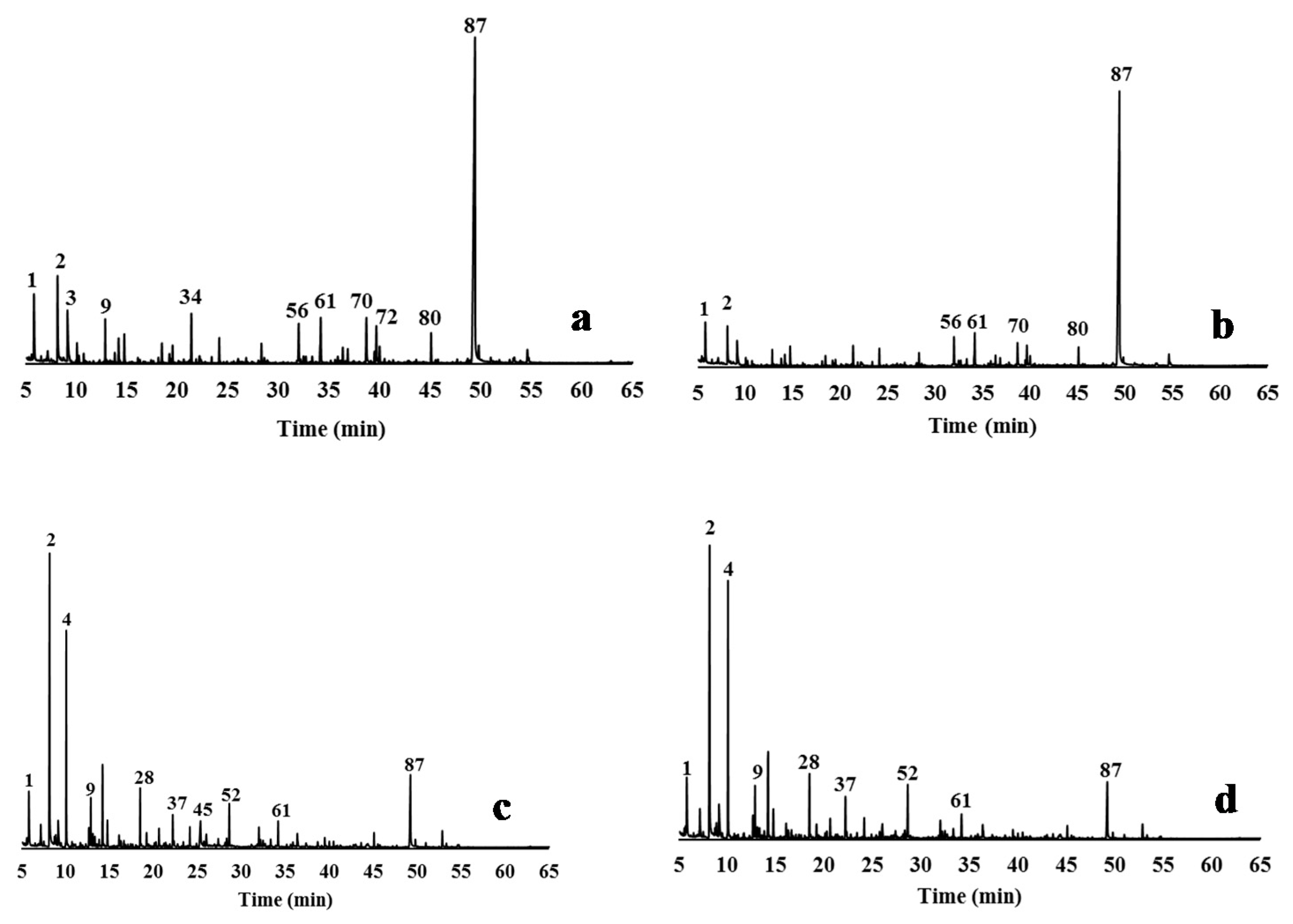
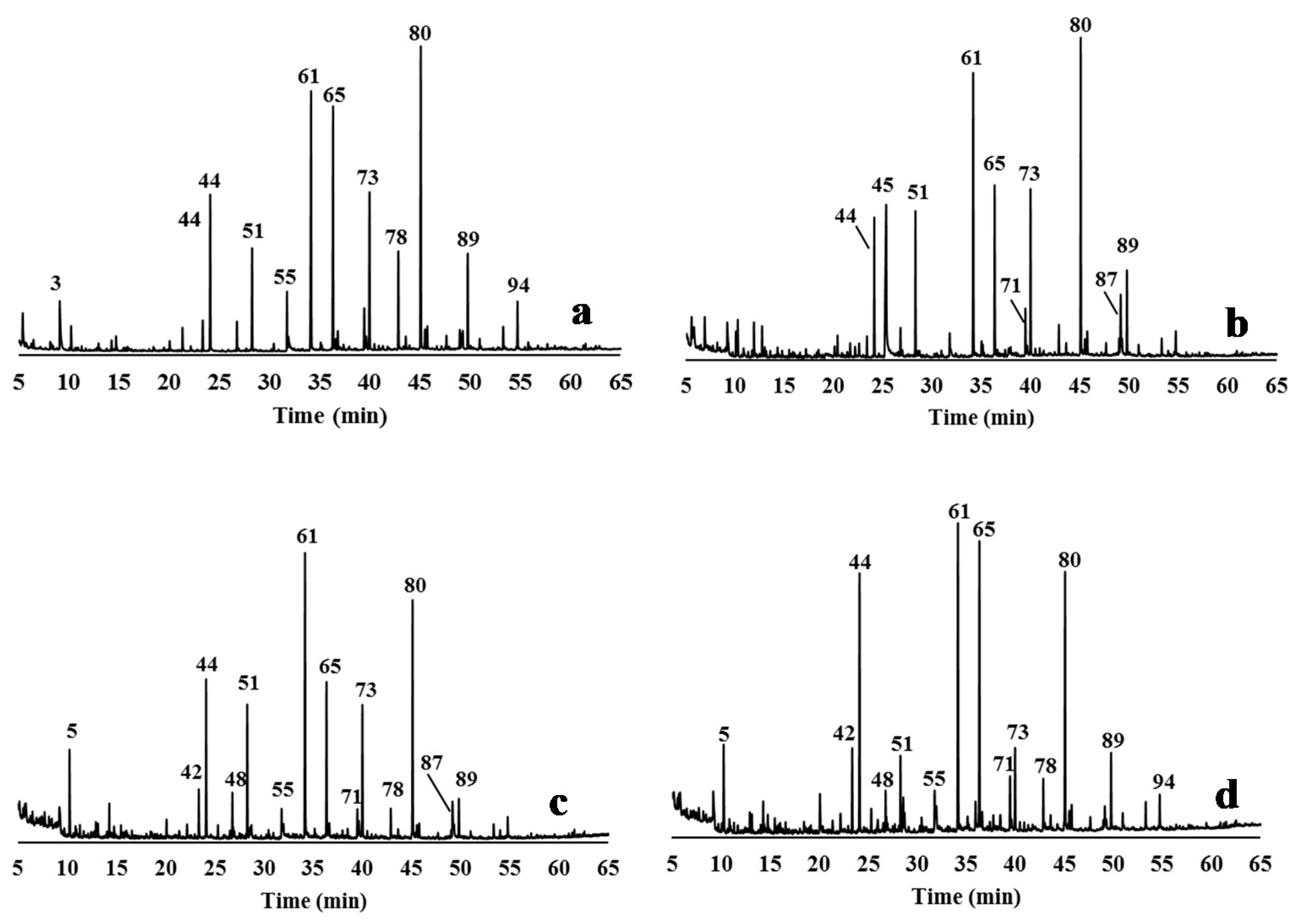
The hydrothermally treated feedstock (CLRtreat), the delignified samples (EO5, AGO3, ASP8), and the isolated lignin from the liquors (EO5, AGO3, ASP7, and ASP8) were characterized by Py-GC/MS. Pyrolysis is a powerful methodology to characterize the monomeric composition of lignin in biomass [69]. Figure 3 and Figure 4 show the pyrograms of the delignified solids and the isolated lignins, respectively, and identify the numbers of the main peaks. Table 2 shows the identification, quantification, and origin of the pyrolysis products.
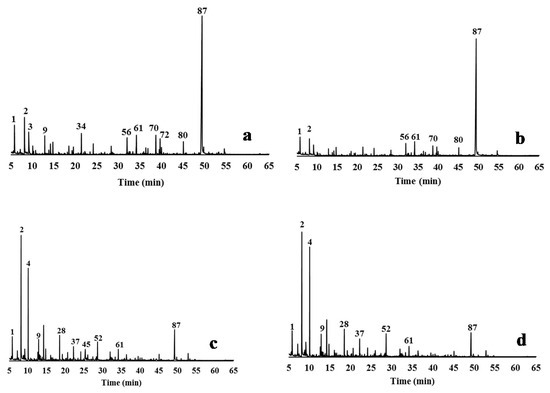
Figure 3.
Py-GC/MS chromatograms of the relevant delignified solids obtained by different delignification processes from CLRext (a) CLRtreat for comparison; (b) EO5 solid; (c) AGO3 solid; (d) ASP8 solid. The peak assignments of the derived pyrolysis products are given in Table 2.
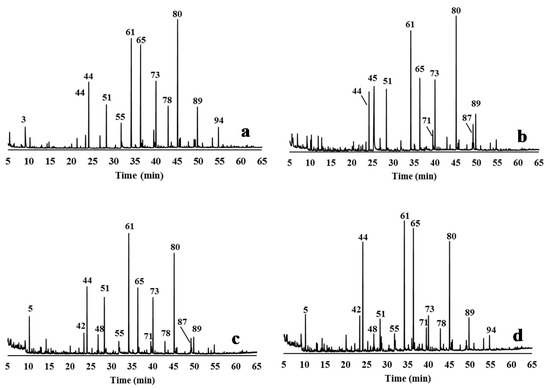
Figure 4.
Py-GC/MS chromatograms of the relevant lignin samples obtained from CLRext by different delignification processes: (a) EO5 lignin; (b) AGO3 lignin; (c) ASP7 lignin; (d) ASP8 lignin. The peak assignments of the derived pyrolysis products are given in Table 2.

Table 2.
Identification of the pyrolysis products (as % of total area) of the hydrothermally treated C. ladanider residues (CLR treat), of delignified solid samples by the three processes-EO (sample EO5), AGO (sample AGO3), and ASP (sample S8), and of lignin precipitated from the delignification liquors of AGO (sample AGO3) and ASP (samples ASP7 and ASP8). The origin of the pyrolysis products is given as derived from carbohydrates (c), lignin hydrophenyl units (H), guaiacyl units (G), syringyl units (S), and undetermined lignin source (NDL), as well as from proteins (P) and other compounds (O).
The pyrograms of the solids remaining after delignification show a strong reduction of the lignin-derived products when compared to the lignin pyrograms (Figure 3 and Figure 4). In fact, the samples recovered from the liquor presented more lignin-derived pyrolysis products (from 49% to 78% of the total area) when compared to the delignified solids (from 7.7% to 11.5% of the total area). The proportion of S, G, and H units, and of the S/G and S/G/H ratios were calculated from the total monomeric phenols identified in the pyrograms (Table 2).
CLRtreat has a S:G:H monomeric composition of 1:0.8:0.1, and a S/G ratio of 1.2. A predominance of syringyl units was found in for xylem, with a G/S ratio of 0.60 and a predominance of guaiacyl units in the pith of C. ladanifer with a G/S of 1.29 was determined in other studies [70].
The lignin composition remaining in the delignified solids was changed after all the treatments. S-lignin represented 2.4% of pyrolysis products of the ASP8 sample, 3.7% of AGO3, and 4.2% of EO5 vs. 5.1% of the undelignified biomass. Compounds such as 4-methylsyringol (peak 73) and 4-vinylsyringol (peak 80) were preferentially attacked by the alkali treatments, contributing to a more pronounced decrease of the S-units in the delignified solids of AGO and ASP than of the EO process. This behavior is consistent with the higher reactivity of syringyl lignin in delignification processes [71]. Otherwise, there was an increase of G and H-type units in the delignified solids when compared to CLRtreat (respectively, from 4.2% to 4.7–5.6% and from 0.6% to 1.1–1.5%). The increase of G and H units and the decrease of S-units during delignification of Eucalyptus globulus was also reported by Lourenço et al. [72].
Regarding the isolated lignins, the S/G ratio values were 1.9, 1.8, 1.0, and 1.3 for EO5, GO3, ASP7, and ASP8, respectively. Syringol derivatives were the dominant group in the pyrolyzed lignin samples of EO5, AGO3, and ASP7 (44.9%, 30.4%, and 32.4 % of the total lignin, respectively), with 4-vinylsyringol as the main constituent (10.5–13.2%), although guaiacol derivatives were obtained in similar proportion in ASP7 (32.8%). Differences in the content of some compounds between ASP7 and ASP8 lignins were also observed, e.g., 4-vinylguaiacol (14.7% and 4%, respectively) and 4-vinylsyringol (11.7% and 5.7%, respectively). The main H-units were phenol (peak 42), p-cresol (peak 48), and 2,3-dihydrobenzofuran (peak 60), which represented from 6.3% of ASP7 to 9.7% of EO5). Other compounds with not determined lignin source (NDL) were also identified: toluene (peak 5), 1,3-dimethyl-benzene (peak 10), 1,4-dimethyl-benzene (peak 13), and styrene (peak 19), reaching a total of 6.4% in ASP7 lignin.
The pyrograms of the delignified solids presented also some peaks that originated from carbohydrates (cellulose and hemicellulose) (Figure 3). The higher intensity of the cellulose products in these pyrograms is an effect of the selective fractionation of the delignification processes [73]. EO caused only a slight compositional change in the solid, while AGO and ASP produced solids with greater changes when compared to the CLRtreat e.g., hydroxyacetaldehyde (peak 2), 2-hydroxypropanone (peak 4), and 1,6-anhydro-β-D-glucopyranose (levoglucosan, peak 87). The pyrograms (Figure 3a,b) show that levoglucosan was the main carbohydrate-derived pyrolysis detected in CLRtreat and EO5 (42.7% and 51%, respectively) since it is the major product of cellulose pyrolysis [74,75,76]. This compound decreased sharply in ASP8 and AGO3 solids (dropped to ≤7%) (Figure 3c,d) and was quite reduced in all the lignin samples (≤3%) (Figure 4a–d). On the other hand, hydroxyacetaldehyde (peak 2) and 2-hydroxypropanone (peak 4) strongly increased in ASP8 and AGO3, which can be explained by the decline of levoglucosan associated with reactions involving opening and reforming of the pyranoid ring [77].
As expected, carbohydrates-derived pyrolysis products represented low percentages in the lignins isolated from the liquid stream (from 3.7% to 13.4%); levoglucosan was the main carbohydrate-derived product in GO3 (3.0%) and ASP7 (2.6%).
The results obtained showed that the type of the delignification process and the reaction time have an impact on the selectivity of the reactions involved and therefore on the type and composition of lignin-derived compounds present in the delignified solids and the solubilized lignins.
4. Conclusions
Delignification of C. ladanifer residues after hydrothermal treatment by organosolv processes was more effective with the alkali-catalyzed glycerol organosolv process than with the ethanol organosolv, which preserved the residual cellulose (>88%) but exhibited lower enzymatic saccharification yields due to the low delignification achieved. The aqueous sodium hydroxide process was the most efficient for the selective separation of lignin and cellulose as well as for the enzymatic hydrolysis of the delignified biomass.
The delignification reactions were selective regarding the different lignin units with S-units being preferentially removed, therefore leaving residual lignins in the solids that were enriched in G- and H-units, the effect being highest for the ASP. Low molecular weight phenolic compounds were also present in all the delignification liquors and may have potential use as a bioactive agent.
Cistus ladanifer, besides being a recognized source of essential oils, can be further processed and integrated as a biorefinery feedstock allowing the production of lignin-derivatives and glucan-rich solids to be used for the production of other added-value products.
Author Contributions
Conceptualization, J.A.-F., H.P. and F.C.; methodology, J.A.-F., F.M., A.L. and L.B.R.; validation, F.C., H.P., M.C.F.; formal analysis, J.A.-F., A.L., L.B.R., L.C.D. and F.C.; investigation, J.A.-F.; resources, J.A.-F.; M.C.F.; F.C.; data curation, J.A.-F., A.L., L.B.R., L.C.D. and F.C.; writing—original draft preparation, J.A.-F. and F.C.; writing—review and editing, A.L., L.B.R., L.C.D., M.C.F., H.P. and F.C.; visualization, J.A.-F., FC.; supervision, M.C.F., H.P. and F.C.; project administration, F.C., H.P., M.C.F., funding acquisition, F.C., L.C.D., M.C.F., H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES–Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil, doctoral scholarship–Process 9109/13-7 and by the QREN Project “Biomassa Endógena”. The work was carried out at the Biomass and Bioenergy Research Infrastructure (BBRI), funded by the BBRI-LISBOA-01-0145-FEDER-022059 project that is supported by the Operational Programme for Competitiveness and Internationalization (PORTUGAL2020), by Lisbon Portugal Regional Operational Programme (Lisboa 2020) and by North Portugal Regional Operational Programme (Norte 2020) under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Centro de Estudos Florestais and Mediterranean Institute for Agriculture, Environment and Development are research units funded by FCT-Fundação para a Ciência e a Tecnologia (UID/AGR/00239/2020 and UIDB/05183/2020, respectively).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable for studies not involving humans.
Acknowledgments
Ana Lourenço acknowledges a research contract funded by FCT (DL 57/2016/CP1382/CT0007). The authors thank Patrícia Moniz, Cláudia Tavares and Céu Penedo for laboratory help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Patón, D.; Azocar, P.; Tovar, J. Growth and productivity in forage biomass in relation to the age assessed by dendrochronology in the evergreen shrub Cistus ladanifer (L.) using different regression models. J. Arid Environ. 1998, 38, 221–235. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass. In Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; Prepared for the U.S. Department of Energy under Contract DE-AC05-76RL01830; Pacific Northwest National Laboratory: Richland, WA, USA, 2007; Volume 2. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-16983.pdf (accessed on 2 April 2020).
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications. Bioenergy Res. Adv. Appl. 2014, 315–336. [Google Scholar] [CrossRef]
- Smolarski, N. High-Value Opportunities for Lignin: Unlocking Its Potential Lignin Potential; Frost & Sullivan: San Antonio, TX, USA, 2012; pp. 1–15. [Google Scholar]
- Erdocia, X.; Prado, R.; Corcuera, M.Á.; Labidi, J. Effect of different organosolv treatments on the structure and properties of olive tree pruning lignin. J. Ind. Eng. Chem. 2014, 20, 1103–1108. [Google Scholar] [CrossRef]
- Sridach, W. The environmentally benign pulping process of non-wood fibers. Suranaree J. Sci. Technol. 2010, 17, 105–123. [Google Scholar]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 7 December 2011. [Google Scholar]
- Cybulska, I.; Brudecki, G.; Schmidt, J.E.; Tomsen, M.H. Organosolv fractionation of palm tree residues. Energy Procedia 2015, 75, 742–747. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Moniz, P.; Serralheiro, C.; Matos, C.T.; Boeriu, C.G.; Frissen, A.E.; Duarte, L.C.; Roseiro, L.B.; Pereira, H.; Carvalheiro, F. Membrane separation and characterisation of lignin and its derived products obtained by a mild ethanol organosolv treatment of rice straw. Process Biochem. 2018, 65, 136–145. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.X.; Sun, R.C.; Fowler, P.; Baird, M.S. Comparative study of organosolv fignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef]
- Alriols, M.G.; Tejado, A.; Blanco, M.; Mondragon, I.; Labidi, J. Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem. Eng. J. 2009, 148, 106–114. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Benjelloun-Mlayah, B.; Huijgen, W.J.J.; de Wild, P.J.; Gosselink, R.J.A.; Gerritsma, J.; Courtin, C.M. Biorefining of wheat straw using an acetic and formic acid based organosolv fractionation process. Bioresour. Technol. 2014, 156, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery residues from Cistus ladanifer (rockrose) as feedstock for the production of added-value phenolic compounds and hemicellulosic oligosaccharides. Bioenergy Res. 2019, 12, 347–358. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal treatments of Cistus ladanifer industrial residues obtained from essential oil distilleries. Waste Biomass Valorization 2019, 10, 1303–1310. [Google Scholar] [CrossRef]
- Pérez, P.; Saúl, L.; Ciria, M.P. Distribución Geográfica, Caracterización Ecológica y Evaluación de Cistus laurifolius y Cistus ladanifer. Estudios Sobre el Matorral Como Recurso Energético; VidaRural: Lisboa, Portugal, 2011; pp. 66–70. [Google Scholar]
- Carrión-Prieto, P.; Martín-Ramos, P.; Maria, T.M.R.; Hernández-Navarro, S.; Garrido-Laurnaga, F.; Eusébio, M.E.S.; Martín-Gil, J. Vibrational and thermal studies of essential oils derived from Cistus ladanifer and Erica arborea shrubs. Nat. Prod. Commun. 2017, 12, 119–122. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 1–19. [Google Scholar] [CrossRef]
- Biolandes. Cistus Labdanum in Andalusia. Available online: https://www.biolandes.com/en-cistus-labdanum.php?voyage=o&lg=en (accessed on 1 February 2021).
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report No. NREL/TP-510-42618; NREL: Golden, CO, USA, 2008; pp. 1–15.
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Faix, O.; Fortmann, I.; Bremer, J.; Meier, D. Thermal degradation products of wood. Holz Roh Werkst 1991, 49, 213–219. [Google Scholar] [CrossRef]
- Ralph, J.; Hatfield, R.D. Pyrolysis-Gc-Ms Characterization of Forage Materials. J. Agric. Food Chem. 1991, 39, 1426–1437. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory analytical procedure (LAP); Technical Report No. NREL/TP-510-42629; NREL: Golden, CO, USA, 2008.
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Berghem, L.E.R.; Pettersson, L.G.; AxiÖ-Fredriksson, U.-B. The mechanism of enzymatic cellulose degradation. Eur. J. Biochem. 1975, 53, 55–62. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, M.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N.; Curvelo, A.A.S.; de Azevedo, E.R.; Guimarães, F.E.G.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Hu, T.; Zhao, X.; Liu, D. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features. Appl. Energy 2015, 150, 224–232. [Google Scholar] [CrossRef]
- Sun, F.F.; Wang, L.; Hong, J.; Ren, J.; Du, F.; Hu, J.; Zhang, Z.; Zhou, B. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 2015, 187, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hundt, M.; Schnitzlein, K.; Schnitzlein, M.G. Alkaline polyol pulping and enzymatic hydrolysis of hardwood: Effect of pulping severity and pulp composition on cellulase activity and overall sugar yield. Bioresour. Technol. 2013, 136, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Novo, L.P.; Gurgel, L.V.A.; Marabezi, K.; Curvelo, A.A.d.S. Delignification of sugarcane bagasse using glycerol-water mixtures to produce pulps for saccharification. Bioresour. Technol. 2011, 102, 10040–10046. [Google Scholar] [CrossRef]
- Meighan, B.N.; Lima, D.R.S.; Cardoso, W.J.; Baêta, B.E.L.; Adarme, O.F.H.; Santucci, B.S.; Pimenta, M.T.B.; de Aquino, S.F.; Gurgel, L.V.A. Two-stage fractionation of sugarcane bagasse by autohydrolysis and glycerol organosolv delignification in a lignocellulosic biorefinery concept. Ind. Crops Prod. 2017, 108, 431–441. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Zhai, S.; Liang, C.; Huang, C.; Lai, C.; Yong, Q. Improving enzymatic hydrolysis efficiency of wheat straw through sequential autohydrolysis and alkaline post-extraction. Bioresour. Technol. 2018, 251, 374–380. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Wang, Y.; Min, D.; Yong, Q. Associating cooking additives with sodium hydroxide to pretreat bamboo residues for improving the enzymatic saccharification and monosaccharides production. Bioresour. Technol. 2015, 193, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nagula, K.N.; Pandit, A.B. Process intensification of delignification and enzymatic hydrolysis of delignified cellulosic biomass using various process intensification techniques including cavitation. Bioresour. Technol. 2016, 213, 162–168. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Liu, J.; Wang, Q.; Wang, C.; Yin, Z.; Wu, C. Lactic acid production from Sophora flavescens residues pretreated with sodium hydroxide: Reutilization of the pretreated liquor during fermentation. Bioresour. Technol. 2017, 241, 915–921. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.K.; Kim, H.Y.; Jeong, H.S.; Kim, J.Y.; Yeo, H.; Choi, I.G. Effect of ethanol organosolv pretreatment factors on enzymatic digestibility and ethanol organosolv lignin structure from Liriodendron tulipifera in specific combined severity factors. Renew. Energy 2016, 87, 599–606. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H. Organosolv pretreatment by crude glycerol from oleochemicals industry for enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2008, 99, 5474–5479. [Google Scholar] [CrossRef]
- Martín, C.; Puls, J.; Saake, B.; Schreiber, A. Effect of glycerol preatreatment on component recovery and enzymatic hydrolysis of sugarcane bagasse. Cell. Chem. Technol. 2011, 45, 487–494. [Google Scholar]
- Pan, X.J.; Gilkes, N.; Saddler, J.N. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Zha, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Arroyo, S.; Barrajon-Catalan, E.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a Cistus ladanifer aqueous extract. Phytochem. Anal. 2010, 21, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Convers. Biorefin. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Govindasami, T.; Pandey, A.; Palanivelu, N.; Pandey, A. Synthesis, characterization and antibacterial activity of biologically important vanillin related hydrazone derivatives. Int. J. Org. Chem. 2011, 1, 71–77. [Google Scholar] [CrossRef]
- Tsuda, H.; Uehara, N.; Iwahori, Y.; Asamoto, M.; Ligo, M.; Nagao, M.; Matsumoto, K.; Ito, M.; Hirono, I. Chemopreventive effects of β-carotene, α-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f] qumoline in the rat. Jpn. J. Cancer Res. 1994, 85, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Vanillin Market Size Worth $724.5 Million by 2025 Growth Rate: 7.0%. Available online: https://www.grandviewresearch.com/press-release/global-vanillin-market (accessed on 10 October 2020).
- Bernatova, I. Biological activities of (−)−epicatechin and (−)−epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Braunhut, S.J.; Bruno, F.; McIntosh, D.; Samuelson, L.; Kumar, J. Biocatalytically oligomerized epicatechin with potent and specific anti-proliferative activity for human breast cancer cells. Molecules 2008, 13, 2704–2716. [Google Scholar] [CrossRef]
- Rizvi, S.I.; Zaid, M.A. Insulin-like effect of (−)epicatechin on erythrocyte membrane acetylcholinesterase activity in type 2 diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2001, 28, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Rahuman, A.A.; Bagavan, A.; Geetha, K.; Kamaraj, C.; Elango, G. Evaluation of medicinal plant extracts and isolated compound epicatechin from Ricinus communis against Paramphistomum cervi. Parasitol. Res. 2012, 111, 1629–1635. [Google Scholar] [CrossRef]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharmacol. 2011, 81, 1211–1218. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Chai, X.; Luo, Y.; Guo, T.; Ying, H. Regulation of ρ -coumaric acid tolerance in Clostridium beijerinckii by disturbing the intracellular electron transport chain. Process Biochem. 2018, 68, 43–52. [Google Scholar] [CrossRef]
- Grabar, T.B.; Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Ingram, L.O. Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(−)−lactate fermentations by recombinant Escherichia coli. Biotechnol. Lett. 2006, 28, 1527–1535. [Google Scholar] [CrossRef]
- Lourenço, A.; Pereira, H. Chapter 3: Compositional Variability of Lignin in Biomass. In Lignin Trends Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Micco, V.; Aronne, G. Anatomical features, monomer lignin composition and accumulation of phenolics in 1-year-old branches of the Mediterranean Cistus ladanifer L. Bot. J. Linn. Soc. 2007, 361–371. [Google Scholar] [CrossRef]
- Tsutsumi, B.Y.; Kondo, R.; Sakai, K.; Imamura, H. The Difference of Reactivity between Syringyl Lignin and Guaiacyl Lignin in Alkaline Systems. Holzforschung 1995, 49, 423–428. [Google Scholar] [CrossRef]
- Lourenço, A.; Gominho, J.; Marques, A.V.; Pereira, H. Variation of lignin monomeric composition during kraft pulping of Eucalyptus globulus heartwood and sapwood. J. Wood Chem. Technol. 2013, 33, 1–18. [Google Scholar] [CrossRef]
- Oudia, A.; Mészáros, E.; Jakab, E.; Simões, R.; Queiroz, J.; Ragauskas, A.; Novák, L. Analytical pyrolysis study of biodelignification of cloned Eucalyptus globulus (EG) clone and Pinus pinaster Aiton kraft pulp and residual lignins. J. Anal. Appl. Pyrolysis 2009, 85, 19–29. [Google Scholar] [CrossRef]
- Li, S.; Lyons-Hart, J.; Banyasz, J.; Shafer, K. Real-time evolved gas analysis by FTIR method: An experimental study of cellulose pyrolysis. Fuel 2001, 80, 1809–1817. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Product distribution from fast pyrolysis of glucose-based carbohydrates. J. Anal. Appl. Pyrolysis 2009, 86, 323–330. [Google Scholar] [CrossRef]
- Lourenço, A.; Gominho, J.; Marques, A.V.; Pereira, H. Py-GC/MS(FID) assessed behavior of polysaccharides during kraft delignification of Eucalyptus globulus heartwood and sapwood. J. Anal. Appl. Pyrolysis 2013, 101, 142–149. [Google Scholar] [CrossRef]
- Liao, Y.F.; Wang, S.R.; Ma, X.Q. Study of reaction mechanisms in cellulose pyrolysis. Prepr. Pap. Chem. Soc. Div. Fuel Chem. 2004, 49, 407–411. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).