Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching
Abstract
1. Introduction
2. Materials and Methods
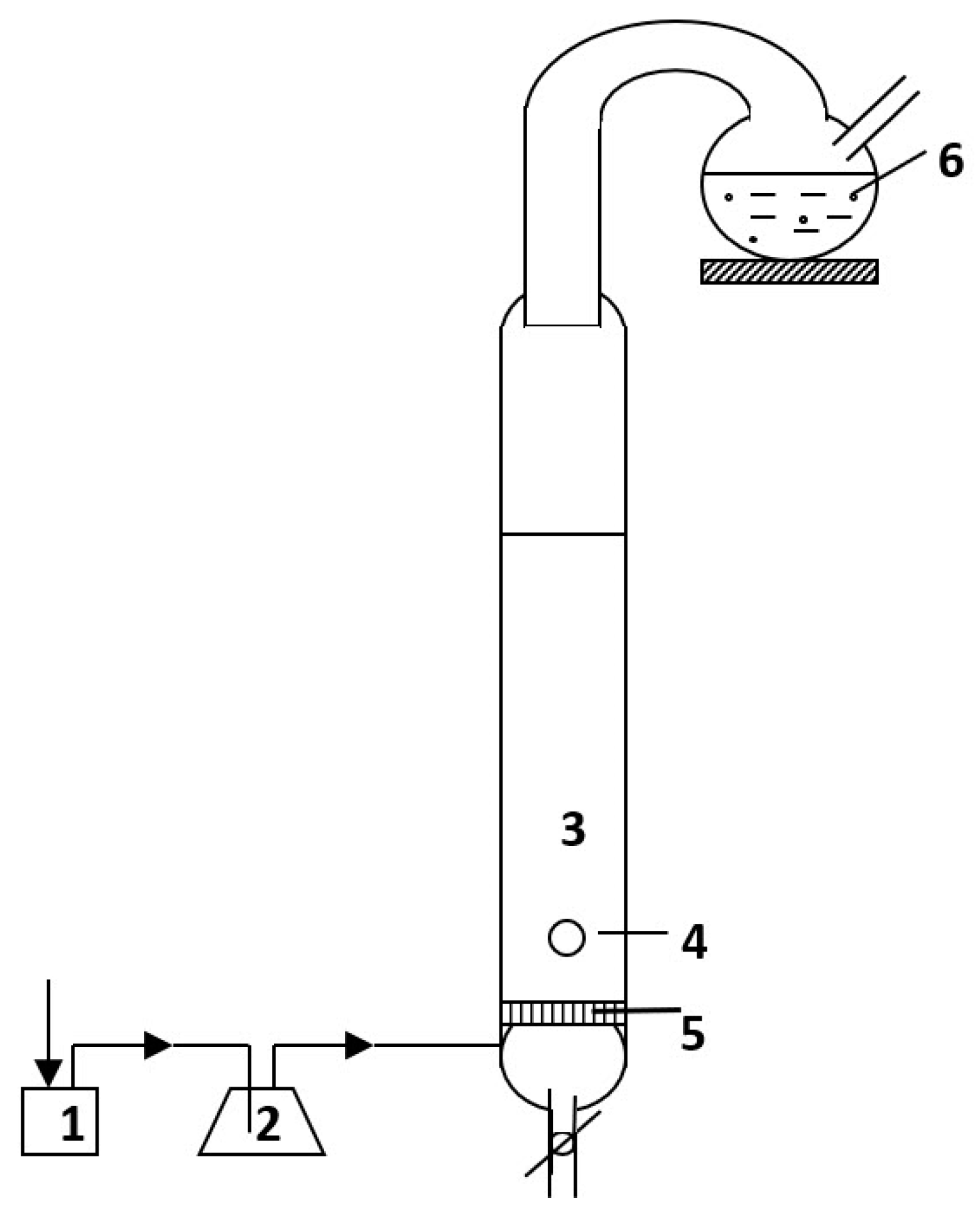
2.1. Apparatus and Measuring Equipment
2.2. Characteristics of Reagents and Tested Solutions
2.3. Research Methodology
3. Results and Discussion
3.1. Ion Flotations from Model Aqueous Solutions
- Parameters influencing Zn(II) and Mn(II) flotation removal with collector 32
- Effect of pH solutions
- Effect of foaming agent presence
- Parameters influencing Zn(II) and Mn(II) flotation removal with collector 33
- Effect of pH solution
- The influence of a foaming agent
3.2. Real Solution
4. Conclusions
- The main parameters which affect Zn(II) and Mn(II) flotation are the type of collector, feed solution pH, concentration of collector and/or foaming agent, and process duration.
- The collective Zn(II) and Mn(II) removal from dilute aqueous solutions in the ion flotation process with using collector 32 or 33 depended on the pH solution. High efficiency Zn(II) removal with collector 32 is possible in the pH range from 2.0 to 7.0 in a 10 min ion flotation process. It has been shown that the maximal percent removal of Zn(II) increased from 80.4% (at pH = 2.0) to 99.7% (at pH = 7.0). In the same experiments, results for Mn(II) removal were as follow: 75.4% and 73.8% for pH 2.0 and 7.0, respectively. On the other hand, effective removal of Zn(II) using second collector, 33, required alkaline solutions, and the observed removal was in the range of 99.1% (pH = 10.0) and 91.6% (pH = 11.0). In the same conditions, results for Mn(II) were 73.2% (pH = 10.0) and 59.4% (pH = 11.0).
- The addition of foaming agent 29 affected Zn(II) removal in particular. Despite a significant improvement in the interfacial surface in the solution and a stable foam over the solution, the addition of 29 caused a decreased removal of both metals. Comparing the results for Zn(II) removal (using collector 32), it can be noticed that addition of 29 lowers Zn(II) removal by half (from 80.4% to 40.1%) at pH = 2.0. On the other hand, in an alkaline solution (using collector 32), the presence of a foaming agent increased the Zn(II) removal, at pH = 9.0, from 50.7% to 96.3%, at pH = 10.0, from 37.1% to 77.2%, and at pH = 11.0, from 60.1% to 86.8%. It was probably related to the observed higher foaming intensity during the flotation process. However, in the case of the second metal, it did not significantly affect the Mn(II) removal. Generally, in some cases, the addition of an agent is beneficial, especially in an alkaline feed solution.
- The time of conducting the ion flotation process also had a significant influence on both metals removal. Comparing the results for Zn(II) and Mn(II) removal in 5 and 20 min processes, it can be concluded that the prolongation of the flotation time affects the results negatively, especially in the case of Zn(II), e.g., removal of Zn(II) with collector 32 in a 5 min process at pH = 5.0 was always over 80%, while in the 20 min process, it did not exceed 20%. Too long a process duration (above 10 min) negatively affects the removal of both metals.
- It was found that the ion flotation process with collectors 32 or 33 can be an alternative to currently known processes in the recovery of metals from waste chemical energy sources. It is also possible to use ion flotation in a hybrid process, as one of the stages of the currently used hydrometallurgical recycling processes. There is also potential to regenerate a waste stream of a solution that would be purified and recycled back into the process.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bigum, M.; Damgaard, A.; Scheutz, C.; Christensen, T.H. Environmental impacts and resource losses of incinerating misplaced household special wastes (WEEE, batteries, ink cartridges and cables). Resour. Conserv. Recycl. 2017, 122, 251–260. [Google Scholar] [CrossRef]
- Kohl, C.A.; Gomes, L.P. Physical and chemical characterization and recycling potential of desktop computer waste, without screen. J. Clean Prod. 2018, 184, 1041–1051. [Google Scholar] [CrossRef]
- Polat, O.; Capraz, O.; Gungor, A. Modelling of WEEE recycling operation planning under uncertainty. J. Clean Prod. 2018, 180, 769–779. [Google Scholar] [CrossRef]
- Zabłocka-Malicka, M.; Szczepaniak, W.; Rutkowski, P.; Ochromowicz, K.; Leśniewicz, A.; Chęcmanowski, J. Decomposition of the ISA-card under steam for valorized polymetallic raw material. J. Anal. Appl. Pyrolysis 2018, 130, 256–268. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Li, J. An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE. Resour. Conserv. Recycl. 2017, 126, 228–239. [Google Scholar] [CrossRef]
- Li, J.; Lopez, B.N.; Liu, L.; Zhao, N.; Yu, K.; Zheng, L. Regional or global WEEE recycling. Where to go? Waste Manag. 2013, 33, 923–934. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Deep, A.; Szulejko, J.E.; Vellingiri, K.; Kumar, S.; Kwon, E.E.; Yun, S.-T. Recovery of nanomaterials from battery and electronic wastes: A new paradigm of environmental waste management. Renew. Sustain. Energy Rev. 2018, 82, 3694–3704. [Google Scholar] [CrossRef]
- Urbańska, W. Recovery of Co, Li and Ni from spent Li-ion batteries by inorganic and/or orgnic reducer assistend leaching metod. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Petranikova, M.; Ebin, B.; Mikhailova, S.; Steenari, B.-M.; Ekberg, C. Investigation of the effects of thermal treatment on the leachability of Zn and Mn from discarded alkaline and Zn C batteries. J. Clean Prod. 2018, 170, 1195–1205. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Prisciandaro, M.; Medici, F.; Vegliò, F. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J. Power Sources 2017, 362, 202–218. [Google Scholar] [CrossRef]
- Gil, S.; Bialik, W.; Saternus, M.; Fornalczyk, A. Thermal Balance of the magneto-hydro-dynamic pump for recovery of platinum group metals from spent auto catalysts. Arch. Metall. Mater. 2016, 61, 253–256. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A. Hydrometallurgical recovery of metals: Ce, La, Co, Fe, Mn, Ni and Zn from the stream of used Ni-MH cells. Waste Manag. 2018, 77, 213–219. [Google Scholar] [CrossRef]
- Dominguez-Benetton, X.; Varia, J.C.; Pozo, G.; Modin, O.; Ter Heijne, A.; Fransaer, J.; Rabaey, K. Metal recovery by microbial electro-metallurgy. Prog. Mater. Sci. 2018, 94, 435–461. [Google Scholar] [CrossRef]
- Mylarappa, M.; Venkata Lakshmi, V.; Vishnu Mahesh, K.R.; Nagaswarupa, H.P.; Prashantha, S.C.; Shravana Kumara, K.N.; Siddeswara, D.M.K.; Raghavendra, N. Resource Recovery and Material Characterization of Metals from Waste Li-ion Batteries by an Eco-Friendly Leaching Agent. Mater. Today Proc. 2017, 4, 12215–12222. [Google Scholar] [CrossRef]
- Torkaman, R.; Asadollahzadeh, M.; Torab-Mostaedi, M.; Ghanadi Maragheh, M. Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process. Sep. Purif. Technol. 2017, 186, 318–325. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Rajesh Kumar, J.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Bi, P.Y.; Dong, H.R.; Dong, J. The recent progress of solvent sublation. J. Chromatogr. A 2010, 1217, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Sobianowska, K.; Walkowiak, W.; Kozłowski, C. Principles and applications of solvent sublation—A review. Ars. Separatoria. Acta 2009, 7, 23–38. [Google Scholar]
- Grudniewska, K.L. Ion Flotation Cs+, Sr2+, Ba2+ and Co2+ Ion Flotation from Dilute Aqueous Solutions with Macrocyclic Compounds; Wrocław University of Technology: Wrocław, Poland, 2012. [Google Scholar]
- Lemlich, R. Adsorptive Bubble Separation Techniques; Elsevier Science & Technology: Saint Louis, MO, USA, 2012. [Google Scholar]
- Sobianowska-Turek, A.; Ulewicz, M.; Sobianowska, K. Ion flotation and solvent sublation of zinc (II) and manganese (II) in the presence of proton-ionizable lariat ethers. Physicochem. Probl. Miner. Process. 2016, 52, 1048–1060. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Bartsch, R.A. Ion flotation of zinc (II) and cadmium (II) with proton-ionizable lariat ethers—Effect of cavity size. Sep. Purif. Technol. 2006, 48, 264–269. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Brandt, K.; Porwolik-Czomperlik, I. Ion Flotation of Zinc (II) and Cadmium (II) in the Presence of Side-Armed Diphosphaza-16-Crown-6 Ethers. Sep. Sci. Technol. 2003, 38, 633–645. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Jang, Y.; Kim, J.S.; Bartsch, R.A. Ion flotation of cadmium (II) and zinc (II) in the presence of proton-ionizable lariat ethers. Anal. Chem. 2003, 75, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, P.; Ulewicz, M.; Robak, W.; Walkowiak, W. Lariat ethers with a novel proton-ionisable groups: New generation of collectors in ion flotation process. Int. J. Environ. Waste Manag. 2011, 8. [Google Scholar] [CrossRef]
- Kozłowski, C.A.; Ulewicz, M.; Walkowiak, W. Removal of zinc and cadmium ions from aqueous chloride solutions by ion flotation and liquid membranes. Physicochem. Probl. Miner. Process. 2000, 34, 141–151. [Google Scholar]
- Charewicz, W.; Hoławiecka, B.; Walkowiak, W. Selective Flotation of Zinc(II) and Silver(I) Ions from Dilute Aqueous Solutions; Wrocław University of Technology: Wrocław, Poland, 1998. [Google Scholar]
- Walkowiak, W. Mechanism of Selective Ion Flotation. 1. Selective Flotation of Transition Metal Cations. Sep. Sci. Technol. 1991, 26, 559–568. [Google Scholar] [CrossRef]
- Jurkiewicz, K. The removal of zinc from solutions by foam separation, I. Foam separation of complex zinc anions. Int. J. Miner. Process. 1990, 28, 173–187. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Szczepaniak, W.; Żurek, A.; Satora, A.; Starowicz, A. The Method of Utilizing the Waste Mass of Zn-C and Zn-Mn Batteries Left after the Removal of the Ferromagnetic Fraction. PL Patent 220853 B1, 25 August 2011. [Google Scholar]
- Polat, H.; Erdogan, D. Heavy metal removal from waste waters by ion flotation. J Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Commision, E. Batteries & Accumulators. Available online: https://ec.europa.eu/environment/waste/batteries/index.htm (accessed on 3 February 2021).
| Structural Formula | Number of the Chemical Compound | -R | -X | -Y- |
|---|---|---|---|---|
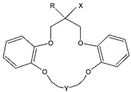 | 1 | -C4H9 | -O(CH2)3SO3Na | -(CH2)2O(CH2)2O(CH2)2O(CH2)2- |
| 2 | -C7H15 | |||
| 3 | -C10H21 | |||
| 4 | -C7H15 | -(CH2)2O(CH2)2O(CH2)2- | ||
| 5 | -(CH2)2O(CH2)2- | |||
| 6 | -CH2CONHSO2CF3 | -(CH2)2O(CH2)2O(CH2)2- | ||
| 7 | -OCH2COOH | |||
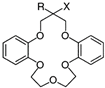 | 8 | -H | -O(CH2)3SO3Na | - |
| 9 | -C3H7 | -O(CH2)3SO3Na | - | |
| 10 | -C10H21 | -O(CH2)3SO3Na | - | |
| 11 | -C10H21 | -OCH2CO2H | - | |
| 12 | -C4H9 | ‑OCH2PO(OH)(OC2H5) | - | |
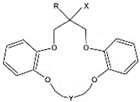 | 13 | -C10H21 | -OCH2COOH | -(CH2)2- |
| 14 | -C10H21 | -O(CH2)3SO3Na | -(CH2)2O(CH2)2- | |
| 15 | -C3H7 | -O(CH2)3SO3Na | -(CH2)2O(CH2)2- | |
| 16 | -H | -O(CH2)3SO3Na | -(CH2)2O(CH2)2- | |
| 17 | -C4H9 | -OCH2COOH | -(CH2)2O(CH2)2- | |
| 18 | -H | -OCH2PO(OH)(OC2H5) | -(CH2)2O(CH2)2- | |
| 19 | -CH3 | -OCH2COOH | ‑(CH2)2O(CH2)2O(CH2)2‑ | |
| 20 | -C2H5 | -OCH2COOH | -(CH2)2O(CH2)2O(CH2)2- | |
| 21 | -C3H7 | -OCH2COOH | -(CH2)2O(CH2)2O(CH2)2- | |
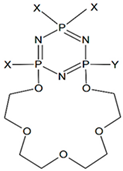 | 22 | - | 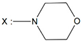 | -NH(CH2)3OH |
| 23 | - | 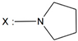 | -NH(CH2)3OH | |
| 24 | - |  | 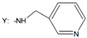 | |
| 25 | - | -O(CH2CH2O)3CH3 | ||
| 26 | - | 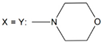 | ||
| 27 | - | 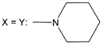 | ||
| 28 | - | 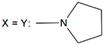 | ||
| Structural Formula | Chemical Compound Name | Number/Function of Chemical Compound |
|---|---|---|
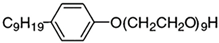 | 1,1,3,3-tetramethylbutyl phenyl polyethylene glycol ether | 29 foaming agent |
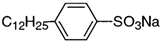 | sodium dodecylbenzenesulfonate | 30 anionic collector |
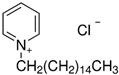 | cetylpyridinium chloride | 31 cationic collector |
| Structural Formula | Total Formula | Chemical Compound Name | Molar Mass, [u] | Number/Function of Chemical Compound |
|---|---|---|---|---|
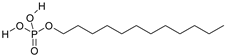 | C12H27O4P | m-dodecylphosphoric acid | 266.31 | 32 collector |
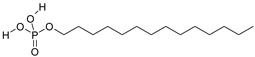 | C14H31O4P | m-tetradecylphosphoric acid | 294.36 | 33 collector |
| pH | Without Foaming Agent 29 | With Foaming Agent 29 | ||||
|---|---|---|---|---|---|---|
| WZn(II),% | WMn(II),% | SZn/Mn | WZn(II),% | WMn(II),% | SZn/Mn | |
| 2.0 | 80.4 | 75.4 | 1.07 | 40.1 | 53.1 | 0.76 |
| 4.0 | 82.1 | 55.9 | 1.47 | 99.6 | 19.4 | 5.13 |
| 5.0 | 92.9 | 43.6 | 2.13 | 77.4 | 16.1 | 4.81 |
| 7.0 | 99.7 | 73.8 | 1.35 | 91.7 | 8.4 | 10.9 |
| 9.0 | 50.7 | 68.8 | 0.74 | 96.3 | 3.3 | 29.2 |
| 10.0 | 37.1 | 54.7 | 0.68 | 77.2 | 60.4 | 1.28 |
| 11.0 | 60.1 | 53.9 | 1.12 | 86.8 | 54.4 | 1.60 |
| pH | Without Foaming Agent 29 | With Foaming Agent 29 | ||||
|---|---|---|---|---|---|---|
| WZn(II),% | WMn(II),% | SZn/Mn | WZn(II),% | WMn(II),% | SZn/Mn | |
| 2.0 | 83.3 | 59.9 | 1.39 | 38.3 | 57.8 | 0.66 |
| 4.0 | 80.4 | 87.5 | 0.92 | 81.7 | 85.2 | 0.96 |
| 5.0 | 90.4 | 20.2 | 4.48 | 82.4 | 94.5 | 0.87 |
| 7.0 | 67.1 | 87.5 | 0.77 | 98.6 | 67.6 | 1.46 |
| 9.0 | 58.5 | 76.6 | 0.76 | 61.1 | 74.2 | 0.82 |
| 10.0 | 99.1 | 73.2 | 1.35 | 59.5 | 72.7 | 0.82 |
| 11.0 | 91.6 | 59.4 | 1.54 | 89.5 | 31.0 | 2.89 |
| pH | WZn(II), % | WMn(II), % | SZn/Mn |
|---|---|---|---|
| 4.0 | 94.2 | 15.0 | 6.28 |
| 7.0 | 89.0 | 10.0 | 8.90 |
| 9.0 | 97.0 | 4.3 | 22.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobianowska-Turek, A.; Grudniewska, K.; Maciejewski, P.; Gawlik-Kobylińska, M. Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching. Energies 2021, 14, 1335. https://doi.org/10.3390/en14051335
Sobianowska-Turek A, Grudniewska K, Maciejewski P, Gawlik-Kobylińska M. Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching. Energies. 2021; 14(5):1335. https://doi.org/10.3390/en14051335
Chicago/Turabian StyleSobianowska-Turek, Agnieszka, Katarzyna Grudniewska, Paweł Maciejewski, and Małgorzata Gawlik-Kobylińska. 2021. "Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching" Energies 14, no. 5: 1335. https://doi.org/10.3390/en14051335
APA StyleSobianowska-Turek, A., Grudniewska, K., Maciejewski, P., & Gawlik-Kobylińska, M. (2021). Removal of Zn(II) and Mn(II) by Ion Flotation from Aqueous Solutions Derived from Zn-C and Zn-Mn(II) Batteries Leaching. Energies, 14(5), 1335. https://doi.org/10.3390/en14051335






