sp2–sp3 Hybrid Porous Carbon Materials Applied for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Characterization
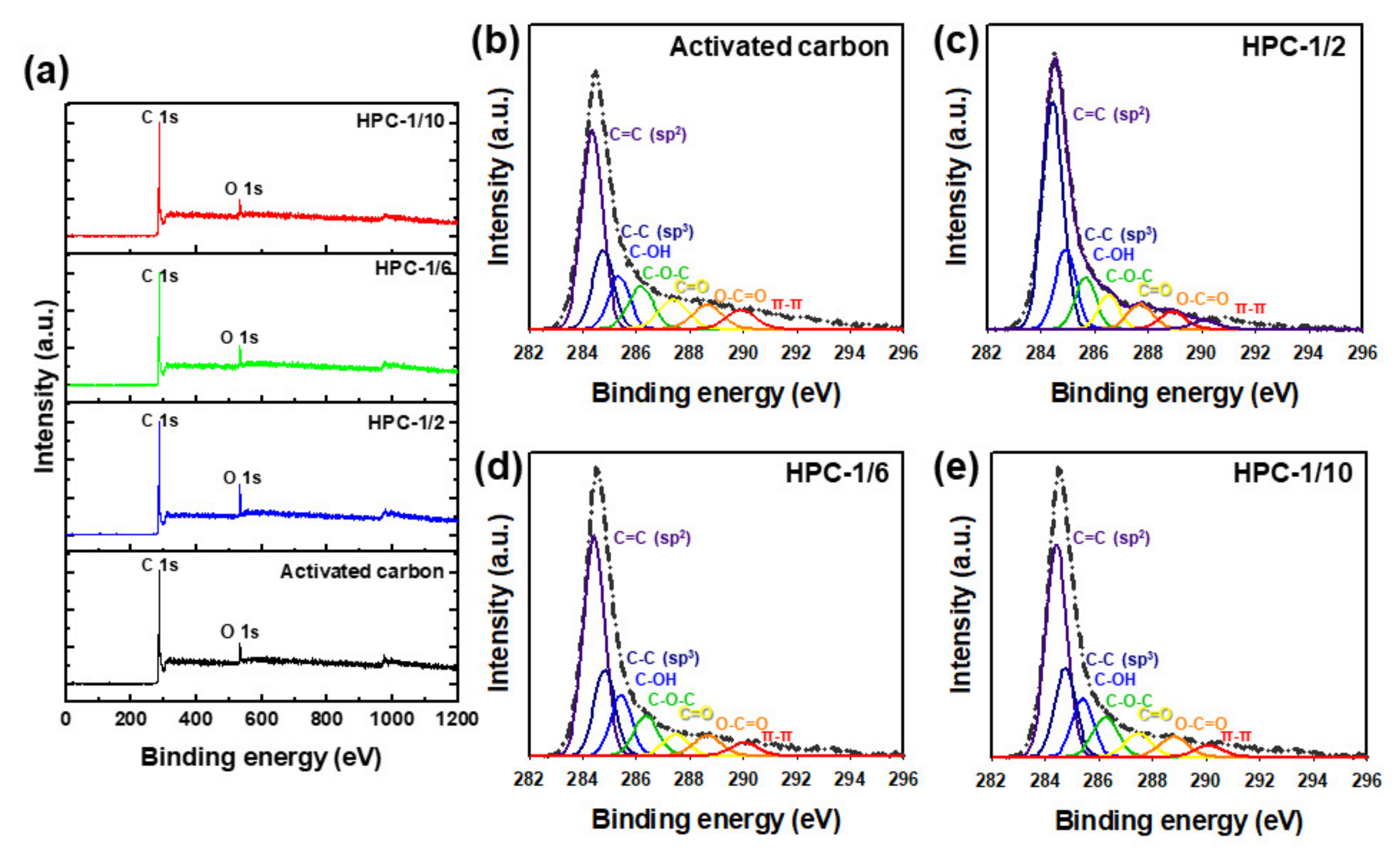
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Shi, W.; Lu, G.; Huang, X.; Yan, Q.; Zhang, Q.; Zhang, H. Preparation of novel 3D graphene networks for supercapacitor applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiao, P.; Chen, H.; Zhang, H.; Zhang, Q.; Chen, Y. Polymeric graphene bulk materials with a 3D cross-linked monolithic graphene network. Adv. Mater. 2019, 31, 1802403. [Google Scholar] [CrossRef]
- Nishihara, H.; Kyotani, T. Templated nanocarbons for energy storage. Adv. Mater. 2012, 24, 4473–4498. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon nanomaterials in different dimensions for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1600278. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, A.; Dhar, A.; Biswas, S.; Sharma, V.; Burada, P.S.; Chandra, A. Theoretical model for magnetic supercapacitors—From the electrode material to electrolyte ion dependence. J. Phys. Chem. C 2020, 124, 26613–26624. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, X.; Chen, G.Z. Nanoporous versus nanoparticulate carbon-based materials for capacitive charge storage. Energy Environ. Mater. 2020, 3, 247–264. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Paul, R.; Du, F.; Dai, L.; Ding, Y.; Wang, Z.L.; Wei, F.; Roy, A. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Adv. Mater. 2019, 31, 1805598. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Han, J.; Zhang, C.; Ling, G.; Kang, F.; Yang, Q.H. Dimensionality, Function and Performance of Carbon Materials in Energy Storage Devices. Adv. Energy Mater. 2021, 2100775. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Miyashiro, D.; Hamano, R.; Umemura, K. A review of applications using mixed materials of cellulose, nanocellulose and carbon nanotubes. Nanomaterials 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonough, J.R.; Choi, J.W.; Yang, Y.; La Mantia, F.; Zhang, Y.; Cui, Y. Carbon nanofiber supercapacitors with large areal capacitances. Appl. Phys. Lett. 2009, 95, 243109. [Google Scholar] [CrossRef]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-doped and oxygen-functionalized nanocarbons for high-performance supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Bhat, V.S.; Kanagavalli, P.; Sriram, G.; John, N.S.; Veerapandian, M.; Kurkuri, M.; Hegde, G. Low cost, catalyst free, high performance supercapacitors based on porous nano carbon derived from agriculture waste. J. Energy Storage 2020, 32, 101829. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.; Hedhili, M.N.; Alshareef, H.N. Shape-controlled porous nanocarbons for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 5236–5243. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, P.; Xie, J.; Yang, Z.; Huang, Y.; Hu, J.; Wan, Y.; Xu, Y. Uniformly dispersed freestanding carbon nanofiber/graphene electrodes made by a scalable biological method for high-performance flexible supercapacitors. Advt. Funct. Mater. 2018, 28, 1803075. [Google Scholar] [CrossRef]
- Azhar, A.; Yamauchi, Y.; Allah, A.E.; Alothman, Z.A.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Wang, J.; Zakaria, M.B. Nanoporous iron oxide/carbon composites through in-situ deposition of prussian blue nanoparticles on graphene oxide nanosheets and subsequent thermal treatment for supercapacitor applications. Nanomaterials 2019, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Luxembourg, D.; Py, X.; Didion, A.; Gadiou, R.; Vix-Guterl, C.; Flamant, G. Chemical activations of herringbone-type nanofibers. Microporous Mesoporous Mater. 2007, 98, 123–131. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lim, S.; Song, Y.; Ota, Y.; Qiao, W.; Tanaka, A.; Mochida, I. KOH activation of carbon nanofibers. Carbon 2004, 42, 1723–1729. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.; Pell, W. Power limitations of supercapacitor operation associated with resistance and capacitance distribution in porous electrode devices. J. Power Sources 2002, 105, 169–181. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.; Wang, H.; Wang, W.; Jin, X.; Wang, H.; Zhou, H.; Lin, T. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 2021, 482, 228986. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Kiran, S.K.; Shukla, S.; Struck, A.; Saxena, S. Surface enhanced 3D rGO hybrids and porous rGO nano-networks as high performance supercapacitor electrodes for integrated energy storage devices. Carbon 2020, 158, 527–535. [Google Scholar] [CrossRef]
- Kötz, R.; Hahn, M.; Gallay, R. Temperature behavior and impedance fundamentals of supercapacitors. J. Power Sources 2006, 154, 550–555. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.F.; Del Bosque, A.; Artigas-Arnaudas, J.; Muñoz, B.K.; Sánchez, M.; Ureña, A. A proof of concept of a structural supercapacitor made of graphene coated woven carbon fibers: EIS study and mechanical performance. Electrochim. Acta 2021, 370, 137746. [Google Scholar] [CrossRef]



| Sample | ABET (m2 g−1) | Vtotal (cm3/g) | VMicro (cm3/g) | VMeso & Macro (cm3/g) |
|---|---|---|---|---|
| Activated carbon | 1902 | 0.88 | 0.75 (86.7%) | 0.13 (13.3%) |
| HPC-1/2 | 945 | 0.43 | 0.37 (85.8%) | 0.06 (14.2%) |
| HPC-1/6 | 1816 | 0.86 | 0.72 (84.8%) | 0.14 (15.2%) |
| HPC-1/10 | 1969 | 0.92 | 0.77 (85.1%) | 0.15 (14.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, J.S.; Kang, W.-s.; Roh, K.C. sp2–sp3 Hybrid Porous Carbon Materials Applied for Supercapacitors. Energies 2021, 14, 5990. https://doi.org/10.3390/en14195990
Chae JS, Kang W-s, Roh KC. sp2–sp3 Hybrid Porous Carbon Materials Applied for Supercapacitors. Energies. 2021; 14(19):5990. https://doi.org/10.3390/en14195990
Chicago/Turabian StyleChae, Ji Su, Won-seop Kang, and Kwang Chul Roh. 2021. "sp2–sp3 Hybrid Porous Carbon Materials Applied for Supercapacitors" Energies 14, no. 19: 5990. https://doi.org/10.3390/en14195990
APA StyleChae, J. S., Kang, W.-s., & Roh, K. C. (2021). sp2–sp3 Hybrid Porous Carbon Materials Applied for Supercapacitors. Energies, 14(19), 5990. https://doi.org/10.3390/en14195990







