Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications
Abstract
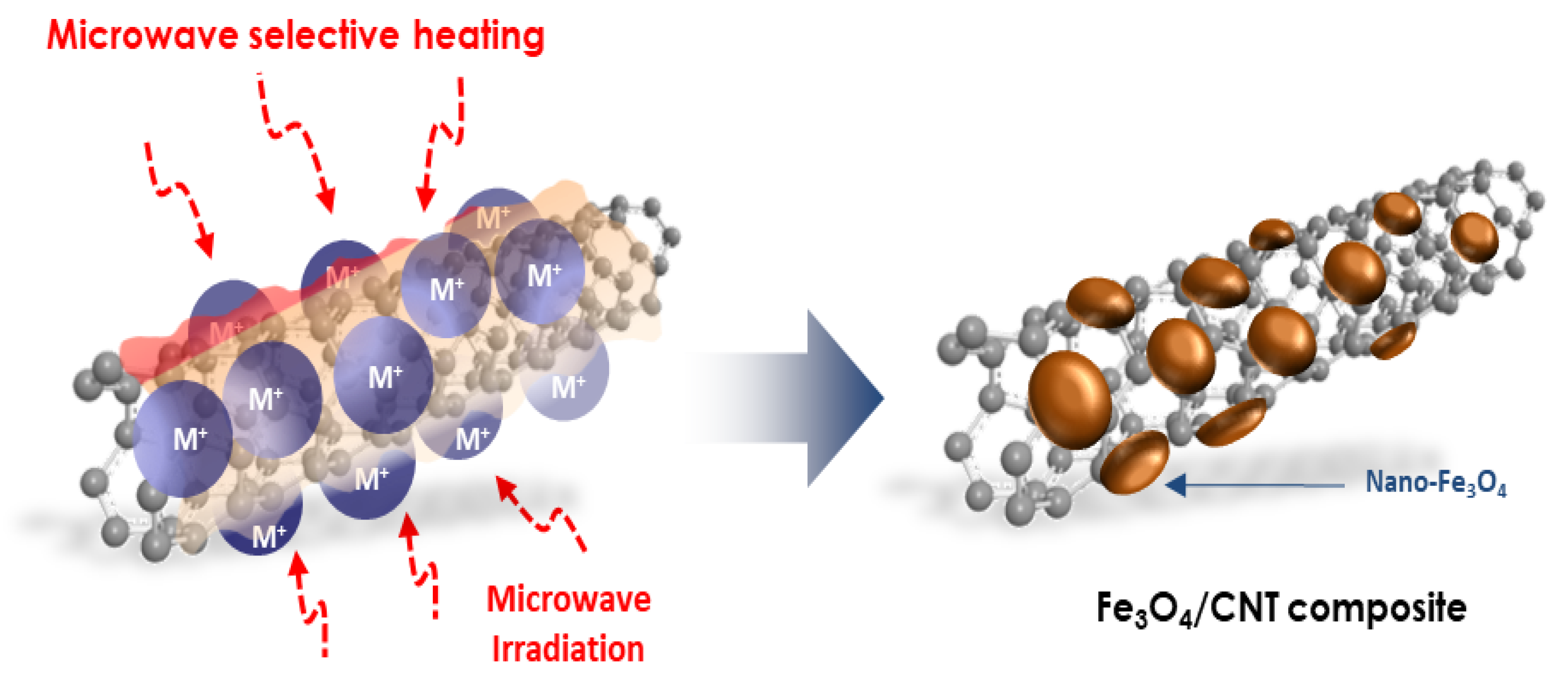
1. Introduction
2. Materials and Methods
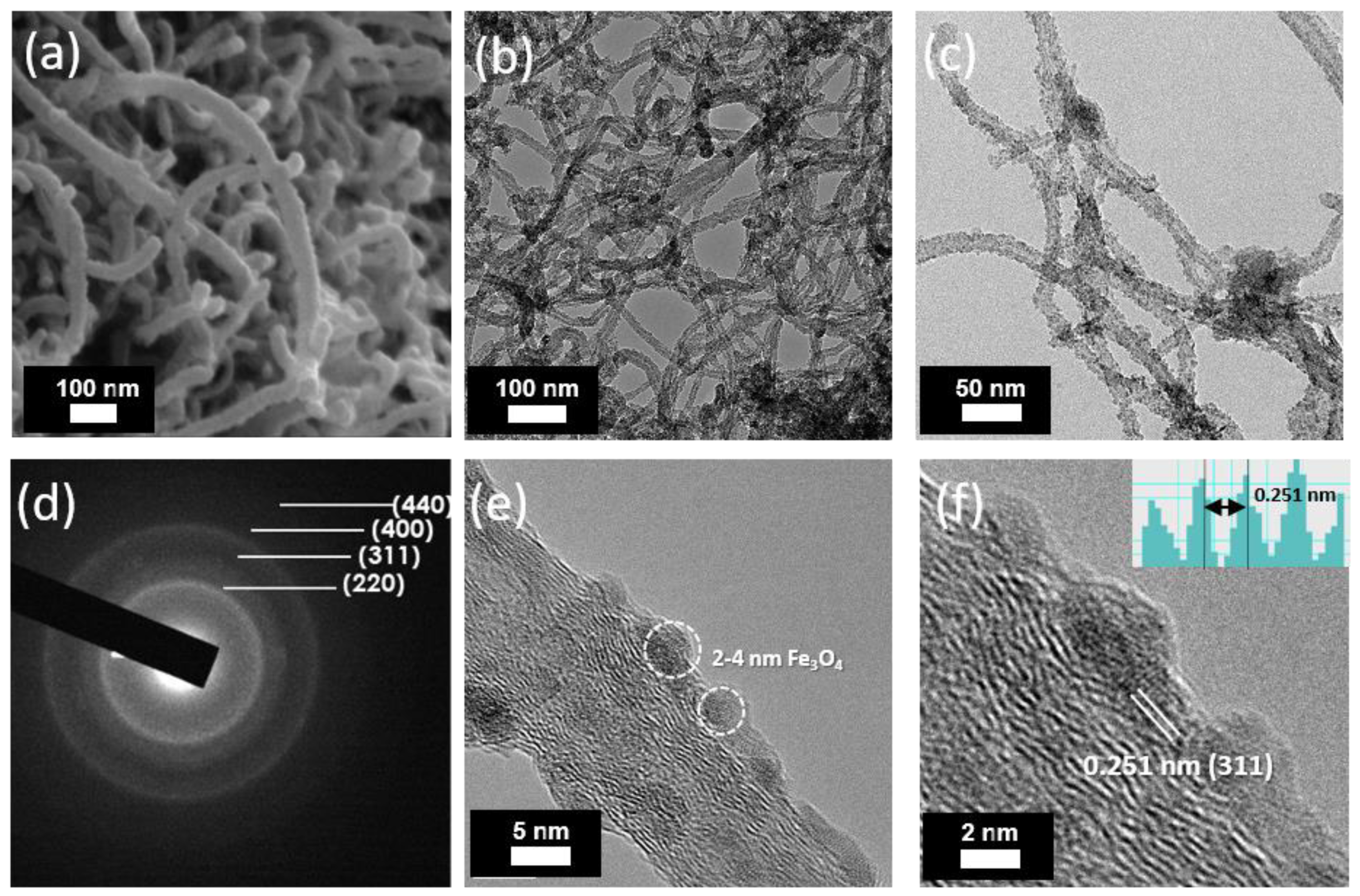
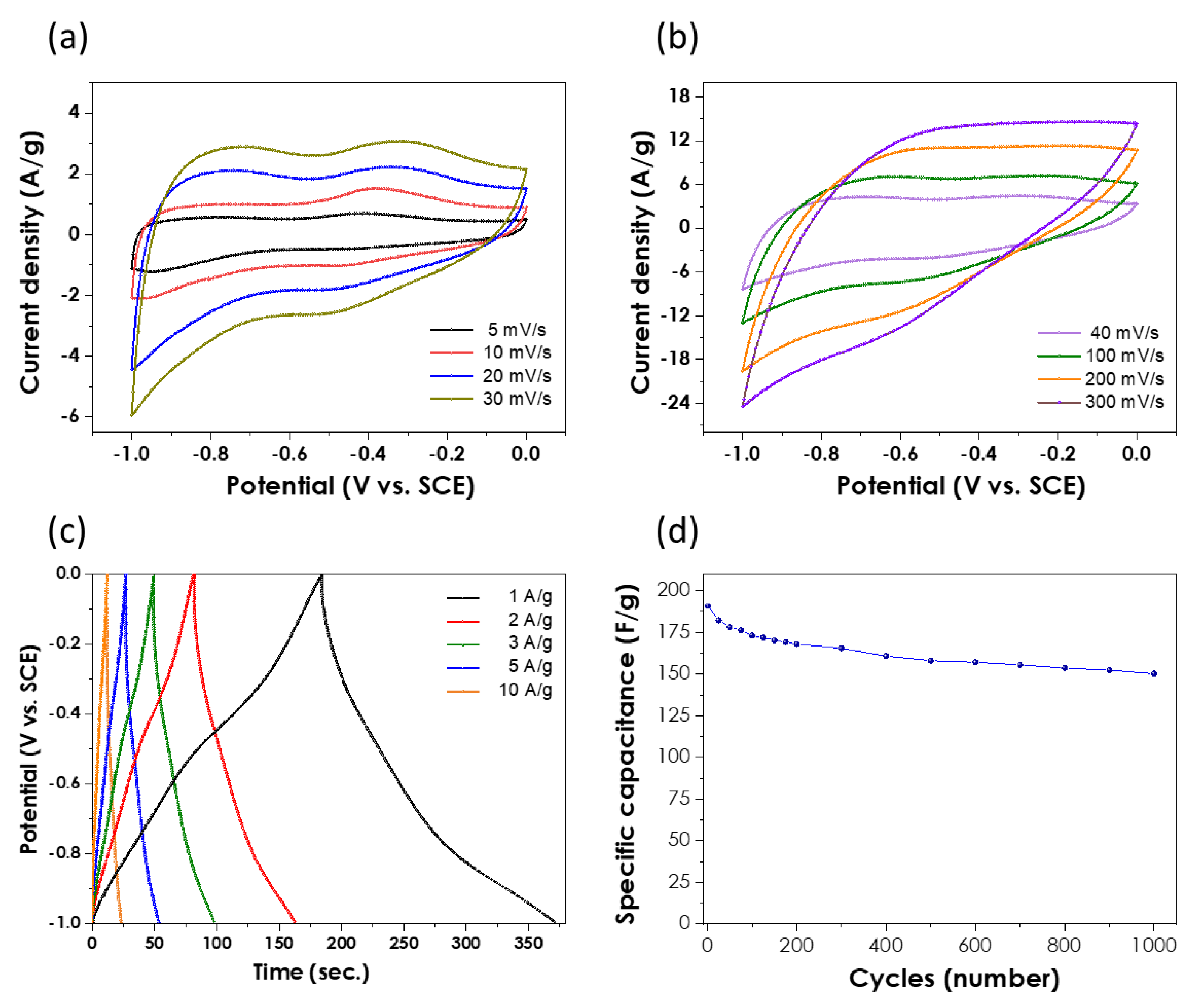
3. Results and Discussion
2FeIIO + SO32− ↔ (FeIIIO) + SO32−(FeIIIO) + 2e−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhao, X.S. On the Configuration of Supercapacitors for Maximizing Electrochemical Performance. Chem. Sus. Chem. 2021, 5, 818–841. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Yao, B.; Liu, T.; Li, Y. Recent advances in chemical methods for activating carbon and metal oxide based electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 17151–17173. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.-I.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An Overview of the Applications of Graphene-Based Materials in Supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. Energy Chem. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Wang, K.; Bi, R.; Huang, M.; Lv, B.; Wang, H.; Li, C.; Wu, H.; Zhang, Q. Porous Cobalt Metal–Organic Frameworks as Active Elements in Battery–Supercapacitor Hybrid Devices. Inorg. Chem. 2020, 59, 6808–6814. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Mahmood, Q.; Park, H.S. Surface functional groups of carbon nanotubes to manipulate capacitive behaviors. Nanoscale 2013, 5, 12304–12309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.C. Carbon Nanotubes for Photoconversion and Electrical Energy Storage. Chem. Rev. 2010, 110, 6856–6872. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Ji, X.; Chen, Q.; Banks, C.E. Electrochemical capacitors utilising transition metal oxides: An update of recent developments. RSC Adv. 2011, 1, 1171–1178. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yuen, M.-F.; Zhang, J.; Li, Y.; Chen, X.; Zhang, W. Hierarchical composite electrodes of nickel oxide nanoflake 3D graphene for high-performance pseudocapacitors. Adv. Funct. Mater. 2014, 24, 6372–6380. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous Transition Metal Oxides for Supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, H.-K.; Kim, M.-S.; Roh, K.C.; Kim, K.-B. A study of the effects of synthesis conditions on Li5FeO4/carbon nanotube composites. Sci. Rep. 2017, 7, 46530. [Google Scholar] [CrossRef]
- Beyer, H.; Meini, S.; Tsiouvaras, N.; Piana, M.; Gasteiger, H.A. Thermal and electrochemical decomposition of lithium peroxide in non-catalyzed carbon cathodes for Li-air batteries. Phys. Chem. Chem. Phys. 2013, 15, 11025–11037. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Ryu, S.; Brus, L.E.; Heinz, T.F. Probing the Intrinsic Properties of Exfoliated Graphene: Raman Spectroscopy of Free-Standing Monolayers. Nano Lett. 2009, 9, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Sinan, N.; Unur, E. Fe3O4/carbon nanocomposite: Investigation of capacitive & magnetic properties for supercapacitor applications. Mater. Chem. Phys. 2016, 183, 571–579. [Google Scholar]
- Yu, R.; Jiang, C.F.; Chu, W.; Ran, M.F.; Sun, W.J. Decoration of CNTs’ surface by Fe3O4 nanoparticles: Influ-ence of ultrasonication time on the magnetic and structural properties. Chin. Chem. Lett. 2017, 28, 302–306. [Google Scholar] [CrossRef]
- Guan, D.; Gao, Z.; Yang, W.; Wang, J.; Yuan, Y.; Wang, B.; Zhang, M.; Liu, L. Hydrothermal synthesis of carbon nanotube/cubic Fe3O4 nanocomposite for enhanced performance supercapacitor electrode material. Mater. Sci. Eng. B 2013, 178, 736–743. [Google Scholar] [CrossRef]
- Kim, H.-K.; Roh, K.C.; Kim, K.-B. In Situ Electrochemical dilatometric study of Fe3O4/Reduced graphene oxide nanocomposites as anode material for lithium ion batteries. J. Electrochem. Soc. 2015, 162, A2308. [Google Scholar] [CrossRef]
- Su, J.; Cao, M.; Ren, L.; Hu, C. Fe3O4–Graphene Nanocomposites with Improved Lithium Storage and Magnetism Properties. J. Phys. Chem. C 2011, 115, 14469–14477. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, G.; Zhang, H.; Li, S. A Facile Route to Controllable Synthesis of Fe3O4/Graphene Composites and Their Application in Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2014, 9, 46–60. [Google Scholar]
- Ghasemi, S.; Ahmadi, F. Effect of surfactant on the electrochemical performanceof graphene/iron oxide electrode for supercapacitor. J. Power Sources 2015, 289, 129–137. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.J. Roles of nanosized Fe3O4 on supercapacitive properties of carbon nanotubes. Curr. Appl. Phys. 2011, 11, 462–466. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Sure, J.; Vishnu, D.S.M.; Jo, S.J.; Lee, W.C.; Ahmad, I.A.; Kim, H.-K. Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications. Energies 2021, 14, 2908. https://doi.org/10.3390/en14102908
Park SK, Sure J, Vishnu DSM, Jo SJ, Lee WC, Ahmad IA, Kim H-K. Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications. Energies. 2021; 14(10):2908. https://doi.org/10.3390/en14102908
Chicago/Turabian StylePark, Sul Ki, Jagadeesh Sure, D. Sri Maha Vishnu, Seong Jun Jo, Woo Cheol Lee, Ibrahim A. Ahmad, and Hyun-Kyung Kim. 2021. "Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications" Energies 14, no. 10: 2908. https://doi.org/10.3390/en14102908
APA StylePark, S. K., Sure, J., Vishnu, D. S. M., Jo, S. J., Lee, W. C., Ahmad, I. A., & Kim, H.-K. (2021). Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications. Energies, 14(10), 2908. https://doi.org/10.3390/en14102908






