Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales
Abstract
:1. Introduction
2. Materials and Methods
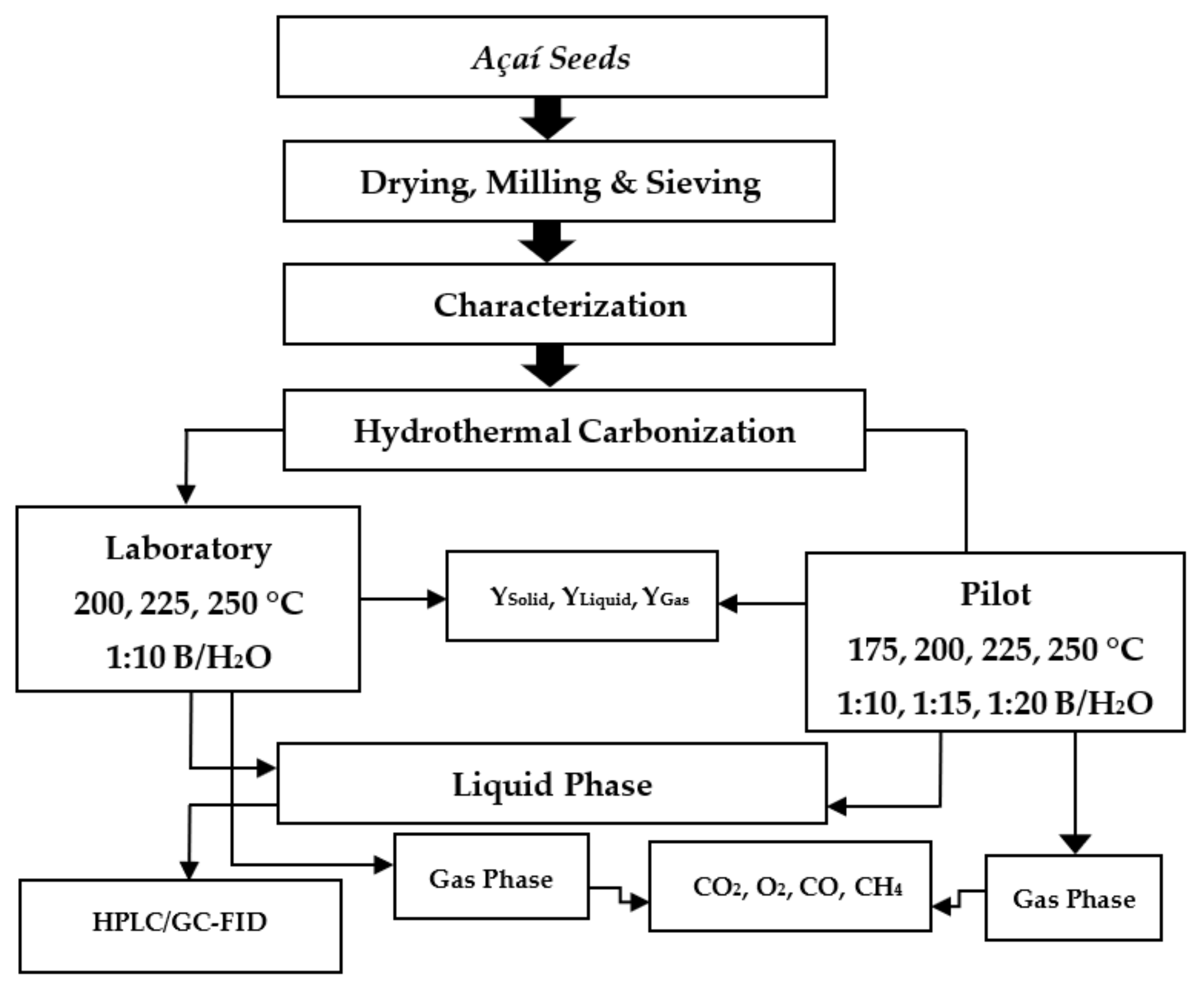
2.1. Methodology
2.2. Materials, Pre-Treatment, and Characterization of Açaí Seeds in Nature
2.3. Experimental Apparatus and Procedures
2.3.1. Experimental Apparatus and Procedures in Pilot Scale
2.3.2. Experimental Apparatus and Procedures in Laboratory Scale
2.4. Compositional Analysis of Reaction Products
2.4.1. Aqueous Phase
2.4.2. Gaseous Phase
2.5. Steady-State Material Balance by Hydrothermal Carbonization
3. Results
3.1. Hydrothermal Processing of Açaí Seeds
3.1.1. Material Balances, Operating Conditions, and Yields of Reaction Products
Influence of Temperature on the Yields of Reaction Products
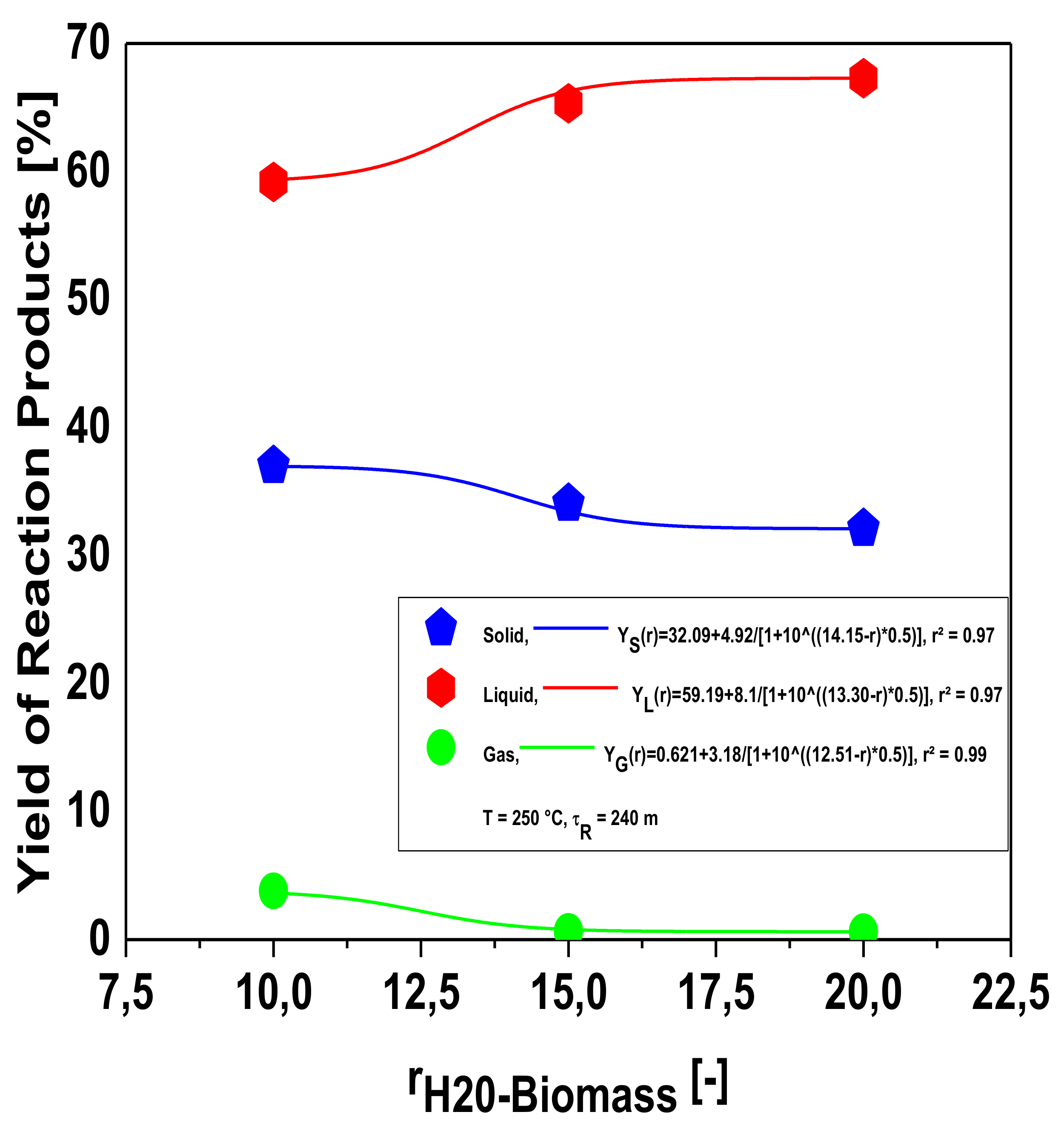
Influence of Biomass-to-Water Ratio on the Yields of Reaction Products
3.1.2. Chemical Composition of Gas Reaction Products
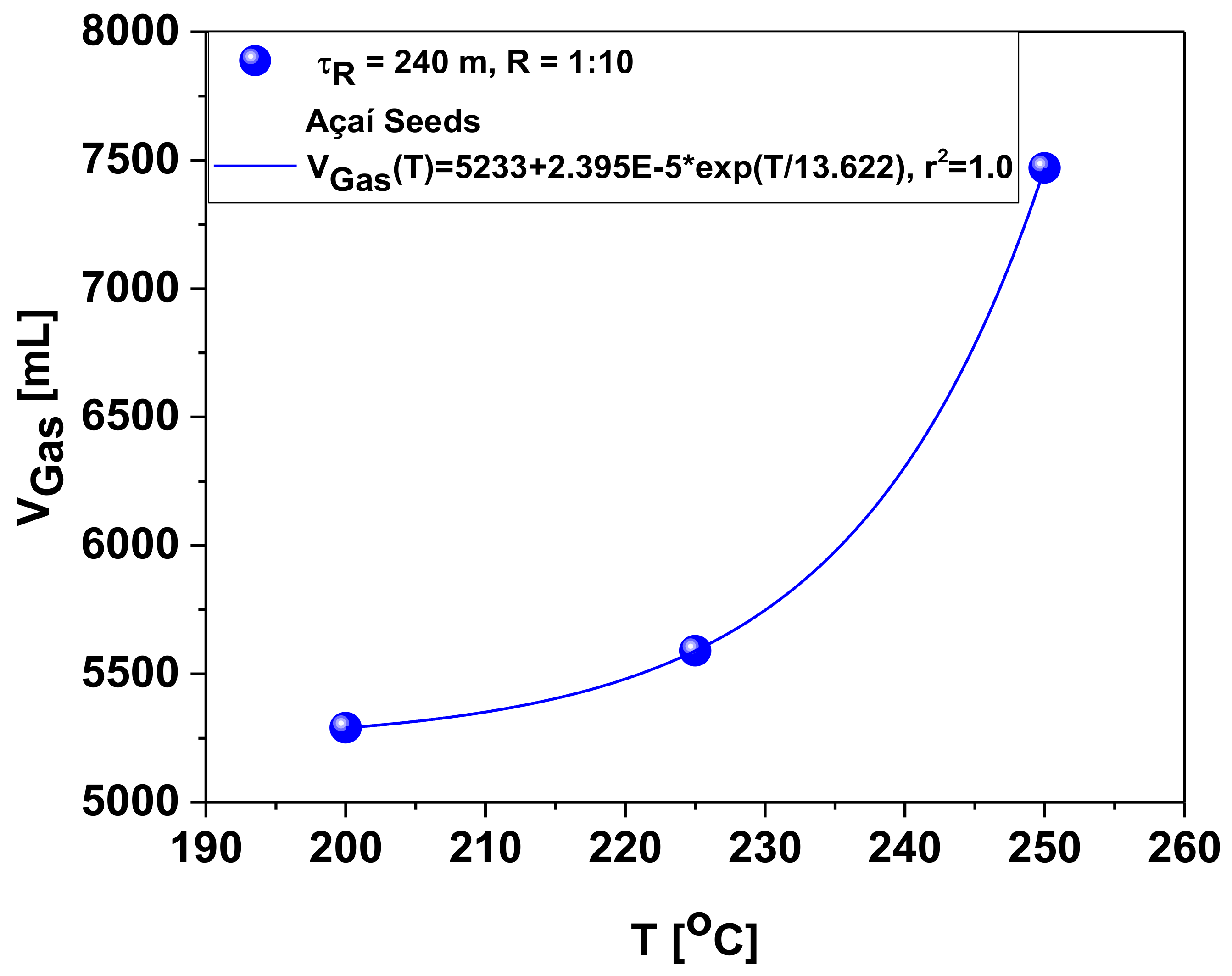
Influence of Temperature on the Chemical Composition of Gas Reaction Products
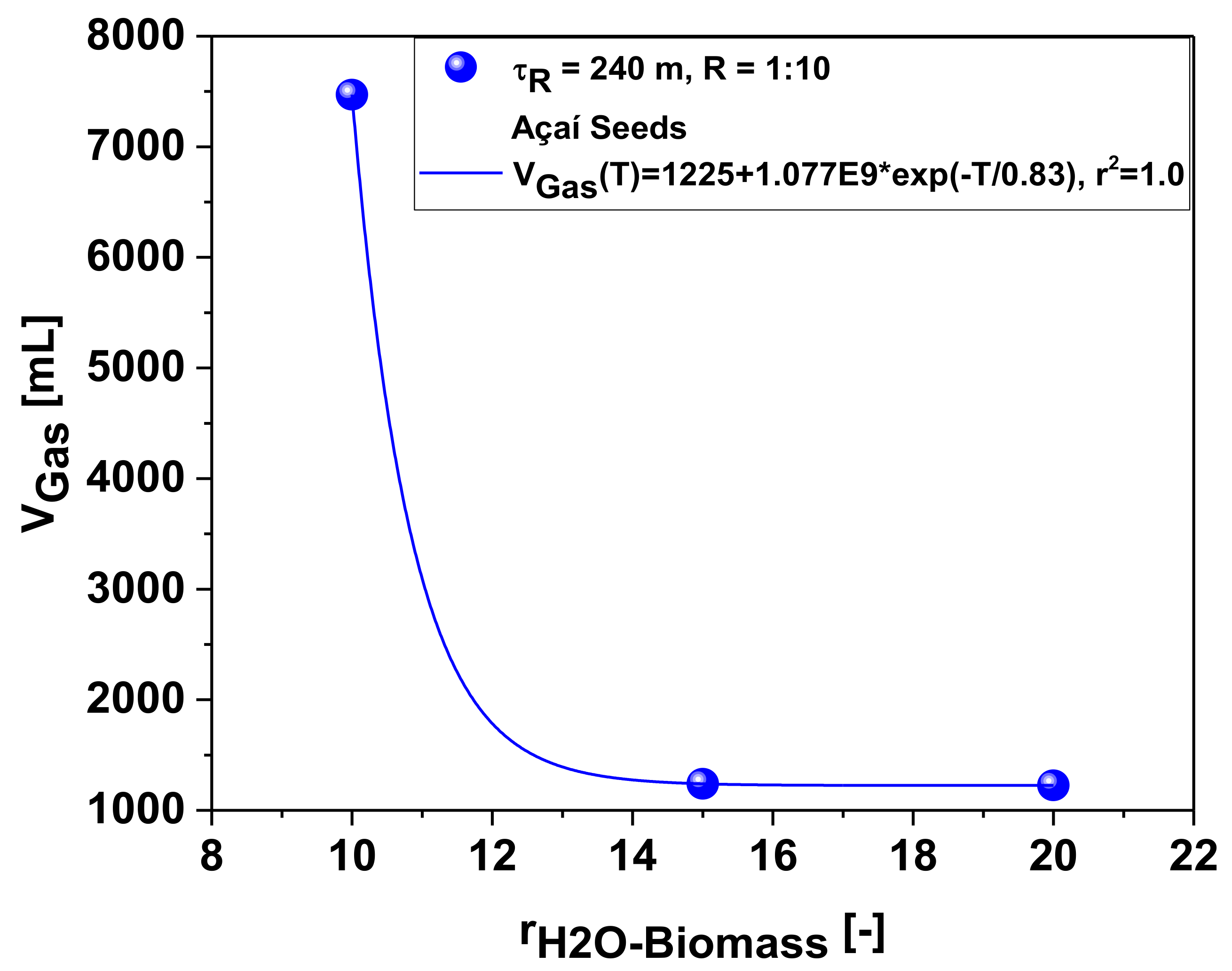
Influence of H2O-to-Biomass Ratio on the Volume of Gas Reaction Products
3.1.3. Chemical Composition of Organic Compounds in the Aqueous Phase
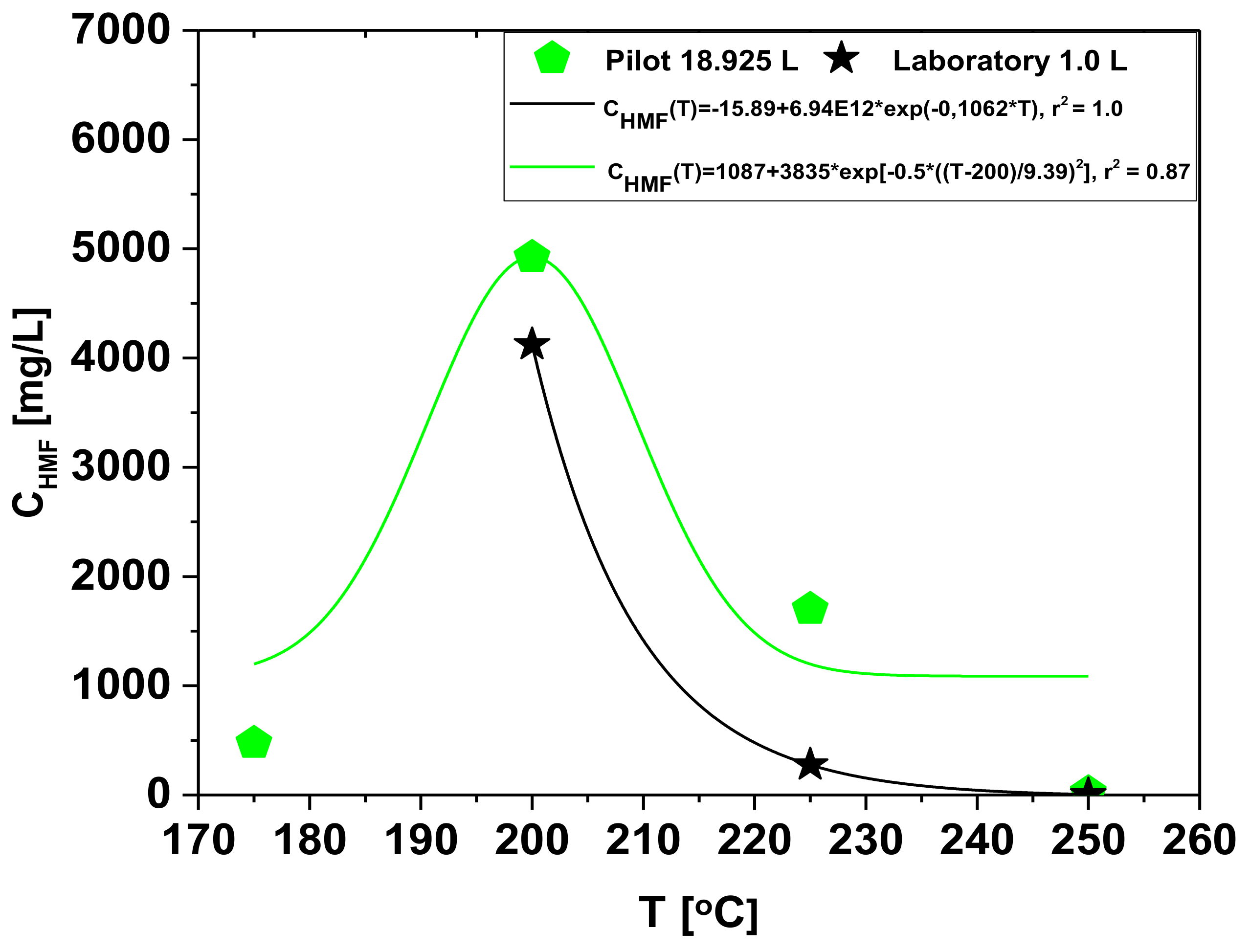
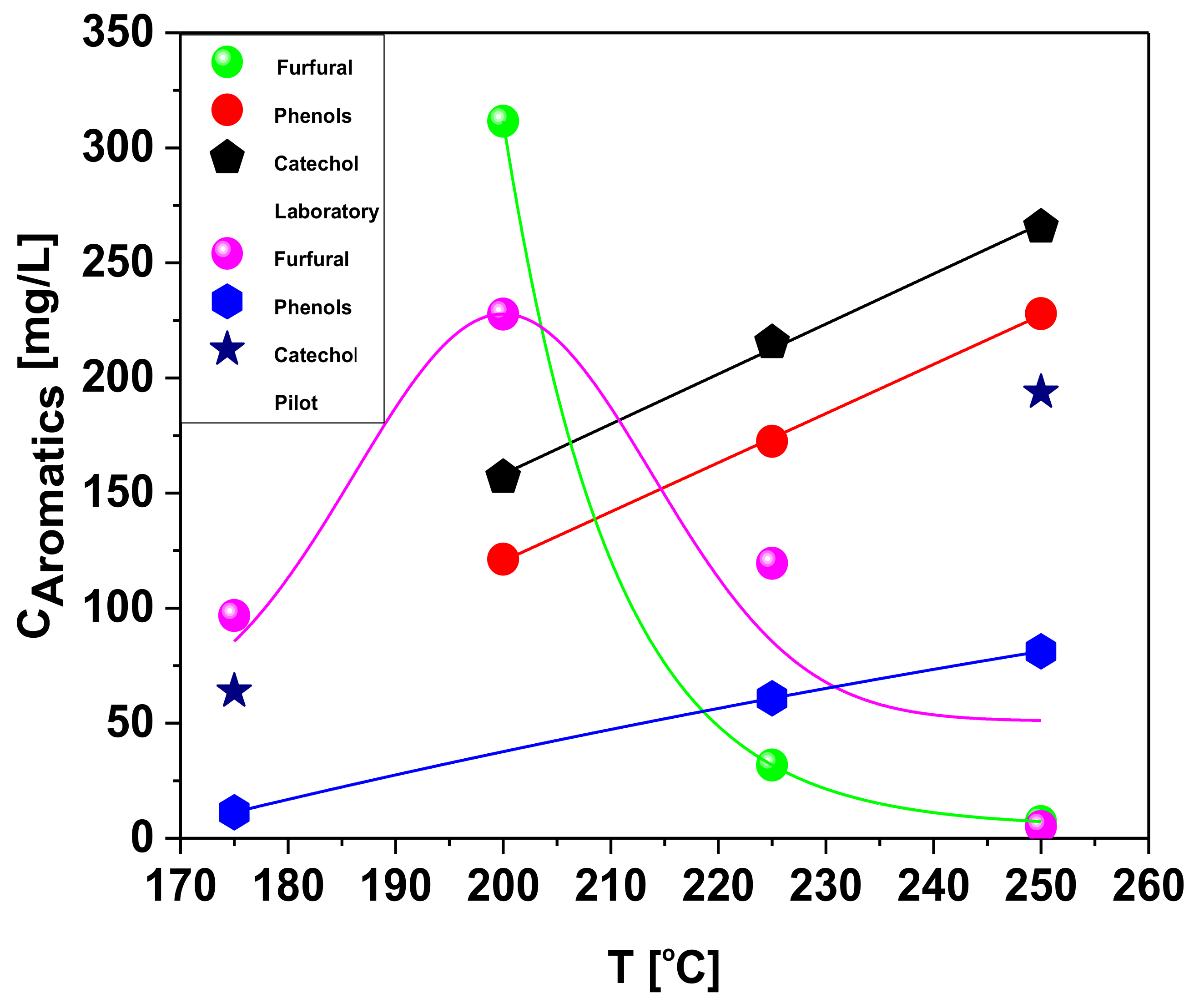
Effect of Temperature on the Chemical Composition of Organic Compounds in the Aqueous Phase
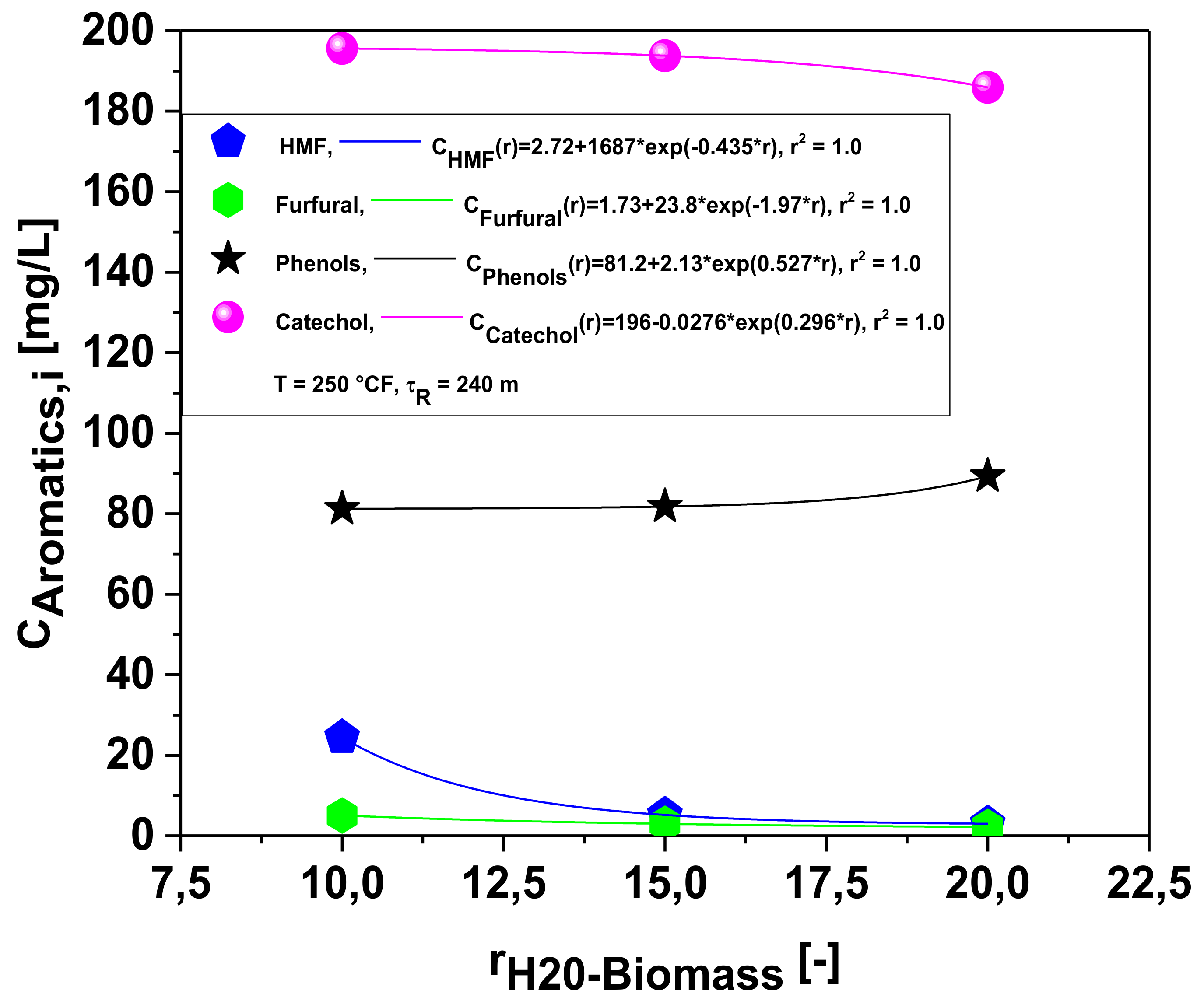
Effect of Biomass-to-Water Ratio on the Chemical Composition of Organic Compounds in the Aqueous Phase
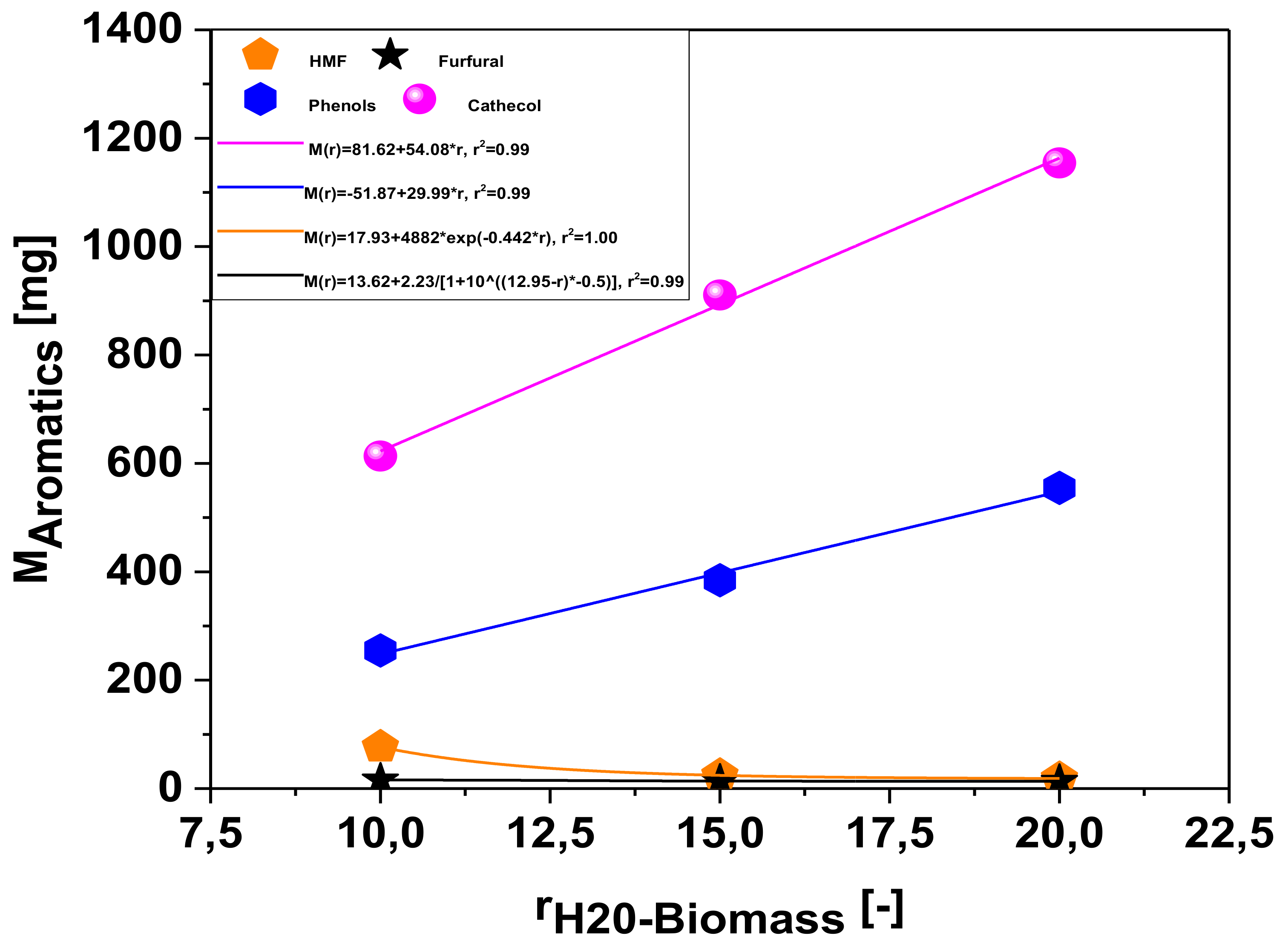
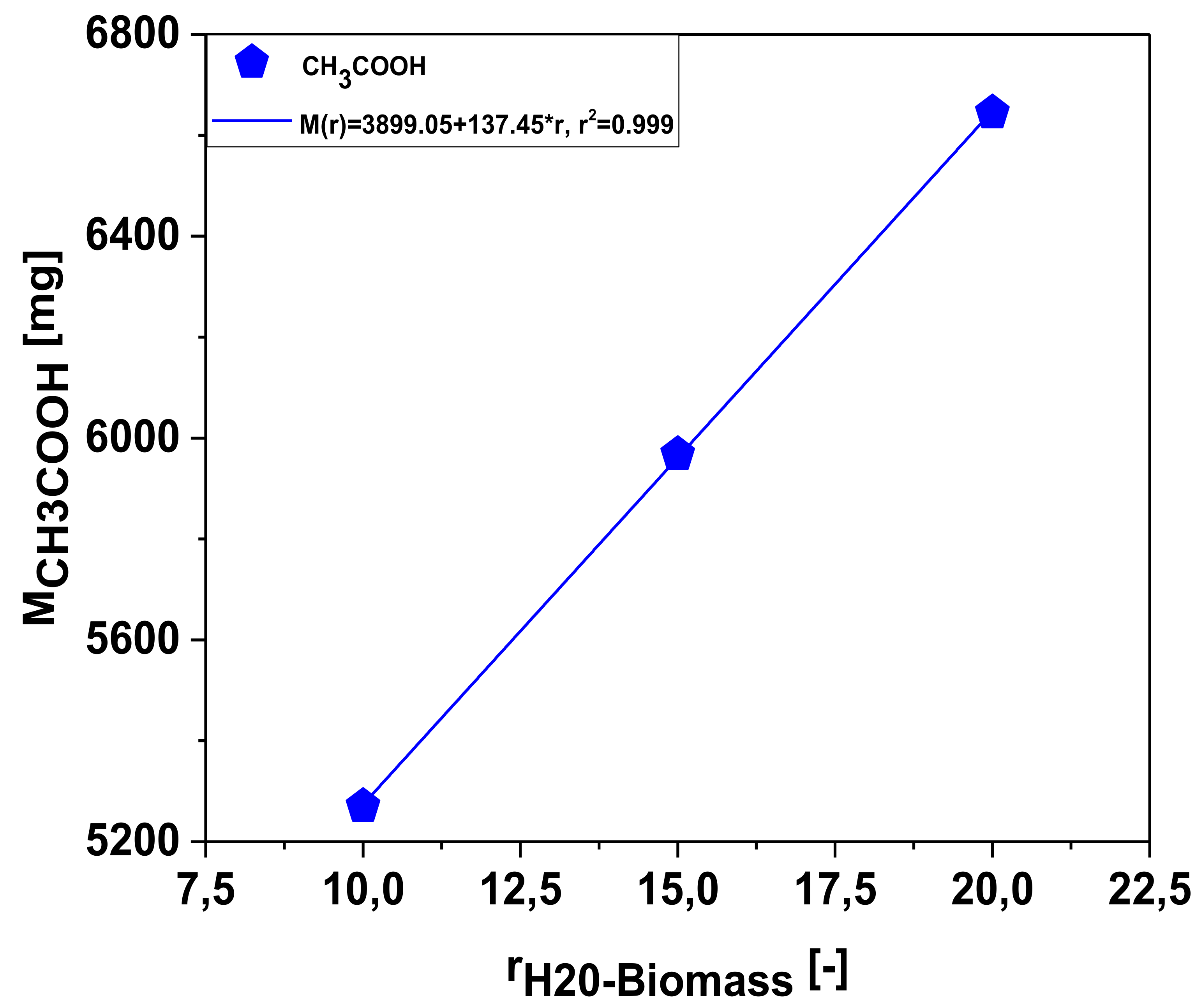
Effect of Biomass-to-Water Ratio on the Mass Production of Chemicals in the Aqueous Phase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindolfo, M.M.; De Matos, G.S.B.; Pereira, W.V.D.S.; Fernandes, A.R. Productivity and nutrition of fertigated açaí palms according to boron fertilization. Rev. Bras. Frutic. 2020, 42, e601. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea. Mart.)—A phytochemical and pharmacologicalassessment of the species’ health claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; van Damme, P. Effect of a health claim and personal characteristics on consumer acceptance offruit juices with different concentrations of açaí (Euterpe oleracea Mart.). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Del Pozo-Insfran, D.; Brenes, C.H.; Brenes, C.H.; Talcott, S.T.; Talcott, S.T. Phytochemical Composition and Pigment Stability of Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef]
- Pessoa, J.D.C.; Silva, P.V.D.S.E. Effect of temperature and storage on açaí (Euterpe oleracea) fruit water uptake: Simulation of fruit transportation and pre-processing. Fruits 2007, 62, 295–302. [Google Scholar] [CrossRef]
- Pompeu, D.; da Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.F.D.; de Almeida, M.D.C.; da Silva, J.A.P.; Araújo, L.L.; Cardozo, N.S.M.; Santana, R.M.C. Thermal treatment of açaí (Euterpe oleracea) fiber for composite reinforcement. Polímeros 2020, 30, e2020003. [Google Scholar] [CrossRef]
- Bufalino, L.; Guimaraes, A.A.; de Silva, B.M.; de Souza, R.L.F.; de Melo, I.C.N.A.; de Oliveira, D.N.P.S.; Trugilho, P.F. Local variability of yield and physical properties of açaí waste and improvement of its energetic attributes by separation of lignocellulosic fibers and seeds. J. Renew. Sustain. Energy 2018, 10, 053102. [Google Scholar] [CrossRef]
- De Lima, A.C.P.; Bastos, D.L.R.; Camarena, M.A.; Bon, E.P.S.; Cammarota, M.C.; Teixeira, R.S.S.; Gutarra, M.L.E. Physicochemical characterization of residual biomass (seed and fiber) from açaí (Euterpe oleracea) processing and assessment of the potential for energy production and bioproducts. Biomass Convers. Biorefin. 2019, 11, 925–935. [Google Scholar] [CrossRef]
- Pessoa, J.D.C.; Arduin, M.; Martins, M.A.; De Carvalho, J.E.U. Characterization of açaí (E. oleracea) fruits and its processing residues. Braz. Arch. Biol. Technol. 2010, 53, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.D.M.; Rebelo, V.S.M.; Martorano, L.G.; Giacon, V.M. Caracterização de partículas de açaí visando seu potencial uso na construção civil. Matéria 2019, 24. [Google Scholar] [CrossRef]
- De Castro, D.A.R.; Ribeiro, H.J.D.S.; Ferreira, C.C.; Cordeiro, M.D.A.; Guerreiro, L.H.H.; Pereira, A.M.; dos Santos, W.; Santos, M.C.; De Carvalho, F.B.; Silva, J.O.C., Jr.; et al. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. Fractionation 2019. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Flora, J.R.; Caicedo, J.M.; Berge, N.D. Investigating the role of feedstock properties and process con-ditions on products formed during the hydrothermal carbonization of organics using regression techniques. Bioresour. Technol. 2015, 187, 263–274. [Google Scholar] [CrossRef]
- Machado, N.; de Castro, D.; Santos, M.; Araújo, M.; Lüder, U.; Herklotz, L.; Werner, M.; Mumme, J.; Hoffmann, T. Process analysis of hydrothermal carbonization of corn Stover with subcritical H2O. J. Supercrit. Fluids 2018, 136, 110–122. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Balasubramanian, R. Hydrothermal carbonization of waste biomass for energy generation. Procedia Environ. Sci. 2012, 16, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin. 2012, 3, 113–126. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Teri, G.; Luo, L.; Savage, P. Hydrothermal Treatment of Protein, Polysaccharide, and Lipids Alone and in Mixtures. Energy Fuels 2014, 28, 7501–7509. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Masson, E.; Celzard, A.; Fierro, V. Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment. Ind. Crop. Prod. 2018, 124, 919–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, W.; Guo, H.; Shi, S.; Dai, J. Preparation and Characterization of Carbon Microspheres From Waste Cotton Textiles By Hydrothermal Carbonization. J. Renew. Mater. 2019, 7, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, I.; Irmak, S.; Hesenov, A.; Erbatur, O. Hydrolysis of kenaf (Hibiscus cannabinus L.) stems by catalytical thermal treatment in subcritical water. Biomass Bioenergy 2010, 34, 1578–1585. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densi-fying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal car-bonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Murillo, A.; Roman, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.; Schendel, F.J.; Von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biomass Bioenergy 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Oktaviananda, C.; Rahmawati, R.F.; Prasetya, A.; Purnomo, C.W.; Yuliansyah, A.T.; Cahyono, R.B. Effect of temperature and biomass-water ratio to yield and product characteristics of hydrothermal treatment of biomass. In Proceedings of the AIP Conference Proceedings, Bydgoszcz, Poland, 9–11 May 2018; AIP Publishing Center: Yogyakarta, Indonesia, 2017; p. 020029. [Google Scholar] [CrossRef] [Green Version]
- Putra, H.; Damanhuri, E.; Dewi, K.; Pasek, A.D. Hydrothermal carbonization of biomass waste under low temperature condition. In Proceedings of the 2nd International Conference on Engineering and Technology for Sustainable Development (ICET4SD 2017), Yogyakarta, Indonesia, 13–14 September 2017; Volume 154, p. 01025. [Google Scholar] [CrossRef] [Green Version]
- Castello, D.; Kruse, A.; Fiori, L. Biomass gasification in supercritical and subcritical water: The effect of the reactor material. Chem. Eng. J. 2013, 228, 535–544. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Axel, F.; Felix, R.; Andrea, K. Experimental comparison of hydrothermal and vapothermal carbonization. Fuel Process. Technol. 2013, 115, 261–269. [Google Scholar]
- Zhang, B.; Huang, H.J.; Ramaswamy, S. Reaction kinetics of the hydrothermal treatment of lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef]
- Li, L.; Diederick, R.; Flora, J.R.; Berge, N.D. Hydrothermal carbonization of food waste and associated packaging materials for energy source generation. Waste Manag. 2013, 33, 2478–2492. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammsan, B.; Worasuwannarak, N. Effects of temperature and holding time during tor-refaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Becker, R.; Dorgerloh, U.; Paulke, E.; Mumme, J.; Nehls, I. Hydrothermal Carbonization of Biomass: Major Organic Components of the Aqueous Phase. Chem. Eng. Technol. 2014, 37, 511–518. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lueder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Dorgerloh, U.; Helmis, M.; Mumme, J.; Diakité, M.; Nehls, I. Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour. Technol. 2013, 130, 621–628. [Google Scholar] [CrossRef]
- Jung, D.; Zimmermann, M.; Kruse, A. Hydrothermal Carbonization of Fructose: Growth Mechanism and Kinetic Model. ACS Sustain. Chem. Eng. 2018, 6, 13877–13887. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Koehler, R.; Kopinke, F.D. Hydrothermal Carbonization of Glucose, Fructose, and Xylose-Identification of Organic Products with Medium Molecular Masses. ACS Sustainable Chem. Eng. 2017, 5, 6420–6428. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Takigawa, M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Mechanism and Kinetics of Cellobiose Decomposition in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 1998, 37, 357–361. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.; Komesu, A.; Maciel Filho, R. Hydrothermal Pretreatment for Enhancing Enzymatic Hydrolysis of Seeds of Açaí (Euterpe oleracea) and Sugar Recovery. Chem. Eng. Trans. 2014, 37, 785–792. [Google Scholar]
- Bauer, S.K.; Cheng, F.; Colosi, L.M. Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery. Energies 2019, 12, 729. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Toor, S.; Nielsen, A.; Pedersen, T.; Rosendahl, L. Bio-Crude Production through Recycling of Pretreated Aqueous Phase via Activated Carbon. Energies 2021, 14, 3488. [Google Scholar] [CrossRef]
- Standards, T. Acid-Insoluble Lignin in Wood and Pulp Tappi Method T 222 Om-06; Tappi Press: Atlanta, GA, USA, 2006. [Google Scholar]
- Buffiere, P.; Loisel, D.; Van Soest, P.J. Dosage des Fibres; Inra-Lbe Laboratoire De Biotechnologie De L’environnement: Narbonne, France, 2007; pp. 1–14. [Google Scholar]








| Temperature (°C) | ||||
|---|---|---|---|---|
| Process Parameters | 175 | 200 | 225 | 250 |
| Mass of Açaí Seeds [g] | 300.00 | 299.82 | 299.98 | 300.16 |
| Mass of H2O [g] | 2997.60 | 3000.20 | 3001.30 | 2999.90 |
| Mechanical Stirrer Speed [rpm] | 90 | 90 | 90 | 90 |
| Initial Temperature [°C] | 30 | 30 | 30 | 30 |
| Heating Rate [°C/min] | 2 | 2 | 2 | 2 |
| Process Time [min] | 240 | 240 | 240 | 240 |
| Mass of Slurry [g] | 3252.20 | 3240.20 | 3216.50 | 3167.40 |
| Volume of Gas [mL], T = 25 °C, P = 1 atm | 0 | 5290 | 5590 | 7470 |
| Mass of Gas [g] | 0 | 7.564 | 8.231 | 11.408 |
| Process Loss (I) [g] | 45.40 | 59.82 | 84.78 | 132.66 |
| Input Mass of Slurry (Pressing) [g] | 3252.20 | 3240.20 | 3216.50 | 3161.70 |
| Process Loss (II) [g] | 0.00 | 0.00 | 0.00 | 5.70 |
| Mass of Liquid Phase [g] | 2638.53 | 2615.56 | 2637.97 | 2556.96 |
| Mass of Moist Hydro-char [g] | 588.10 | 587.37 | 557.61 | 591.29 |
| Process Loss (III) [g] | 25.57 | 37.27 | 20.92 | 13.41 |
| Mass of Dry Hydro-char [g] | 160.16 | 118.53 | 113.052 | 111.092 |
| (Mass of Liquid Phase + Σ Process Loss + Mass of Moist Hydro-char − Mass of Dry Hydro-char − Mass of Gas) [g] | 3137.44 | 3173.926 | 3179.997 | 3177.52 |
| Process Loss (I + II + III) [g] | 70.97 | 97.09 | 105.70 | 151.77 |
| Mass of LiquidReaction [g] | 139.84 | 173.726 | 178.697 | 177.62 |
| Yield of Hydro-char [wt.%] | 53.39 | 39.534 | 37.686 | 37.011 |
| Yield of Liquid Phase [wt.%] | 46.61 | 57.943 | 59.570 | 59.188 |
| Yield of Gas [wt.%] | 0.000 | 2.523 | 2.744 | 3.801 |
| Decomposition | |||||||
|---|---|---|---|---|---|---|---|
| Centesimal Composition | [wt.%] | Cellulose [wt.%] | Lignin [wt.%] | Hemi-Cellulose [wt.%] | Proteins [wt.%] | Lipids [wt.%] | Fibers [wt.%] |
| 61.25 | 15.00 | 65.00 | 72.00 | 5.00 | 50.00 | ||
| Açaí Seeds [12] | - | - | - | - | - | - | |
| Cellulose | 40.29 | 15.6124 | - | - | - | - | - |
| Lignin | 4.00 | - | 3.40 | - | - | - | - |
| Hemi-cellulose | 5.50 | - | - | 1.925 | - | ||
| Proteins | 6.25 | - | - | - | 1.82 | - | |
| Lipids | 0.61 | - | - | - | - | 0.5795 | - |
| Fibers | 29.79 | - | - | - | - | - | 14.895 |
| Moisture | 10.15 | - | - | - | - | - | - |
| Volatile matter | 0.50 | - | - | - | - | - | - |
| Fixed carbon | 0.83 | - | - | - | - | - | - |
| Ash | 0.15 | - | - | - | - | - | - |
| YHydro-char/Seeds [wt.%] | 37.287 | 15.6124 | 3.400 | 1.925 | 1.820 | 0.5795 | 14.895 |
| Açaí Fibers [7] | - | - | - | - | - | - | |
| Cellulose | 41.37 | 16.080 | - | - | - | - | - |
| Lignin | 40.25 | - | 34.213 | - | - | - | - |
| Hemi-cellulose | 11.54 | - | - | 4.039 | - | - | - |
| Moisture | 8.88 | - | - | - | - | - | - |
| Ash | 1.96 | - | - | - | - | - | - |
| YHydro-char/Fibers [wt.%] | 16.170 | 4.775 | 10.192 | 1.203 | - | - | - |
| Temperature [°C] | |||
|---|---|---|---|
| Process Parameters | 200 | 225 | 250 |
| Mass of Açaí Seeds [g] | 73.50 | 73.50 | 73.50 |
| Moisture Content of Açaí Seeds [wt.%] | 25.23 | 25.23 | 25.23 |
| Mass of H2O in Açaí Seeds [g] | 18.54 | 18.54 | 18.54 |
| Mass of H2O [g] | 531.01 | 531.01 | 531.01 |
| Mechanical Stirrer Speed [rpm] | 90 | 90 | 90 |
| Initial Temperature [°C] | 30 | 30 | 30 |
| Heating Rate [°C/min] | 2 | 2 | 2 |
| Process Time [min] | 240 | 240 | 240 |
| Volume of Gas [mL], T = 25 °C, P = 1 atm | 1395 | 2475 | 3215 |
| CO2 [vol.%] | 48.60 | 44.80 | 35.80 |
| CH4 [vol.%] | 1.90 | 1.20 | 0.60 |
| O2 [vol.%] | 9.20 | 9.90 | 12.10 |
| 100 − Σ (CO2 + CH4 + O2) [vol.%] | 40.30 | 44.10 | 51.50 |
| Mass of Gas [g] | 1.94 | 3.57 | 4.70 |
| Mass of Dry Hydro-char [g] | 30.77 | 29.43 | 28.16 |
| Mass of LiquidReaction [g] | 34.72 | 36.01 | 36.59 |
| Yield of Hydro-char [wt.%] | 41.86 | 40.04 | 38.31 |
| Yield of Liquid Phase [wt.%] | 47.24 | 48.99 | 49.78 |
| Yield of Gas [wt.%] | 2.64 | 4.86 | 6.39 |
| 250 °C | |||
|---|---|---|---|
| Biomass/H2O [-] | |||
| Process Parameters | 1:10 | 1:15 | 1:20 |
| Mass of Açaí Seeds [g] | 300.16 | 300.28 | 300.07 |
| Mass of H2O [g] | 2999.90 | 4502.90 | 6000.80 |
| Mechanical Stirrer Speed [rpm] | 90 | 90 | 90 |
| Initial Temperature [°C] | 30 | 30 | 30 |
| Heating Rate [°C/min] | 2 | 2 | 2 |
| Process Time [min] | 240 | 240 | 240 |
| Mass of Slurry [g] | 3167.40 | 4696.50 | 6217.40 |
| Volume of Gas [mL], T = 25 °C, P = 1 atm | 7470 | 1240 | 1225 |
| Mass of Gas [g] | 11.408 | 1.905 | 1.863 |
| Process Loss (I) [g] | 132.66 | 106.68 | 83.47 |
| Input Mass of Slurry (Pressing) [g] | 3161.70 | 4696.50 | 6209.30 |
| Process Loss (II) [g] | 5.70 | 0.00 | 8.10 |
| Mass of Liquid Phase [g] | 2556.96 | 4077.05 | 5663.60 |
| Mass of Moist Hydro-char [g] | 591.29 | 585.83 | 518.45 |
| Process Loss (III) [g] | 13.41 | 33.62 | 35.35 |
| Mass of Dry Hydro-char [g] | 111.092 | 102.25 | 96.302 |
| (Mass of Liquid Phase + Σ Process Loss + Mass of Moist Hydro-char − Mass of Dry Hydro-char − Mass of Gas) [g] | 3177.52 | 5000.795 | 6210.805 |
| Process Loss (I + II + III) [g] | 151.77 | 140.30 | 126.92 |
| Mass of LiquidReaction [g] | 177.62 | 196.125 | 202.535 |
| Yield of Hydro-char [wt.%] | 37.011 | 34.051 | 32.093 |
| Yield of Liquid Phase [wt.%] | 59.188 | 65.315 | 67.286 |
| Yield of Gas [wt.%] | 3.801 | 0.634 | 0.621 |
| Temperature [°C] | ||||
|---|---|---|---|---|
| Composition [vol.%] | 175 | 200 | 200 | 200 |
| CO2 [vol.%] | 0.00 | 44.40 | 51.30 | 60.60 |
| CH4 [vol.%] | 0.00 | 0.40 | 0.50 | 1.30 |
| O2 [vol.%] | 0.00 | 1.0 | 0.30 | 0.00 |
| 100 − Σ (CO2 + CH4 + O2) [vol.%] | 0.00 | 54.20 | 47.90 | 38.10 |
| Volume of Gas [mL] | 0.00 | 5290 | 5590 | 7470 |
| Volume of CO2 [mL] | 0.00 | 2348.76 | 2867.67 | 4526.82 |
| Volume of CH4 [mL] | 0.00 | 21.16 | 27.95 | 97.11 |
| Volume of O2 [mL] | 0.00 | 52.90 | 16.77 | 0.00 |
| V100 − Σ (CO2 + CH4 + O2) ≈ VCO [mL] | 0.00 | 2867.18 | 2677.61 | 2846.07 |
| Mass of Gas[g] | 0.00 | 7.564 | 8.231 | 11.408 |
| Mass of CO2 [g] | 0.00 | 4.191 | 5.117 | 8.078 |
| Mass of CH4 [g] | 0.00 | 0.014 | 0.018 | 0.063 |
| Mass of O2 [g] | 0.00 | 0.068 | 0.022 | 0.000 |
| Mass of CO [g] | 0.00 | 3.291 | 3.074 | 3.267 |
| Composition of Gas [mol.%] | ||||
| YCO2 | 0.00 | 0.441410 | 0.510306 | 0.603507 |
| YCH4 | 0.00 | 0.004055 | 0.004936 | 0.012943 |
| YO2 | 0.00 | 0.009848 | 0.003017 | 0.000000 |
| Y100 − Σ (CO2 + CH4 + O2) ≈ YCO | 0.00 | 0.544687 | 0.481741 | 0.383550 |
| 250 °C | |||
|---|---|---|---|
| Biomass/H2O [-] | |||
| Composition [vol.%] | 1:10 | 1:15 | 1:20 |
| CO2 [vol.%] | 60.60 | 63.40 | 59.90 |
| CH4 [vol.%] | 1.30 | 3.10 | 1.60 |
| O2 [vol.%] | 0.00 | 0.00 | 0.00 |
| Σ (CO2 + CH4 + O2) | 61.90 | 65.50 | 61.50 |
| 100 − Σ (CO2 + CH4 + O2) [vol.%] | 38.10 | 33.50 | 38.50 |
| Volume of Gas [mL] | - | - | - |
| Volume of CO2 [mL] | 4526.82 | 786.16 | 733.775 |
| Volume of CH4 [mL] | 97.11 | 38.44 | 19.60 |
| Volume of O2 [mL] | 0.00 | 00.00 | 00.00 |
| V100 − Σ (CO2 + CH4 + O2) ≈ VCO [mL] | 2846.07 | 415.40 | 471.625 |
| Temperature [°C] | ||||
|---|---|---|---|---|
| Pilot | ||||
| Concentration of aromatics [mg/L] | 175 | 200 | 225 | 250 |
| HMF: CAS: 67-47-0 | 474.8 | 4923 | 1701 | 24.45 |
| Furfural: CAS:98-01-1 | 96.83 | 227.8 | 119.5 | 5.05 |
| Phenol: CAS: 108-95-2 | 11.34 | - | 60.81 | 81.24 |
| Catechol: CAS: 120-80-9 | 63.86 | - | - | 193.8 |
| Concentration of carboxylic acids [mg/L] | - | - | - | - |
| Acetic acid: CAS: 64-19-7 | 700 | 1280 | 1410 | 1680 |
| Total acetic acid (HAc) | 773.2 | 1394 | 1540 | 1859 |
| Propionic Acid: CAS: 79-09-4 | 90 | 140 | 160 | 220 |
| Laboratory | ||||
| Concentration of aromatics [mg/L] | - | 200 | 225 | 250 |
| HMF: CAS: 67-47-0 | - | 4123 | 275 | 4.56 |
| Furfural: CAS:98-01-1 | - | 311.5 | 31.82 | 7.28 |
| Phenol: CAS: 108-95-2 | - | 121.2 | 172.5 | 227.99 |
| Catechol: CAS: 120-80-9 | - | 156.84 | 215.4 | 365.61 |
| Concentration of carboxylic acids [mg/L] | - | - | - | - |
| Acetic acid: CAS: 64-19-7 | - | 3420 | 3690 | 3810 |
| Total acetic acid (HAc) | - | 3797 | 3996 | 4286 |
| Propionic Acid: CAS: 79-09-4 | - | 240 | 310 | 410 |
| 250 °C | |||
|---|---|---|---|
| Biomass/H2O [-] | |||
| Concentration of aromatics [mg/L] | 1:10 | 1:15 | 1:20 |
| HMF: CAS: 67-47-0 | 24.450 | 5.188 | 3.002 |
| Furfural: CAS:98-01-1 | 5.054 | 2.972 | 2.194 |
| Phenol: CAS: 108-95-2 | 81.24 | 81.78 | 89.33 |
| Catechol: CAS: 120-80-9 | 195.6 | 193.8 | 185.9 |
| Concentration of carboxylic acids [mg/L] | - | - | - |
| Acetic acid: CAS: 64-19-7 | 1680 | 1270 | 1070 |
| Total acetic acid (HAc) | 1859 | 1424 | 1070 |
| Propionic Acid: CAS: 79-09-4 | 220 | 190 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, C.d.M.S.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.d.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies 2021, 14, 5608. https://doi.org/10.3390/en14185608
da Silva CdMS, de Castro DAR, Santos MC, Almeida HdS, Schultze M, Lüder U, Hoffmann T, Machado NT. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies. 2021; 14(18):5608. https://doi.org/10.3390/en14185608
Chicago/Turabian Styleda Silva, Conceição de Maria Sales, Douglas Alberto Rocha de Castro, Marcelo Costa Santos, Hélio da Silva Almeida, Maja Schultze, Ulf Lüder, Thomas Hoffmann, and Nélio Teixeira Machado. 2021. "Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales" Energies 14, no. 18: 5608. https://doi.org/10.3390/en14185608
APA Styleda Silva, C. d. M. S., de Castro, D. A. R., Santos, M. C., Almeida, H. d. S., Schultze, M., Lüder, U., Hoffmann, T., & Machado, N. T. (2021). Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies, 14(18), 5608. https://doi.org/10.3390/en14185608







