The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatments
2.3. Assessments of Physiological Parameters of Biofertilized Plants
2.4. Proximate Analysis
2.5. Fuel Characteristics
2.5.1. Caloric Value
- Thermogravimetric analysis TG, DTA, TGA TG-FTiR, and TG-MS carbonization process for untreated and carbonized willow combustion;
- Proximate and technical analysis of the willow torrefaction process and final products;
- Gas analysis of torgas formed as a result of carbonization: FTiR TG-MS analysis;
- Fuel analysis (caloric value, ash content, moisture content) of willow carbonization process products;
- SEM-EDS ash analysis of carbonized Salix viminalis L. after combustion.
2.5.2. Ash Characteristics of the Torrefied Salix viminalis L. Biomass
2.6. Analyses of Willow Energy Properties and Torrefied Willow Charactersitics
3. Statistical Analysis
4. Results and Discussion
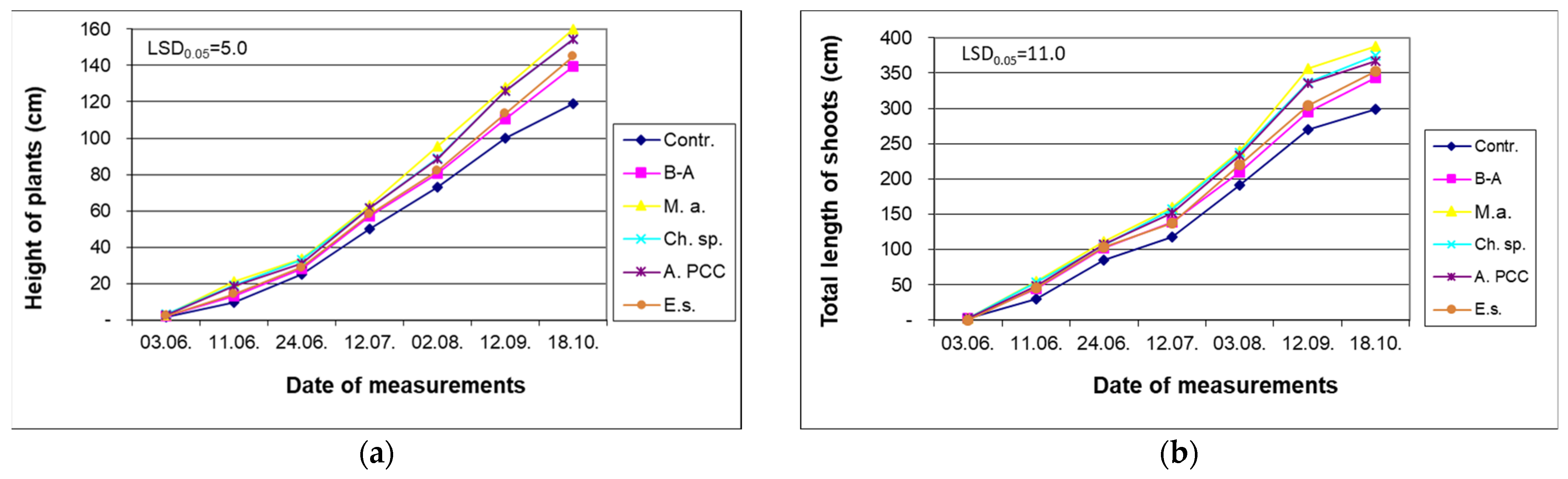
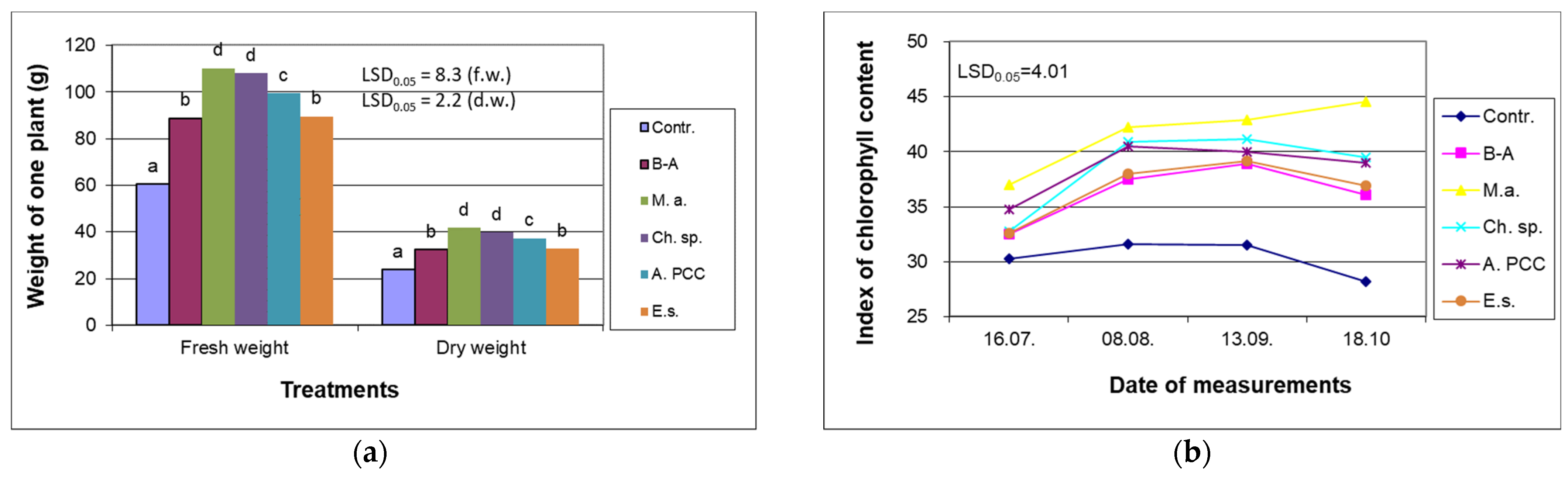
4.1. Impact of Cyanobacteria and Chlorella sp. on Growth of Willow
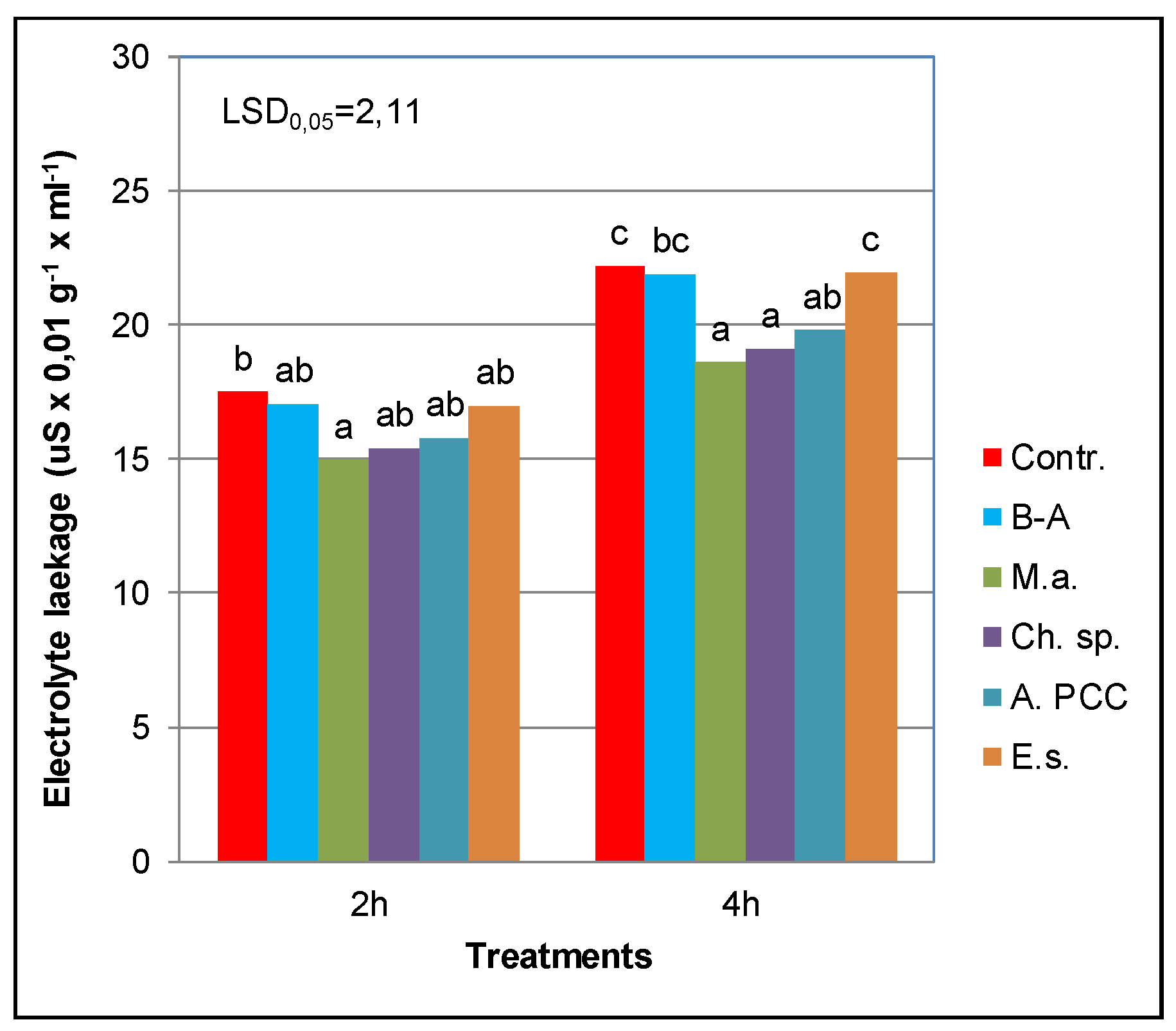
4.2. Effect of Cyanobacteria and Chlorella sp. on Permeability of Cytomembranes and Physiological Activity in Willow Plants
4.3. Effect of Cyanobacteria and Algae on Composition and Energy Value of Willow Biomass
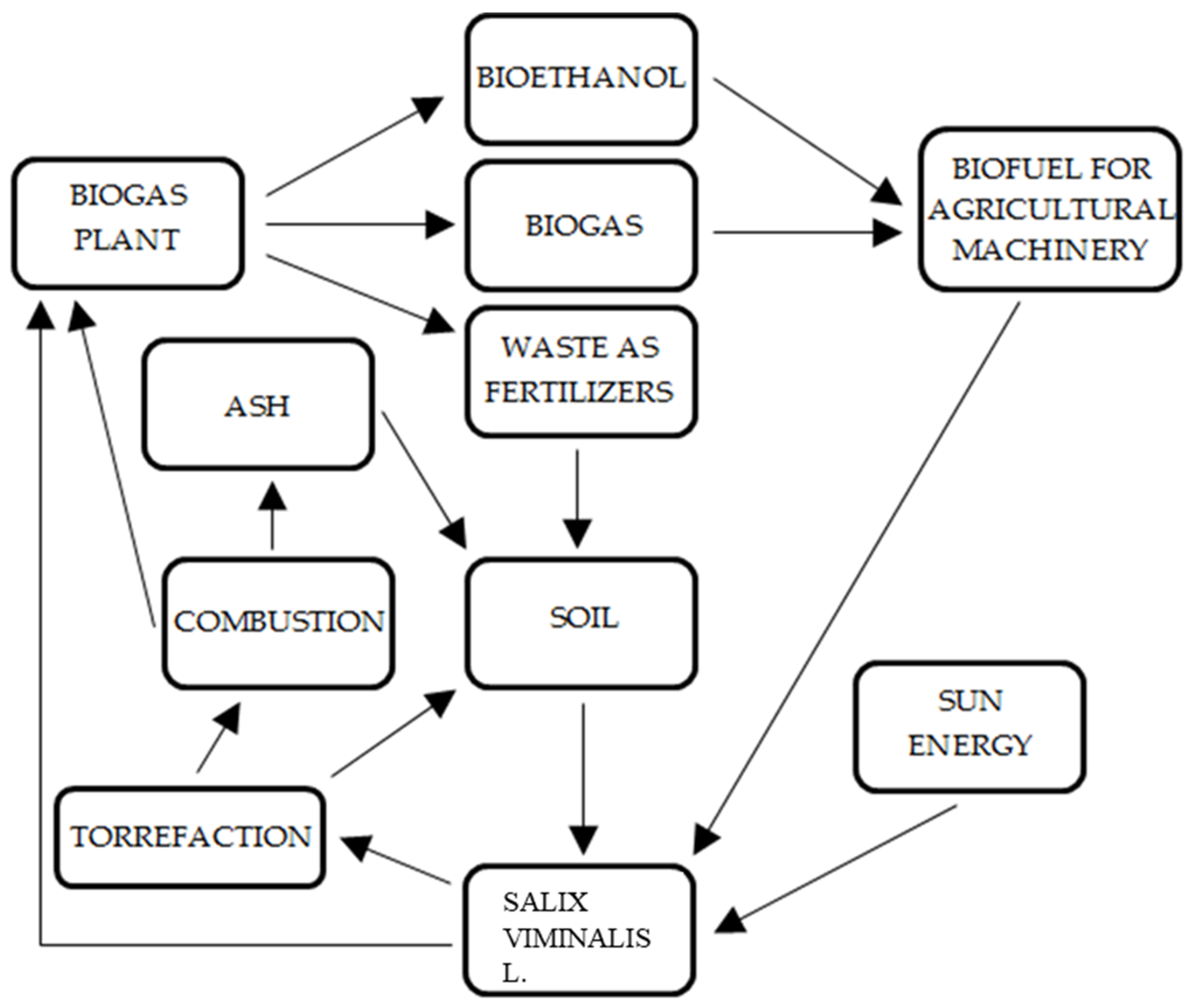
4.4. Circulation of By-Products (Nutrients) in the Closed Circular Production of Salix viminalis L. as a Source of Energy Fuel in Four Closed Cycles
5. Conclusions
- The triple foliar biofertilization with Cyanobacteria (Microcystis aeruginosa MKR 0105, Anabaena sp. PCC 7120) and green algae (Chlorella sp.) significantly increase the willow plant development, and several metabolic events have a key influence on plant growth and biomass yielding, of which energy value can be improved in the torrefaction process.
- The use of non-toxic Cyanobacteria and Chlorella sp. is cost-efficient and environmentally friendly and increases productivity of willow crops when used as biofuel for energy production.
- New physiological possibilities concerning enhancement of natural plant defense is considered one of the most promising strategies for ecological and integrated protection of energy crops cultivated on large areas.
- The developed technology for the production of willow biomass by means of fertilization with algae and increasing its energy properties in the thermo-chemical process enables the production of environmentally friendly energy as an alternative to that obtained from fossil fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulk, M.M. The potential for using Cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur. J. Plant Pathol. 1995, 101, 85–599. [Google Scholar] [CrossRef]
- Adam, M.S. The promotive effect of cyanobacterium Nostoc muscorum on the growth of some crop plants. Acta Microbiol. Pol. 1999, 48, 163–171. [Google Scholar]
- Abd-El-Moniem, E.; Abd-Allah, A.S.E. Effect of green alga cells extract as foliar spray on vegetative growth, yield and berries quality of superior grapevines. Am. Eur. J. Agric. Environ. Sci. 2008, 4, 427–433. [Google Scholar]
- Haroun, S.A.; Hussein, M.H. The promotive effect of algal biofertilizers on growth, protein pattern and some metabolic activities of Lupinus termis plants grown in siliceous soil. Asian J. Plant Sci. 2003, 2, 944–951. [Google Scholar] [CrossRef] [Green Version]
- Masojídek, J.; Prášil, O. The development of microalgal biotechnology in the Czech Republic. J. Ind. Microbiol. Biotechnol. 2010, 37, 1307–1317. [Google Scholar] [CrossRef]
- Chojnacka, A.; Romanowska-Duda, Z.B.; Grzesik, M.; Pszczolkowski, W.; Sakowicz, T. Cyanobacteria as a source of bioactive compounds for crop cultivation. In Taxonomy the Queen of Science—The Beauty of Algae, Book of Abstracts of the 29th International Phycological Conference, Krakow, Poland, 29–31 May 2020; Wolowski, K., Kwandrans, J., Wojtal, A.Z., Eds.; Polish Academy of Sciences: Krakow, Poland, 2010; pp. 81–82. [Google Scholar]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Pszczolkowski, W.; Romanowska-Duda, Z.; Owczarczyk, A.; Grzesik, M.; Sakowicz, T.; Chojnacka, A. Influence of secondary metabolites from Cyanobacteria on the growth and plant development. In Phycological Reports: Current Advances in Algal Taxonomy and Its Applications: Phylogenetic, Ecological and Applied Perspective; Institute of Botany Polish, Academy of Sciences: Krakow, Poland, 2012; pp. 195–203. [Google Scholar]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.Y.; Abomohra, A.E.F. Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Murugesan, A.G.; Wang, S.; Abomohra, A.E.F.; Jovita, P.A.; Kiruthiga, S. Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. J. Clean. Prod. 2020, 247, 119398. [Google Scholar] [CrossRef]
- Piotrowski, K.; Romanowska-Duda, Z.; Messyasz, B. Cultivation of energy crops by ecological methods under the conditions of global climate and environmental changes with the use of diatom extract as a natural source of chemical compounds. Acta Physiol. Plant. 2020, 42, 146. [Google Scholar] [CrossRef]
- Nain, L.; Rana, A.; Joshi, M.; Jadhav, S.D.; Kumar, D.; Shivay, Y.S.; Paul, S.; Prasanna, R. Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil 2010, 331, 217–230. [Google Scholar] [CrossRef]
- Spiller, H.; Gunasekaran, M. Ammonia-excreting mutant strain of the cyanobacterium Anabaena variabilis supports growth of wheat. Appl. Microbiol. Biotechnol. 1990, 33, 477–480. [Google Scholar] [CrossRef]
- Nilsson, M.; Rasmussen, U.; Bergman, B. Competition among symbiotic cyanobacterial Nostoc strains forming artificial associations with rice (Oryza sativa). FEMS Microbiol. Lett. 2005, 245, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Shanan, N.T.; Higazy, A.M. Integrated biofertilization management and Cyanobacteria application to improve growth and flower quality of Matthiola incana. Res. J. Agric. Biologic. Sci. 2009, 5, 1162–1168. [Google Scholar]
- Romanowska-Duda, Z.B.; Grzesik, M.; Owczarczyk, A.; Mazur-Marzec, H. Impact of intra and extracellular substances from Cyanobacteria on the growth and physiological parameters of grapevine (Vitis vinifera). In Book of Abstracts 28.07–02.08.2010, Proceedings of the 20th International Conference on Plant Growth Substance (IPGSA); Universitat Rovira and Virgili: Taragona, Spain, 2010; 118p. [Google Scholar]
- Sahu, D.; Priyadarshani, I.; Rath, B. Cyanobacteria—As potential biofertilizer. J. Microbiol. 2012, 1, 20–26. [Google Scholar]
- Falch, B.S.; Konig, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezzuto, J.M.; Bachmann, H. Biological activities of cyanobacteria: Evaluation of extracts and pure compounds. Planta Med. 1995, 61, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kreitlow, S.; Mundt, S.; Lindequist, U. Cyanobacteria—A potential source of new biologically active substances. J. Biotechnol. 1999, 70, 61–63. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine Cyanobacteria prolifi a source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Gorelova, O.A. Communication of Cyanobacteria with plant partners during association formation. Microbiology 2006, 75, 465–469. [Google Scholar] [CrossRef]
- Metting, B.; Zimmerman, W.J.; Crouch, I.; Van Staden, J. Agronomic uses of seaweed and microalgae. In Introduction to Applied Phycology; Akatsuka, I., Ed.; SPB Academic Publishing: Hague, The Netherlands, 1990; pp. 589–628. [Google Scholar]
- Marczak-Grzesik, M.; Budzyń, S.; Tora, B.; Szufa, S.; Kogut, K.; Burmistrz, P. Low-Cost Organic Adsorbents for Elemental Mercury Removal from Lignite Flue Gas. Energies 2021, 14, 2174. [Google Scholar] [CrossRef]
- Grzesik, M.; Górnik, K.; Janas, R.; Lewandowki, M.; Romanowska-Duda, Z.; van Duijn, B. High efficiency stratification of apple cultivar Ligol seed dormancy by phytohormones, heat shock and pulsed radio frequency. J. Plant Physiol. 2017, 219, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane. Front. Sci. Front. Plant Sci. 2019, 9, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Sielski, J.; Grzesik, M.; Romanowska-Duda, Z.; Piotrowski, K.; Lewandowska, W. Acquisition of Torrefied Biomass from Jerusalem Artichoke Grown in a Closed Circular System Using Biogas Plant Waste. Molecules 2020, 25, 3862. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescencje parameters as an early indicator of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Duda, Z.; Janas, R.; Grzesik, M. Application of Phytotoxkit in the quick assessment of ashes suitability as fertilizers in sorghum crops. Int. Agrophys. 2019, 33. [Google Scholar] [CrossRef]
- Knypl, J.S.; Kabzińska, E. Growth, phosphatase and ribonuclease activity in phosphate deficient Spirodela oligorrhiza cultures. Biochem. Physiol. Pflanzen. 1977, 171, 279–287. [Google Scholar] [CrossRef]
- Gornik, K.; Grzesik, M. Effect of Asahi SL on China aster ‘Aleksandra’ seed yield, germination and some metabolic events. Acta Physiol. Plant. 2002, 24, 379–383. [Google Scholar] [CrossRef]
- Jaworski, E.G. Nitrate reductase assay in intact plant tissues. Biochem. Biophys. Res. Commun. 1971, 43, 1274–1279. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, R.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. biomass grow and nutrients removal from liquid phase of digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physico-chemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Norrie, J.; Keathley, J.P. Benefits of ascophyllum nodosum marine-plant extract applications to ´Thompson Seedless’ grape production. Acta Hort. 2006, 727, 243–248. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; Tantaway, A.S.; Hafez, M.M.; Habib, H.A.M. Seaweed extract improves growth, yield and quality of different watermelon hybrids. Res. J. Agric. Biol. Sci. 2010, 6, 161–168. [Google Scholar]
- El Modafar, C.; Elgadda, M.; El Boutachfaitib, R.; Abouraicha, E.; Zehhara, N.; Petit, E.; El Alaoui-Talibia, Z.; Courtoisb, B.; Courtoisb, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hort. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Wolska, A.; Małecka, A. Influence of blue-green algae as nitrogen fertilizer supplier in regulation of water status in grapevines under stress conditions. In Proceedings of the Water Transport and Aquaporins in Grapevines, Alcudia, Spain, 20–23 October 2004; p. 10. [Google Scholar]
- Grzesik, M.; Romanowska-Duda, Z.B. Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with Cyanobacteria and microalgae. Pol. J. Environ. Study 2014, 23, 89–96. [Google Scholar]
- Hussain, A.; Hasnain, S. Comparative assessment of the efficacy of bacterial and cyanobacterial phytohormones in plant tissue culture. World J. Microbiol. Biotechnol. 2012, 28, 1459–1466. [Google Scholar] [CrossRef]
- Glick, B.R.; Patten, C.L.; Holguim, G.; Penrose, D.M. Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria; Imperial College Press: London, UK, 1999; p. 267. [Google Scholar]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef]
- Johansson, C.; Bergman, B. Early events during the establishment of Gunnera–Nostoc symbiosis. Planta 1992, 188, 403–413. [Google Scholar] [CrossRef]
- Obreht, Z.; Kerby, N.W.; Gantar, M.; Rowell, P. Effects of root-associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biol. Fertil. Soils 1993, 15, 68–72. [Google Scholar] [CrossRef]
- Saadatnia, H.; Riahi, H. Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ. 2009, 55, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.T. Cyanobacteria: A potential nitrogen source in rice fields. Texas Rice 2006, 6, 9–10. [Google Scholar]
- Romanowska-Duda, Z.; Mankiewicz, J.; Tarczyńska, M.; Walter, Z.; Zalewski, M. The effect of toxic cyanobacteria (blue-green algae) on water plants and animal cells. Pol. J. Environ. Stud. 2002, 11, 561–566. [Google Scholar]
- Romanowska-Duda, Z.; Tarczyńska, M. The influence of Microcystin-LR and hepatotoxic cyanobacterial extract on water plant (Spirodela oligorrhiza). Environ. Toxicol. 2002, 17, 434–440. [Google Scholar] [CrossRef]
- El-Shabasi, M.S.; Mohamed, S.M.; Mahfouz, S.A. Effect of Foliar Spray with Amino Acids on Growth, Yield and Chemical Composition of Garlic Plants. In Proceedings of the 6th Arabian Conference, Horticulture, Ismailia, Egypt, 20–22 March 2005. [Google Scholar]
- Awad, E.M.M.; Abd El-Hameed, A.M.; Shall, Z.S. Effect of glycine, lysine and nitrogen fertilizer; rates on growth, yield and chemical composition of potato. J. Agric. Sci. Mansoura Univ. 2007, 32, 8541–8551. [Google Scholar]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J. Hort. Sci. Biotechnol. 2009, 84, 131–137. [Google Scholar] [CrossRef]
- Cheng, K.J.; Ingram, J.M.; Costerton, J.W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J. Bacteriol. 1971, 107, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Mule, M.C.Z.; De Caire, G.Z.; De Cano, M.S.; Palma, R.M.; Colombo, K. Effect of cyanobacterial inoculation and fertilizers on rice seedlings and post harvest soil structure. Comm. Soil Sci. Plant Anal. 1999, 30, 97–107. [Google Scholar] [CrossRef]
- De-Caire, G.Z.; De Cano, M.S.; Palma, R.M.; De Mule, C.Z. Changes in soi enzymes activity by cyanobacterial biomass and exopolysaccharides. Soil Biol. Biochem. 2000, 32, 1985–1987. [Google Scholar] [CrossRef]
- Grzesik, M.; Romanowska-Duda, Z.B. The effect of potential climatic changes, Cyanobacteria, Biojodis and Asahi SL on development of the Virginia fanpetals (Sida hermaphrodita) plants. Pamiętnik Pulawski 2009, 151, 483–491. [Google Scholar]
- Tazisong, I.A.; Senwo, Z.N.; He, Z.Q. Phosphatase Hydrolysis of Organic Phosphorus Compounds. Adv. Enzym. Res. 2015, 3, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Dick, W.A.; Tabatabai, M.A. Significance and potential uses of soil enzymes. In Soil Microbial Ecology: Application in Agricultural and Environmental Management; Metting, F.B., Ed.; Dekker: New York, NY, USA, 1993; pp. 95–125. [Google Scholar]
- Lehmann, K.; Hause, B.; Altmann, D.; Köck, M. Tomato ribonuclease LX with the functional endoplasmic reticulum retention motif HDEF is expressed during programmed cell death processes, including xylem differentiation, germination, and senescence. Plant Physiol. 2001, 127, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Sindelarova, M.; Sindelar, L.; Wilhelmova, N.; Prochazkova, D. Changes in key enzymes of viral-RNA biosynthesis in chloroplasts from PVY and TMV infected tobacco plants. Biol. Plant. 2005, 49, 471–474. [Google Scholar] [CrossRef]
- Srivastava, S.; Emery, R.J.N.; Kurepin, L.V.; Reid, D.M.; Fristensky, B.; Kav, N.N.V. Pea PR 10.1 is a ribonuclease and its transgenic expression elevates cytokinin levels. Plant Growth Regul. 2006, 49, 17–25. [Google Scholar] [CrossRef]
- Booker, F.L. Influence of ozone on ribonuclease activity in wheat (Triticum aestivum) leaves. Physiol. Plant. 2004, 120, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Ghalavand, A.; Aghaalikhani, M. Study the efficacies of green manure application as chickpea per plant. Eng. Technol. 2010, 46, 233–236. [Google Scholar]
- Al-Khiat, S.H.A. Effect of Cyanobacteria as a Soil Conditioner and Biofertilizer on Growth and Some Biochemical Characteristics of Tomato (Lycopersicon esculentum L.) Seedlings; Faculty of Science, King Saud University: Riyadh, Arab Saudi, 2006; pp. 1–190. [Google Scholar]
- Swarnalakshmi, K.; Prasanna, C.R.; Kumar, A.; Pattnaik, S.C.; Chakravarty, K.C.; Shiv, Y.S.A.; Singh, B.R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Hegazi, A.Z.; Mostafa, M.S.S.; Ahmed, H.M.I. Influence of different cyanobacterial application methods on growth and seed production of common bean under various levels of mineral nitrogen fertilization. Nat. Sci. 2010, 8, 183–194. [Google Scholar]
- Wielgosiński, G.; Czerwińska, J.; Szufa, S. Municipal Solid Waste Mass Balance as a Tool for Calculation of the Possibility of Implementing the Circular Economy Concept. Energies 2021, 14, 1811. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Czerwińska, J.; Lewandowski, A.; Lewandowska, W.; Sielski, J.; Dzikuć, M.; Wróbel, M.; Jewiarz, M.; et al. Sustainable Drying and Torrefaction Processes of Miscanthus for Use as a Pelletized Solid Biofuel and Biocarbon-Carrier for Fertilizers. Molecules 2021, 26, 1014. [Google Scholar] [CrossRef]
- Szufa, S. Use of superheated steam in the process of biomass torrefaction. Przem. Chem. 2020, 99, 1797–1801. (In Polish) [Google Scholar]
- Romanowska-Duda, Z.; Piotrowski, K.; Wolska, B.; Dębowski, M.; Zieliński, M.; Dziugan, P.; Szufa, S. Stimulating effect of ash from Sorghum on the growth of Lemnaceae—A new source of energy biomass. In Renewable Energy Sources: Engineering, Technology, Innovation; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 341–349. ISBN 978-3-030-13887-5. [Google Scholar] [CrossRef]
- Ławińska, K.; Szufa, S.; Modrzewski, R.; Obraniak, A.; Wężyk, T.; Rostocki, A.; Olejnik, T.P. Obtaining Granules from Waste Tannery Shavings and Mineral Additives by Wet Pulp Granulation. Molecules 2020, 25, 5419. [Google Scholar] [CrossRef]
- Krzystek, L.; Wajszczuk, K.; Pazera, A.; Matyka, M.; Slezak, R.; Ledakowicz, S. The influence of plant cultivation methods on biogas production: Energy efficiency. In Proceedings of the WasteEng2018 Conference, Prague, Czech Republic, 2–5 July 2018; pp. 710–720. [Google Scholar]
- Dzikuć, M.; Kuryło, P.; Dudziak, R.; Szufa, S.; Dzikuć, M.; Godzisz, K. Selected Aspects of Combustion Optimization of Coal in Power Plants. Energies 2020, 13, 2208. [Google Scholar] [CrossRef]
- Szufa, S.; Wielgosiński, G.; Piersa, P.; Czerwińska, J.; Dzikuć, M.; Adrian, Ł.; Lewandowska, W.; Marczak, M. Torrefaction of Straw from Oats and Maize for Use as A Fuel and Additive to Organic Fertilizers—TGA Analysis, Kinetics as Products for Agricultural Purposes. Energies 2020, 13, 2064. [Google Scholar] [CrossRef] [Green Version]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the Drying Temperature and Grinding Technique on Biomass Grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- Szufa, S.; Dzikuć, M.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Lewandowska, W.; Marczak, M.; Błaszczuk, A.; Piwowar, A. Torrefaction of oat straw to use as solid biofuel, an additive to organic fertilizers for agriculture purposes and activated carbon—TGA analysis, kinetics. E3S Web Conf. 2020, 154, 02004. [Google Scholar] [CrossRef] [Green Version]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Ratajczyk-Szufa, J. Torrefaction process of millet and cane using batch reactor. In Renewable Energy Sources: Engineering, Technology, Innovation, Springer Proceedings in Energy; Wróbel, M., Jewiarz, M., Szlęk, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 371–379. [Google Scholar]
- Szufa, S.; Adrian, Ł.; Piersa, P.; Romanowska-Duda, Z.; Grzesik, M.; Cebula, A.; Kowalczyk, S. Experimental studies on energy crops torrefaction process using batch reactor to estimate torrefaction temperature and residence time. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2018; pp. 365–373. ISBN 978-3-319-72370-9. [Google Scholar] [CrossRef]
- Szufa, S.; Romanowska-Duda, B.Z.; Grzesik, M. Torrefaction process of the Phragmites Communis growing in soil contaminated with cadmium. In Proceedings of the 20th European Biomass Conference and Exibition, Milan, Italy, 18–22 June 2014; pp. 628–634, ISBN 978-88-89407-54-7. [Google Scholar] [CrossRef]
- Ławińska, K.; Szufa, S.; Obraniak, A.; Olejnik, T.; Siuda, R.; Kwiatek, J.; Ogrodowczyk, D. Disc Granulation Process of Carbonation Lime Mud as a Method of Post-Production Waste Management. Energies 2020, 13, 3419. [Google Scholar] [CrossRef]
- Siuda, R.; Kwiatek, J.; Szufa, S.; Obraniak, A.; Piersa, P.; Adrian, Ł.; Modrzewski, R.; Ławińska, K.; Siczek, K.; Olejnik, T.P. Industrial Verification and Research Development of Lime–Gypsum Fertilizer Granulation Method. Minerals 2021, 11, 119. [Google Scholar] [CrossRef]
- Luo, H.; Niedzwiecki, L.; Arora, A.; Mościcki, K.; Pawlak-Kruczek, H.; Krochmalny, K.; Baranowski, M.; Tiwari, M.; Sharma, A.; Sharma, T.; et al. Influence of Torrefaction and Pelletizing of Sawdust on the Design Parameters of a Fixed Bed Gasifier. Energies 2020, 13, 3018. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Arora, A.; Gupta, A.; Saeed, M.A.; Niedzwiecki, L.; Andrews, G.; Phylaktou, H.; Gibbs, B.; Newlaczyl, A.; Livesey, P.M. Biocoal—Quality control and assurance. Biomass Bioenergy 2020, 135, 105509. [Google Scholar] [CrossRef]
- Krochmalny, K.; Niedzwiecki, L.; Pelińska-Olko, E.; Wnukowski, M.; Czajka, K.; Tkaczuk-Serafin, M.; Pawlak-Kruczek, H. Determination of the marker for automation of torrefaction and slow pyrolysis processes—A case study of spherical wood particles. Renew. Energy 2020, 161, 350–360. [Google Scholar] [CrossRef]
- Obraniak, A.; Orczykowska, M.; Olejnik, T.P. The effects of viscoelastic properties of the wetting liquid on the kinetics of the disc granulation process. Powder Technol. 2019, 342, 328–334. [Google Scholar] [CrossRef]



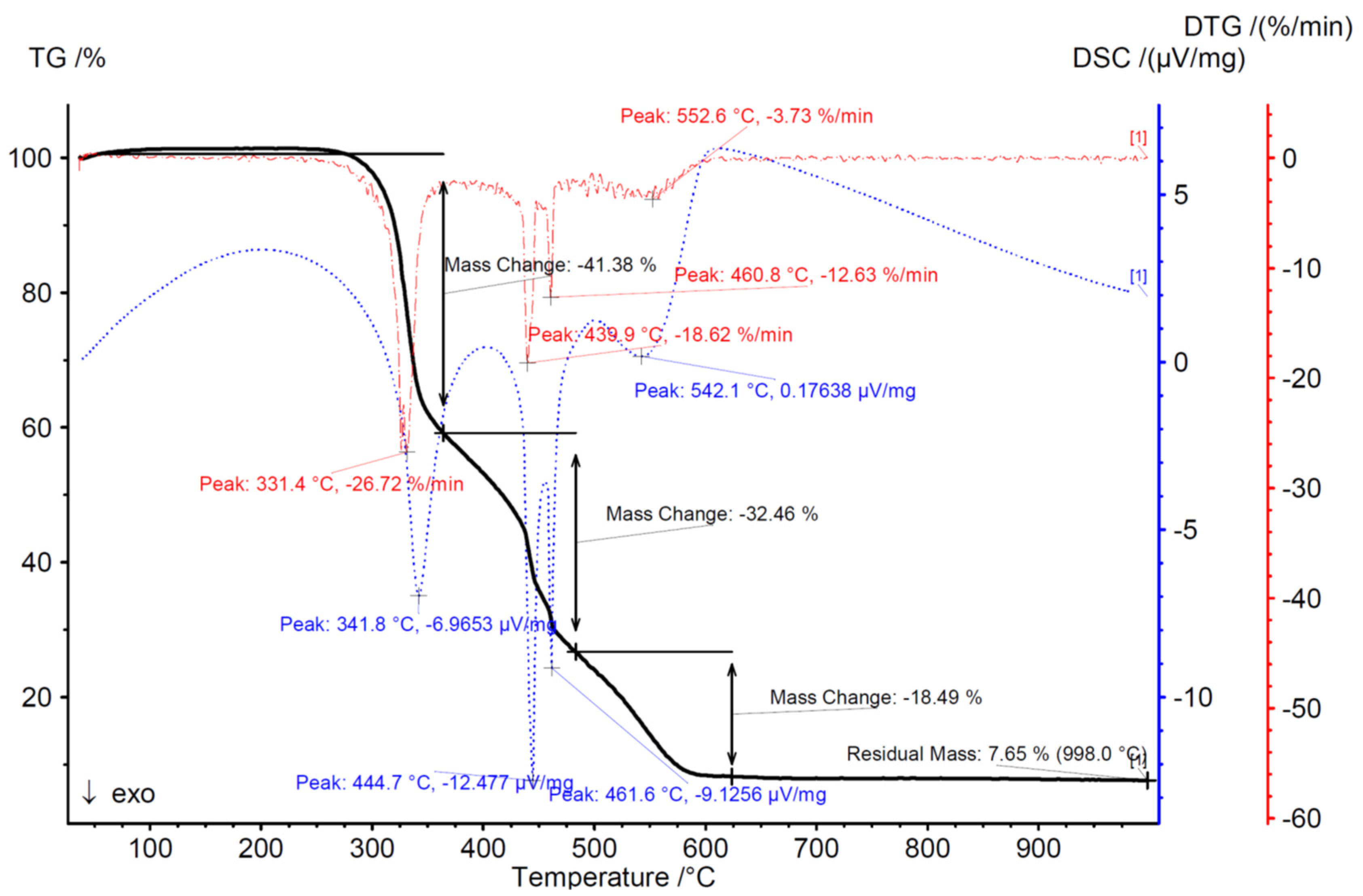
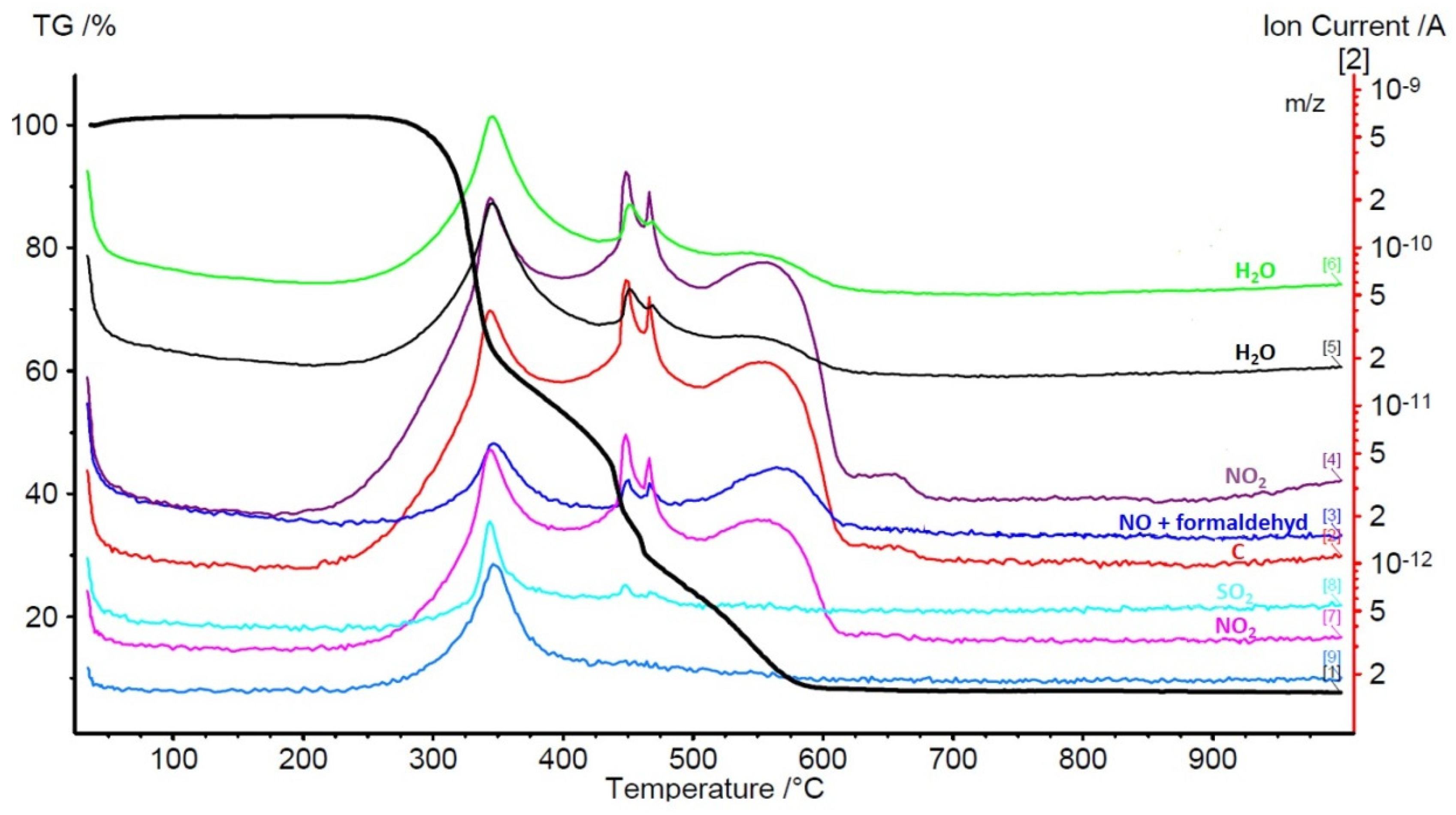
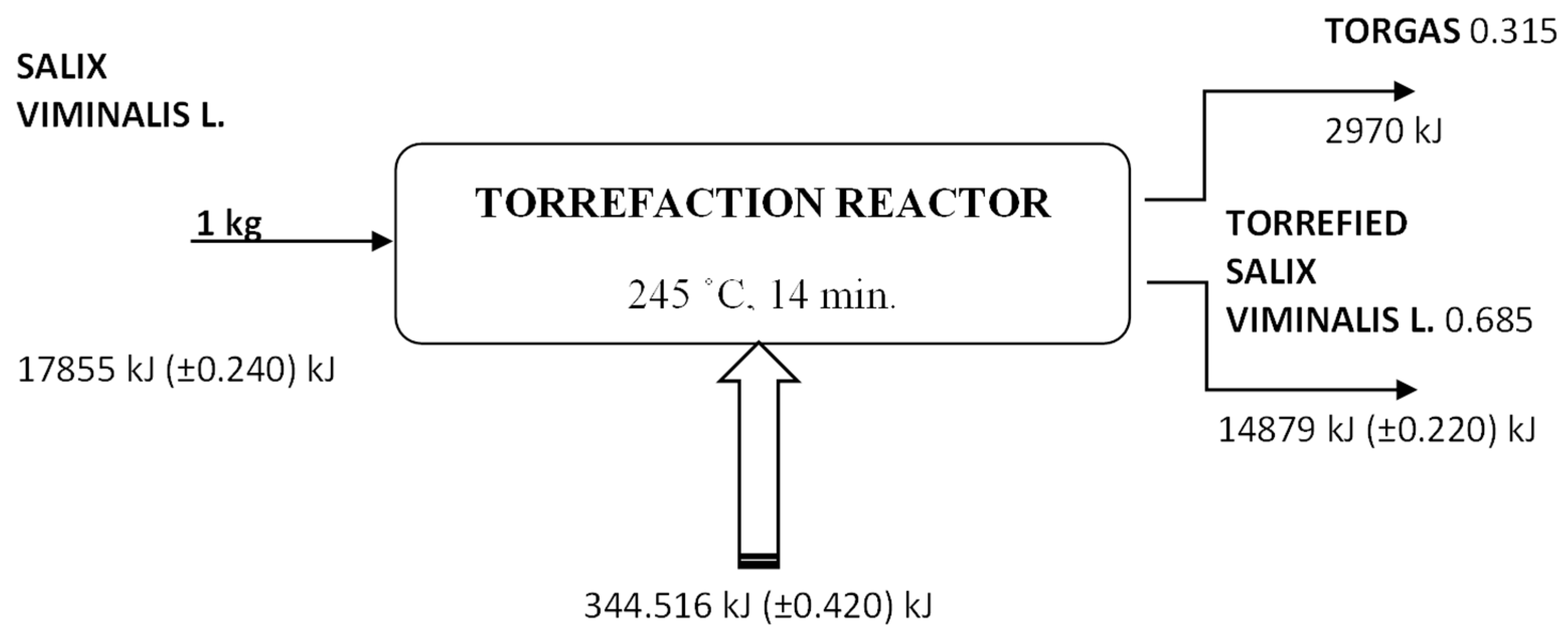
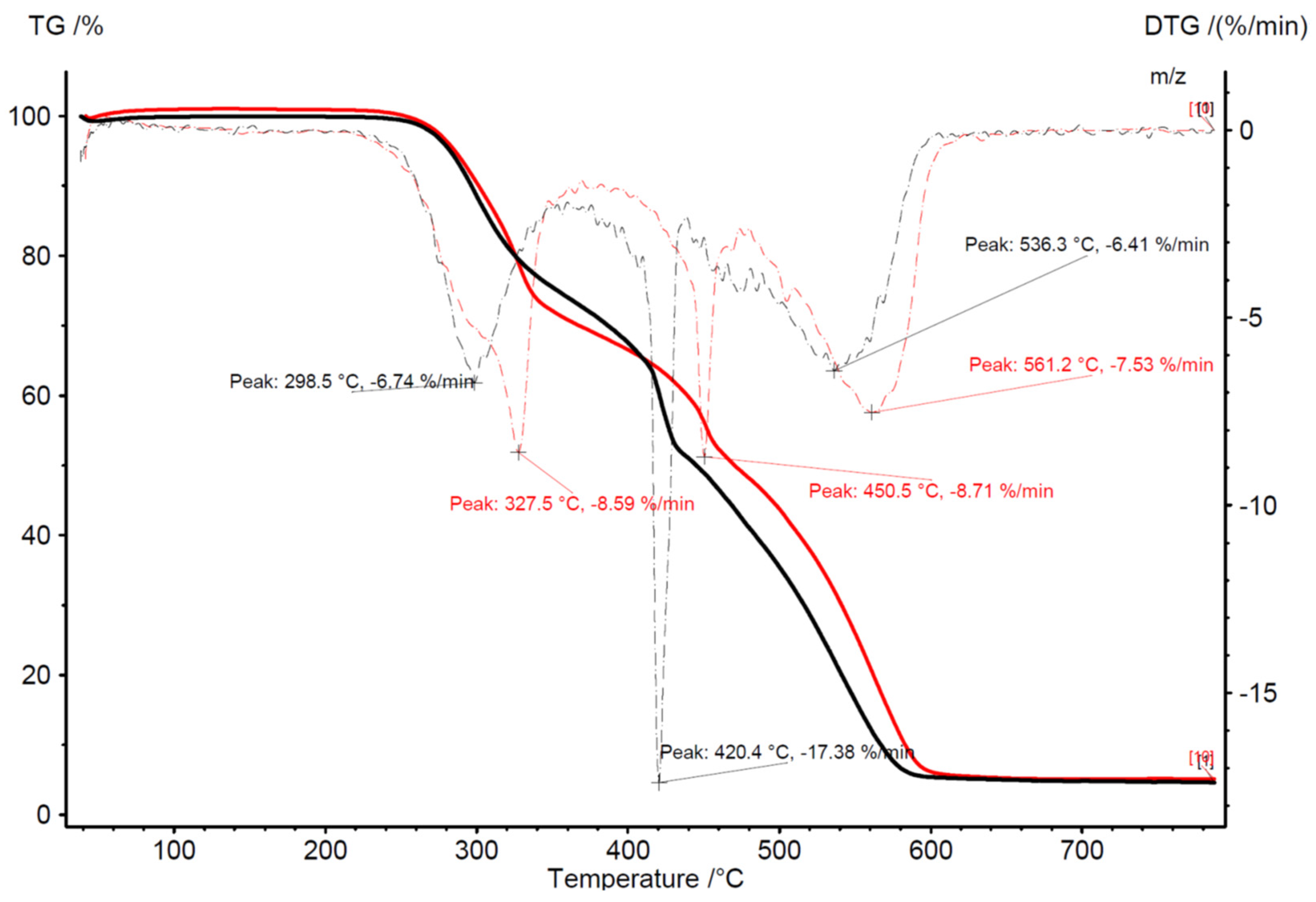


| N | P | K | Ca | Mg | Fe | Mn | Cu | Zn | B | Dry Mass |
|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 Dry Weight | % | |||||||||
| 1.01 | 930 | 3829 | 25,734 | 1553 | 656 | 45.1 | 23.5 | 29.0 | 60.1 | 25.3 |
| Applied Preparate, Cyanobacteria and Algae | Day and Month of Measurements | ||||
|---|---|---|---|---|---|
| 1 June | 11 June | 22 June | 12 July | 3 August | |
| Control | 1.2 a * | 2.2 a | 2.3 a | 2.5 a | 2.6 a |
| Bio-Algeen S90 | 1.4 b | 2.3 b | 2.7 b | 2.9 b | 2.9 b |
| Microcistis aeruginosa MKR 0105 | 1.5 b | 2.5 d | 3.1 cd | 3.7 d | 3.7 c |
| Chlorella sp. | 1.5 b | 2.4 c | 3.0 c | 3.4 c | 3.6 c |
| Anabaena sp. PCC 7120 | 1.4 b | 2.4 c | 2.9 c | 3.4 c | 3.5 c |
| Environmental sample | 1.3 a | 2.3 b | 2.7 b | 2.9 b | 2.9 b |
| LSD 0.05 ** | 0.1 | 0.08 | 0.1 | 0.2 | 0.2 |
| Applied Preparate, Cyanobacteria and Algae | Activity of Selected Enzymes | ||||
|---|---|---|---|---|---|
| Phosphorylase Acid (mU g−1 f.w.) | Phosphorylase Alkaline (mU g−1 f.w.) | RNase (mU g−1 f.w.) | Dehydrogenases (mg Formazan · g leaf −1) | Nitrate Reductase (μmol NO2 g−1 f.w. h−1) | |
| Control | 0.54 a * | 0.14 a | 2.6 a | 0.77 a | 0.90 a |
| Bio-Algeen S90 | 0.76 b | 0.25 b | 3.75 b | 1.22 b | 1.32 b |
| Microcistis aeruginosa | 0.94 c | 0.38 c | 4.81 d | 1.62 d | 1.72 d |
| Chlorella sp. | 0.87 c | 0.33 c | 4.43 c | 1.44 c | 1.59 c |
| Anabaena PCC 7120 | 0.86 c | 0.33 c | 4.41 c | 1.43 c | 1.52 c |
| Environmental sample | 0.67 b | 0.25 b | 3.76 b | 1.23 b | 1.35 b |
| LSD 0.05 ** | 0.09 | 0.05 | 0.40 | 0.14 | 0.12 |
| Applied Preparate Cyanobacteria or Algae | Characteristics of Willow Plant Quality | ||||||
|---|---|---|---|---|---|---|---|
| Increase in the Length of Shoots % | Content of Macroelements in Plants | Calorific Value in the Operating State kJ kg−1 | Heat of Combustion in the Analytical State kJ kg−1 | The Ash Content in the Working State % | |||
| N% | P mg kg d.w−1 | K mg kg d.w−1 | |||||
| Control | 100.0 a * | 3.40 a | 2 166 a | 31 910 a | 15 247 a | 18 722 a | 1.60 a |
| Bio-Algeen S90 | 110.5 b | 3.48 b | 2 178 ab | 31 920 a | 15 140 a | 18 799 a | 1.59 a |
| Microcistis aeruginosa | 125.1 d | 3.64 c | 2 210 c | 31 980 b | 15 223 a | 18 769 a | 1.60 a |
| Chlorella sp. | 120.9 c | 3.48 b | 2 201 bc | 31 962 b | 15 224 a | 18 699 a | 1.61 a |
| Anabaena PCC 7120 | 118.2 c | 3.58 c | 2 199 bc | 31 960 b | 15 199 a | 18 701 a | 1.62 a |
| Environmental sample | 113.7 b | 3.47 ab | 2 176 ab | 31 922 a | 15 187 a | 18 745 a | 1.61 a |
| LSD 0.05 ** | 4.0 | 0.7 | 28.9 | 34.3 | 112.1 | 102.2 | 0.3 |
| Sample nr. | Mass Reduction, g | Mass Loss, % | Residential Time, min. | Torrefaction Temp., °C |
|---|---|---|---|---|
| 1 | 20/13.17 | 34.15 | 15 | 243.89 |
| 2 | 20/13.92 | 30.40 | 14 | 244.67 |
| 3 | 20/13.70 | 31.50 | 16 | 238.99 |
| 4 | 20/13.97 | 30.15 | 14 | 245.00 |
| 5 | 20/12.69 | 36.55 | 17 | 241.99 |
| 6 | 20/12.36 | 38.20 | 20 | 230.02 |
| 7 | 20/12.03 | 39.85 | 18 | 230.47 |
| Energy Crop | Moisture (%) | C ad, (%) | N ad, (%) | H ad, (%) | S ad, (%) | Cl, (%) | Volatile ad (%) | Ash (%) | High Heating Value, (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Salix viminalis L. | 7.12 | 45.69 | 0.52 | 6.37 | 0.05 | 0.115 | 91.29 | 7.12 | 19.79 |
| Torrefied Salix viminalis L: | |||||||||
| (244.67 °C, 14 min) | 4.05 | 52.51 | 0.19 | 5.85 | 0.05 | 0.014 | 73.37 | 3.01 | 23.48 |
| (243.89 °C, 15 min) | 3.93 | 53.20 | 0.19 | 5.85 | 0.05 | 0.014 | 72.81 | 3.2 | 24.02 |
| (245.0 °C, 14 min) | 3.78 | 54.53 | 0.19 | 5.72 | 0.05 | 0.014 | 72.27 | 3.46 | 25.10 |
| Assessed Material | C | O | K | Ca | Mg | Fe | Si | P | S | Cl | Dry Mass |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [Atomic, %] | [%] | ||||||||||
| Ash composition from torrefied Salix viminalis L. (average values) | 23.75 | 45.82 | 22.01 | 2.60 | 0.84 | 0.02 | 0.29 | 3.07 | 0.17 | 1.43 | 100.00 |
| Standard deviation, σ | 2.99 | 2.19 | 2.85 | 0.49 | 0.30 | 0.00 | 0.10 | 0.60 | 0.05 | 0.40 | |
| Ash composition from untreated Salix viminalis L. (average values) | 33.50 | 33.08 | 21.09 | 2.22 | 0.98 | 0.11 | 0.46 | 1.16 | 0.19 | 3.13 | 100.00 |
| Standard deviation, σ | 3.28 | 4.04 | 3.82 | 0.78 | 0.30 | 0.04 | 0.42 | 0.62 | 0.05 | 1.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies 2021, 14, 5262. https://doi.org/10.3390/en14175262
Romanowska-Duda Z, Szufa S, Grzesik M, Piotrowski K, Janas R. The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies. 2021; 14(17):5262. https://doi.org/10.3390/en14175262
Chicago/Turabian StyleRomanowska-Duda, Zdzislawa, Szymon Szufa, Mieczysław Grzesik, Krzysztof Piotrowski, and Regina Janas. 2021. "The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process" Energies 14, no. 17: 5262. https://doi.org/10.3390/en14175262
APA StyleRomanowska-Duda, Z., Szufa, S., Grzesik, M., Piotrowski, K., & Janas, R. (2021). The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies, 14(17), 5262. https://doi.org/10.3390/en14175262







