Abstract
In the bottom ash (BA) of municipal solid waste incineration, the content of iron and aluminum is relatively high. The efficiency of eddy current extraction of non-ferrous metals (including aluminium) routinely used in incineration plants is limited. The determination of the form of occurrence of aluminium or aluminium-rich components in BA is important in terms of its recovery or utilisation. BA from a newly built incineration plant in Poland was analysed using chemical analysis, X-ray diffraction, optical microscopy, and scanning electron microscopy with chemical microanalysis. Samples of water-quenched BA were analysed. For comparison, a non-quenched sample (collected above a water tank) was analysed. The obtained results indicate that aluminium-rich components in BA are present in both the melt phase and quench phase. In the melt phase (glassy material), the content of aluminium is low (usually below 2 wt%). Aluminium-rich components present in glass, inherited after aluminium products are usually oxidised, and occur as platy or irregular forms. Aluminium components in the quench phase are significantly transformed with the common presence of Cl− and SO42− phases formed during reaction with the quench water. Secondary phases form simple or complex rims around metallic or slightly oxidised cores, of which the size is significantly reduced during transformations. The variety in the forms of aluminium occurrence in BA makes its recovery challenging and inefficient. The reduced content of metallic aluminium indicates that the potential for hydrogen generation of BA is low.
1. Introduction
Globally, aluminium is the second-most produced metal after iron (steel) and its demand is expected to continue growing [1,2]. The production of aluminium is energy-consuming (ca. 3.5% of global electricity) and is thus related to significant CO2 emissions (ca. 1% of global greenhouse gas emissions are related to industrial and energy production processes) [2]. The recycling of aluminium is important not only because of the conservation of aluminium ore deposits. While the production of aluminium from scraps is related to much lower energy consumption and emission of CO2, an additional advantage is strongly related to the purity of aluminium scraps [2,3,4].
The global metal content in solid waste is ca. 4% and varies by income level (from ca. 6% in high-income countries to 2% in middle- and low-income countries) and in terms of geography [4,5]. Aluminium as a component frequently found in everyday use products is commonly present in municipal solid waste and its content in waste is related to the effectiveness of curbside recycling. Aluminium in municipal solid waste can also be related to the production of various goods. When metallic aluminium is deposited in landfill sites, it can react with alkaline water to produce hydrogen. Heat production in this exothermic reaction can lower or stop microbial activity and ignite or pyrolyse municipal waste [6]. Hydrogen can then react with CO2 to produce methane [7].
Aluminium for recycling can be collected at the pre-user stage or at the end-of-life stage of aluminium-containing products through curbside collection, the separation from waste, and from municipal waste incineration (MSWI) residues. Metals from MSWI bottom ash (BA) can be extracted using magnetic methods (ferrous metals) or eddy-current separation (non-ferrous metals including aluminium) [8,9]. The efficiency of using magnetic and eddy-current separation in MSWI BA is relatively low. The content of metals after processing using these methods remains significant. Only 30% of aluminium can be recovered from BA using these technologies [10].
The content of iron and aluminium in MSWI BA is usually high, but the ratio of their content differs significantly [11]. According to Biganzoli et al. [12], the content of ferrous and non-ferrous metals in the BA varies in the 7–15% and 1–2% range, respectively, with the domination of aluminium (ca. 60%) in non-ferrous metals’ fraction. MSWI BA with the dominance of aluminium over Fe is also noted [13,14]. The ratio of iron to aluminium in BA can decrease with the decreasing size fraction of the material [13].
Aluminium separation can be difficult because of the complex chemical form of occurrence and small size of aluminium-rich inclusions in slag. The effectiveness of aluminium extraction using eddy-current separation from MSWI BA is related to the grain size of the ash and is usually lower for more fine-grained ash [12]. According to Biganzoli and Grasso [12], the recovery of aluminium is related to the structure and mechanical properties of the BA material.
Difficulty with the separation of metallic aluminium from MSWI BA suggests other applications of its potential. Hydrogen generation from the metallic aluminium–water reaction in MSWI BA was postulated by several authors [15,16,17,18]. Hydrogen production in the reduction of water using aluminium or its alloys is often considered as a possibility for energy generation [19]. Published data related to the efficiency of hydrogen production during BA aluminium–water reaction differ significantly. Biganzoli et al. [20] report hydrogen production from 5.7 to 10.8 L/kg of dry BA according to the sample. Larsson [20] discusses the role of numerous factors in the process and his results are from 0 to ca. 5 L of hydrogen per 1 kg of ash. A higher value of 39.4 l per 1 kg of BA was obtained by Saffarzadeh et al. [17]. Hydrogen can also be generated from MSWI fly ash’s reaction with water [21] or as a result of photocatalytic reactions [22], as shown in Rumayor et al. 2022.
The spontaneous reaction of aluminium-containing MSWI ash with water can result in the uncontrolled accumulation of hydrogen (or hydrogen-containing gas mixture) and explosions in ash bunkers [20,21,23]. Aluminium in MSWI residue can reduce its use as an addition to concrete owing to the production of hydrogen, which lowers the mechanical properties of concrete [13,16,24,25].
The aim of this paper is to present the form of occurrence of aluminium in MSWI BA from incineration plants in Poland built in the second decade of the 21st century and to discuss the possibility of its utilisation. The form of occurrence and degree of transformation of metallic aluminium determine the potential for its extraction or hydrogen production. The study is based on a detailed phase analysis of BA.
2. Materials and Methods
Several samples of BA were collected from an MSWI plant in Poland. The sampling details are listed in Table 1. Samples were collected in two cycles and several samples from each cycle were studied.

Table 1.
List of the studied samples.
The chemical composition of the BA samples was analysed using inductively coupled plasma mass spectrometry and inductively coupled plasma atomic emission spectroscopy, performed by Bureau Veritas Minerals in Vancouver, Canada. Loss of ignition (LOI) was determined by the mass difference after heating to 1000 °C. Sulphur and carbon content were determined using the Leco method.
X-ray diffraction was used for the mineralogical characterisation of the BA samples. The analyses were performed at the Institute of Geological Sciences, Jagiellonian University in Krakow, Poland, using a Philips X’Pert APD type diffractometer with a PW 3020 vertical goniometer equipped with a curved graphite crystal monochromator (CuKα radiation, analytical range 2–64° 2Θ, step 0.02°, counting time 2 s/step) (Almelo, The Netherlands). Phase identification was obtained using Philips X’Pert software (associated with the ICDD database).
Optical microscopy was employed for the observation of the samples’ mineral composition and structure using transmitting and reflected light.
A field emission scanning electron microscope (FE-SEM, HITACHI S-4700; Japan) fitted with an energy-dispersive spectrometer (EDS, NORAN NSS; Madison, USA) at the Institute of Geological Sciences, Jagiellonian University in Krakow, Poland, was used for detailed analysis of the BA samples’ phase and chemical composition. Both rough and polished preparations were coated with carbon or gold. An accelerating voltage of 20 kV and current of 10 μA were used for imaging and chemical analysis. Secondary electrons (SEs) and backscattered electrons (BSEs) were utilised for imaging. For the chemical analysis in spots, a 100 s acquisition time and the standardless method of quantification were used. Mapping of the selected chemical elements’ distribution was performed for the imaging of aluminium-rich compounds and their reaction products’ distribution.
3. Results and Discussion
3.1. Chemical Composition of BA
The results of the analyses of samples (Table 1 and Table 2) indicate variation in the chemical composition of the studied samples. In the chemical composition of the samples presented as the content of oxides, SiO2 dominates in all samples (56–63 wt%), followed by CaO as the second-most-important component (11.56–15.30 wt%). The variation can be related to changes in composition in the waste stream, as well as the BA treatment (Table 1). The ratio of Al/Fe for all samples is greater than 1 (1.01–2.86), except for samples RZ5 and RZ9 at 0.98 and 0.99, respectively. The BA sample collected without quenching exhibits the lowest LOI value. Samples after three months of ageing are characterised by significantly higher LOI values. The removal of ferrous and non-ferrous metals results in a lowering of the content of iron and aluminium (Table 2). For Fe, the process seems to be more effective (see sample RZ3, Table 2).

Table 2.
Chemical composition of BA samples (content of all components in wt%; except for Cu, Zn, and Pb, in mgkg−1; LOI—loss of ignition; samples’ symbols—see Table 1).
The hand picking of fragmented glass, ceramics, and metallic fragments results in a decrease in the SiO2 content and an increase in the Al2O3, Fe2O3, and CaO content (Table 1 and Table 2). These changes are related to the high frequency of glass (from 20 to over 52 wt%), ceramics (from 2.8 to 28 wt%), and metal fragments (from 0.6 to 3.9 wt%).
3.2. Mineral Composition of BA
Besides amorphous glassy material, the analysed BA contains quartz, wollastonite, diopside, calcite, anhydrite, mullite, and feldspar. Quartz and other minerals (e.g., zircon) are inherited from waste material, while others are formed in high-temperature processes.
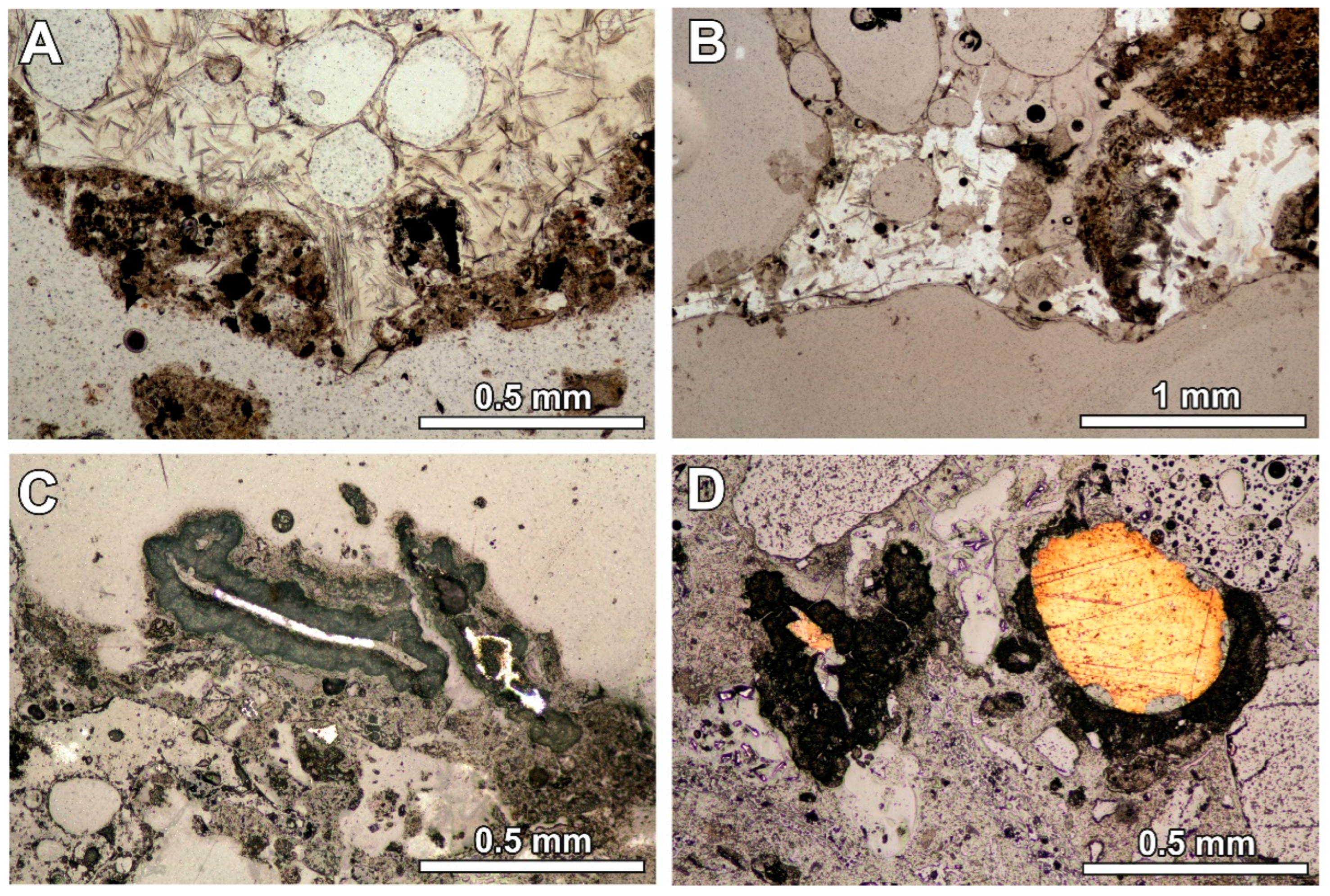
MSWI BA usually contains two phases—the phase formed during the solidification of melt and the phase formed during the quenching of the slag in water tanks [17,23]. In the studied BA, both phases are present (Figure 1A). The quench phase occurs around the glassy material. The quench phase was also noticed in the RZ11 sample (collected without quenching), but its content is significantly lower (Figure 1B), which is also confirmed by the lower LOI value (Table 2).
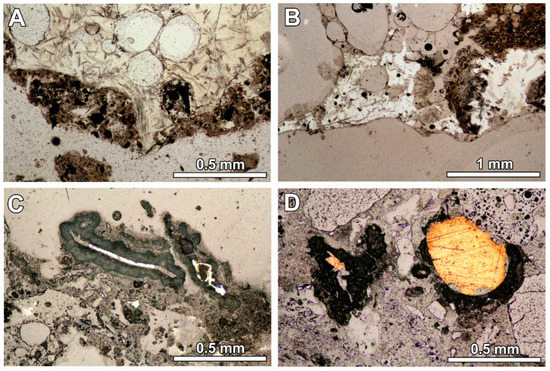
Figure 1.
(A) Glassy melt product with elongated crystals and quench product at the margin or as a separated grain (optical microscope image, transmitting light; sample RZ4); (B) glassy melt product with elongated crystals with the quench phase inside (optical microscope image, transmitting light; sample RZ11); (C) metallic fragment in the quench product surrounded by the reaction rim (optical microscope image, reflected light; sample RZ2); (D) reaction rim around the metallic fragment—the rim is absent at the point of contact with the glassy fragment (optical microscope image, reflected light; sample RZ10).
3.3. Form of Occurrence of Aluminium in BA
Aluminium is present in the studied samples in different forms. Below, we briefly present typical examples.
The content of aluminium in the glass phase in BA is commonly below 2 wt% (Figure 2A, Table 3), although higher values are also noted (ca. 4 wt%; Figure 2B, Table 3). Glass is dominated by silicon and calcium. Glass often contains detrital quartz grains, which are cracked during the rapid changes in temperature (Figure 2A), and rarely other detrital components (e.g., zircon, Figure 2A). Differences in the composition of the melt phase formed during waste incineration (and different aluminium content) are related to local variation in the waste composition.
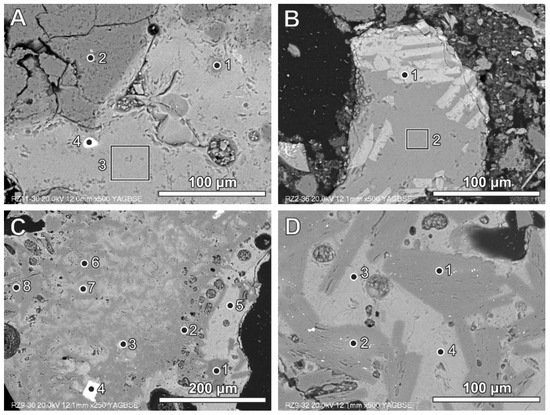
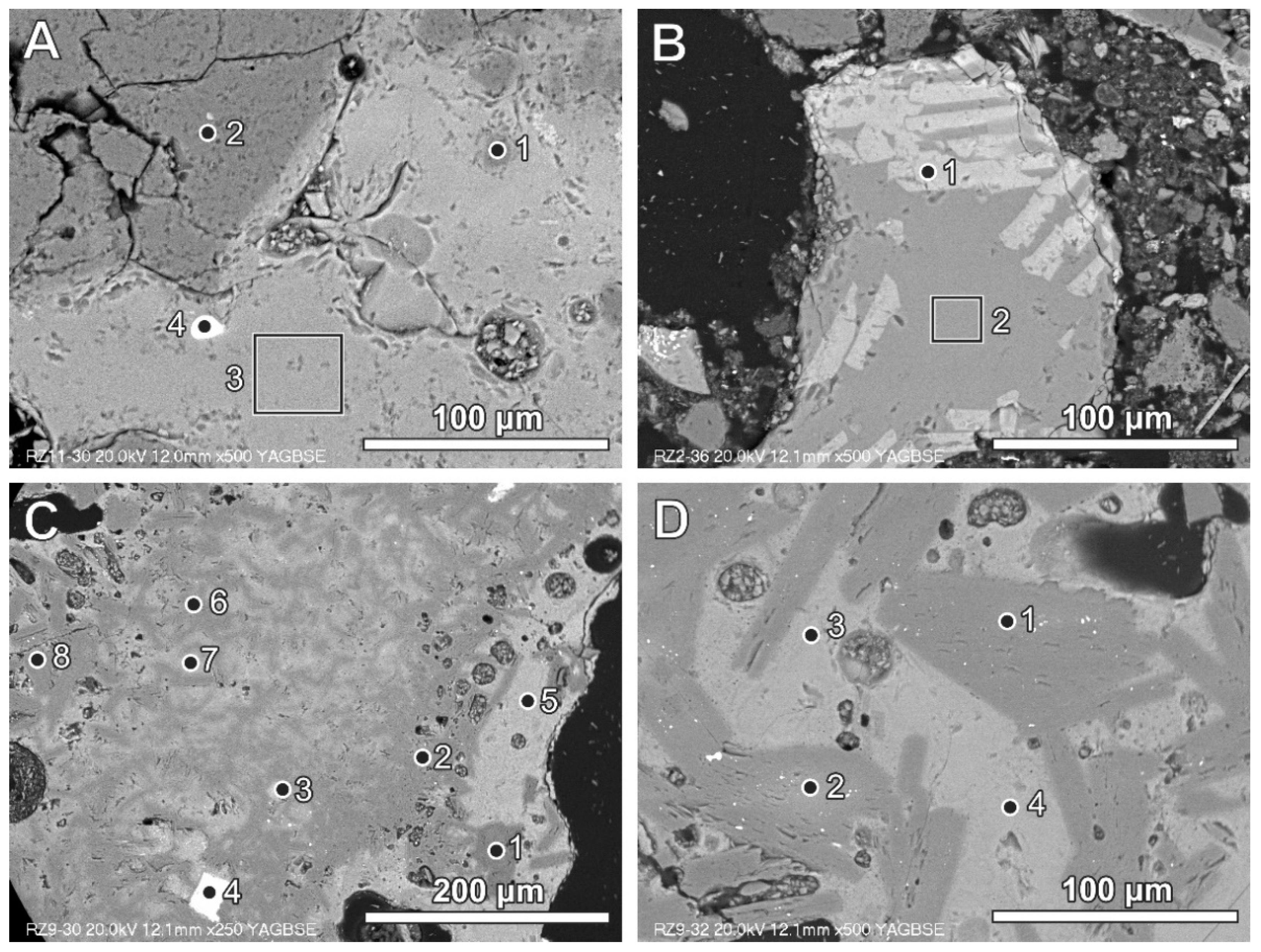
Figure 2.
(A) Glassy fragment in BA (bright grey) with quartz grains (dark grey) and zircon grain (white)—numbers of spots and area correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ11); (B) glass containing wollastonite crystals (bright lathes)—numbers of spots and area correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ2); (C) small Al-rich plates (dark grey) dispersed in glass (brighter grey)—numbers of spots and area correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ9); (D) Al-rich plates dispersed in the Al-, Si-, and Ca-rich phase—numbers of spots and area correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ9).
Aluminium can be noted in glass in the form of remains inherited after different products (e.g., aluminium foil or other aluminium packing materials). Aluminium-rich phases of this type commonly occur as platy forms of different size, often below 50 μm (Figure 2C,D; Table 3). The glass phase between aluminium-rich elongated fragments is also relatively rich in aluminium (Figure 2C,D; Table 3). This could be related to local enrichment of the melt phase in aluminium or partly to the analytical ambiguity caused by a mixed X-ray signal from the area situated in close proximity to aluminium-rich fragments. The aluminium-rich fragments (Figure 2C) differ in chemical composition from almost calcium-free (spot 1) to Ca-enriched (spots 2, 6, and 8), but with a low content of silicon to aluminium-, calcium-, and silicon-containing phases of variable composition (spots 7 and 5). Phases containing aluminium with calcium (and oxygen) represent Al-rich calcium aluminate (with non-stoichiometric composition) or a mixture of different calcium aluminates. Phases containing aluminium, silicon, and calcium locally exhibit the presence of iron, titanium, and magnesium (Figure 2C, Table 3). Similar aluminium-containing phases, but occurring in plates larger than 50 mm, are presented in Figure 2D.
Aluminium-rich components also occur as irregularly shaped forms present in glassy material (Figure 3 and Figure 4).
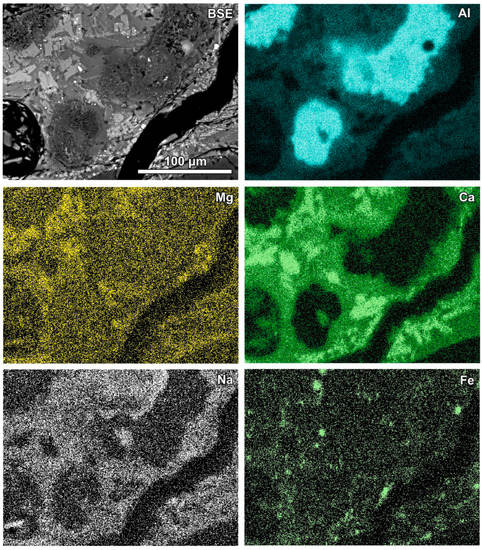
Figure 3.
Al-rich irregular fragments (dark grey in the SEM BSE image) in glass rich in wollastonite-type crystals; BSE and selected elements’ distribution images (sample RZ10).
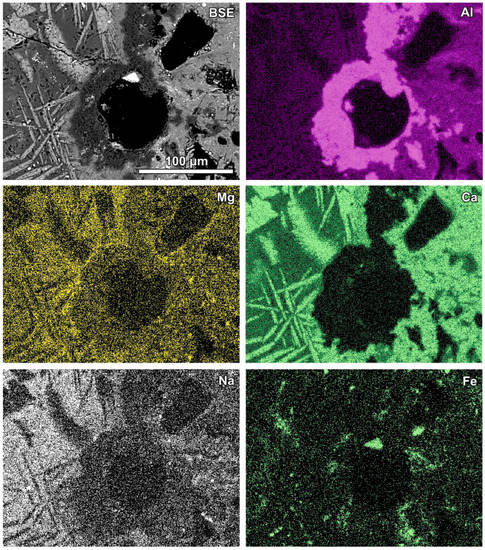
Figure 4.
Al-rich irregular fragments (dark grey in the SEM BSE image) around voids in glassy material; BSE and selected elements’ distribution images (sample RZ11).

Table 3.
EDS chemical analyses in spots and areas indicated in Figure 2 and Figure 5 (content in wt%; “-”— not determined).
| Figure (Sample) | Spot | Si | Al | Fe | Ti | Mg | Ca | Mn | Ba | K | Na | O | S | Cl | P | Zr | Cu | Cr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figure 2A (RZ11) | 1 | 66.69 | - | 0.20 | - | - | 0.22 | - | - | - | - | 32.89 | - | - | - | - | - | - | - |
| 2 | 66.08 | - | - | - | - | - | - | - | - | - | 33.92 | - | - | - | - | - | - | - | |
| 3 | 45.81 | 1.66 | 0.87 | 0.13 | 1.67 | 12.48 | - | 1.37 | 1.67 | 7.56 | 26.6 | 0.18 | - | - | - | - | - | - | |
| 4 | 16.88 | - | - | - | - | - | - | - | - | 0.22 | 15.4 | - | - | - | 67.50 | - | - | - | |
| Figure 2B (RZ2) | 1 | 30.47 | - | - | - | - | 48.83 | - | - | - | - | 20.70 | - | - | - | - | - | - | - |
| 2 | 39.83 | 3.65 | 1.19 | 0.91 | 1.08 | 12.21 | 4.08 | 10.85 | 25.61 | - | - | 0.59 | - | - | - | - | |||
| Figure 2C (RZ9) | 1 | 0.11 | 71.05 | 1.77 | - | - | 0.29 | 0.36 | - | - | - | 25.78 | - | - | 0.64 | - | - | - | - |
| 2 | 0.56 | 61.65 | - | - | - | 12.44 | - | - | - | - | 25.35 | - | - | - | - | - | - | - | |
| 3 | 21.57 | 15.80 | 5.33 | 9.18 | 0.31 | 12.17 | 1.86 | - | 0.78 | 0.61 | 13.1 | 0.13 | - | 9.19 | - | 9.97 | - | - | |
| 4 | 28.09 | 40.05 | 25.72 | - | - | 0.20 | 5.94 | - | - | - | - | - | - | - | - | - | - | - | |
| 5 | 15.08 | 26.26 | 2.30 | 1.01 | 1.15 | 27.37 | - | - | 0.74 | 1.39 | 24.09 | - | - | 0.61 | - | - | - | - | |
| 6 | 1.06 | 60.62 | - | - | - | 11.40 | - | - | - | - | 26.92 | - | - | - | - | - | - | - | |
| 7 | 7.80 | 46.10 | - | - | - | 25.75 | - | - | - | - | 19.07 | 0.19 | - | 1.09 | - | - | - | - | |
| 8 | 1.23 | 61.54 | - | - | - | 10.01 | - | - | - | - | 27.22 | - | - | - | - | - | - | - | |
| Figure 2D (RZ9) | 1 | - | 62.46 | - | - | - | 10.75 | - | - | - | - | 26.79 | - | - | - | - | - | - | - |
| 2 | 0.07 | 60.02 | - | - | - | 8.71 | - | - | - | - | 31.20 | - | - | - | - | - | - | - | |
| 3 | 26.91 | 12.55 | 2.43 | 4.18 | 1.77 | 23.38 | - | - | 1.01 | 2.67 | 24.65 | - | - | 0.45 | - | - | - | - | |
| 4 | 25.56 | 12.55 | 2.86 | 4.08 | 2.01 | 24.53 | - | - | 0.82 | 2.57 | 24.43 | - | - | - | 0.59 | - | - | - | |
| Figure 5A (RZ10) | 1 | - | 98.93 | - | - | - | 0.24 | - | - | - | - | 0.83 | - | - | - | - | - | - | - |
| 2 | 1.14 | 52.66 | - | 0.91 | - | 0.65 | - | - | 0.60 | 1.37 | 40.53 | 0.71 | 1.29 | - | 0.14 | - | - | - | |
| 3 | 0.88 | 51.60 | 1.36 | - | - | 6.81 | - | - | 0.26 | 0.52 | 36.49 | 1.24 | 0.84 | - | - | - | - | - | |
| 4 | 1.25 | 8.29 | - | - | - | 38.75 | - | - | - | - | 35.34 | 15.93 | 0.44 | - | - | - | - | - | |
| 5 | 0.13 | 12.89 | - | - | - | 50.61 | - | - | - | - | 20.52 | 0.16 | 15.69 | - | - | - | - | - | |
| Figure 5B (RZ11) | 1 | 1.44 | 92.97 | - | - | - | - | - | - | - | - | 5.59 | - | - | - | - | - | - | - |
| 2 | 6.54 | 61.73 | 10.79 | - | - | - | 19.88 | - | - | - | 0.45 | - | - | - | - | - | 0.61 | - | |
| 3 | 2.22 | 36.07 | - | - | - | 3.69 | 0.44 | - | - | - | 54.73 | 0.42 | - | - | - | - | - | 2.43 | |
| 4 | - | 40.85 | - | - | - | 0.53 | - | - | - | - | 58.62 | - | - | - | - | - | - | - | |
| Figure 5C (RZ3) | 1 | 0.60 | 42.31 | 1.98 | 0.36 | - | 11.97 | - | - | 0.30 | 0.19 | 36.92 | 3.14 | 2.23 | - | - | - | - | - |
| 2 | - | 56.81 | 0.81 | - | - | 3.20 | - | - | 0.23 | - | 36.84 | 0.37 | 1.74 | - | - | - | - | - | |
| 3 | 1.06 | 13.54 | - | - | - | 48.21 | - | - | - | - | 21.30 | 0.29 | 15.6 | - | - | - | - | - | |
| 4 | 0.43 | 35.74 | - | - | - | 17.73 | - | - | - | - | 41.48 | 0.28 | 4.34 | - | - | - | - | - | |
| 5 | 7.29 | 5.71 | 43.79 | 1.62 | 0.80 | 22.56 | 0.93 | - | - | - | 16.84 | - | 0.46 | - | - | - | - | - | |
| 6 | 18.03 | 11.49 | 0.63 | - | 1.23 | 48.07 | - | - | - | - | 20.55 | - | - | - | - | - | - | - | |
| Figure 5D (RZ3) | 1 | 0.41 | 13.36 | - | - | - | 47.39 | - | - | - | - | 26.72 | 0.38 | 11.74 | - | - | - | - | - |
| 2 | 2.17 | 8.30 | - | - | 0.58 | 42.17 | - | - | 1.10 | 1.10 | 29.35 | 12.37 | 2.57 | 0.29 | - | - | - | - |
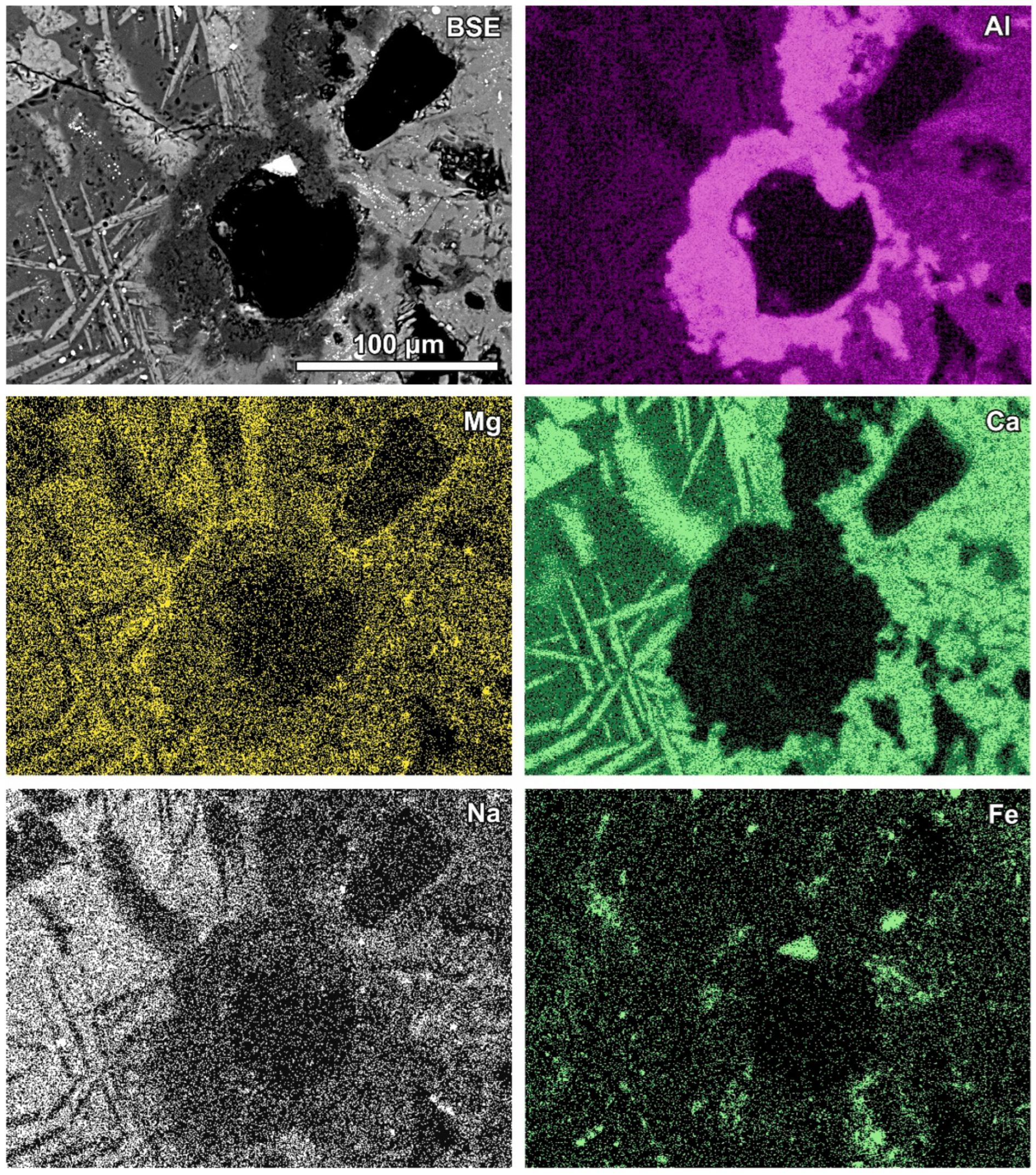
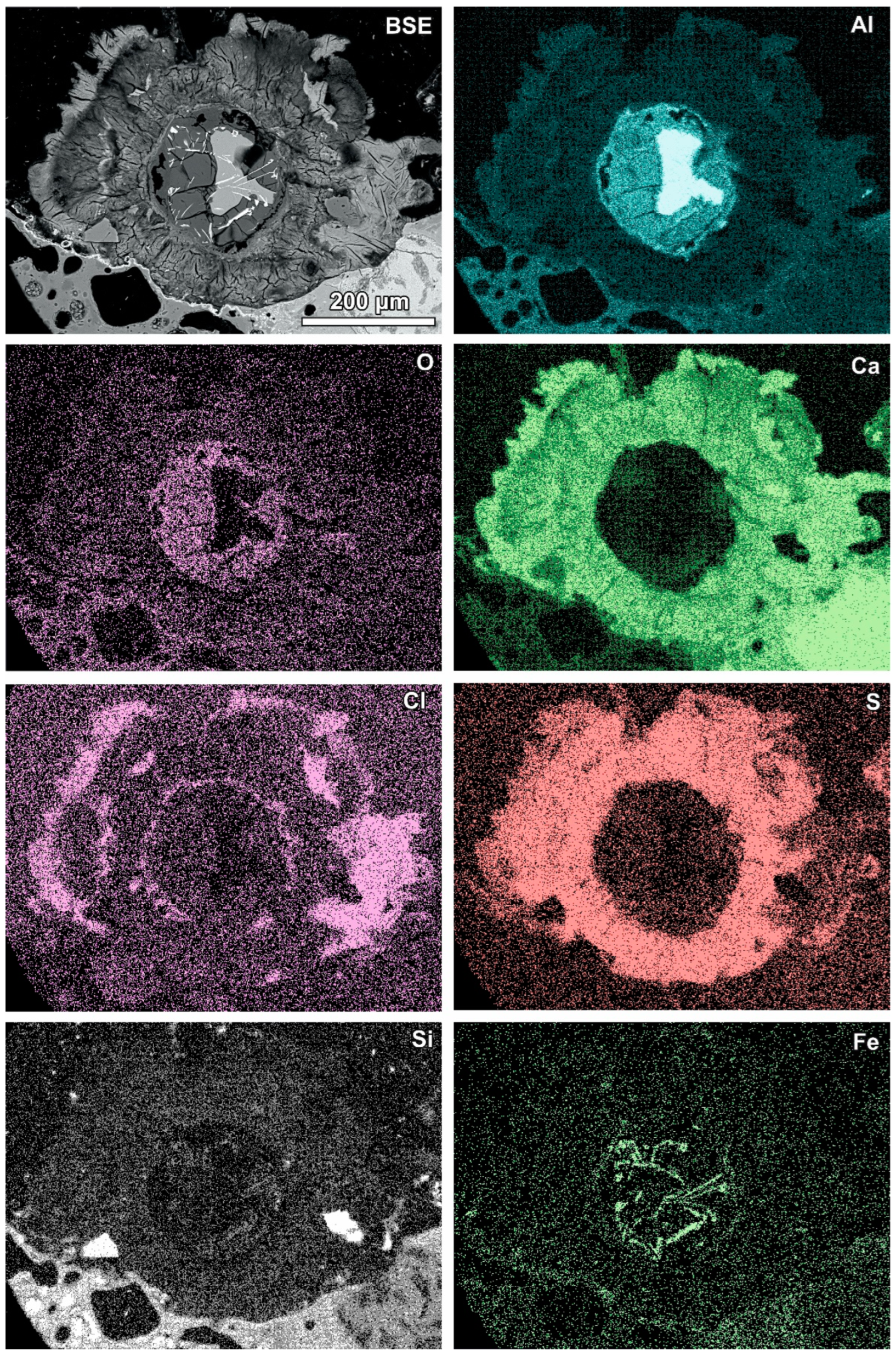
Aluminium-rich components in the quench component or in fine-grained ash material are characterised by circular reaction rims (Figure 5, Table 3). Different stages of the transformation process can be observed from slightly advanced with a metallic aluminium core inside to complete substitution by secondary products (Figure 5). Figure 5A represents a relatively rare case of reaction rims around almost non-oxidised metallic aluminium (spot 1, Table 3). The metallic aluminium is surrounded by the oxidised phase (spot 2, Figure 5A), probably aluminium hydroxide (gibbsite). The rim is characterised by an irregular net of cracks (Figure 5A) and veins of the iron-rich phase exsolved in aluminium passing from the metallic aluminium core, similarly to the structure described by [17]. The rim also contains chlorine, sulphur, and a low content of aluminium. The next rim is very thin and more rich in calcium (spot 3). The following rim is relatively broad (up to 100 μm) and characterised by a high content of sulphur and calcium, but lower in comparison with the internal rims’ content of aluminium (spot 4, Figure 5A). A high content of S and Ca can suggest the occurrence of ettringite or calcium sulphate in the mixture with the calcium and aluminium phase, while the presence of other components in the mixture cannot be excluded (e.g., kuzelite Ca4Al2(OH)12[SO4]·6H2O or Kuzel’s salt 3Ca·Al2O3·½ CaSO4·½ CaCl2·11H2O). The outermost rim contains less sulphur, but significantly more chlorine (spot 5, Figure 5A). The composition of this rim corresponds to the Friedel’s salt. The formation of the Friedel’s salt in calcium-aluminate-rich material in the reaction with chloride solution is a typical process occurring in cements [26,27]. The transition from a sulphur-rich rim to a chlorine-dominated rim can be related to an increase in the chlorine concentration in the solution or to a depletion of S during rim formation.
A relatively simple reaction rim composed of oxidised aluminium (possibly hydroxide) is presented in Figure 5B (spot 2 and 3; Table 3). The rim is formed around slightly oxidised aluminium (spot 1, Figure 5B; Table 3). Compared with the previous example (Figure 5A), a lack of calcium-, sulphur-, or chlorine-containing rims is visible. The RZ11 sample was collected without quenching in a water tank, thus it solidified without contact with calcium-, sulphur-, and chlorine-containing solution.
At a more advanced stage of exchange reactions, the core of the Al-rich form is composed of material containing aluminium, calcium, oxygen, sulphur, and chlorine (spot 1, Figure 5C; Table 3). Only small fragments of the material poor in calcium are noted (spot 1, Figure 5C; Table 3). The following zones are characterised by a variable proportion between aluminium and calcium, with a relatively broad zone of the composition similar to the Friedel’s salt (spot 3, Figure 5C; Table 3). The irregular accumulation of material rich in Fe or Si can be noted (spot 5 and 6, Figure 5C; Table 3).
In the final stage of the exchange reaction, patches of the material of a composition similar to the Friedel’s salt (spot 1, Figure 5D; Table 3) are present, including S-rich material (phase of a chemical composition similar to ettringite) (area 2, Figure 5D; Table 3).
The abovementioned results indicate that of most importance to the transformation of aluminium (or aluminium-rich) metallic components in BA is the composition of the quench water. Newly formed phases around the metallic core are rich in calcium, chlorine, or sulphur, which indicates a high concentration of these components in the solution. The BA is a Ca-rich material with CaO content varying between 11.56 and 15.36 wt% (Table 2), the second-most-dominant element after SiO2. It is possible to assume that Ca2+ can be present in the solution in a relatively high concentration after the reaction of the molten slag with the quench water. The sulphur content is between 0.26 and 0.55 wt% (Table 2), which is related to the primary content of sulphur in the BA and the exchange efficiency during the slag–water reaction. According to [27,28], the presence of the Friedel’s salt or chlorides can be related to the significant concentration of Cl− and SO42− in quench water.
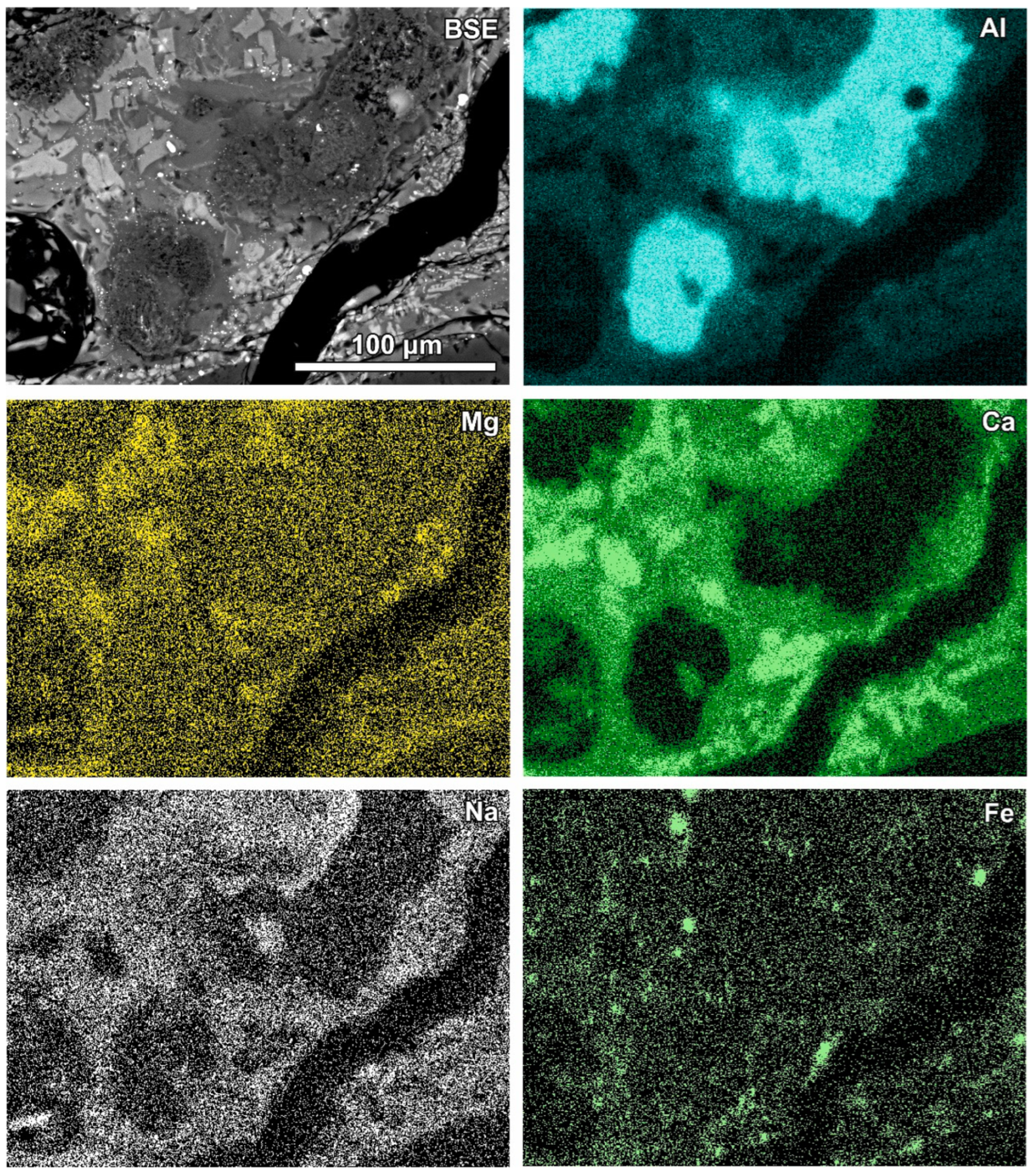
Newly formed phases are usually present as more or less circular rims (Figure 6, Figure 7 and Figure 8). A reaction rim composed mainly of material containing calcium and sulphur (probably sulphates) is presented in Figure 6. The content of aluminium in this material is low. The rim containing more chlorine is incomplete and very thin. A more complex reaction zone contains two chlorine- and two sulphur-rich rims (Figure 7). One chlorine-rich rim occurs directly on an oxidised aluminium core, while the second is separated from the first by a rim rich in sulphur. The rims rich in chlorine contain more aluminium in comparison with the sulphur-rich rims. The most complex noted reaction zone contains three chlorine-rich rims separated by sulphur-rich material (Figure 8). Similarly to the previous example, the rims rich in chlorine are enriched in aluminium in contrast to the sulphur-containing material.
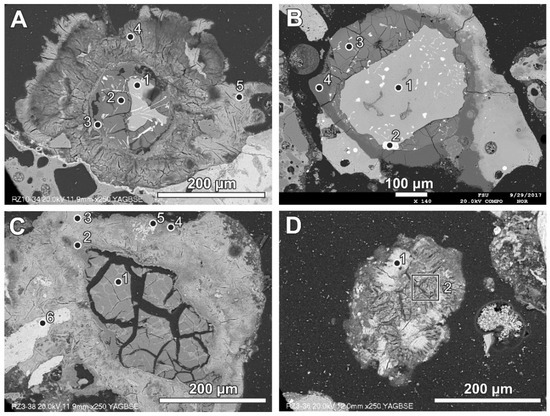
Figure 5.
(A) Reaction rims around metallic Al (also see Figure 8)—numbers of spots correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ10); (B) relatively simple reaction rim around a slightly oxidised Al core—numbers of spots correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ11); (C,D) advanced stages of Al components’ transformation—numbers of spots and areas correspond to the analyses presented in Table 3 (SEM BSE images, sample RZ3).
Figure 5.
(A) Reaction rims around metallic Al (also see Figure 8)—numbers of spots correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ10); (B) relatively simple reaction rim around a slightly oxidised Al core—numbers of spots correspond to the analyses presented in Table 3 (SEM BSE image, sample RZ11); (C,D) advanced stages of Al components’ transformation—numbers of spots and areas correspond to the analyses presented in Table 3 (SEM BSE images, sample RZ3).
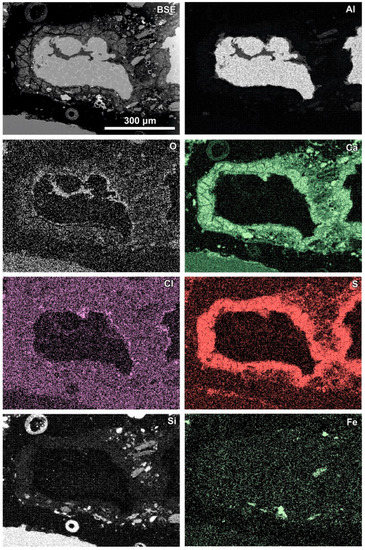
Figure 6.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ3.
Figure 6.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ3.
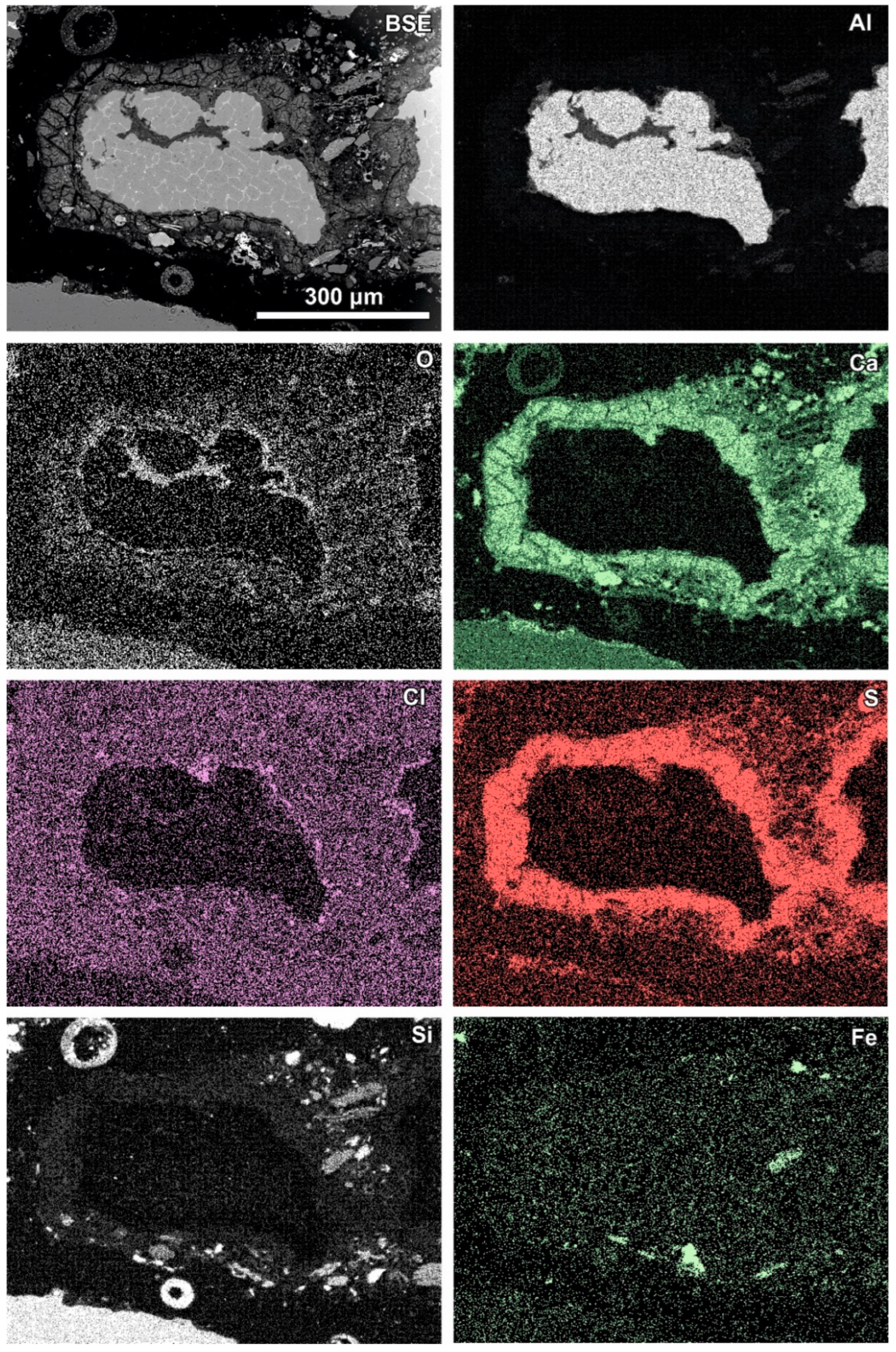
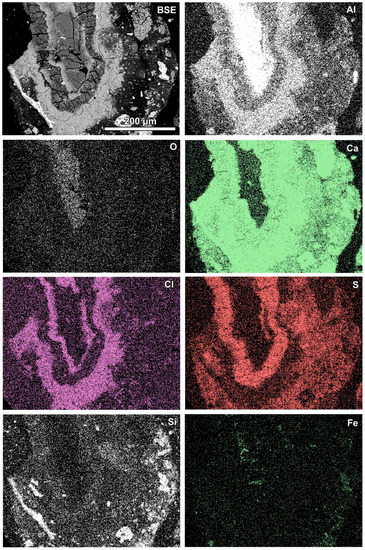
Figure 7.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ4.
Figure 7.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ4.
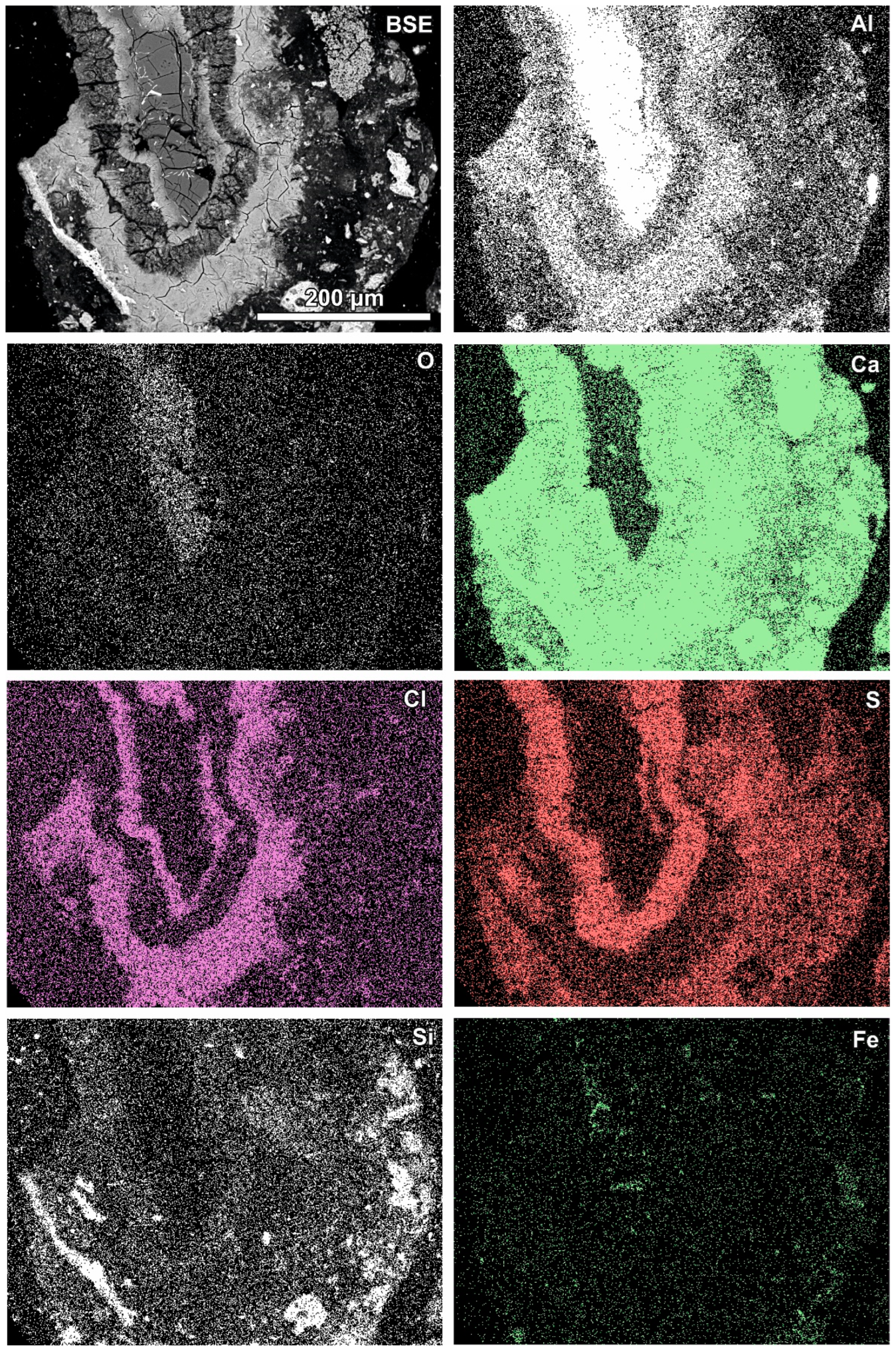
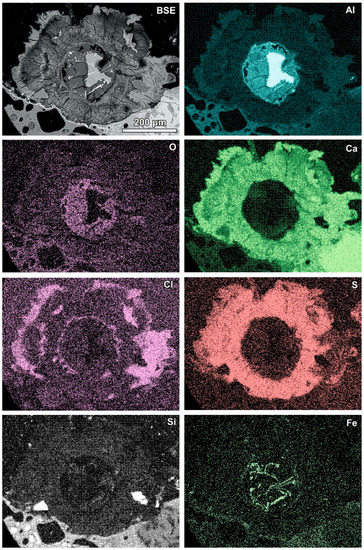
Figure 8.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ10.
Figure 8.
SEM BSE image of the reaction zone and distribution of selected elements—sample RZ10.
Variation in the composition of the reaction zones forming regular circular structures is probably caused by fluctuation in the quench water composition during the addition of a new portion of molten slag. The range of fluctuations can differ during the progression of the waste incineration process, which results in differences between different samples.
The reaction zones formed around aluminium-rich cores are generally similar to those described in the literature, but differ owing to the much higher content of Ca, S, and Cl [17,29]. The described reaction zones correspond to the corrosion textures analysed by [15], formed in the presence of salt in pore water. Yang et al. [30] compared air-cooled and water-quenched MSWI BA. The difference between the reaction zones in the RZ11 sample (without water quenching) and other samples is much more significant. This can be related to a higher concentration of elements in the quench water or a longer residence time of the slag in the quench water tank.
The described forms of occurrence of aluminium in the BA (in glass representing solidified melt phase) and in the quench phase exhibit a very strong variation in composition and size, which indicates that the attempts of aluminium recovery appear to be inefficient.
Other utilisation methods of aluminium metallic components in BA can be considered. Several authors describe hydrogen production during the reaction of metallic aluminium and water [16,17,18]. The usage of the described BA for hydrogen production was not tested, but a high degree of transformation of metallic aluminium to various secondary phases significantly reduced the possible effectiveness of the process. It is worth mentioning that metallic aluminium or slightly oxidised aluminium is shielded against contact with water by secondary products (see the process of passivation described by [17]). It is possible to conclude that hydrogen was spontaneously produced in the reaction of the slag with quench water and dispersed in the atmosphere.
MSWI BA can be used as an additive for concrete or in road construction [31,32]. However, the presence of metallic aluminium is a factor limiting this application because of the potential for hydrogen gas emission after contact with water in the alkaline environment and the lowering of the mechanical properties of the material [33,34,35]. The evolution of hydrogen gas before the adding of MSWI BA to the concrete mixture allows hydrogen bubbles in concrete to be avoided [36,37]. It is possible to conclude that the studied material had previously passed the hydrogen-generation stage.
4. Conclusions
- Aluminium-rich components occurring in the studied MSWI BA contain both the melt phase and quench phase.
- Aluminium-rich components occurring in the solid phase formed from melt (i.e., in glass) are often oxidised and occur in platy or irregular forms. The Al content in the glass phase is low (usually below 2 wt%).
- Aluminium components in the quench phase are significantly transformed with an abundance of Cl and SO42− phases, which is a result of the reaction with water in a tank during cooling. The volume of metallic or slightly oxidised cores is strongly reduced.
- The broad variation in the composition of aluminium components makes its recovery challenging and inefficient.
- The high degree of transformation of metallic aluminium fragments indicates that the potential for hydrogen generation is limited.
Author Contributions
Conceptualization, M.M., M.K. and P.K.; methodology, M.M., M.K., B.K. and P.K.; validation, M.M. and M.K.; investigation, P.K., B.K., M.K. and M.M.; resources, P.K., M.K., B.K. and M.M.; data curation, P.K. and M.K.; writing—original draft preparation, M.M.; writing—review and editing, M.M.; supervision, M.M. and M.K.; project administration, M.M. and M.K.; funding acquisition M.M. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Polish National Science Centre (NCN) grant no. UMO-2014/15/B/ST10/04171. The Open Access publication of this paper has been supported by a grant from the Faculty of Geography and Geology under the Strategic Programme Excellence Initiative at Jagiellonian University.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Waldemar Obcowski for the preparation of figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, G.; Müller, D.B. Addressing sustainability in the aluminum industry: A critical review of life cycle assessments. J. Clean. Prod. 2012, 35, 108–117. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Mapping the Global Flow of Aluminum: From Liquid Aluminum to End-Use Goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Gökelma, M.; Vallejo-Olivares, A.; Tranell, G. Characteristic properties and recyclability of the aluminium fraction of MSWI bottom ash. Waste Manag. 2021, 130, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mehr, J.; Haupt, M.; Skutan, S.; Morf, L.; Adrianto, L.R.; Weibel, G.; Hellweg, S. The environmental performance of enhanced metal recovery from dry municipal solid waste incineration bottom ash. Waste Manag. 2021, 119, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development, World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Calder, G.V.; Stark, T.D. Aluminum reactions and problems in municipal solid waste landfills. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2010, 14, 258–265. [Google Scholar] [CrossRef]
- Buryan, P.; Hlinčík, T. Aluminium-containing municipal-waste ash and the greenhouse effect. Pol. J. Environ. Stud. 2020, 29, 1095–1100. [Google Scholar] [CrossRef]
- Berkhout, S.P.M.; Oudenhoven, B.P.M.; Rem, P.C. Optimizing non-ferrous metal value from MSWI bottom ashes. J. Environ. Prot. 2011, 2, 564–570. [Google Scholar] [CrossRef]
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Grosso, M.; Biganzoli, L.; Rigamonti, L. A quantitative estimate of potential aluminium recovery from incineration bottom ashes. Resour. Conserv. Recycl. 2011, 55, 1178–1184. [Google Scholar] [CrossRef]
- Astrup, T.; Muntoni, A.; Polettini, A.; Pomi, R.; Van Gerven, T.; Van Zomeren, A. Treatment and reuse of Incineration bottom ash. In Environmental Materials and Waste, Resource Recovery and Pollution Prevention; Prasad, M.N.V., Shih, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 607–645. [Google Scholar] [CrossRef]
- Biganzoli, L.; Grosso, M. Aluminium recovery from waste incineration bottom ash, and its oxidation level. Waste Manag. Res. 2013, 31, 954–959. [Google Scholar] [CrossRef]
- Alam, Q.; Hendrix, Y.; Thijs, L.; Lazaro, A.Q.; Schollbach, K.; Brouwers, H.J.H. Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash. J. Clean. Prod. 2019, 211, 874–883. [Google Scholar] [CrossRef]
- Czop, M.; Łaźniewska-Piekarczyk, B.; Kajda-Szczęsniak, M. Analysis of the Possibility of Using Slags from the Thermal Treatment of Municipal Waste as Potential Component of Cement—Case Study. Materials 2021, 14, 6491. [Google Scholar] [CrossRef] [PubMed]
- Heuss-Aßbichler, S.; Magel, G.; Fehr, K.T. Abiotic hydrogen production in fresh and altered MSWI-residues: Texture and microstructure investigation. Waste Manag. 2010, 30, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Ilyas, A.; van Praagh, M.; Persson, K.M.; Grosso, M. Aluminium recovery vs. hydrogen production as resource recovery options for fine MSWI bottom ash fraction. Waste Manag. 2013, 33, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, A.; Arumugam, N.; Shimaoka, T. Aluminum and aluminum alloys in municipal solid waste incineration (MSWI) bottom ash: A potential source for the production of hydrogen gas. Int. J. Hydrogen Energy 2016, 41, 820–831. [Google Scholar] [CrossRef]
- Nithiya, A.; Saffarzadeh, A.; Shimaoka, T. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (MSWI) bottom ash. Waste Manag. 2017, 73, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Ramírez, J.M.; Marroquín de Jesús, Á.; Jiménez-Sandoval, O.; Pless, R.C. Hydrogen Generation by Treatment of Aluminium Metal with Aqueous Solutions: Procedures and Uses. In Hydrogen Energy-Challenges and Perspectives; Minic, D., Ed.; InTech: London, UK, 2012; pp. 55–76. [Google Scholar] [CrossRef]
- Larsson, R. Energy Recovery of Metallic Aluminium in MSWI Bottom Ash Different Approaches to Hydrogen Production from MSWI Bottom Ash: A Case Study. Masters’s Thesis, Tekniska Högskolan Umeå Universitet, Umeå, Sweden, 2014; 24p. [Google Scholar]
- Mizutani, S.; Sakai, S.; Takatsuki, H. Investigation of hydrogen generation from municipal solid waste incineration fly ash. J. Mater. Cycles Waste Manag. 2000, 2, 16–23. [Google Scholar]
- Rumayor, M.; Corredor, J.; Rivero, M.J.; Ortiz, I. Prospective Life Cycle Assessment of Hydrogen Production by Waste Photoreforming. J. Clean. Prod. 2022, 336, 130430. [Google Scholar] [CrossRef]
- Arm, M.; Lindeberg, J.; Rodin, Å.; Öhrström, A.; Backman, R.; Öhman, M.; Boström, D. Gasbildning i Aska (Gas Generation in Incinerator Ash); Värmeforsk Service AB: Stockholm, Sweden, 2006; 158p. [Google Scholar]
- Joseph, A.M.; Snellings, R.; van den Heede, P.; Matthys, S.; De Belie, N. The Use of Municipal SolidWaste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef]
- Bawab, J.; Khatib, J.; Kenai, S.; Sonebi, M. A Review on Cementitious Materials Including Municipal Solid Waste Incineration Bottom Ash (MSWI-BA) as Aggregates. Buildings 2021, 11, 179. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Inkaew, K.; Saffarzadeh, A.; Shimaoka, T. Modeling the formation of the quench product in municipal solid waste incineration (MSWI) bottom ash. Waste Manag. 2016, 52, 159–168. [Google Scholar] [CrossRef]
- Saffarzadeh, A.; Arumugam, N.; Shimaoka, T. Hydrogen generation from aluminum-water reactions in municipal solid waste incineration (MSWI) bottom ash. In Proceedings of the 6th International Symposium on Energy from Biomass and Waste, Venice, Italy, 14–17 November 2016; CISA Publisher: Venice, Italy, 2016. [Google Scholar]
- Yang, S.; Saffarzadeh, A.; Shimaoka, T.; Kawano, T.; Kakuta, Y. The impact of thermal treatment and cooling methods on municipal solid waste incineration bottom ash with an emphasis on Cl. Environ. Technol. 2016, 37, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Hjelmar, O.; Holma, J.; Crillesen, K. Utilisation of MSWI bottom ash as sub-base in road construction: First results from a large-scale test site. J. Hazard. Mater. 2007, A139, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.H.; Nam, B.H.; An, J.; Youn, H. Municipal Solid Waste Incineration (MSWI) Ashes as Construction Materials—A Review. Materials 2020, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Rübner, K. The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component. Cem. Concr. Res. 2006, 36, 1434–1443. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Municipal solid waste incineration bottom ash recycling in concrete: Preliminary approach with Oporto wastes. Constr. Building Mater. 2022, 323, 126548. [Google Scholar] [CrossRef]
- Vaičienė, M.; Simanavičius, E. The Effect of Municipal Solid Waste Incineration Ash on the Properties and Durability of Cement Concrete. Materials 2022, 15, 4486. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Cassago, D.; Curzio, A.Q.; Collepardi, M. MSWI ashes as mineral additions in concrete. Cem. Concr. Res. 2004, 34, 1899–1906. [Google Scholar] [CrossRef]
- Alderete, N.M.; Joseph, A.M.; Van den Heede, P.; Matthys, S.; De Belie, N. Effective and sustainable use of municipal solid waste incineration bottom ash in concrete regarding strength and durability. Resour. Conserv. Recycl. 2021, 167, 105356. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).