Dominant Processing Factors in Two-Step Fabrication of Pure Sulfide CIGS Absorbers
Abstract
:1. Introduction
2. Materials and Methods
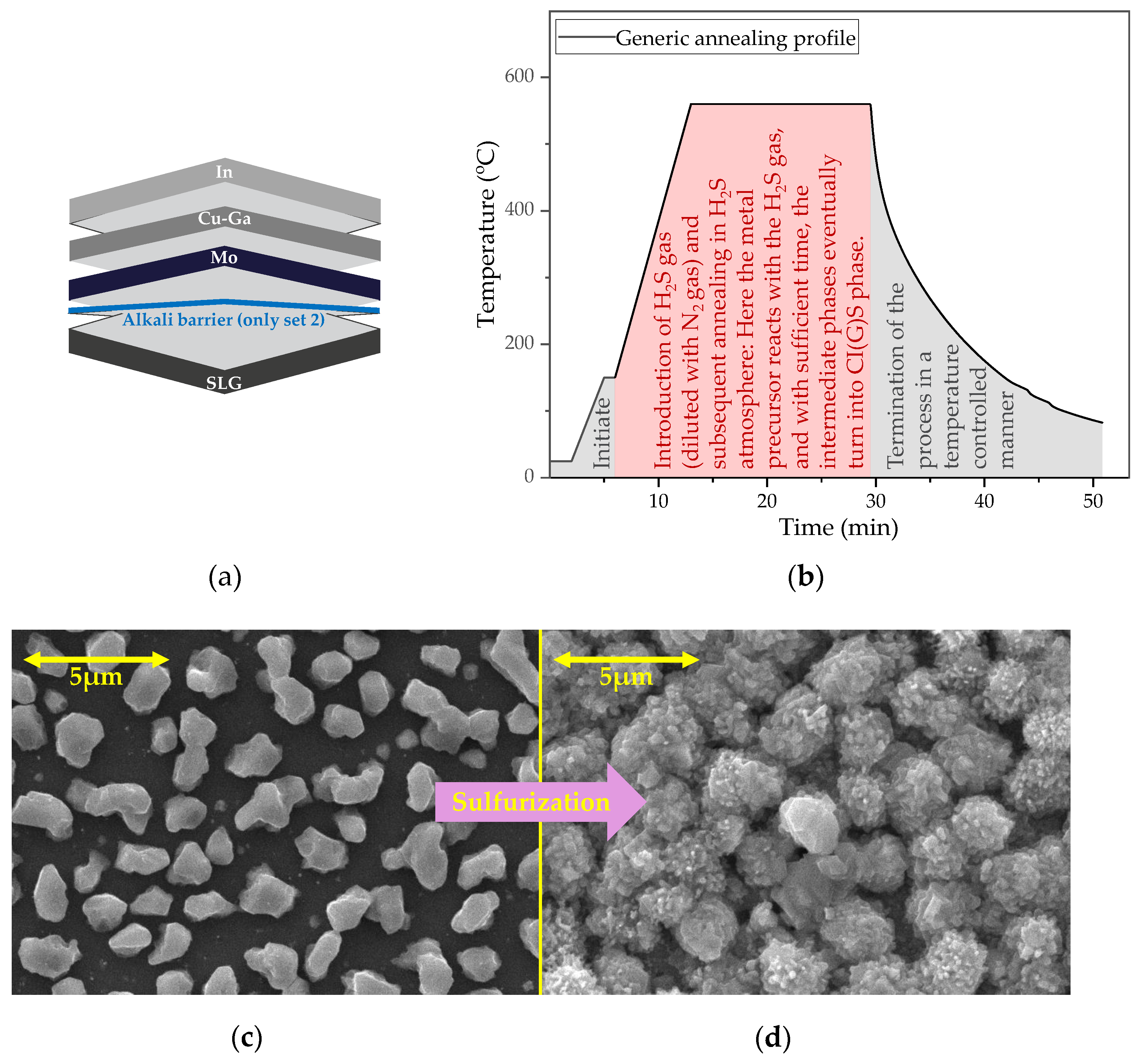
2.1. The Precursors
2.2. The Absorber Layers
2.3. The Solar Cell Devices
3. Results and Discussion
3.1. The Absorber Layers
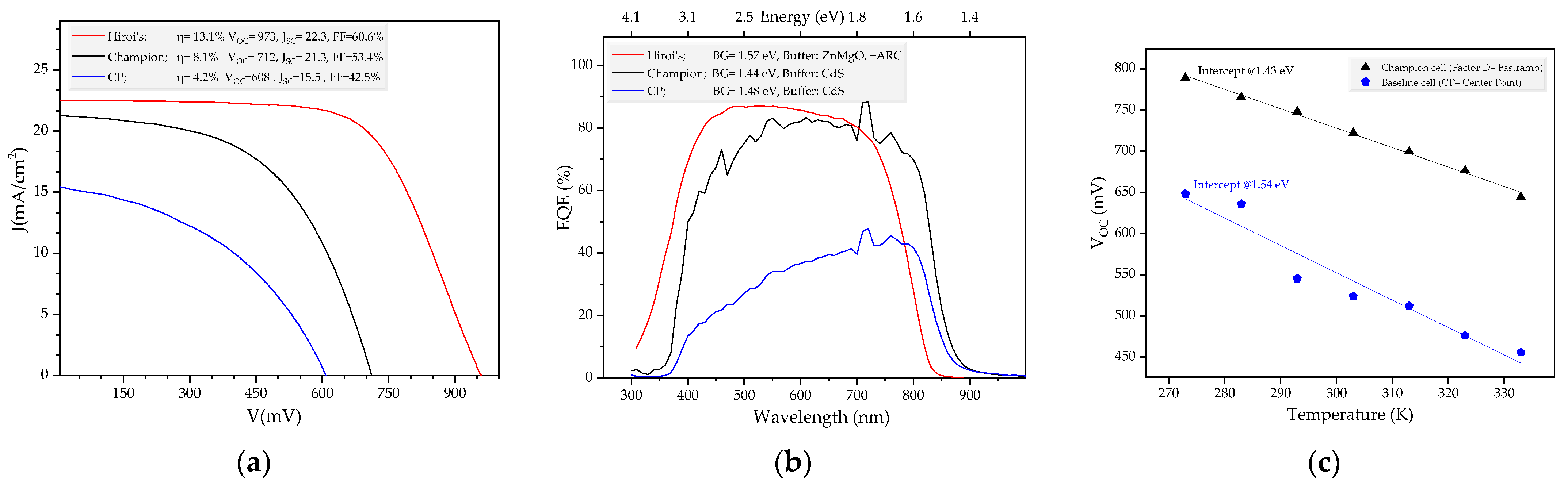
3.2. The solar Cell Devices
3.3. Curvature in Response
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kageshima, Y.; Shiga, S.; Kumagai, H.; Teshima, K.; Domen, K.; Nishikiori, H. Photoelectrochemical Properties of Particulate CuGaSe2 and CuIn0. 7Ga0. 3Se2 Photocathodes in Nonaqueous Electrolyte. Bull. Chem. Soc. Jpn. 2020, 93, 942–948. [Google Scholar] [CrossRef]
- Yuan, J.; Hao, C. Solar-Driven Photoelectrochemical Reduction of Carbon Dioxide to Methanol at CuInS2 Thin Film Photocathode. Sol. Energy Mater. Sol. Cells 2013, 108, 170–174. [Google Scholar] [CrossRef]
- Feurer, T.; Reinhard, P.; Avancini, E.; Bissig, B.; Löckinger, J.; Fuchs, P.; Carron, R.; Weiss, T.P.; Perrenoud, J.; Stutterheim, S. Progress in Thin Film CIGS Photovoltaics–Research and Development, Manufacturing, and Applications. Prog. Photovolt. Res. Appl. 2017, 25, 645–667. [Google Scholar] [CrossRef]
- Tell, B.; Shay, J.L.; Kasper, H.M. Electrical Properties, Optical Properties, and Band Structure of CuGaS2 and CuInS2. Phys. Rev. B 1971, 4, 2463–2471. [Google Scholar] [CrossRef]
- Tinoco, T.; Rincón, C.; Quintero, M.; Pérez, G.S. Phase Diagram and Optical Energy Gaps for CuInyGa1−ySe2 Alloys. Phys. Status Solidi A 1991, 124, 427–434. [Google Scholar] [CrossRef]
- Guillemoles, J.; Rau, U.; Kronik, L.; Schock, H.; Cahen, D. Cu(In,Ga)Se2 Solar Cells: Device Stability Based on Chemical Flexibility. Adv. Mater. 1999, 11, 957–961. [Google Scholar] [CrossRef]
- de Wild-Scholten, M.J. Energy Payback Time and Carbon Footprint of Commercial Photovoltaic Systems. Sol. Energy Mater. Sol. Cells 2013, 119, 296–305. [Google Scholar] [CrossRef]
- Jordan, D.C.; Kurtz, S.R.; VanSant, K.; Newmiller, J. Compendium of Photovoltaic Degradation Rates. Prog. Photovolt. Res. Appl. 2016, 24, 978–989. [Google Scholar] [CrossRef]
- Brenner, W.; Adamovic, N. Thin-Film CIGS Solar Modules for Design Driven Applications in the Frame of the FP7 NMP Project SolarDesign. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Oatija, Croatia, 25–29 May 2015; pp. 131–136. [Google Scholar]
- Kazmerski, L.L.; Sanborn, G.A. CuInS2 Thin-film Homojunction Solar Cells. J. Appl. Phys. 1977, 48, 3178–3180. [Google Scholar] [CrossRef]
- Hiroi, H.; Iwata, Y.; Adachi, S.; Sugimoto, H.; Yamada, A. New World-Record Efficiency for Pure-Sulfide Cu(In,Ga)S2 Thin-Film Solar Cell With Cd-Free Buffer Layer via KCN-Free Process. IEEE J. Photovolt. 2016, 6, 760–763. [Google Scholar] [CrossRef]
- Nishimura, T.; Kim, S.; Chantana, J.; Kawano, Y.; Ishizuka, S.; Minemoto, T. Application of Al-Doped (Zn, Mg)O on Pure-Sulfide Cu(In, Ga)S2 Solar Cells for Enhancement of Open-Circuit Voltage. Sol. Energy Mater. Sol. Cells 2019, 202, 110157. [Google Scholar] [CrossRef]
- Shukla, S.; Sood, M.; Adeleye, D.; Peedle, S.; Kusch, G.; Dahliah, D.; Melchiorre, M.; Rignanese, G.-M.; Hautier, G.; Oliver, R.; et al. Over 15% Efficient Wide-Band-Gap Cu(In,Ga)S2 Solar Cell: Suppressing Bulk and Interface Recombination through Composition Engineering. Joule 2021, 1816–1831. [Google Scholar] [CrossRef]
- Kim, S.; Nagai, T.; Tampo, H.; Ishizuka, S.; Shibata, H. Large Open-Circuit Voltage Boosting of Pure Sulfide Chalcopyrite Cu(In,Ga)S2 Prepared Using Cu-Deficient Metal Precursors. Prog. Photovolt. Res. Appl. 2020, 28, 816–822. [Google Scholar] [CrossRef]
- He, G.; Yan, C.; Li, J.; Yuan, X.; Sun, K.; Huang, J.; Sun, H.; He, M.; Zhang, Y.; Stride, J.A.; et al. 11.6% Efficient Pure Sulfide Cu(In,Ga)S2 Solar Cell through a Cu-Deficient and KCN-Free Process. ACS Appl. Energy Mater. 2020, 3, 11974–11980. [Google Scholar] [CrossRef]
- Merdes, S.; Abou-Ras, D.; Mainz, R.; Klenk, R.; Lux-Steiner, M.C.; Meeder, A.; Schock, H.W.; Klaer, J. CdS/Cu(In,Ga)S2 Based Solar Cells with Efficiencies Reaching 12.9% Prepared by a Rapid Thermal Process. Prog. Photovolt. Res. Appl. 2013, 21, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Lomuscio, A.; Rödel, T.; Schwarz, T.; Gault, B.; Melchiorre, M.; Raabe, D.; Siebentritt, S. Quasi-Fermi-Level Splitting of Cu-Poor and Cu-Rich CuInS2 Absorber Layers. Phys. Rev. Appl. 2019, 11, 054052. [Google Scholar] [CrossRef] [Green Version]
- Kwon, I.; Nagai, T.; Ishizuka, S.; Tampo, H.; Shibata, H.; Kim, S.; Kim, Y. Improving the Performance of Pure Sulfide Cu(InGa)S2 Solar Cells via Injection Annealing System. Curr. Appl. Phys. 2021, 22, 71–76. [Google Scholar] [CrossRef]
- Kato, T. Cu(In,Ga)(Se,S)2 Solar Cell Research in Solar Frontier: Progress and Current Status. Jpn. J. Appl. Phys. 2017, 56, 04CA02. [Google Scholar] [CrossRef]
- Salomé, P.M.P.; Rodriguez-Alvarez, H.; Sadewasser, S. Incorporation of Alkali Metals in Chalcogenide Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 143, 9–20. [Google Scholar] [CrossRef]
- Oikkonen, L.; Ganchenkova, M.; Seitsonen, A.; Nieminen, R.M. Effect of Sodium Incorporation into CuInSe2 from First Principles. J. Appl. Phys. 2013, 114, 083503. [Google Scholar] [CrossRef] [Green Version]
- Kamikawa, Y.; Nishinaga, J.; Shibata, H.; Ishizuka, S. Efficient Narrow Band Gap Cu(In,Ga)Se2 Solar Cells with Flat Surface. ACS Appl. Mater. Interfaces 2020, 12, 8. [Google Scholar] [CrossRef]
- Abou-Ras, D.; Wagner, S.; Stanbery, B.J.; Schock, H.-W.; Scheer, R.; Stolt, L.; Siebentritt, S.; Lincot, D.; Eberspacher, C.; Kushiya, K.; et al. Innovation Highway: Breakthrough Milestones and Key Developments in Chalcopyrite Photovoltaics from a Retrospective Viewpoint. Thin Solid Film 2017, 633, 2–12. [Google Scholar] [CrossRef]
- Deprédurand, V.; Tanaka, D.; Aida, Y.; Carlberg, M.; Fèvre, N.; Siebentritt, S. Current Loss Due to Recombination in Cu-Rich CuInSe2 Solar Cells. J. Appl. Phys. 2014, 115, 044503. [Google Scholar] [CrossRef]
- Nakada, T.; Iga, D.; Ohbo, H.; Kunioka, A. Effects of Sodium on Cu(In,Ga)Se2-Based Thin Films and Solar Cells. Jpn. J. Appl. Phys. 1997, 36, 732–737. [Google Scholar] [CrossRef]
- Hergert, F.; Hock, R.; Weber, A.; Purwins, M.; Palm, J.; Probst, V. In Situ Investigation of the Formation of Cu(In,Ga)Se2 from Selenised Metallic Precursors by X-Ray Diffraction—The Impact of Gallium, Sodium and Selenium Excess. J. Phys. Chem. Solids 2005, 66, 1903–1907. [Google Scholar] [CrossRef]
- Hiroi, H.; Iwata, Y.; Sugimoto, H.; Yamada, A. Progress Toward 1000-MV Open-Circuit Voltage on Chalcopyrite Solar Cells. IEEE J. Photovolt. 2016, 6, 1630–1634. [Google Scholar] [CrossRef] [Green Version]
- Jäger, T.; Romanyuk, Y.E.; Bissig, B.; Pianezzi, F.; Nishiwaki, S.; Reinhard, P.; Steinhauser, J.; Schwenk, J.; Tiwari, A.N. Improved Open-Circuit Voltage in Cu(In,Ga)Se2 Solar Cells with High Work Function Transparent Electrodes. J. Appl. Phys. 2015, 117, 225303. [Google Scholar] [CrossRef] [Green Version]
- Klenk, R.; Bakehe, S.; Kaigawa, R.; Neisser, A.; Reiß, J.; Lux-Steiner, M.C. Optimising the Open-Circuit Voltage of Cu(In,Ga)S2 Solar Cells—Design and Analysis. Thin Solid Film 2004, 451–452, 424–429. [Google Scholar] [CrossRef]
- Neisser, A.; Hengel, I.; Klenk, R.; Matthes, T.W.; Álvarez-García, J.; Pérez-Rodíguez, A.; Romano-Rodíguez, A.; Lux-Steiner, M.-C. Effect of Ga Incorporation in Sequentially Prepared CuInS2 Thin Film Absorbers. Sol. Energy Mater. Sol. Cells 2001, 67, 97–104. [Google Scholar] [CrossRef]
- Hegedus, S.S.; Shafarman, W.N. Thin-Film Solar Cells: Device Measurements and Analysis. Prog. Photovolt. Res. Appl. 2004, 12, 155–176. [Google Scholar] [CrossRef]
- Dodge, Y. The Concise Encyclopedia of Statistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 0387317422. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]




| Codes | Processing Parameter | Level | ||
|---|---|---|---|---|
| Low (-) | Baseline (CP) | High (+) | ||
| A | Anneal temp 1 (°C) | 520 | 560 | 600 |
| B | Anneal dur 2 (min) | 5 | 15 | 45 |
| C | H2S press 3 (mbar) | 5 | 200 | 200 * |
| D | Ramp rate (°C/s) | 0.2 | 1 | 5 |
| E | H2S intro temp (°C) | 50 | 150 | 350 |
| Metric | Sample | ||
|---|---|---|---|
| +Bar | -Bar | All | |
| Lifetime | 2.13 ns (CV = 20%) | 2.54 ns (CV = 36%) | 2.33 ns (CV = 30%) |
| Roughness | 310 nm (CV = 10%) | 280 nm (CV = 17%) | 292 nm (CV = 14%) |
| Barrier | +Bar | -Bar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Metric | Lifetime | Roughness | Lifetime | Roughness | |||||
| Level | - | + | - | + | - | + | - | + | |
| Factor codes - See Table 1 for reference | A | 30 | 19 | 18 | 4 | 13 | 8 | 7 | 5 |
| B | 15 | 7 | 4 | 3 | 14 | 3 | 13 | 2 | |
| C | 11 | 3 | 34 | 0.1 | 19 | 40 | 140 | 40 | |
| D | 11 | 2 | 7 | 4 | 11 | 16 | 30 | 1 | |
| E | 30 | 14 | 4 | 22 | 45 | 6 | 18 | 36 | |
| Barrier | +Bar | -Bar | |||
|---|---|---|---|---|---|
| Metric | Lifetime | Roughness | Lifetime | Roughness | |
| Factor codes | A | −71 | 29 | ~0 | 54 |
| B | 26 | −15 | 66 | −37 | |
| C | 22 | 20 | 15 | 76 | |
| D | −99 | 29 | 69 | −86 | |
| E | −7 | 94 | 35 | 86 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamtaei, S.; Brammertz, G.; Meuris, M.; Poortmans, J.; Vermang, B. Dominant Processing Factors in Two-Step Fabrication of Pure Sulfide CIGS Absorbers. Energies 2021, 14, 4737. https://doi.org/10.3390/en14164737
Hamtaei S, Brammertz G, Meuris M, Poortmans J, Vermang B. Dominant Processing Factors in Two-Step Fabrication of Pure Sulfide CIGS Absorbers. Energies. 2021; 14(16):4737. https://doi.org/10.3390/en14164737
Chicago/Turabian StyleHamtaei, Sarallah, Guy Brammertz, Marc Meuris, Jef Poortmans, and Bart Vermang. 2021. "Dominant Processing Factors in Two-Step Fabrication of Pure Sulfide CIGS Absorbers" Energies 14, no. 16: 4737. https://doi.org/10.3390/en14164737
APA StyleHamtaei, S., Brammertz, G., Meuris, M., Poortmans, J., & Vermang, B. (2021). Dominant Processing Factors in Two-Step Fabrication of Pure Sulfide CIGS Absorbers. Energies, 14(16), 4737. https://doi.org/10.3390/en14164737







