Thermal Stability and Reliability Test of Some Saturated Fatty Acids for Low and Medium Temperature Thermal Energy Storage
Abstract
:1. Introduction
2. Material Preparation and Investigational Approach
2.1. Material Preparation
2.2. Thermal Cycle Test Unit
2.3. Differential Scanning Calorimeter (DSC)
2.4. Thermogravimetric Analysis (TGA)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. DSC Analysis
3.2. Thermal Cycle Test Analysis
3.3. Thermogravimetric Analysis (TGA)
3.4. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CA | Capric acid |
| LA | Lauric acid |
| MA | Myristic acid |
| SA | Stearic acid |
| PA | Palmitic acid |
| DSC | Differential scanning calorimetry |
| TGA | Thermal gravimetric analysis |
| FTIR | Fourier transform infrared spectroscopy |
| MT | Melting temperature |
| LHF | Latent heat of fusion |
| FT | Freezing temperature |
| LHC | Latent heat of crystallization |
References
- Nitsas, M.T.; Koronaki, I.P. Thermal Analysis of Pure and Nanoparticle-Enhanced PCM—Application in Concentric Tube Heat Exchanger. Energies 2020, 13, 3841. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Ternary mixture of fatty acids as phase change materials for thermal energy storage applications. Energy Rep. 2016, 2, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Karaipekli, A.; Sari, A. Capric-myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew. Energy 2008, 33, 2599–2605. [Google Scholar] [CrossRef]
- Nascimento Porto, T.; Delgado, J.M.P.Q.; Guimarães, A.S.; Fernandes Magalhães, H.L.; Moreira, G.; Brito Correia, B.; Freire de Andrade, T.; Barbosa de Lima, A.G. Phase Change Material Melting Process in a Thermal Energy Storage System for Applications in Buildings. Energies 2020, 13, 3254. [Google Scholar] [CrossRef]
- Okogeri, O.; Stathopoulos, V.N. What about greener phase change materials? A review on biobased phase change materials for thermal energy storage applications. Int. J. Thermofluids 2021, 10, 100081. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Gasia, J.; Martin, M.; Solé, A.; Barreneche, C.; Cabeza, L. Phase Change Material Selection for Thermal Processes Working under Partial Load Operating Conditions in the Temperature Range between 120 and 200 °C. Appl. Sci. 2017, 7, 722. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Thermal cycling test of few selected inorganic and organic phase change materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Sari, A. Thermal reliability test of some fatty acids as PCMs used for solar thermal latent heat storage applications. Energy Convers. Manag. 2003, 44, 2277–2287. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A. Thermal cycle test of binary mixtures of some fatty acids as phase change materials for building applications. Energy Build. 2015, 99, 196–203. [Google Scholar] [CrossRef]
- Anand, A.; Shukla, A.; Kumar, A.; Buddhi, D.; Sharma, A. Cycle test stability and corrosion evaluation of phase change materials used in thermal energy storage systems. J. Energy Storage 2021, 39, 102664. [Google Scholar] [CrossRef]
- Mousa, M.M.; Bayomy, A.M.; Saghir, M.Z. Experimental and Numerical Study on Energy Piles with Phase Change Materials. Energies 2020, 13, 4699. [Google Scholar] [CrossRef]
- Sharma, S.D.; Buddhi, D.; Sawhney, R.L. Accelerated thermal cycle test of latent heat-storage materials. Sol. Energy 1999, 66, 483–490. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, J.L.; He, S.M.; Wu, K.Z.; Liu, X. Di Thermal studies on the solid-liquid phase transition in binary systems of fatty acids. Thermochim. Acta 2001, 369, 157–160. [Google Scholar] [CrossRef]
- Sari, A.; Sari, H.; Önal, A. Thermal properties and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials. Energy Convers. Manag. 2004, 45, 365–376. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Chen, C.; Xu, T.; Fang, Y.; Zhang, Z. A capric–palmitic–stearic acid ternary eutectic mixture/expanded graphite composite phase change material for thermal energy storage. Compos. Part A Appl. Sci. Manuf. 2016, 87, 138–145. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Thermal properties and heat storage analysis of palmitic acid-TiO 2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Wen, R.; Zhang, X.; Huang, Z.; Fang, M.; Liu, Y.; Wu, X.; Min, X.; Gao, W.; Huang, S. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 273–279. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Wen, X.; Yin, H.; Liu, J. A novel CNT encapsulated phase change material with enhanced thermal conductivity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2018, 184, 82–90. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Zahir, M.H.; Mohamed, S.A. Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 353–361. [Google Scholar] [CrossRef]
- Anand, A.; Shukla, A.; Kumar, A.; Sharma, A. Development and characterization of ternary mixture series of medium- and long-chain saturated fatty acids for energy applications. Energy Storage 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Koukou, M.K.; Vrachopoulos, M.G.; Tachos, N.S.; Dogkas, G.; Lymperis, K.; Stathopoulos, V. Experimental and computational investigation of a latent heat energy storage system with a staggered heat exchanger for various phase change materials. Therm. Sci. Eng. Prog. 2018, 7, 87–98. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, A.; Shukla, M.; Chen, C.R. Development of thermal energy storage materials for biomedical applications. J. Med. Eng. Technol. 2015, 39, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cordobilla, R.; Calvet, T.; Cuevas-Diarte, M.A.; Gbabode, G.; Negrier, P.; Mondieig, D.; Oonk, H.A.J. Polymorphism of even saturated carboxylic acids from n-decanoic to n-eicosanoic acid. New J. Chem. 2007, 31, 947. [Google Scholar] [CrossRef] [Green Version]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Novel phase change materials for thermal energy storage: Evaluation of tropical tree fruit oils. Biotechnol. Rep. 2019, 24, e00359. [Google Scholar] [CrossRef]
- Sarı, A.; Kaygusuz, K. Some fatty acids used for latent heat storage: Thermal stability and corrosion of metals with respect to thermal cycling. Renew. Energy 2003, 28, 939–948. [Google Scholar] [CrossRef]



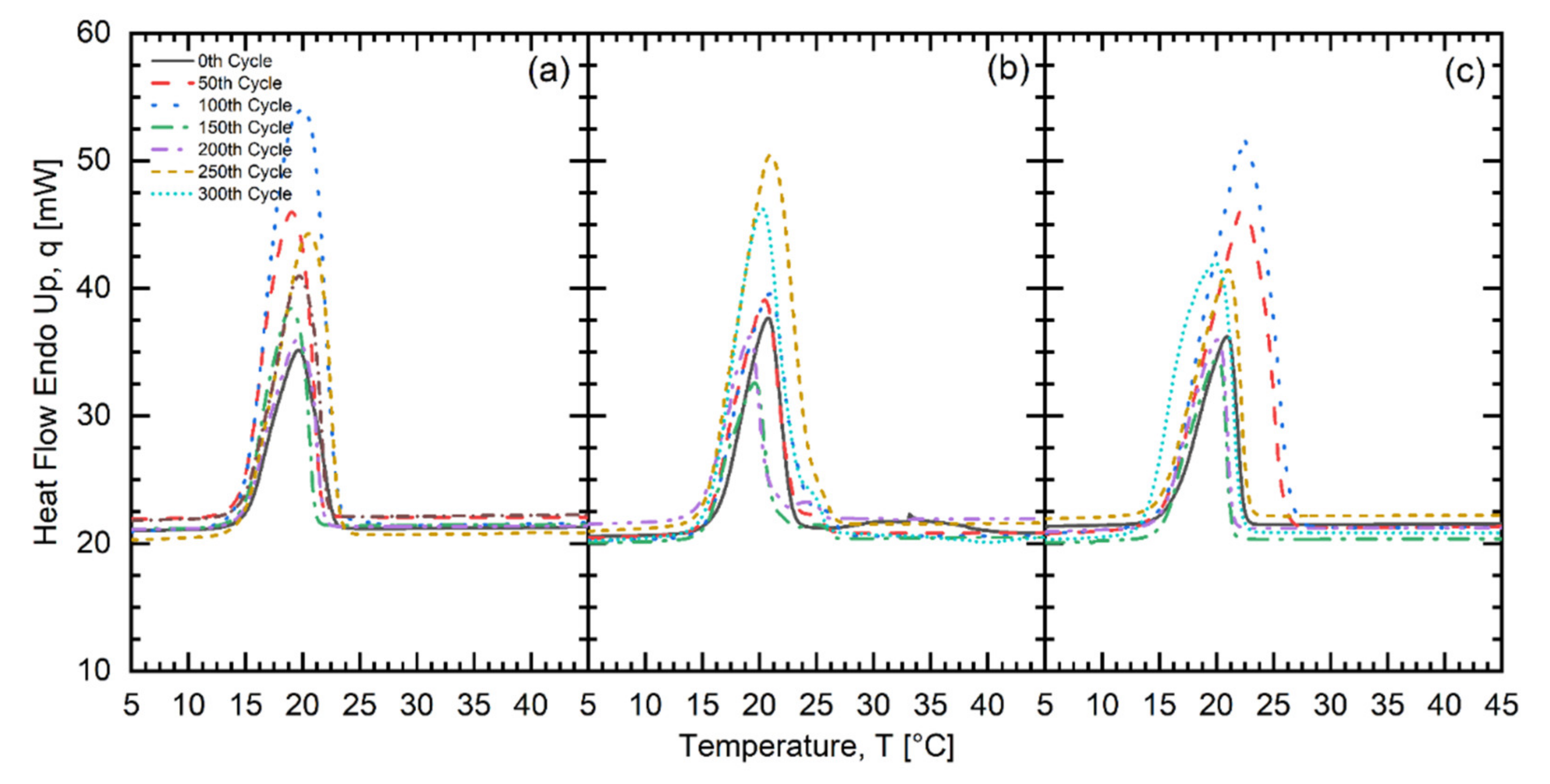

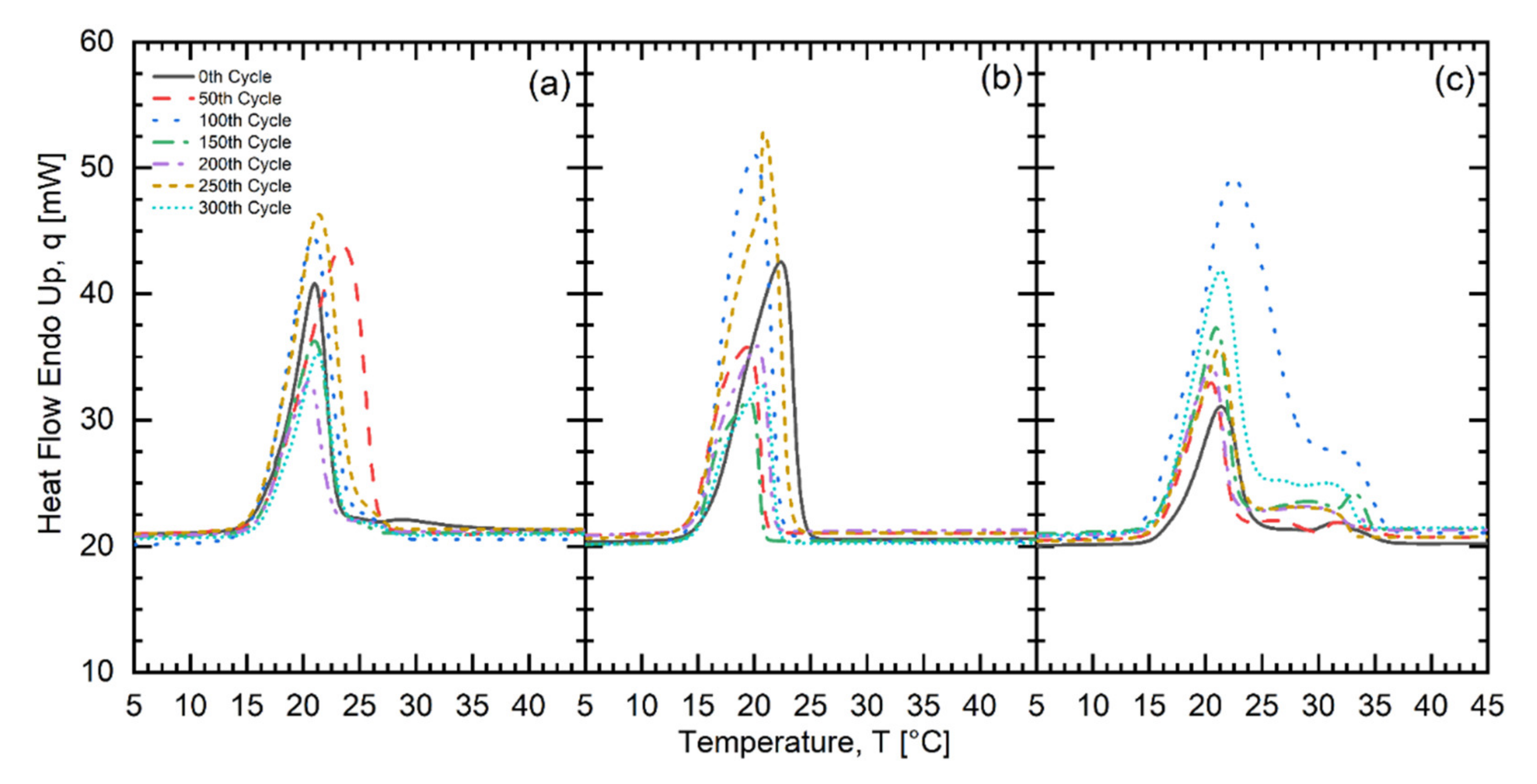


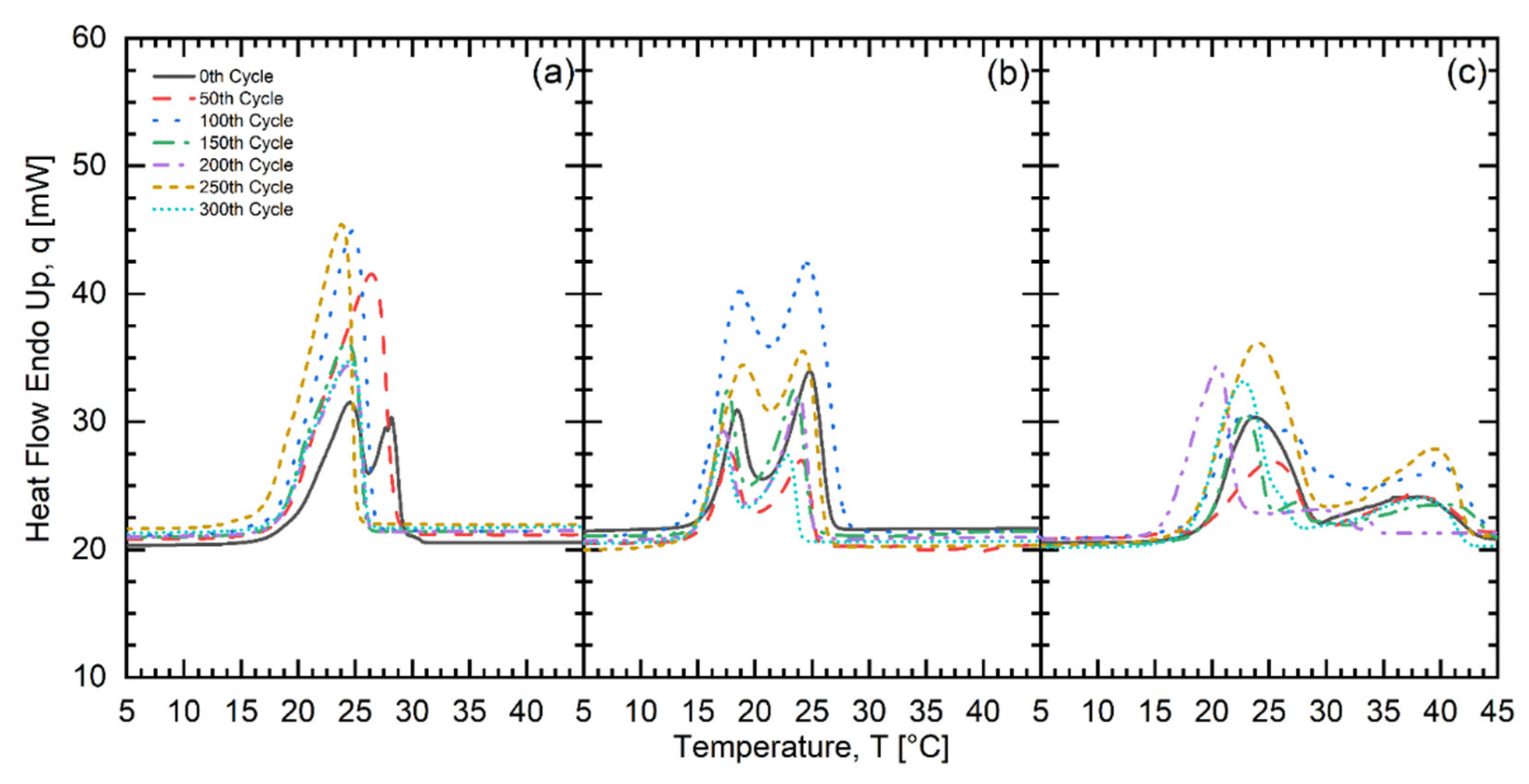
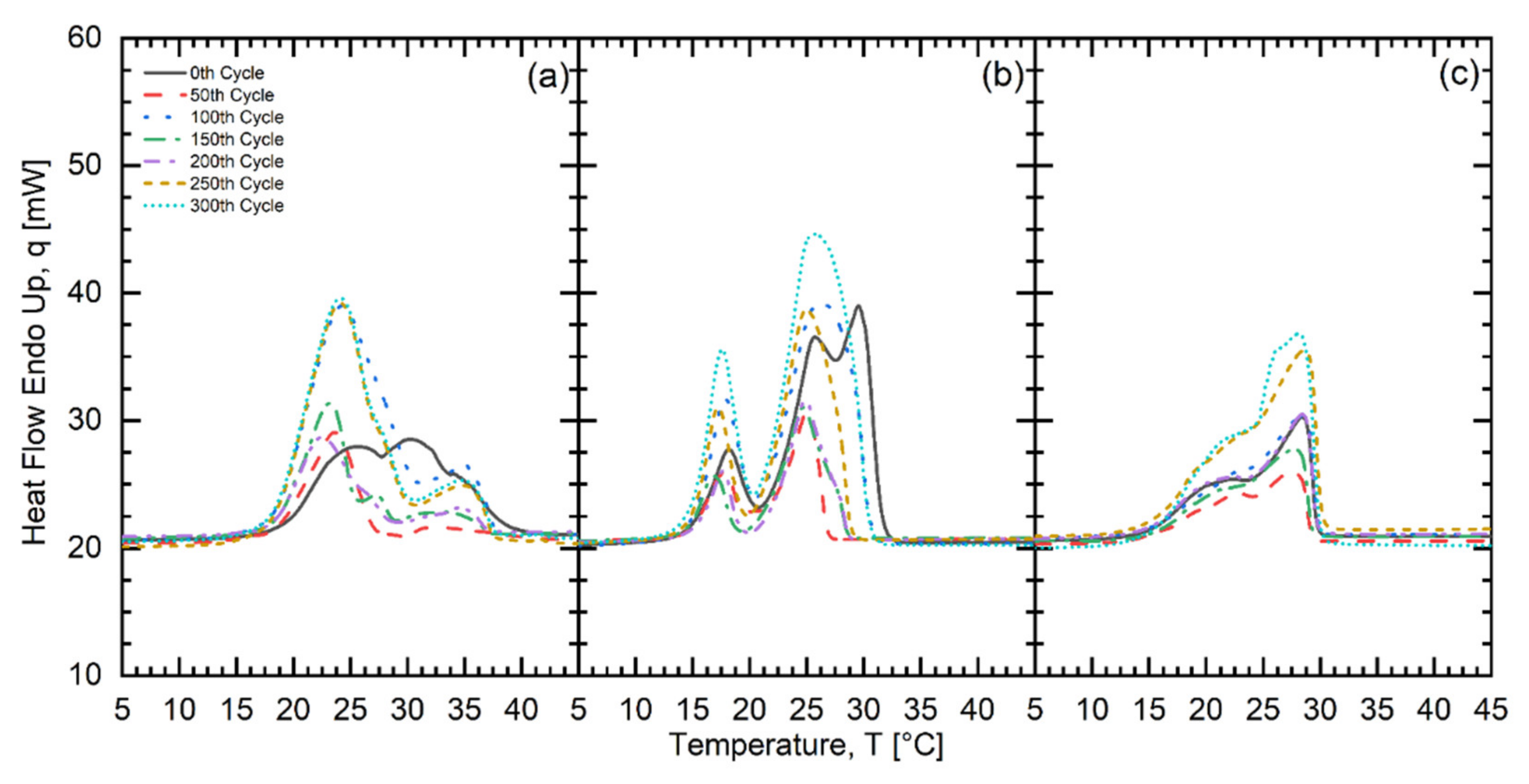
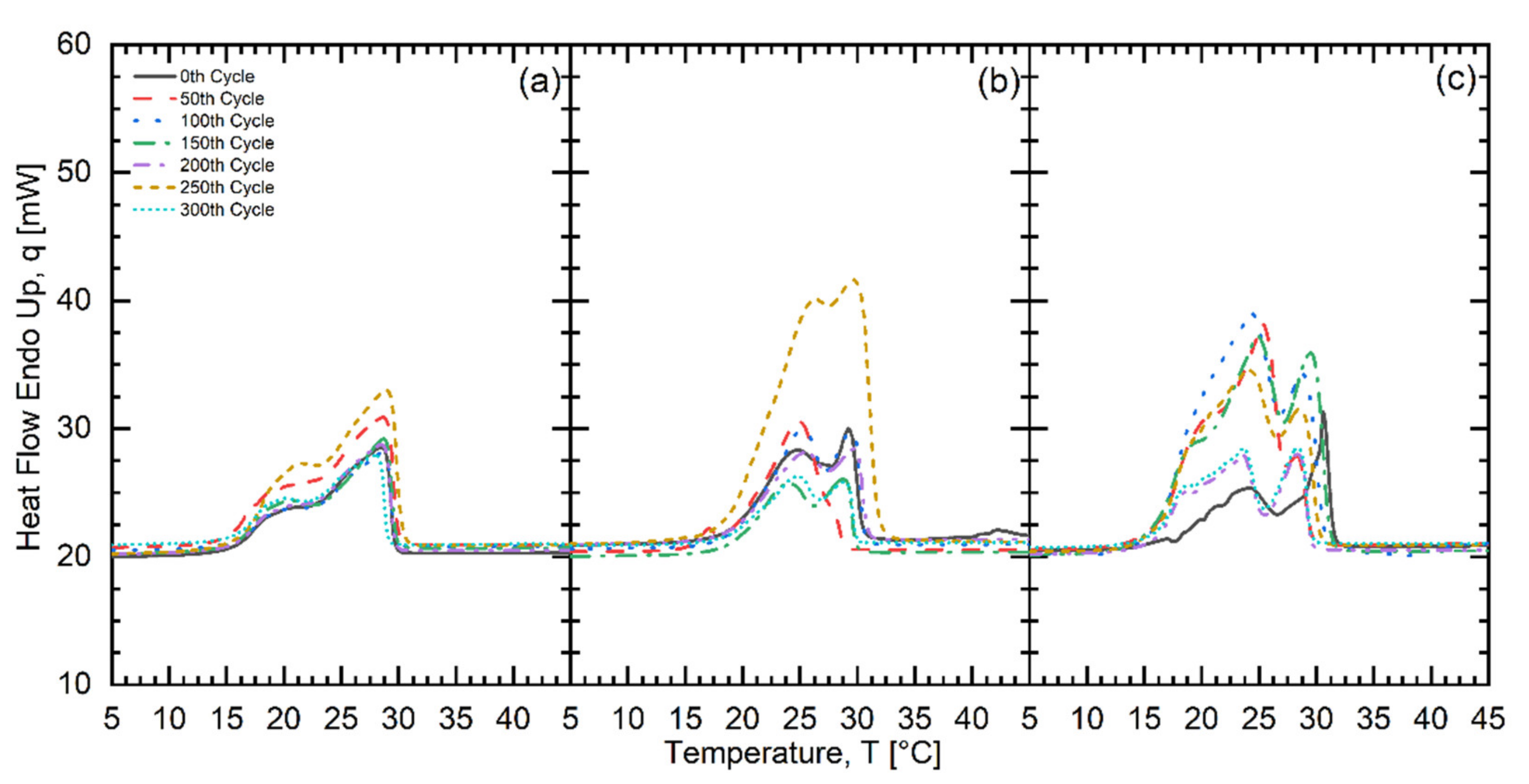
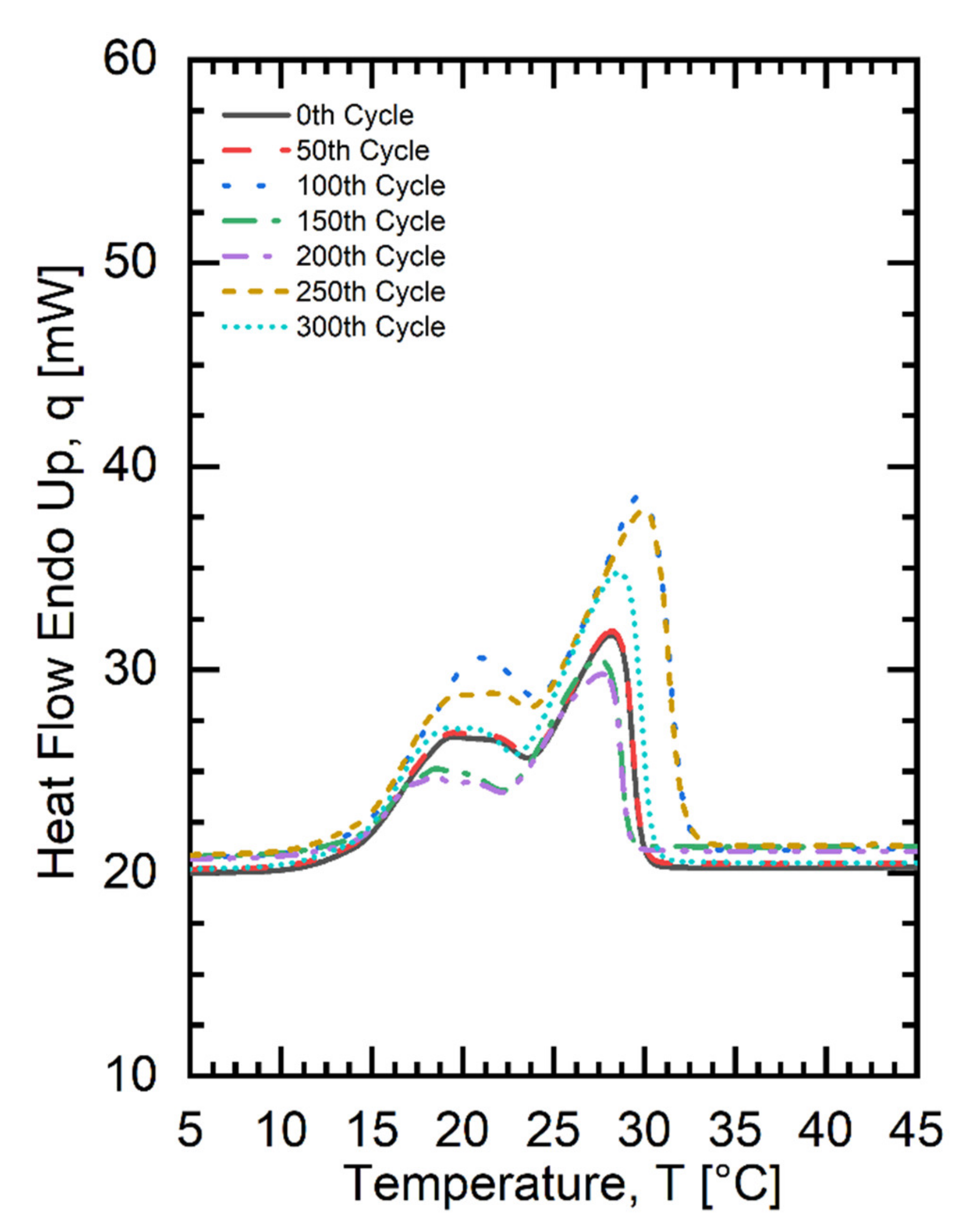


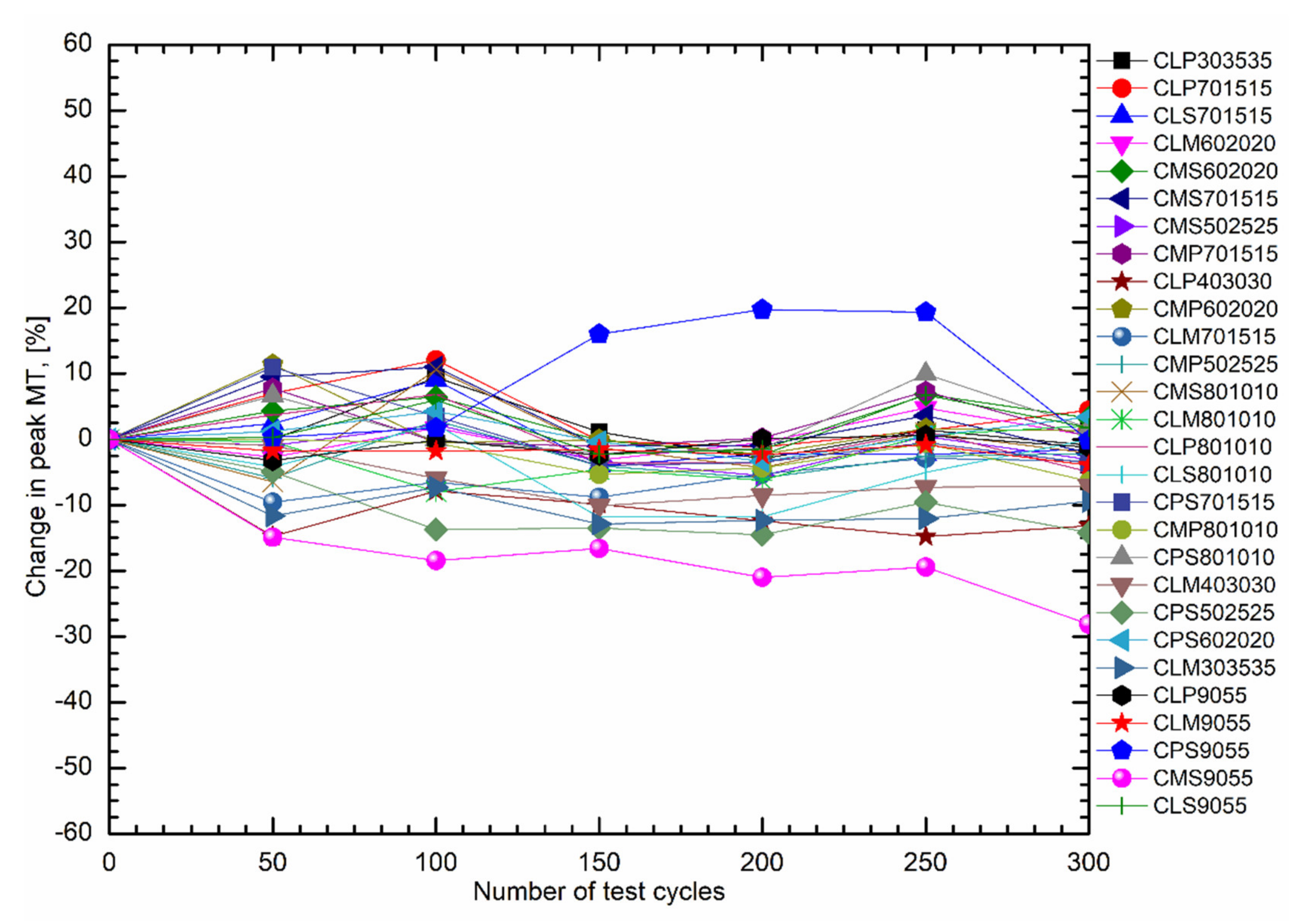
| Fatty Acid | Melting Temperature Range (°C) | * Onset (°C) | * Peak (°C) | * Latent Heat of Fusion (kJ/kg) | * Freezing Point (°C) | * Latent Heat of Crystallization (kJ/kg) | Purity (%) | ** Cost (USD/kg) |
|---|---|---|---|---|---|---|---|---|
| CA | 29–31 | 30.61 | 33.03 | 154.42 | 27.87 | 157.97 | 98.5 | 18.06 |
| LA | 44–46 | 43.50 | 45.93 | 175.77 | 40.42 | 179.72 | 99.0 | 4.12 |
| MA | 51–54 | 53.76 | 56.83 | 168.27 | 50.29 | 174.95 | 98.0 | 5.31 |
| PA | 60–63 | 61.62 | 64.25 | 206.11 | 58.93 | 208.67 | 99.0 | 4.80 |
| SA | 68–69 | 54.83 | 57.73 | 180.79 | 51.70 | 180.05 | 99.0 | 3.35 |
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Cost (USD/kg) | Laboratory Code/Name * |
|---|---|---|---|---|
| 15.27 | 19.01 | 151.92 | 8.54 | CLP303535 |
| 14.13 | 19.51 | 108.13 | 13.98 | CLP701515 |
| 13.85 | 19.56 | 82.97 | 13.76 | CLS701515 |
| 15.87 | 19.59 | 156.38 | 12.72 | CLM602020 |
| 15.96 | 19.63 | 175.16 | 12.57 | CMS602020 |
| 15.25 | 20.30 | 169.74 | 13.94 | CMS701515 |
| 15.54 | 20.34 | 148.95 | 11.19 | CMS502525 |
| 16.59 | 20.83 | 154.24 | 14.16 | CMP701515 |
| 13.99 | 20.89 | 116.65 | 9.71 | CLP403030 |
| 17.15 | 21.02 | 138.54 | 12.86 | CMP602020 |
| 14.61 | 21.41 | 103.22 | 14.06 | CLM701515 |
| 17.00 | 21.80 | 170.37 | 11.56 | CMP502525 |
| 16.23 | 21.81 | 178.45 | 15.31 | CMS801010 |
| 16.81 | 22.30 | 172.65 | 15.39 | CLM801010 |
| 15.60 | 22.43 | 99.43 | 15.34 | CLP801010 |
| 15.62 | 22.49 | 162.89 | 15.19 | CLS801010 |
| 19.48 | 23.73 | 146.65 | 13.86 | CPS701515 |
| 23.16 | 24.18 | 131.72 | 15.46 | CMP801010 |
| 19.09 | 24.79 | 162.40 | 15.26 | CPS801010 |
| 24.03 | 26.09 | 154.25 | 10.05 | CLM403030 |
| 21.78 | 26.52 | 183.11 | 11.06 | CPS502525 |
| 19.51 | 23.30 | 164.80 | 12.47 | CPS602020 |
| 25.75 | 28.40 | 136.52 | 8.72 | CLM303535 |
| 20.46 | 28.41 | 140.03 | 16.70 | CLP9055 |
| 20.83 | 29.16 | 124.64 | 16.72 | CLM9055 |
| 19.83 | 24.83 | 128.09 | 16.66 | CPS9055 |
| 28.05 | 29.83 | 160.71 | 16.69 | CMS9055 |
| 21.81 | 28.10 | 144.91 | 16.63 | CLS9055 |
| No. of Test Cycles | CLP303535 | CLP701515 | CLS701515 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 15.27 | 19.01 | 151.92 | 14.13 | 19.51 | 108.13 | 13.85 | 19.56 | 82.97 |
| 50 | 15.29 | 19.13 | 117.83 | 13.77 | 20.87 | 145.90 | 13.87 | 20.03 | 133.58 |
| 100 | 15.24 | 20.80 | 148.49 | 15.35 | 21.86 | 153.13 | 13.90 | 21.32 | 161.24 |
| 150 | 14.55 | 19.22 | 154.10 | 13.67 | 19.47 | 165.26 | 13.78 | 18.78 | 139.32 |
| 200 | 14.29 | 18.44 | 201.69 | 13.55 | 19.28 | 124.64 | 13.61 | 19.11 | 135.02 |
| 250 | 14.50 | 19.28 | 149.89 | 13.72 | 19.77 | 156.30 | 13.65 | 19.11 | 140.87 |
| 300 | 14.35 | 18.84 | 184.29 | 17.61 | 20.37 | 104.25 | 13.57 | 19.26 | 162.78 |
| No. of Test Cycles | CLM602020 | CMS602020 | CMS701515 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion) (kJ/kg) | |
| 0 | 15.87 | 19.59 | 156.38 | 15.96 | 19.63 | 175.16 | 15.25 | 20.30 | 169.74 |
| 50 | 14.78 | 19.06 | 143.87 | 15.54 | 20.48 | 141.07 | 15.40 | 22.23 | 129.96 |
| 100 | 14.90 | 19.93 | 167.22 | 15.66 | 20.89 | 141.62 | 20.15 | 22.53 | 147.37 |
| 150 | 14.93 | 18.97 | 157.23 | 15.37 | 19.60 | 137.07 | 15.27 | 20.09 | 139.37 |
| 200 | 14.98 | 19.49 | 137.95 | 16.62 | 19.16 | 163.73 | 15.21 | 20.11 | 132.27 |
| 250 | 15.14 | 20.52 | 150.65 | 15.29 | 20.97 | 163.98 | 15.09 | 21.03 | 128.18 |
| 300 | 15.18 | 19.70 | 152.61 | 15.46 | 20.26 | 168.89 | 13.90 | 19.88 | 147.25 |
| No. of Test Cycles | CMS502525 | CMP701515 | CLP403030 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 15.54 | 20.34 | 148.95 | 16.59 | 20.83 | 154.24 | 13.99 | 20.89 | 116.65 |
| 50 | 15.74 | 20.15 | 154.87 | 16.58 | 22.45 | 152.18 | 14.10 | 17.81 | 151.31 |
| 100 | 16.29 | 20.79 | 155.05 | 16.99 | 20.75 | 190.75 | 13.70 | 19.25 | 171.31 |
| 150 | 15.38 | 19.66 | 160.55 | 15.58 | 20.60 | 161.37 | 13.94 | 18.83 | 136.16 |
| 200 | 15.27 | 19.21 | 146.43 | 16.63 | 20.87 | 131.49 | 13.93 | 18.30 | 152.94 |
| 250 | 15.38 | 20.43 | 179.66 | 16.09 | 22.35 | 138.25 | 14.30 | 17.80 | 98.48 |
| 300 | 15.35 | 19.71 | 153.71 | 16.39 | 20.90 | 157.65 | 13.87 | 18.14 | 128.18 |
| No. Of Test Cycles | CMP602020 | CLM701515 | CMP502525 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 17.15 | 21.02 | 138.53 | 14.61 | 21.41 | 103.22 | 17.00 | 21.80 | 170.37 |
| 50 | 16.69 | 23.41 | 139.77 | 14.43 | 19.36 | 162.95 | 15.91 | 20.51 | 163.12 |
| 100 | 16.10 | 20.90 | 162.64 | 15.25 | 20.01 | 151.81 | 16.25 | 22.42 | 195.33 |
| 150 | 16.35 | 21.01 | 141.97 | 14.85 | 19.52 | 129.20 | 16.64 | 20.92 | 187.82 |
| 200 | 16.14 | 20.54 | 142.20 | 14.88 | 20.28 | 128.87 | 16.07 | 20.52 | 168.11 |
| 250 | 16.64 | 21.36 | 140.84 | 19.69 | 20.79 | 158.40 | 16.13 | 21.24 | 135.43 |
| 300 | 17.03 | 21.29 | 159.96 | 14.94 | 20.70 | 188.39 | 16.36 | 21.32 | 153.09 |
| No. of Test Cycles | CMS801010 | CLM801010 | CLP801010 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 16.23 | 21.81 | 178.45 | 16.81 | 22.30 | 172.65 | 15.60 | 22.43 | 99.43 |
| 50 | 16.30 | 20.40 | 153.01 | 15.22 | 22.22 | 127.09 | 15.55 | 23.26 | 115.80 |
| 100 | 15.98 | 24.12 | 134.22 | 15.17 | 20.52 | 152.50 | 15.35 | 23.96 | 142.89 |
| 150 | 16.16 | 21.53 | 145.04 | 15.66 | 21.28 | 147.54 | 15.70 | 21.62 | 167.38 |
| 200 | 16.08 | 20.87 | 146.60 | 15.71 | 20.91 | 140.13 | 15.29 | 21.62 | 151.95 |
| 250 | 16.14 | 22.02 | 145.75 | 16.70 | 22.43 | 146.46 | 14.64 | 22.70 | 139.50 |
| 300 | 13.37 | 21.35 | 166.74 | 15.17 | 22.76 | 139.83 | 15.63 | 21.32 | 143.45 |
| No. of Test Cycles | CLS801010 | CPS701515 | CMP801010 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 15.62 | 22.49 | 162.89 | 19.48 | 23.73 | 146.65 | 23.16 | 24.18 | 131.72 |
| 50 | 13.67 | 21.55 | 122.01 | 19.36 | 26.33 | 130.92 | 17.26 | 24.18 | 137.29 |
| 100 | 14.38 | 22.99 | 165.52 | 18.79 | 24.56 | 134.48 | 18.85 | 24.04 | 175.86 |
| 150 | 18.02 | 19.83 | 148.10 | 18.81 | 22.78 | 146.36 | 18.33 | 22.89 | 136.21 |
| 200 | 13.45 | 19.84 | 165.31 | 20.12 | 22.90 | 134.25 | 18.61 | 23.13 | 157.99 |
| 250 | 17.36 | 21.36 | 116.50 | 18.70 | 23.61 | 166.75 | 18.48 | 24.03 | 163.11 |
| 300 | 13.81 | 22.49 | 122.93 | 19.04 | 22.87 | 118.06 | 17.53 | 22.63 | 169.37 |
| No. of Test Cycles | CPS801010 | CLM403030 | CPS502525 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 19.09 | 24.79 | 162.40 | 24.03 | 26.09 | 154.25 | 21.78 | 26.52 | 183.11 |
| 50 | 18.92 | 26.43 | 161.77 | 15.50 | 25.84 | 65.09 | 19.16 | 25.21 | 144.49 |
| 100 | 18.86 | 24.77 | 157.77 | 14.86 | 24.54 | 148.40 | 18.22 | 22.88 | 179.26 |
| 150 | 18.40 | 24.27 | 176.39 | 15.91 | 23.45 | 162.47 | 19.47 | 22.94 | 180.33 |
| 200 | 18.43 | 24.44 | 152.36 | 15.54 | 23.86 | 150.74 | 17.02 | 22.67 | 168.15 |
| 250 | 18.10 | 27.25 | 141.34 | 14.81 | 24.19 | 121.17 | 18.15 | 23.98 | 181.72 |
| 300 | 18.50 | 25.22 | 157.80 | 14.80 | 24.23 | 130.90 | 18.29 | 22.76 | 173.89 |
| No. of Test Cycles | CPS602020 | CLM303535 | CLP9055 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 19.51 | 23.30 | 164.80 | 25.75 | 28.40 | 136.52 | 20.46 | 28.41 | 140.03 |
| 50 | 19.09 | 23.58 | 149.41 | 19.64 | 25.09 | 131.13 | 20.35 | 27.49 | 124.76 |
| 100 | 18.38 | 24.29 | 133.72 | 20.66 | 26.32 | 206.21 | 20.16 | 28.37 | 163.88 |
| 150 | 18.52 | 23.21 | 147.14 | 18.70 | 24.74 | 148.36 | 15.74 | 27.73 | 138.33 |
| 200 | 18.28 | 22.52 | 151.09 | 15.41 | 24.90 | 136.38 | 21.61 | 28.42 | 146.53 |
| 250 | 18.18 | 23.40 | 170.50 | 15.15 | 24.97 | 152.38 | 19.74 | 28.58 | 155.39 |
| 300 | 18.20 | 24.09 | 178.85 | 15.22 | 25.72 | 160.19 | 23.83 | 28.07 | 164.27 |
| No. of Test Cycles | CLM9055 | CPS9055 | CMS9055 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 20.83 | 29.16 | 124.64 | 19.83 | 24.83 | 128.09 | 28.05 | 29.83 | 160.71 |
| 50 | 15.25 | 28.65 | 133.90 | 19.57 | 24.90 | 152.34 | 23.65 | 25.39 | 236.78 |
| 100 | 20.16 | 28.64 | 135.32 | 19.86 | 25.26 | 147.42 | 16.22 | 24.33 | 163.31 |
| 150 | 21.03 | 28.70 | 143.17 | 20.88 | 28.80 | 148.49 | 15.58 | 24.88 | 158.01 |
| 200 | 19.77 | 28.48 | 139.37 | 19.57 | 29.73 | 136.21 | 15.85 | 23.57 | 167.37 |
| 250 | 16.53 | 28.92 | 145.50 | 19.34 | 29.63 | 156.04 | 16.12 | 24.03 | 139.40 |
| 300 | 15.66 | 28.01 | 147.96 | 19.36 | 24.78 | 125.54 | 16.44 | 21.44 | 168.29 |
| No. of Test Cycles | CLS9055 | ||
|---|---|---|---|
| Onset (°C) | Peak (°C) | Latent Heat of Fusion (kJ/kg) | |
| 0 | 21.81 | 28.10 | 144.91 |
| 50 | 21.04 | 28.18 | 128.35 |
| 100 | 21.84 | 29.77 | 144.91 |
| 150 | 21.27 | 27.45 | 195.55 |
| 200 | 20.94 | 27.67 | 177.99 |
| 250 | 20.98 | 29.99 | 142.98 |
| 300 | 20.85 | 28.59 | 135.42 |
| Laboratory Code/Name | Tonset (°C) | Tmax (°C) | Toffset (°C) | Mass Loss (%) |
|---|---|---|---|---|
| CLP303535 | 185.19 | 226.50 | 258.00 | 97.93 |
| CLP701515 | 195.22 | 224.58 | 248.40 | 88.14 |
| CLS701515 | 190.45 | 226.40 | 250.57 | 92.83 |
| CLM602020 | 185.30 | 229.70 | 255.10 | 96.87 |
| CMS602020 | 189.73 | 230.30 | 270.88 | 101.75 |
| CMS701515 | 180.80 | 223.27 | 264.36 | 80.26 |
| CMS502525 | 186.02 | 220.91 | 255.10 | 91.95 |
| CMP701515 | 189.01 | 225.40 | 258.82 | 86.25 |
| CLP403030 | 207.05 | 232.30 | 250.57 | 98.13 |
| CMP602020 | 189.67 | 212.61 | 237.44 | 96.89 |
| CLM701515 | 183.25 | 221.25 | 251.59 | 91.92 |
| CMP-502525 | 216.75 | 232.80 | 242.32 | 93.99 |
| CMS801010 | 191.56 | 208.95 | 226.47 | 92.46 |
| CLM801010 | 206.18 | 213.62 | 220.93 | 95.67 |
| CLP801010 | 186.74 | 223.30 | 243.04 | 97.30 |
| CLS801010 | 182.20 | 213.00 | 228.81 | 100.0 |
| CPS701515 | 173.95 | 211.70 | 252.84 | 91.17 |
| CMP801010 | 187.46 | 221.10 | 240.77 | 94.42 |
| CPS801010 | 182.36 | 222.70 | 264.03 | 84.90 |
| CLM403030 | 183.75 | 226.60 | 238.50 | 89.90 |
| CPS502525 | 193.55 | 230.10 | 285.84 | 96.46 |
| CPS602020 | 170.24 | 211.30 | 259.54 | 94.57 |
| CLM303535 | 203.24 | 248.80 | 263.36 | 84.27 |
| CLP9055 | 188.29 | 224.50 | 239.33 | 97.59 |
| CLM9055 | 179.22 | 216.60 | 233.35 | 92.14 |
| CPS9055 | 182.21 | 217.80 | 240.78 | 93.25 |
| CMS9055 | 176.95 | 217.90 | 239.33 | 97.92 |
| CLS9055 | 185.30 | 220.10 | 234.80 | 101.37 |
| Laboratory Code/Name | Variation in Melting Peak (%) | Variation in the Latent Heat of the Fusion (%) | Mean Latent Heat of Fusion (kJ/kg) | Categorization Based on Thermal Cycle Stability * |
|---|---|---|---|---|
| CLP303535 | −3.00–9.42 | −2.26–32.00 | 165.06 | + |
| CLP701515 | −1.18–12.05 | −3.59–52.83 | 136.80 | ++ |
| CLS701515 | −3.99–9.00 | −61.00–96.16 | 136.54 | +++ |
| CLM602020 | −3.16–4.75 | −11.78–7.12 | 152.27 | + |
| CMS602020 | −2.39–6.83 | −21.75–0.00 | 155.93 | + |
| CMS701515 | −2.07–10.99 | −24.48–00.00 | 142.02 | + |
| CMS502525 | −5.56–2.21 | −1.69–20.62 | 157.03 | + |
| CMP701515 | −1.10–7.78 | −14.75–23.67 | 155.13 | + |
| CLP403030 | −14.79–0.00 | −15.58–46.86 | 136.43 | ++ |
| CMP602020 | −2.28–11.37 | 0–17.00 | 146.56 | + |
| CLM701515 | −9.57–0.00 | 0.00–82.51 | 146.12 | +++ |
| CMP502525 | −5.92–2.84 | −20.51–14.65 | 167.61 | + |
| CMS801010 | −6.46–10.59 | −24.79–0.00 | 152.83 | + |
| CLM801010 | −7.98–2.06 | −26.39–0.00 | 146.60 | + |
| CLP801010 | −4.95–6.82 | 0.00–68.34 | 137.20 | ++ |
| CLS801010 | −11.83–2.22 | −28.47–1.61 | 143.32 | + |
| CPS701515 | −4.00–10.96 | −19.50–13.71 | 139.64 | + |
| CMP801010 | −6.41–0.00 | 0.00–33.51 | 153.08 | + |
| CPS801010 | −2.10–9.92 | −12.97–8.61 | 158.55 | + |
| CLM403030 | −34.07–0.00 | −57.80–5.33 | 133.29 | ++ |
| CPS502525 | −14.52–0.00 | −21.09–0.00 | 172.99 | + |
| CPS602020 | −3.35–4.24 | −18.86–8.53 | 156.50 | + |
| CLM303535 | −12.89–0.00 | −3.95–31.05 | 153.02 | + |
| CLP9055 | −3.24–0.60 | −10.90–17.31 | 147.60 | + |
| CLM9055 | −3.94–0.00 | 0.00–18.71 | 138.55 | + |
| CPS9055 | −0.20–19.73 | −1.99–21.82 | 142.02 | ++ |
| CMS9055 | −28.13–0.00 | −13.26–47.33 | 170.55 | ++ |
| CLS9055 | −2.31–6.72 | −11.42–34.94 | 152.87 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, A.; Kant, K.; Shukla, A.; Chen, C.-R.; Sharma, A. Thermal Stability and Reliability Test of Some Saturated Fatty Acids for Low and Medium Temperature Thermal Energy Storage. Energies 2021, 14, 4509. https://doi.org/10.3390/en14154509
Anand A, Kant K, Shukla A, Chen C-R, Sharma A. Thermal Stability and Reliability Test of Some Saturated Fatty Acids for Low and Medium Temperature Thermal Energy Storage. Energies. 2021; 14(15):4509. https://doi.org/10.3390/en14154509
Chicago/Turabian StyleAnand, Abhishek, Karunesh Kant, Amritanshu Shukla, Chang-Ren Chen, and Atul Sharma. 2021. "Thermal Stability and Reliability Test of Some Saturated Fatty Acids for Low and Medium Temperature Thermal Energy Storage" Energies 14, no. 15: 4509. https://doi.org/10.3390/en14154509
APA StyleAnand, A., Kant, K., Shukla, A., Chen, C.-R., & Sharma, A. (2021). Thermal Stability and Reliability Test of Some Saturated Fatty Acids for Low and Medium Temperature Thermal Energy Storage. Energies, 14(15), 4509. https://doi.org/10.3390/en14154509








