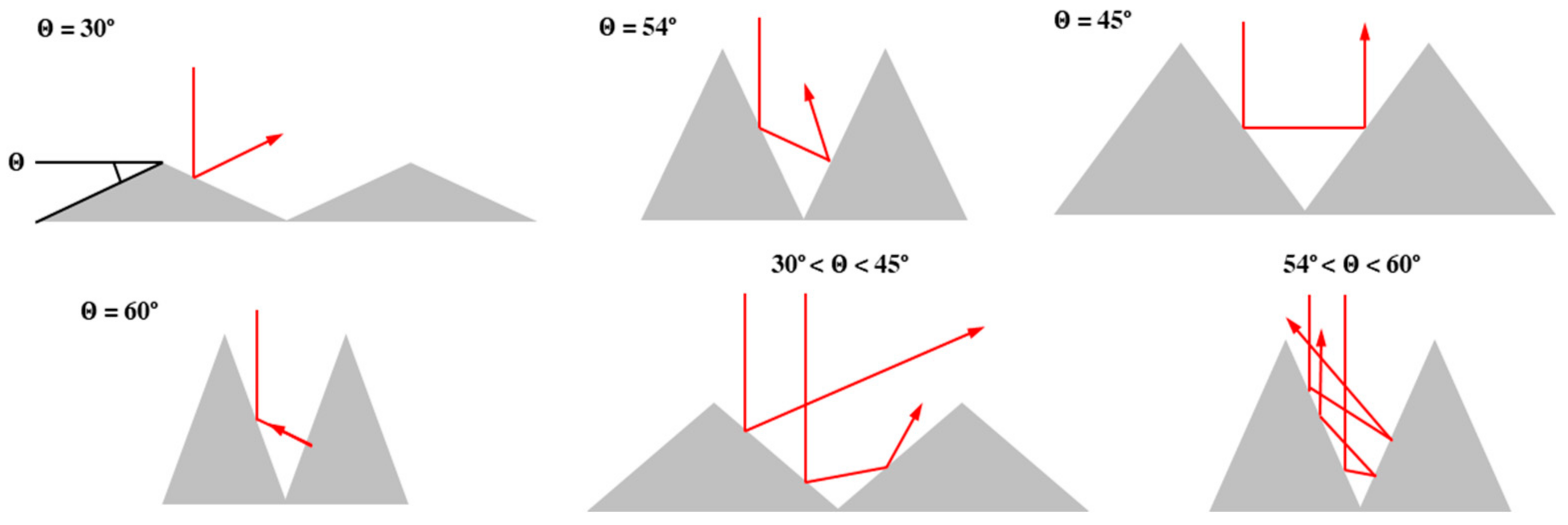
3.1. Conventional Etching (Alkali Solution)
The pyramid formation method using an alkali solution is most commonly used, but has a limitation in that it cannot obtain specific angles. Accordingly, it is possible to observe the characteristics of the reflectance for each wavelength according to the change of the pyramid size rather than the change of the pyramid angle. In this study, the structural and optical properties of the pyramid were analyzed using KOH as alkali solution to form the structure of the micro pyramid. The approximate etching depth for the surface etching of a single crystal Si wafer can be calculated by the following equation using the etching weight of the wafer:
The etching weight represents a value obtained by subtracting the weight of the wafer after etching from the weight of the wafer before etching, and the Si density was calculated by applying the Si density value of 2.33 g/cm
3 at room temperature. In the surface etching using KOH, both sides of the wafer are etched at the same time, so the approximate etching depth is half of the total etched amount. Through the above equation, the etching depth of the wafer according to the KOH and IPA concentrations is expressed as shown in
Figure 3a. As the KOH concentration increases, the etching depth generated at the same time increases, because the etching rate increases as the KOH concentration increases. As the etch rate increases, the size of the hydrogen droplet increases quickly, making it difficult to form a pyramid on the surface, and pyramid uniformity is lower with a higher concentration. As the concentration of the IPA increases, the etching depth tends to decrease, which seems to make the concentration of KOH in the H
2O lower. However, in practice, IPA acts as a surfactant, reducing the surface tension of the solution and removing hydrogen droplets. Accordingly, rather than lowering the concentration, it acts as a factor that interferes with the etching reaction by lowering the etching rate on the surface. In addition, since IPA takes away the heat of the reaction to vaporize, etching is slow and the pyramid is not formed, and accordingly, it was confirmed that the size of the pyramid cannot be increased [
23,
24].
Figure 3b shows the reflectances of the wafer according to the concentrations of solutions such as KOH and IPA.
Figure 4 shows the SEM images of the wafer surface according to the concentrations of solutions such as KOH and IPA. In the above experiment, KOH concentration plays a leading role in the formation of the pyramid, and IPA helps the formation of the pyramid under the proper concentration conditions, but when the concentration of IPA increases, a non-uniform pyramid showing high reflectance is formed. It was confirmed that the size of the pyramid increased as the concentration of KOH increased, and it was observed that the reflectance greatly decreased with IPA concentrations of 7.5% and 9% in conditions of the KOH concentrations of 2% and 3%, respectively. When the KOH concentration was 2%, if the IPA concentration increases from 7.5 to 9%, the size of the pyramid was very non-uniform and the reflectance was increased. In a pyramid structure having a uniform size, the reflected light is relatively constant, but in a pyramid structure with a large difference, light is not reflected, and the reflectance increases. The highest reflectance was confirmed under the conditions of a KOH concentration of 3% and IPA concentration of 7.5%. This is because the pyramid formed initially at a fast etching rate grows large, and the pyramid formed later grows smaller due to the slow etching rate. Accordingly, as the KOH concentration increases, the pyramid size becomes non-uniform. It can be seen that as the pyramid size increases, the uniformity of the pyramid size decreases.
3.2. Metal-Assisted Chemical Etching (MACE)
Etching processes using a metal catalyst rely on the reduction of the oxidizing agent by the catalyst causing Si atoms near the deposited metal catalyst and the Si interface to be etched, taking into account that the metal catalyst penetrates the Si substrate in a vertical direction, thereby generating pores. The MACE process greatly affects the etching shape and reflectance characteristics of the wafer depending on conditions such as the type of metal catalyst, the deposition thickness of the metal catalyst, the ratio of the etching solution, and the etching time. Among them, process factors such as the ratio of the etching solution based on HF and H2O2 and the etching time control the etching rate to change the shape of the pores of the Si wafer. Accordingly, nano-pyramidal structures having various sizes and shapes can be obtained. In this study, a nano-pyramidal structure was formed through a surface texture process using a Ag metal catalyst, and the effects of the etching conditions on the wafer shape were analyzed.
Figure 5 shows reflectance and cross-section SEM images of the wafer surface according to etching time for the MACE process. At an etch time of 30 s, it was confirmed that the pore depth was 99 nm. Here, it was considered that the etching process had proceeded, however a relatively high reflectance characteristic of 10.12% was demonstrated. This is because the etching time of 30 s was too short, and it was not sufficient to create pores. It was confirmed that the pore depth rapidly increased from etching times of 90 s or more, with reflectance values decreasing to 5% or less. As the etching time increased, an oxide film as SiO
2 was formed at the interface between the Si wafer and the metal particle and was etched by HF, through which the metal particles entered to form a deeper structure. Since the reaction rate was affected by the ratio of the etching solution rather than the etching time, the etching time was fixed at 180 s (s), the condition showing the lowest reflectance, and then the characteristics of the etched wafer were analyzed by varying the ratio of the etching solution. The ratio of the etching solution can be expressed as a mole fraction, and
can be expressed as the following equation:
As shown in the above equation, a larger
value indicates that the HF concentration is relatively high, whereas a smaller
value indicates that the H
2O
2 concentration is relatively high. In this paper, an experiment was conducted by varying the value of
from 0 to 100%, and the characteristics of the wafer were analyzed.
Figure 6 shows the reflectance and cross-section SEM images of the wafer surfaces according to mole fraction (
for the MACE process. It can be seen that the etching process did not proceed, as the reflectance of the wafer was similar to that of the initial reflectance until the
condition of 30%. This is because the content of HF for etching SiO
2 was too low, thus the etching was not sufficiently performed. The reflectance decreased from the condition of
40%, but remained relatively high. Under the condition of
100%, there was no H
2O
2 reduction process because only HF exists in the solution. Accordingly, it was confirmed that the etching process was not performed because the SiO
2 layer could not be generated. From the condition of
80%, the reflectance was rapidly decreased. In order to analyze this, a structural analysis of the wafer after the etching process was performed through SEM images. As shown in
Figure 6, the surface shape of the wafer was very different depending on the value of
, and in the range of 80% <
< 90%, a deep pore shape of 500 nm or more could be observed. In the range of
< 70%, pores were not observed, but it was observed that the wafer surface was etched non-uniformly. The formation of deep pores is determined by the reaction rate by the reduction reaction of H
2O
2. When the
value is large, the H
2O
2 reduction proceeds slowly because the HF ratio is high. The electrons required for H
2O
2 reduction are sufficiently procured from the local region of the metal catalyst and the Si interface. Accordingly, the Si wafer placed under the catalyst particles is etched to form deep and vertical pores. As a result, the surface reflectance of the etched wafer after the MACE process was the lowest at 2.36% under the condition of
of 0.9.
3.3. Reactive Ion Etching (RIE)
In this study, a one-step surface etching process was performed using RIE, and the effect of the process conditions of gas ratio and process time on the shape and reflectance characteristics of the wafer after etching was analyzed. In the RIE process, the gas ratio of SF
6 and O
2 acts as an important factor in determining the shape and size of the pyramid, and has a great influence on the reflectance characteristics. The pyramid structures of various sizes are formed according to the gas ratio, which is related to the density of the Si
xO
yF
z passivation occurring in the initial process. The introduced SF
6 and O
2 generate radicals of F* and O* by plasma, and the etching rate varies depending on the ratio of these radicals. At this time, the Si
xO
yF
z layer formation rate formed on the sidewall can be expressed as υ
pf, and the etching rate of the etched Si
xO
yF
z passivation can be expressed as υ
pe. The etching rate of the Si wafer can be expressed as υ
Si. Etching is not performed when υ
pe is low and υ
pf is high, whereas when high υ
pe and low υ
pf exist a uniform etching shape instead of a pyramid structure is shown. It was confirmed that in the case of low υ
pe and high υ
pf, the Si
xO
yF
z passivation was excessively deposited on the wafer surface, and in the case of high υ
pe and low υ
pf, the Si
xO
yF
z passivation was not sufficiently deposited. That is, the Si
xO
yF
z passivation serves to help the Si wafer to be partially etched because it interferes with the etching by F* radicals on its side. Through this process, the etching shape can be controlled.
Figure 7 and
Figure 8 show the surface shape and reflectance characteristics of the wafer according to the gas ratio and etching time after RIE etching. As shown in
Figure 7, different shapes of pyramids can be observed depending on the different ratios of SF
6 and O
2. It was confirmed when the ratio of O
2 was higher, a pyramid structure with the low aspect ratio was obtained, and the reflectance also increased. When SF
6 and O
2 gas were added at an appropriate ratio, the surface shape may be small and densely generated, resulting in low reflectance, but when the O
2 gas ratio was excessively injected, the mask role was too strong and the surface was etched too little, so that the reflectance increased. As a result, the lowest reflectance of 5.92% was confirmed under the gas ratio of SF
6 and O
2 of 1:3. After fixing the gas ratio to SF
6 and O
2 of 1:2, the experiment was conducted by varying the process time from 200 to 700 s to analyze the effect of the process time. As shown in
Figure 8, when the process time was 200 s, a high reflectance of 14.42% was confirmed because the etching rate did not increase sufficiently. It was confirmed that the reflectance of the wafer gradually decreased through the increased etching rate under a process time of 300 s. Under process time conditions of 400 s or more, the reflectance of wafer was less than approximately 5%. It was confirmed that at the process times of 600 s or more, the size of the pyramid no longer increased even if the process time was increased, and the reflectance also reached a limit and did not decrease any more. As a result, the lowest reflectance of 3.87% was shown under the process time of 400 s.
3.4. Metal-Assisted Chemical Etching Alkali Solution Etching (Alkali Solution + MACE)
In the previous experiment, a one-step etching process was performed using KOH, which is an alkali solution. Through this, it was possible to lower the reflectance from about 30%, which is the initial reflectance of the wafer, to 9.11%. After the one-step etching process was performed, a two-step etching process was performed with the MACE process. The two-step etching process was divided into two stages of deposition and etching, the same as the one-step etching process using MACE. After the deposition of the Ag metal catalyst through a thermal evaporator, a Si wafer-deposited Ag metal was immersed in a solution of a mixture of a solution containing fluorine (F) such as HF and an oxidizing agent such as H
2O
2 to be etched. In this study, the effect on the thickness of the Ag metal catalyst used in the deposition step and the effect of the etching solution ratio in the etching step were analyzed. First, the Ag metal catalyst thickness was fixed to a constant value, and then the effect of the etching solution ratio was analyzed. The thickness of the Ag metal catalyst was fixed to 7 nm, and the etching time was fixed to 3 min in various ratios of etching solution in order to observe the change. The concentration of HF in the etching solution was fixed to 1 M, and the experiment was conducted by increasing the molar ratio of H
2O
2, which was converted into a
as mole fraction. The etching reaction rate was determined by the reduction reaction of H
2O
2, which means that when concentration of HF is greater than the ratio of H
2O
2, the H
2O
2 reduction proceeds slowly. Accordingly, it can be seen that the shape of the Si surface is very different after the surface etching process according to the
of the etching solution.
Figure 9 shows the reflectance and cross-section SEM images of wafer surfaces according to
for the two-step etching process with alkali solution and MACE. As the
value decreases, the reduction of H
2O
2 proceeds faster, and electrons required for this are supplied not only from the interface between the catalyst and the Si wafer, but also from the pore walls at a distance. Accordingly, not only the area under the metal catalyst particles, but also the pore walls of the Si wafer were etched, resulting in a shape of pores in which pore wall was inclined rather than vertical. As a result, a deep and uniform pore shape was displayed under the conditions of
50%, and the lowest reflectance of approximately 1.62% was confirmed.
As a result, the lowest reflectance was obtained under the conditions of a Ag metal catalyst thickness of 7 nm, an etching time of 3 min, and a
50%. After the
was fixed at 50%, the thickness of the Ag metal catalyst was changed in the deposition step to observe its effect. When the thickness of the Ag metal catalyst was less than 5 nm, the etching process was not performed smoothly. Accordingly, the thickness of the Ag metal catalyst was deposited at 7, 10, and 13 nm. The etching was performed under conditions of 1 to 5 min for each thickness.
Figure 10 shows the reflectance and cross-section SEM images of the wafer surfaces according to etching time for two-step etching process with alkali solution and MACE after depositing a Ag metal catalyst according to various thicknesses. The SEM image shows a cross-sectional image of a wafer after depositing a Ag catalyst of a thickness of 13 nm and etching according to the etching time. It was found that the reflectance results of the wafer were all within 5% of one another under the varying thickness conditions of Ag metal catalyst. When forming a nano-sized pyramid on a micro-sized pyramid structure formed using a KOH solution, reflectance gradually decreased as the etching time increased, showing the lowest reflectance of 1.3% in conditions of Ag thickness of 13 nm. As the etching time increased, the depth of the nano-pyramid increased, so that the reflectance was decreased. This means that the better absorption of the incident light was allowed within the nano-pyramid structure of Si. However, if the etching process is performed for more than 3 min, the reflectance gradually increases. This is because as the etching time increases, the pyramid structure of the surface gradually collapses due to excessive etching, and accordingly, the reflectance increases due to the non-uniform characteristics of the wafer surface. As can be seen from
Figure 10, since the size of the pores is determined by the size of the Ag metal particles in the deposition step before etching, it is important to deposit the Ag metal catalyst in the form of nano-particles. As a result of comparing the structural shape of the wafer according to the etching time, it can be seen that the nano-pyramid structure was not formed when etching at a thickness of 7 nm for 1 min. This is because not all Ag particles reacted due to the short etching time. As the etching time gradually increased, the number of pores increased, and a dense structure of the surface of the Si wafer could be observed. This is because as the etching time increased, the oxide film formed at the interface between the Si wafer and the metal particles was etched by HF, and through this, the metal particles entered deeper to form deep and high pores. Accordingly, the shape of the pores is perpendicular to the Si surface, and the height and inclination of the structure affects reflectance, so it was confirmed that the reflectance decreases as the etching time increases [
25,
26]. However, after depositing the Ag metal catalyst of 10 to 13 nm thickness, it was confirmed that the pore depth was shallow and a non-uniform pore shape was confirmed under the etch time conditions of 5 min or more. This is because even though the Ag metal catalyst was sufficiently etched, excessive etching occurred with the longer etching time. Accordingly, etching happened not only in the area where the Ag catalyst was deposited but also in the area where the Ag metal catalyst was not formed, resulting in a deterioration of the surface characteristics. As a result, an etching time of 3 min was found to be optimal for the 13 nm-thick Ag metal catalyst, and the lowest reflectance of approximately 1.3% was confirmed.
Metal removal was performed for 30 min in a solution in which NH
3 and H
2O
2 were mixed in a ratio of 3:1 to remove any remaining Ag particles after the completion of the etching process.
Figure 11 shows the energy dispersive X-ray spectroscopy (EDS) measurement results before and after removing the Ag particles. As shown in the
Figure 11, it was confirmed that Si and Ag components appeared along with peaks after Ag deposition, and it was confirmed that all the particles of Ag were removed after etching.
3.5. Reactive Ion Etching with Alkali Solution Etching (Alkali Solution + RIE)
A one-step surface etching process was performed using an alkali solution, and then a two-step surface etching process was performed using the etched wafer. SF
6 gas, O
2 gas, and Ar gas were used for the RIE process, and the effects of the gas ratio of SF
6 and O
2 and the RF power on the surface shape and reflectance characteristics of the wafer were analyzed. In the previous experiment with one-step etching process using RIE, the F radicals lead the etching and the O radicals passivated the Si wafer. As a result, the balance between etching and passivation induces vertical etching, and through this, as the etching time increases, the etching rate increases. In addition, as the etching proceeds for a longer period of time, the formation of a nano-pyramid structure can be observed, which is composed between the Si and Si
xO
yF
z layers.
Figure 12 shows the reflectance and cross-section SEM images of wafer surfaces according to the gas ratio for two-step etching process with alkali solution and RIE. When the ratio of SF
6 and O
2 was 3:1, an etching rate of 30.81 nm/min was measured, and when the ratio of 2:1 was used this rate was 48.93 nm/min. However, the etching rate started to decrease from the ratio conditions of SF
6:O
2 at 1:1, and the etching rate was zero in the case of the 1:3 ratio, because no etching was performed at all. This can be seen from the chemical reaction equation of RIE dry etching in Equation (3) from Equation (7), given below:
The above equation represents the reactions of SF
6 and O
2 gas in the RIE process using plasma. As shown in Equations (3) and (6), fluorine radicals (F*) are generated due to the physical collision of Ar gas and SF
6 gas, and a chemical reaction between the F radicals and the Si wafer occurs. When the proportion of SF
6 gas increases, the amount of F ions and F* generated by collisions with Ar increases. The generated F ions and F radicals react directly with Si to generate SF
4, which has volatile properties. On the other hand, when the proportion of O
2 gas increases, the etching rate does not increase linearly. This can be seen through Equations (4) and (5). SF
5 and F particles separated by Ar gas are chemically unstable, and recombine into SF
6 within a short time. SF
5O is formed by combining O
2 with SF
5 particles and temporarily prevents the reaction of F ions to recombine to SF
6. Accordingly, until the ratio of SF
6 and O
2 becomes 3:1 to 2:1, the etching rate increases because the O
2 gas contributes to the generation of F particles that directly react to the etching reaction. This can be seen from Equations (4) and (7). O
2 gas is decomposed into monoatoms by plasma, and activated O atoms passivate the Si surface to form a thin film of SiO
2 or SiO
x phase. Since the SiO
2 thin film does not react with the F* particles, the etching rate decreases as the O
2 gas ratio increases As can be seen from
Figure 12,
Figure 12a shows the wafer surface with a densely formed nano-pyramidal due to the strong action of F radicals.
Figure 12c shows that the entire wafer surface was etched. However, since the action of the O radical interferes with the etching, a pyramid surface shape with a low slope was obtained. As a result, under the ratio conditions of SF
6 and O
2 at 3:1, the reflectance greatly decreases due to the effect of the uniform and dense nano-pyramid, and a low reflectance of 1.24% was obtained.
Figure 13 shows the reflectance and cross-section SEM images of wafer surfaces according to RF power for the two-step etching process with alkali solution and RIE. The RF power was changed from 25 to 200 W while injecting the reaction gas. At an RF power of 50 W or less, a pyramid structure was not sufficiently formed on the surface because the physical collision of the reaction gas was weak. Accordingly, high reflectance values of 11.43% and 10.55% were confirmed respectively, and the etch rate was also less than approximately 7 nm/min. It was confirmed that the etching rate increased as the applied RF power increased. This is because when high RP power is applied during atmospheric pressure plasma discharge, and the concentration of active ions increases due to the increase in plasma density thereby increasing the reactivity. Under RF power conditions of 100 W or more, the wafer surface was observed in the form of a pyramid of a needle-like structure. As the RF power increased, the chemical reaction became active and the movement of electrons and ions rapidly increased, and accordingly, the etching rate increased. It was found that the surface reflectance was decreased as the height of the pyramid structure increased and the slope increased after the RIE process. The lowest reflectance of 1.24% was confirmed under the RF power conditions of 100 W. An inverted pyramid structure was formed at an RF power of 125 W. Under RF power conditions of 150 W or more, it was confirmed that the surface of the wafer was over-etched, resulting in a non-uniform pyramidal shape, and thus the surface characteristics of the wafer were degraded and the reflectance increased again.
It was confirmed that the pyramid structure on the surface through the two-step surface etching process uniformly covers the entire area of the wafer.
Figure 14 displays a graph showing reflectance characteristics according to the pyramid structure. The reflectance characteristics were measured in the wavelength range of 400–1000 nm, and the reflectance of the textured wafer surface ranged from 9.11 to 1.2%. In the case of the micro-sized pyramid, the surface reflectance of the wafer was approximately 9%, and the nano-sized pyramid formed through RIE and MACE showed surface reflectances of 3.87% and 2.36%, respectively. In the case of two-step etching through KOH and RIE, the reflectance was approximately 1.24%, and in the case of two-step etching through KOH and MACE, the reflectance was approximately 1.3%. The reflectance values of the Si wafers etched with two-step processes are lower than where one-step processes were used. This is expected to increase solar cell efficiency because optical loss can be reduced by improving reflectance characteristics. In order to analyze the effect of surface reflectance on cell characteristics, the quantum efficiency of the cell fabricated through the process of an emitter, a passivation film formation, and an electrode formation after the surface etching process were measured.
Quantum efficiency characteristics were compared to conduct electrical analysis based on the structural analysis of the emitter layer according to the pyramid structure.
Figure 15 shows a graph of the quantum efficiency characteristics according to various etching process methods. Although the reflectance of the wafer after surface etching was the lowest in the alkali solution and MACE process, it was confirmed that the quantum efficiency value of the etching process with alkali solution alone was higher. In particular, it can be seen that the difference of the quantum efficiency value is large in the short wavelength band.
In order to form an emitter layer that serves to separate electron-hole pairs generated by light energy by an internal electric field, a doping process was performed on a Si wafer, formed with various sized pyramids, using a doping furnace. The doping process was divided into two stages: pre-deposition and drive-in. The pre-deposition process is a process that supplies doping atoms to the semiconductor wafer surface. The drive-in process is a process of diffusing impurity atoms pre-deposited on the wafer surface into the wafer. In general, in the case of a c-Si solar cell, an n-type emitter layer is formed on a p-type wafer using a POCl
3 solution. The POCl
3 solution is maintained within 25 °C and bubbles are generated by nitrogen gas. The generated bubbles contain POCl
3 and are transported into the doping furnace by nitrogen gas. In the process transportation, they mix with oxygen (O
2) gas to produce P
2O
5, which is deposited on the wafer surface. The injection of O
2 gas during the diffusion process causes the growth of an oxide film by a reaction between the P
2O
5 gas and the Si wafer, and this oxide film is generally known as phosphosilicateglass (PSG). This process is represented by the formula as follows. The PSG layer is an oxide film in which phosphorus is diffused relatively thickly in comparison to an oxide film grown during a normal oxidation process under the same temperature and time conditions. Accordingly, after the diffusion process, the PSG film was removed using a 10% diluted HF solution. It is very important to measure the distribution of the doping concentration and the doping profile of the doping depth after the emitter formation process. The doping profile is mainly measured using secondary ion mass spectroscopy (SIMS). This analysis method is difficult to accurately measure and can only be measured using expensive analysis equipment. As such, a selective etching analysis was performed using SEM instead of the SIMS analysis method in this study. This is a method of analysis that uses a relative comparison value by forming a step by the difference in etch rate between the emitter layer and the non-emitter layer.
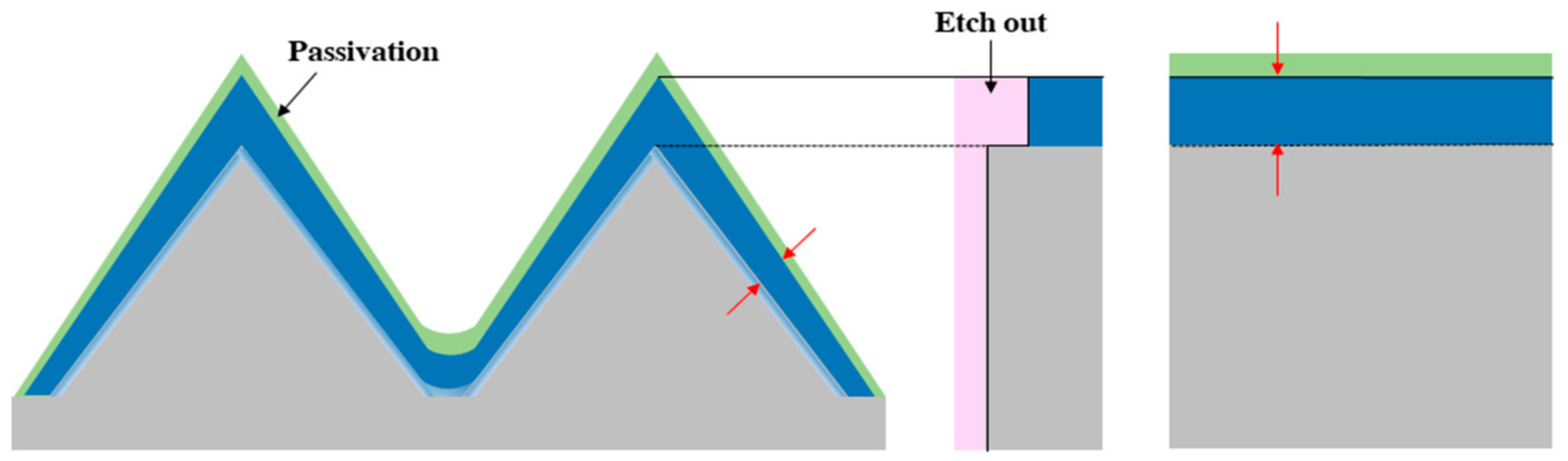
Figure 16 shows a schematic diagram of the selective etching (SE) analysis. The etching solution for SE consisted of HF:HNO
3:CH
3COOH = 1:100:25, and the doped wafer was etched for 5 s. It is only possible to form an accurate step if there exists a passivation layer on the emitter layer, as this prevents additional etching on the top surface. In this paper, the effect of the pyramid structure on the emitter formation process was analyzed based on the results obtained through the previous experiment.
After the surface etching process, the emitter layer was formed using a furnace.
Figure 17 shows a cross-section SEM image of the wafer after the emitter formation process according to various etching process methods. The SEM image is the result of the formation of the n-type emitter and clearly shows the emitter formation structure according to the pyramid structure.
Figure 17a is a result of observing the emitter layer formed on the micro-sized pyramid wafer, where it can be seen that a uniform doped layer is formed. In the nano-sized pyramid wafers of
Figure 17b–e, it can be observed that the emitter layer has a non-uniform formation compared to the micro-sized pyramid structure. This is judged by the influence of the dopant in the deposition step and the diffusion depth in the diffusion step during emitter formation, and can be explained through a schematic diagram of the formation process of the emitter layer, as shown in
Figure 18. To form an emitter on the surface-textured wafer, POCl
3 and O
2 are injected into the furnace and deposited through pre-deposition and post-diffusion steps. The chemical reaction formula for P diffusion using a liquid source is as follows:
POCl
3 and O
2 gas react in a chamber at a high temperature of approximately 800 °C to form a P
2O
5 layer on the wafer surface. This step is called the pre-deposition step. In this state, when high-temperature heat treatment is performed, the P atoms of P
2O
5 diffuse to the Si surface to form an emitter layer. This step is called the post-diffusion step. At this time, the dopant deposited on the wafer surface and the diffusion of the P is greatly influenced by the pyramidal structure of the wafer. In the case of a micro-sized pyramid structured wafer, as shown in
Figure 18a, the thickness of the top and valley portions of the PSG layer is different. This is because the PSG layer is deposited thinly on the top portion of the pyramid structure due to the tensile properties present. In the valley portions of the pyramid structure, a thick PSG layer is deposited due to the compressive properties present. The thicker PSG layer forms a higher P surface concentration, and a deeper emitter layer is formed in the post-diffusion process. In the case of a micro-sized pyramid, it was observed that the side of the pyramid and the valley portion could form an emitter layer of similar thickness, and the top portion had more diffusion of the dopant and the emitter layer was thicker. In the case of a wafer having a nano-sized pyramid structure as shown in
Figure 18b, more valley area is occupied in comparison to the same area of a micro-sized pyramid structure. Like the micro-sized pyramid, a thicker PSG layer is formed in the valley area with the nano-sized pyramid structure. The nano-sized pyramid structure may have a thicker PSG layer than the micro-sized pyramid structure due to the large number of valleys [
27,
28]. Accordingly, the emitter layer was formed deeper than the micro-sized pyramid structure in the nano-sized pyramid structure wafer. Based on the non-uniformity of the emitter layer observed through the SE analysis, it can be concluded that the formation of the emitter layer according to the pyramid structure has an effect on the number of tops and valleys compared to the same area.
As a result, although the reflectance of the wafer after the etching process was the lowest in the KOH + MACE process, the quantum efficiency value of the KOH process alone was measured to be higher. This is because the recombination rate is high due to the low sheet resistance by the deep emitter layer of the nano-pyramidal structure. It is also expected to be as a result of the influence of the non-uniform emitter layer in the nano-pyramidal structure. Subsequently, in order to increase the efficiency of the cell, it can be seen that the surface of the nano-pyramid using the one-step etching process is more advantageous when considering the formation of the emitter layer rather than the surface of the micro-pyramid using the two-step etching process.