Nano-Enhanced Phase Change Materials in Latent Heat Thermal Energy Storage Systems: A Review
Abstract
:1. Introduction to Latent Heat Thermal Energy Storage
1.1. Applications of LHTES Systems
1.2. Types of PCM
1.2.1. Organic and Inorganic
1.2.2. Eutectic PCMs
2. Heat Transfer Enhancement
3. Nano-Enhanced PCM
3.1. Nanoparticles as Sole Enhancement
3.1.1. Water NePCMs with Cu
3.1.2. Paraffin and Alumina
3.1.3. Paraffin and Cu
3.1.4. Paraffin with Carbons
3.1.5. Paraffin with Other NPs
3.1.6. Eutectic Hydrate Salts
3.1.7. PEG and Ag
3.1.8. Fatty Acids
3.1.9. Various Middle Temperature PCMs
3.1.10. NaNO3-KNO3
3.1.11. High-Temperature PCMs
3.2. NePCM and Fins
3.3. NePCMs and Heat Pipes
3.4. Highly Conductive Porous Materials Impregnated with NePCMs
3.5. NPs and Multiple PCMs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Tiari, S.; Qiu, S. Three-dimensional simulation of high temperature latent heat thermal energy storage system assisted by finned heat pipes. Energy Convers. Manag. 2015, 105, 260–271. [Google Scholar] [CrossRef]
- De Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.Y.; Qu, Z.G. Lithium-ion battery thermal management using heat pipe and phase change material during discharge–charge cycle: A comprehensive numerical study. Appl. Energy 2019, 242, 378–392. [Google Scholar] [CrossRef]
- Tariq, S.L.; Ali, H.M.; Akram, M.A.; Janjua, M.M.; Ahmadlouydarab, M. Nanoparticles enhanced phase change materials (NePCMs)-A recent review. Appl. Therm. Eng. 2020, 176, 115305. [Google Scholar] [CrossRef]
- European Energy Research Alliance. High-Temperature Latent Heat Storage [digital]; EERA: Brussels, Belgium, 2018; Available online: https://eera-es.eu/ (accessed on 18 June 2021).
- Huang, Z.; Xie, N.; Luo, Z.; Gao, X.; Fang, X.; Fang, Y.; Zhang, Z. Characterization of medium-temperature phase change materials for solar thermal energy storage using temperature history method. Sol. Energy Mater. Sol. Cells 2018, 179, 152–160. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Singh, V.K.; Bhavsar, R.R.; Chandrasekar, M.; Archita, V. Life cycle assessment of nanoalloy enhanced layered perovskite solid-solid phase change material till 10000 thermal cycles for energy storage applications. J. Energy Storage 2021, 35, 102220. [Google Scholar] [CrossRef]
- Singh, R.P.; Sze, J.Y.; Kaushik, S.C.; Rakshit, D.; Romagnoli, A. Thermal performance enhancement of eutectic PCM laden with functionalised graphene nanoplatelets for an efficient solar absorption cooling storage system. J. Energy Storage 2021, 33, 102092. [Google Scholar] [CrossRef]
- Mehta, D.S.; Solanki, K.; Rathod, M.K.; Banerjee, J. Thermal performance of shell and tube latent heat storage unit: Comparative assessment of horizontal and vertical orientation. J. Energy Storage 2019, 23, 344–362. [Google Scholar] [CrossRef]
- Mahdavi, M.; Qiu, S.; Tiari, S. Improvement of a novel heat pipe network designed for latent heat thermal energy storage systems. Appl. Therm. Eng. 2016, 108, 878–892. [Google Scholar] [CrossRef]
- Tiari, S.; Mahdavi, M.; Qiu, S. Experimental study of a latent heat thermal energy storage system assisted by a heat pipe network. Energy Convers. Manag. 2017, 153, 362–373. [Google Scholar] [CrossRef]
- Tiari, S.; Qiu, S.; Mahdavi, M. Numerical study of finned heat pipe-assisted thermal energy storage system with high temperature phase change material. Energy Convers. Manag. 2015, 89, 833–842. [Google Scholar] [CrossRef]
- Tiari, S.; Qiu, S.; Mahdavi, M. Discharging process of a finned heat pipe–assisted thermal energy storage system with high temperature phase change material. Energy Convers. Manag. 2016, 118, 426–437. [Google Scholar] [CrossRef]
- Aly, K.A.; El-Lathy, A.R.; Fouad, M.A. Enhancement of solidification rate of latent heat thermal energy storage using corrugated fins. J. Energy Storage 2019, 24, 100785. [Google Scholar] [CrossRef]
- Tiari, S.; Hockins, A.; Mahdavi, M. Numerical study of a latent heat thermal energy storage system enhanced by varying fin configurations. Case Stud. Therm. Eng. 2021, 25, 100999. [Google Scholar] [CrossRef]
- Gasia, J.; Maldonado, J.M.; Galati, F.; de Simone, M.; Cabeza, L.F. Experimental evaluation of the use of fins and metal wool as heat transfer enhancement techniques in a latent heat thermal energy storage system. Energy Convers. Manag. 2019, 184, 530–538. [Google Scholar] [CrossRef]
- Reyes, A.; Negrete, D.; Mahn, A.; Sepúlveda, F. Design and evaluation of a heat exchanger that uses paraffin wax and recycled materials as solar energy accumulator. Energy Convers. Manag. 2014, 88, 391–398. [Google Scholar] [CrossRef]
- Tiari, S.; Mahdavi, M. Computational study of a latent heat thermal energy storage system enhanced by highly conductive metal foams and heat pipes. J. Anal. Calorim. 2020, 141, 1741–1751. [Google Scholar] [CrossRef]
- Lakshmi Narasimhan, N. Assessment of latent heat thermal storage systems operating with multiple phase change materials. J. Energy Storage 2019, 23, 442–455. [Google Scholar] [CrossRef]
- Li, W.Q.; Guo, S.J.; Tan, L.; Liu, L.L.; Ao, W. Heat transfer enhancement of nano-encapsulated phase change material (NEPCM) using metal foam for thermal energy storage. Int. J. Heat Mass Transf. 2021, 166, 120737. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145, 118741. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of mPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159, 120083. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B. Determining the heat of fusion and specific heat of microencapsulated phase change material slurry by thermal delay method. Energies 2021, 14, 179. [Google Scholar] [CrossRef]
- Elgafy, A.; Lafdi, K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon 2005, 43, 3067–3074. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Jourabian, M.; Farhadi, M. Melting of nanoparticles-enhanced phase change material (NEPCM) in vertical semicircle enclosure: Numerical study. J. Mech. Sci. Technol. 2015, 29, 3819–3830. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Li, L.; Bu, L.; Wang, T. Numerical investigation on the melting of nanoparticle-enhanced phase change materials (NEPCM) in a bottom-heated rectangular cavity using lattice Boltzmann method. Int. J. Heat Mass Transf. 2015, 81, 415–425. [Google Scholar] [CrossRef]
- Akhmetov, B.; Navarro, M.E.; Seitov, A.; Kaltayev, A.; Bakenov, Z.; Ding, Y. Numerical study of integrated latent heat thermal energy storage devices using nanoparticle-enhanced phase change materials. Sol. Energy 2019, 194, 724–741. [Google Scholar] [CrossRef]
- Abdulateef, A.M.; Jaszczur, M.; Hassan, Q.; Anish, R.; Niyas, H.; Sopian, K.; Abdulateef, J. Enhancing the melting of phase change material using a fins–nanoparticle combination in a triplex tube heat exchanger. J. Energy Storage 2021, 35, 102227. [Google Scholar] [CrossRef]
- Zaidan, M.J.; Alhamdo, M.H. Improvement in heat transfer inside a phase change energy system. Int. J. Mech. Mechatron. Eng. 2018, 18, 33–46. [Google Scholar]
- Farsani, R.Y.; Raisi, A.; Nadooshan, A.A.; Vanapalli, S. Does nanoparticles dispersed in a phase change material improve melting characteristics? Int. Commun. Heat Mass Transf. 2017, 89, 219–229. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; el Qarnia, H. Transient behavior analysis of the melting of nanoparticle-enhanced phase change material inside a rectangular latent heat storage unit. Appl. Therm. Eng. 2017, 112, 720–738. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; el Qarnia, H. Thermal analysis of nanoparticle-enhanced phase change material solidification in a rectangular latent heat storage unit including natural convection. Energy Build. 2017, 153, 1–17. [Google Scholar] [CrossRef]
- Nie, C.; Liu, J.; Deng, S. Effect of geometric parameter and nanoparticles on PCM melting in a vertical shell-tube system. Appl. Therm. Eng. 2021, 184, 116290. [Google Scholar] [CrossRef]
- Hosseini, S.M.J.; Ranjbar, A.A.; Sedighi, K.; Rahimi, M. Melting of nanoprticle-enhanced phase change material inside shell and tube heat exchanger. J. Eng. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Algarni, S.; Mellouli, S.; Alqahtani, T.; Almutairi, K.; Khan, A.; Anqi, A. Experimental investigation of an evacuated tube solar collector incorporating nano-enhanced PCM as a thermal booster. Appl. Therm. Eng. 2020, 180, 115831. [Google Scholar] [CrossRef]
- Nitsas, M.T.; Koronaki, I.P. Thermal analysis of pure and nanoparticle-enhanced PCM—Application in concentric tube heat exchanger. Energies 2020, 13, 3841. [Google Scholar] [CrossRef]
- Aqib, M.; Hussain, A.; Ali, H.M.; Naseer, A.; Jamil, F. Experimental case studies of the effect of Al2O3 and MWCNTs nanoparticles on heating and cooling of PCM. Case Stud. Therm. Eng. 2020, 22, 100753. [Google Scholar] [CrossRef]
- Temel, Ü.N.; Çiftçi, B.Y. Determination of thermal properties of a82 organic phase change material embedded with different type nanoparticles. Farkli Tipte Nanoparçaciklarla Katkilanan A82 Organik Faz Değiştiren Malzemenin Termal. Özellİklerİnİn Belirlenmesi 2018, 38, 75–85. [Google Scholar]
- Murugan, P.; Ganesh Kumar, P.; Kumaresan, V.; Meikandan, M.; Malar Mohan, K.; Velraj, R. Thermal energy storage behaviour of nanoparticle enhanced PCM during freezing and melting. Phase Transit. 2018, 91, 254–270. [Google Scholar] [CrossRef]
- Thalib, M.M.; Manokar, A.M.; Essa, F.A.; Vasimalai, N.; Sathyamurthy, R.; Garcia Marquez, F.P. Comparative study of tubular solar stills with phase change material and nano-enhanced phase change material. Energies 2020, 13, 3989. [Google Scholar] [CrossRef]
- Javadi, H.; Urchueguia, J.F.; Mousavi Ajarostaghi, S.S.; Badenes, B. Numerical study on the thermal performance of a single U-tube borehole heat exchanger using nano-enhanced phase change materials. Energies 2020, 13, 5156. [Google Scholar] [CrossRef]
- Bashar, M.; Siddiqui, K. Experimental investigation of transient melting and heat transfer behavior of nanoparticle-enriched PCM in a rectangular enclosure. J. Energy Storage 2018, 18, 485–497. [Google Scholar] [CrossRef]
- Khatibi, M.; Nemati-Farouji, R.; Taheri, A.; Kazemian, A.; Ma, T.; Niazmand, H. Optimization and performance investigation of the solidification behavior of nano-enhanced phase change materials in triplex-tube and shell-and-tube energy storage units. J. Energy Storage 2021, 33, 102055. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Boyaghchi, F.A. Exergy and economic analyses of nanoparticle-enriched phase change material in an air heat exchanger for cooling of residential buildings. J. Energy Storage 2020, 32, 101705. [Google Scholar] [CrossRef]
- Pasupathi, M.K.; Alagar, K.; Stalin P, M.J.; Matheswaran, M.M.; Aritra, G. Characterization of hybrid-nano/paraffin organic phase change material for thermal energy storage applications in solar thermal systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Liu, F.; Li, Q. Thermal conductivity and thermal mechanism of aluminum nanoparticles/octadecane composite phase change materials from molecular dynamics simulations and experimental study. J. Ovonic Res. 2016, 12, 49–58. [Google Scholar]
- Badakhsh, A.; An, K.-H.; Park, C.W.; Kim, B.-J. Effects of biceramic AlN-SiC microparticles on the thermal properties of paraffin for thermal energy storage. J. Nanomater. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maher, H.; Rocky, K.A.; Bassiouny, R.; Saha, B.B. Synthesis and thermal characterization of paraffin-based nanocomposites for thermal energy storage applications. Therm. Sci. Eng. Prog. 2021, 22, 100797. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Doostani, A.; Chamkha, A.J.; Ismael, M.A. Melting of nanoparticles-enhanced phase-change materials in an enclosure: Effect of hybrid nanoparticles. Int. J. Mech. Sci. 2017, 134, 85–97. [Google Scholar] [CrossRef]
- Liang, L.; Chen, X. Preparation and thermal properties of eutectic hydrate salt phase change thermal energy storage material. Int. J. Photoenergy 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jamalabadi, M.Y.A. Use of nanoparticle enhanced phase change material for cooling of surface acoustic wave sensor. Fluids 2021, 6, 31. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Hamze, S.; Fedele, L.; Bobbo, S.; Estellé, P.; Lugo, L. NePCM based on silver dispersions in poly(ethylene glycol) as a stable solution for thermal storage. Nanomaterials 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Qiu, F.; Zhu, W.; Guo, Y.; Zhang, Y.; Ju, Y.; Feng, R.; Liu, Y.; Chen, Z.; Zhou, J.; et al. Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 193, 237–245. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Guan, W.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Prabakaran, R.; Sidney, S.; Lal, D.M.; Selvam, C.; Harish, S. Solidification of graphene-assisted phase change nanocomposites inside a sphere for cold storage applications. Energies 2019, 12, 3473. [Google Scholar] [CrossRef] [Green Version]
- Santhosh, S.; Satish, M.; Yadav, A.; Anish Madhavan, A. Thermal analysis of Fe2O3—Myristic acid nanocomposite for latent heat storage. Mater. Today Proc. 2021, 52, 687. [Google Scholar] [CrossRef]
- Barreneche, C.; Martín, M.; la Calvo-de Rosa, J.; Majó, M.; Fernández, A.I. Own-synthetize nanoparticles to develop nano-enhanced phase change materials (NEPCM) to improve the energy efficiency in buildings. Molecules 2019, 24, 1232. [Google Scholar] [CrossRef] [Green Version]
- Vivekananthan, M.; Amirtham, V.A. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Mayilvelnathan, V.; Arasu, A.V. Experimental investigation on thermal behavior of graphene dispersed erythritol PCM in a shell and helical tube latent energy storage system. Int. J. Therm. Sci. 2020, 155, 106446. [Google Scholar] [CrossRef]
- Manickam, R.; Kalidoss, P.; Suresh, S.; Venkatachalapathy, S. Erythritol based nano-PCM for solar thermal energy storage. Int. Res. J. Eng. Technol. 2019, 6, 1631–1636. [Google Scholar]
- u Mekrisuh, K.; Giri, S.; Udayraj; Singh, D.; Rakshit, D. Optimal design of the phase change material based thermal energy storage systems: Efficacy of fins and/or nanoparticles for performance enhancement. J. Energy Storage 2021, 33, 102126. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef] [Green Version]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M.; Torre, L. Heat capacity of nanofluids for solar energy storage produced by dispersing oxide nanoparticles in nitrate salt mixture directly at high temperature. Sol. Energy Mater. Sol. Cells 2017, 167, 60–69. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Rahman, S.; Mohd Sabri, M.F.; Arifutzzaman, A. Experimental investigation of thermal stability and enthalpy of eutectic alkali metal solar salt dispersed with MgO nanoparticles. IJTech 2019, 10, 1112. [Google Scholar] [CrossRef] [Green Version]
- Saranprabhu, M.K.; Rajan, K.S. Magnesium oxide nanoparticles dispersed solar salt with improved solid phase thermal conductivity and specific heat for latent heat thermal energy storage. Renew. Energy 2019, 141, 451–459. [Google Scholar] [CrossRef]
- Myers, P.D.; Alam, T.E.; Kamal, R.; Goswami, D.Y.; Stefanakos, E. Nitrate salts doped with CuO nanoparticles for thermal energy storage with improved heat transfer. Appl. Energy 2016, 165, 225–233. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Crescenzi, T.; Torre, L.; Kenny, J.M. A new phase change material based on potassium nitrate with silica and alumina nanoparticles for thermal energy storage. Nanoscale Res. Lett. 2015, 10, 984. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Wang, Z.; Wu, Y.; Xu, P.; Ding, Y.; Chang, C.; Ma, C. Performance enhancement of bromide salt by nano-particle dispersion for high-temperature heat pipes in concentrated solar power plants. Appl. Energy 2019, 237, 171–179. [Google Scholar] [CrossRef]
- Han, D.; Lougou, B.G.; Xu, Y.; Shuai, Y.; Huang, X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl. Energy 2020, 264, 114674. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Erfani Moghaddam, M.A.; Asadi, A.; Mogharrebi, A.R.; Jafari, B.; Hasani, M.R.; Ganji, D.D. Effect of two different fins (longitudinal-tree like) and hybrid nano-particles (MoS2-TiO2) on solidification process in triplex latent heat thermal energy storage system. Alex. Eng. J. 2021, 60, 1967–1979. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Keshteli, A.N.; Bach, Q.-V. Solidification of PCM within a tank with longitudinal-Y shape fins and CuO nanoparticle. J. Mol. Liq. 2020, 317, 114188. [Google Scholar] [CrossRef]
- Nakhchi, M.E.; Hatami, M.; Rahmati, M. A numerical study on the effects of nanoparticles and stair fins on performance improvement of phase change thermal energy storages. Energy 2021, 215, 119112. [Google Scholar] [CrossRef]
- Li, F.; Almarashi, A.; Jafaryar, M.; Hajizadeh, M.R.; Chu, Y.-M. Melting process of nanoparticle enhanced PCM through storage cylinder incorporating fins. Powder Technol. 2021, 381, 551–560. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, H.; Luo, Z. PCM charging process accelerated with combination of optimized triangle fins and nanoparticles. Int. J. Therm. Sci. 2019, 140, 466–479. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E.C. Simultaneous energy storage and recovery in the triplex-tube heat exchanger with PCM, copper fins and Al2O3 nanoparticles. Energy Convers. Manag. 2019, 180, 949–961. [Google Scholar] [CrossRef]
- Bondareva, N.S.; Gibanov, N.S.; Sheremet, M.A. Computational study of heat transfer inside different PCMs enhanced by Al2O3 nanoparticles in a copper heat sink at high heat loads. Nanomaterials 2020, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Koukou, M.K.; Dogkas, G.; Vrachopoulos, M.G.; Konstantaras, J.; Pagkalos, C.; Lymperis, K.; Stathopoulos, V.; Evangelakis, G.; Prouskas, C.; Coelho, L.; et al. Performance evaluation of a small-scale latent heat thermal energy storage unit for heating applications based on a nanocomposite organic PCM. ChemEngineering 2019, 3, 88. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, M.; Tiari, S.; Pawar, V. A numerical study on the combined effect of dispersed nanoparticles and embedded heat pipes on melting and solidification of a shell and tube latent heat thermal energy storage system. J. Energy Storage 2020, 27, 101086. [Google Scholar] [CrossRef]
- Tiari, S.; Mahdavi, M.; Pawar, V. Heat transfer analysis of a low-temperature heat pipe-assisted latent heat thermal energy storage system with nano-enhanced PCM. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018; pp. 1–10. [Google Scholar]
- Tiari, S.; Mahdavi, M.; Thakore, V.; Joseph, S. Thermal analysis of a high-temperature heat pipe-assisted thermal energy storage system with nano-enhanced phase change material. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018; pp. 1–10. [Google Scholar]
- Tiari, S.; Rose, O.L.; Mahdavi, M. Discharging process of a high-temperature heat pipeassisted thermal energy storage system with nanoenahnced phase change material. In Proceedings of the 4th Thermal and Fluids Engineering Conference (TFEC), Las Vegas, NV, USA, 14–17 April 2019; pp. 1–10. [Google Scholar]
- Ren, Q.; Meng, F.; Guo, P. A comparative study of PCM melting process in a heat pipe-assisted LHTES unit enhanced with nanoparticles and metal foams by immersed boundary-lattice Boltzmann method at pore-scale. Int. J. Heat Mass Transf. 2018, 121, 1214–1228. [Google Scholar] [CrossRef]
- Yu, J.; Yu, Z.C.; Tang, C.L.; Chen, X.; Song, Q.F.; Kong, L. Preparation and characterization of composite phase change materials containing nanoparticles. Kemija U Industriji Časopis Kemičara I Kemijskih Inženjera Hrvatske 2016, 65, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Senobar, H.; Aramesh, M.; Shabani, B. Nanoparticles and metal foams for heat transfer enhancement of phase change materials: A comparative experimental study. J. Energy Storage 2020, 32, 101911. [Google Scholar] [CrossRef]
- Buonomo, B.; di Pasqua, A.; Ercole, D.; Manca, O. Numerical study of latent heat thermal energy storage enhancement by nano-PCM in aluminum foam. Inventions 2018, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Zhang, Y.; Chen, X.; Song, X.; Tang, K. Experimental study of an enhanced phase change material of paraffin/expanded graphite/nano-metal particles for a personal cooling system. Materials 2020, 13, 980. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.J.; Lee, S.O.; Lee, U.S.; Shin, H.K. Latent heat storage and thermal efficacy of carboxymethyl cellulose carbon foams containing Ag, Al, carbon nanotubes, and graphene in a phase change material. Nanomaterials 2019, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Zhang, C.; Lu, Y.; Kong, Q.; Wei, H.; Yang, Y.; Gao, Q.; Wu, Y.; Sciacovelli, A. Comprehensive performance of composite phase change materials based on eutectic chloride with SiO2 nanoparticles and expanded graphite for thermal energy storage system. Renew. Energy 2021, 172, 1120–1132. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Hashim, E.T.; Talebizadehsardari, P.; Nsofor, E.C. Solidification enhancement with multiple PCMs, cascaded metal foam and nanoparticles in the shell-and-tube energy storage system. Appl. Energy 2020, 257, 113993. [Google Scholar] [CrossRef]
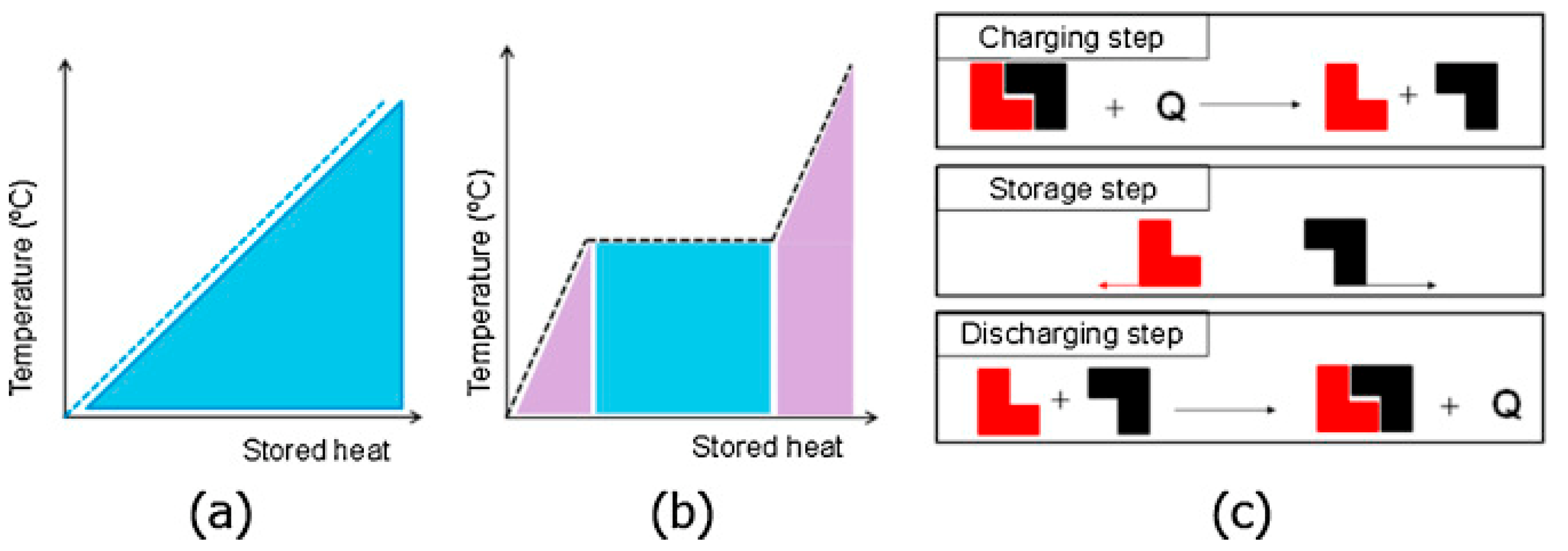
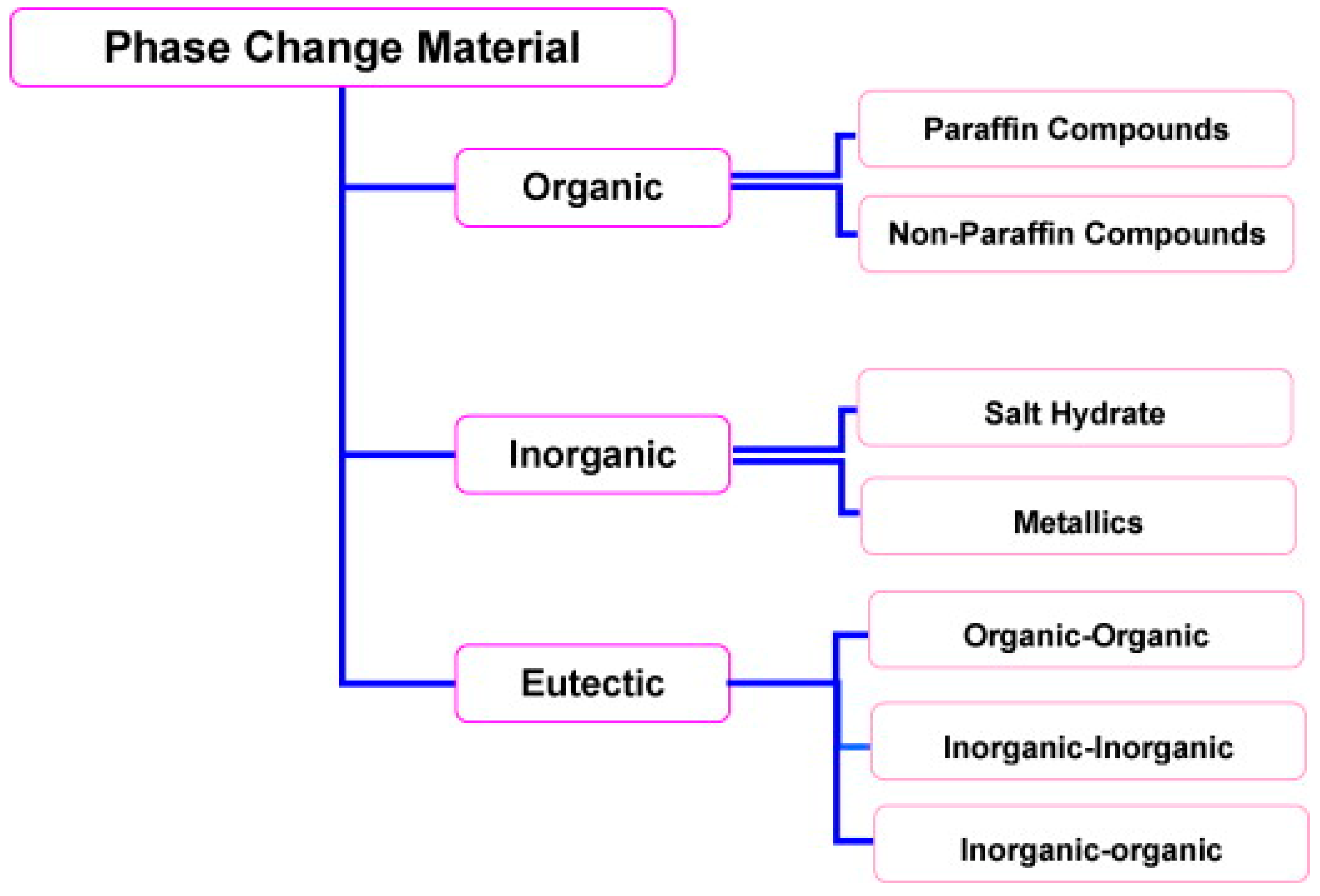
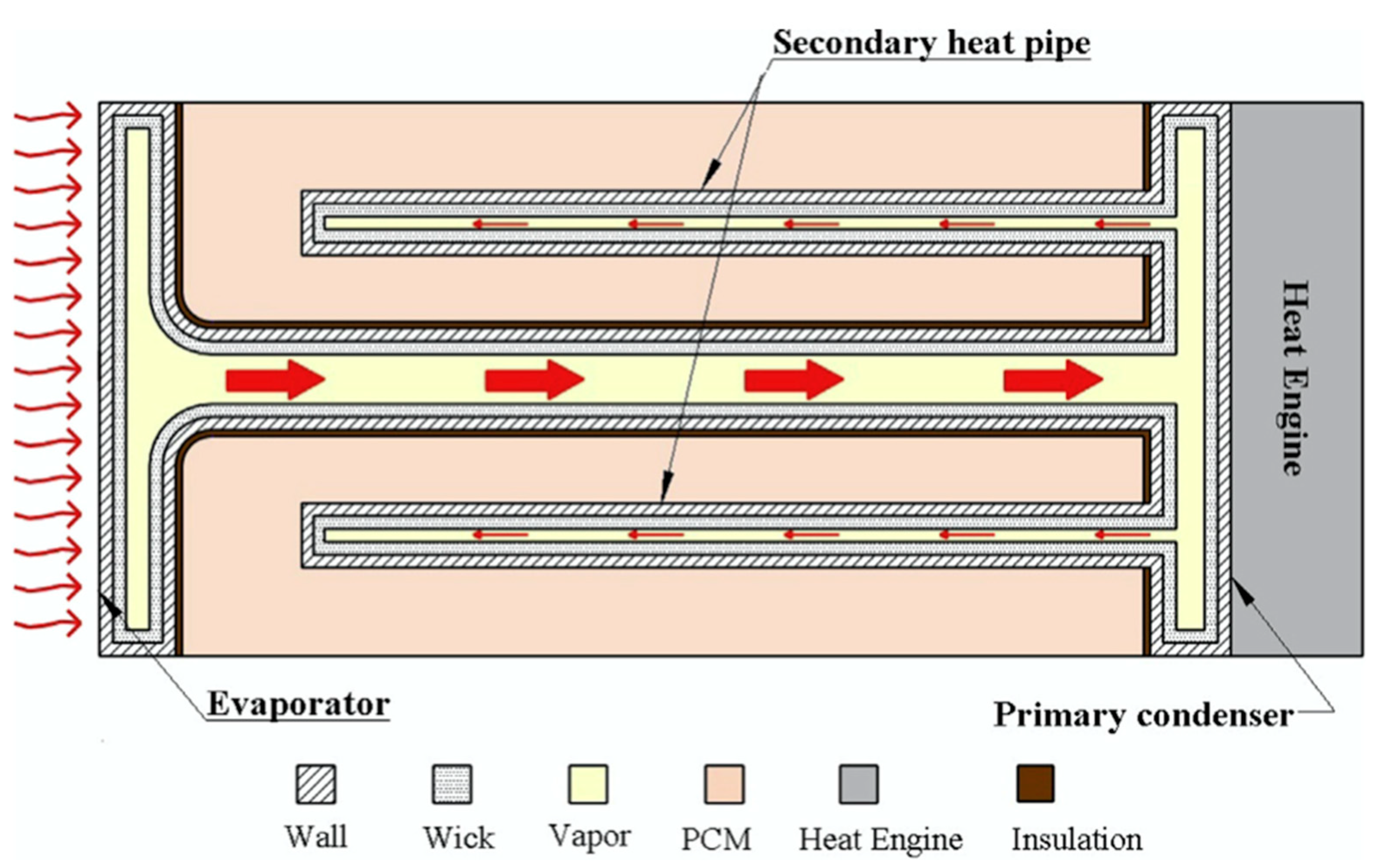
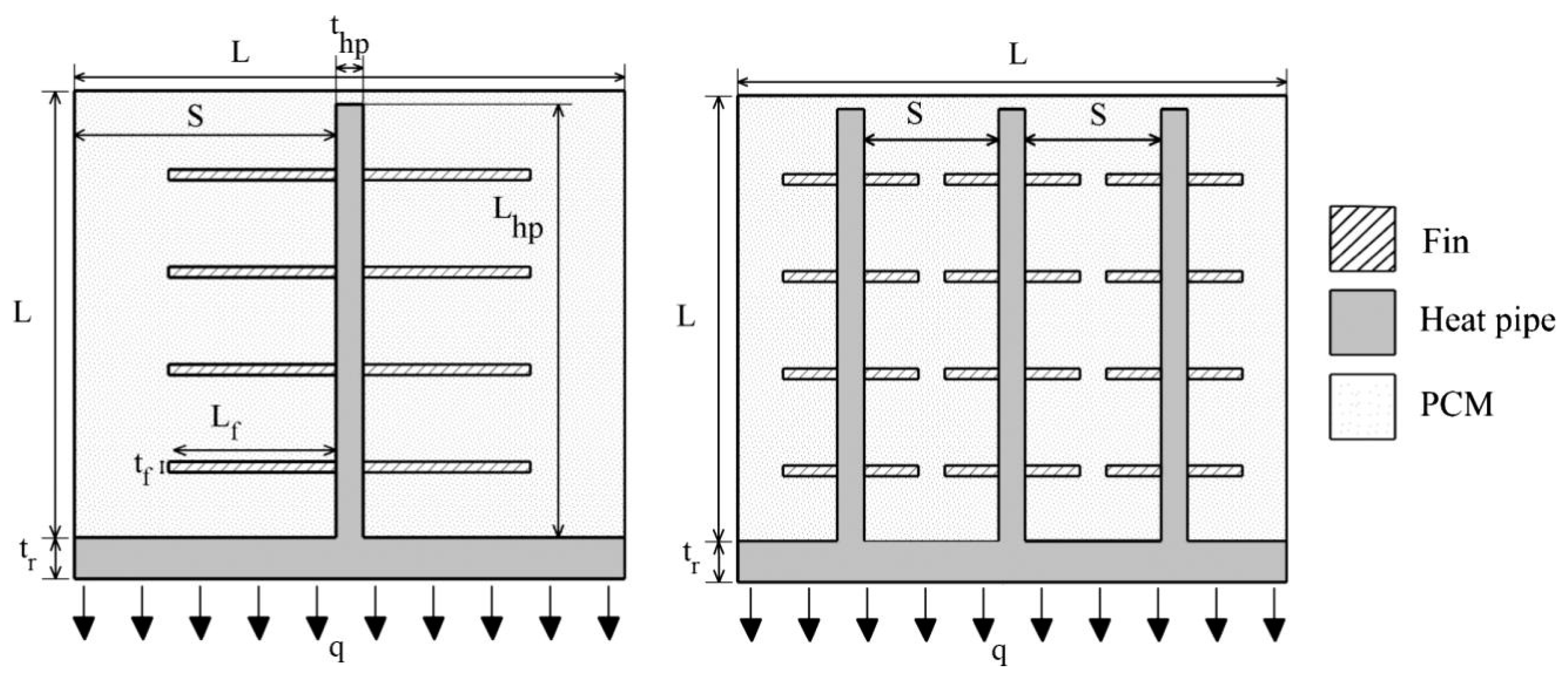
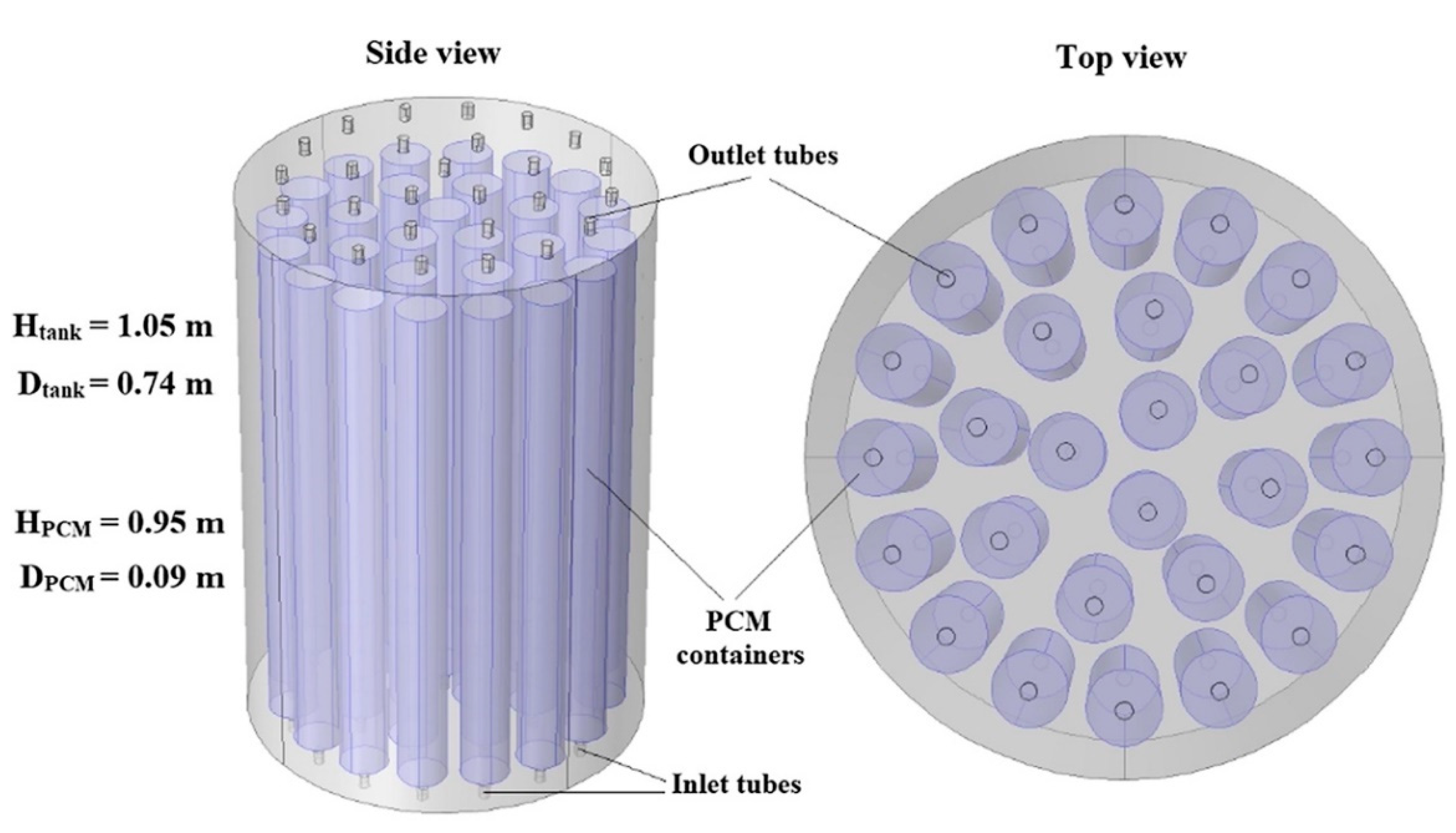
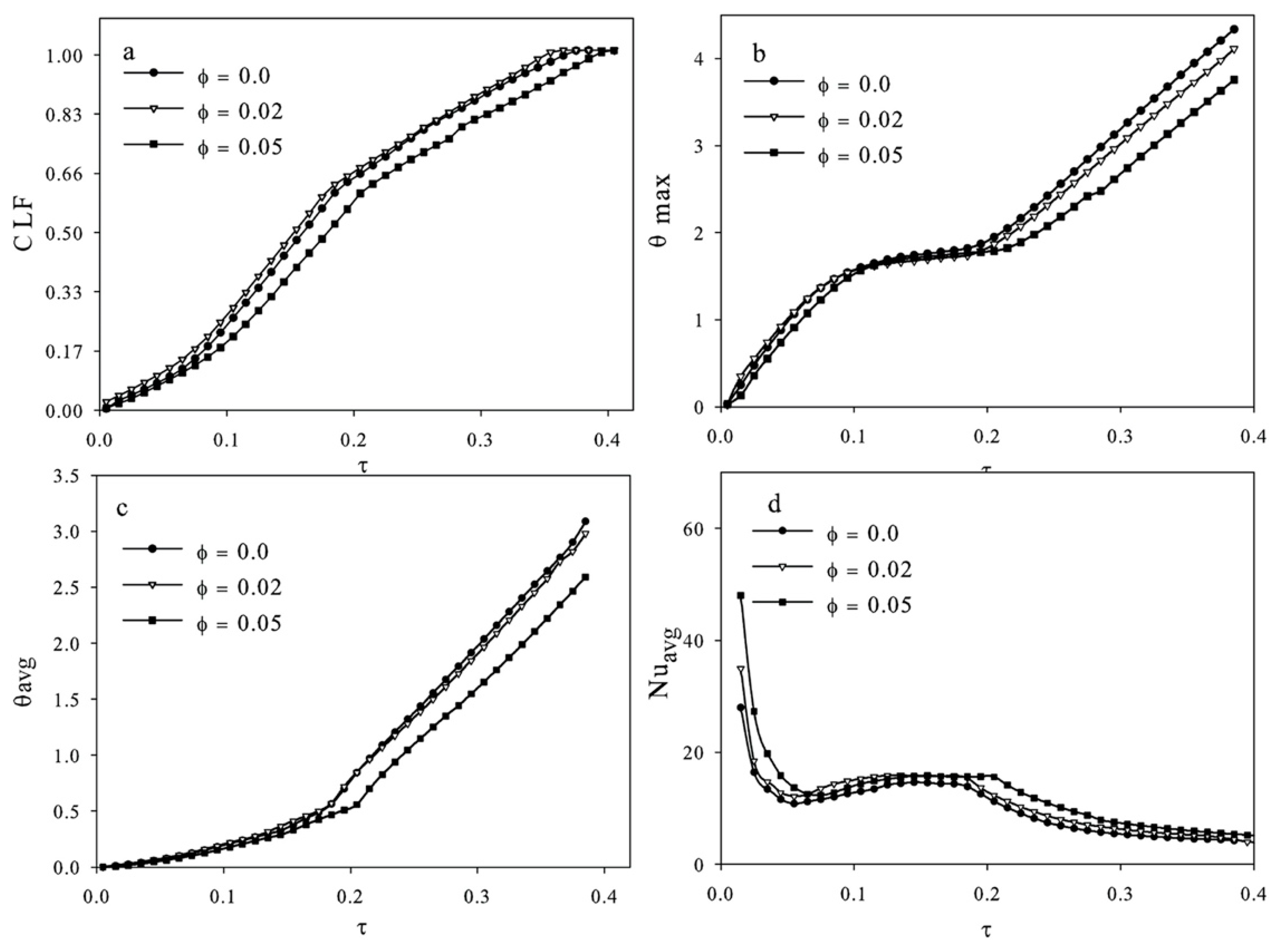
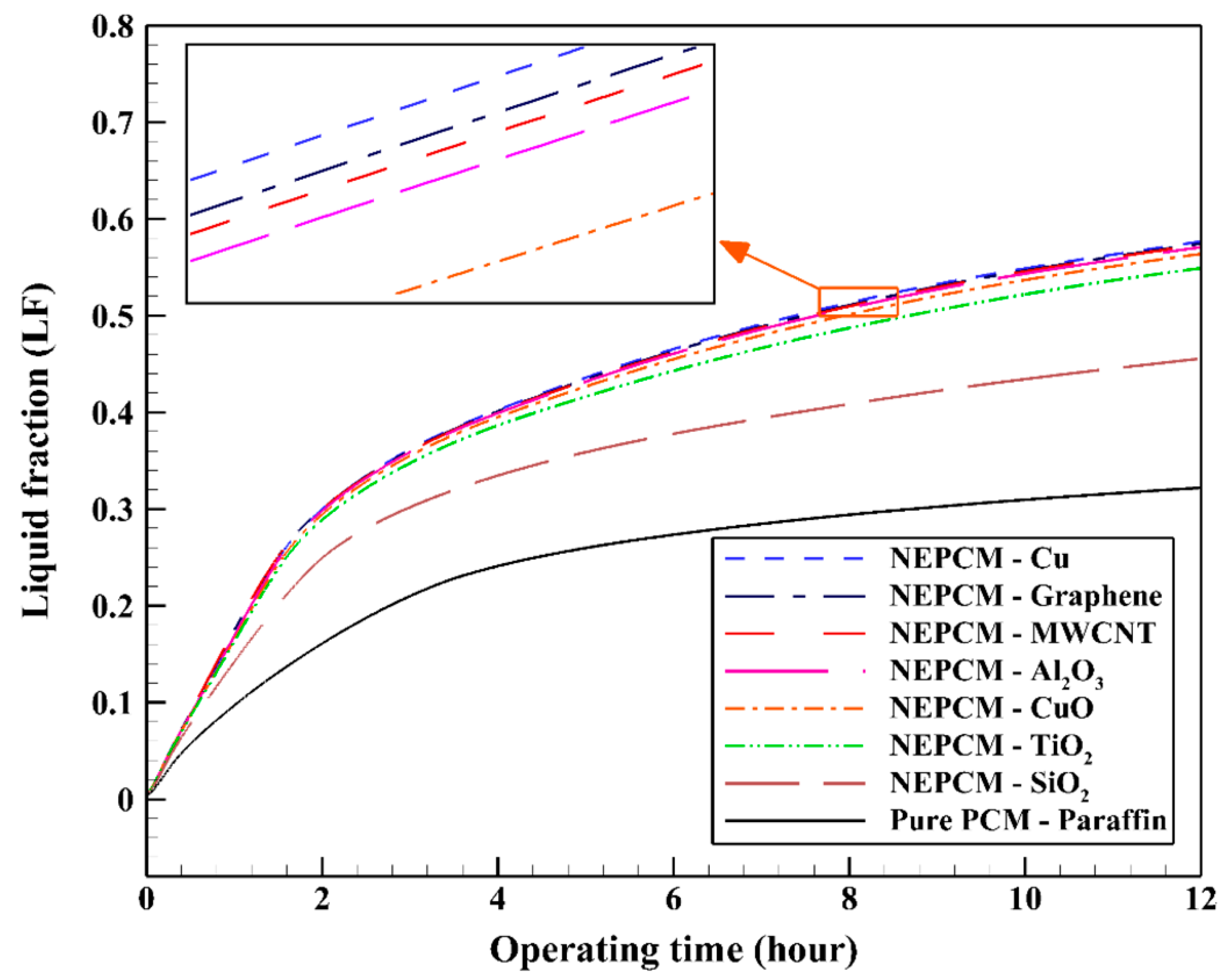
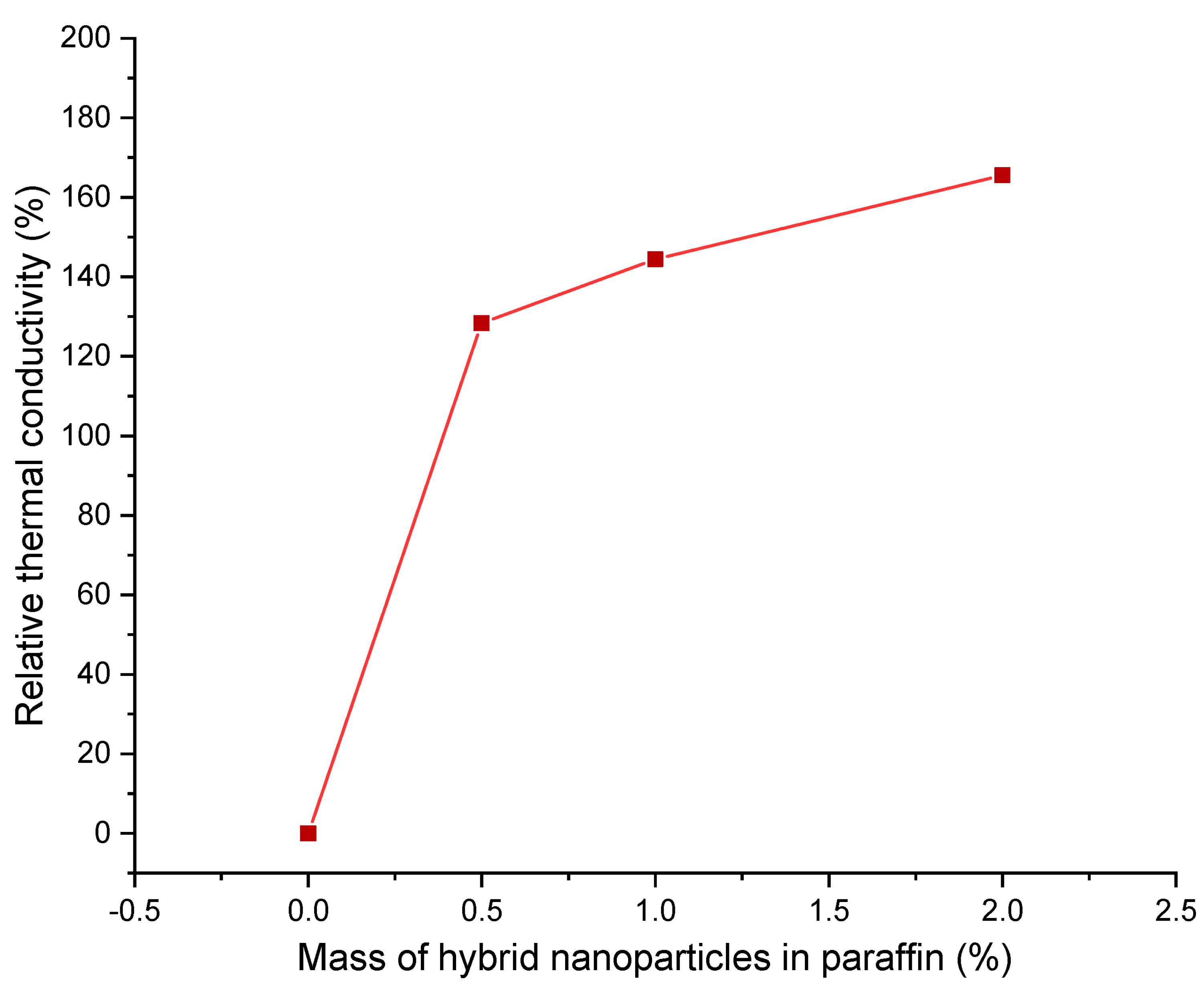

| Authors | PCM Melting Point (°C) | Nanoparticle Type | Phase Change Process | Study Case | Result |
|---|---|---|---|---|---|
| Elgafy and Lafdi (2005) [27] | Paraffin wax (67 °C) Low | Carbon nanofibers | Solidification | Experimental and analytical (1D) | The nanofibers enhanced the PCM performance |
| Khodadadi and Hosseinizadeh (2007) [28] | Water (0 °C) Low | Cu | Solidification | Numerical | The NPs enhanced the heat transfer and solidification |
| Jourabian and Farhadi (2015) [29] | Ice (0 °C) Low | Cu | Melting | Numerical (2D) | NPs increased the thermal conductivity |
| Feng et al. (2015) [30] | Water (0 °C) Low | Cu | Melting | Numerical (2D) | The Cu NPs enhanced the system |
| Akhmetov et al. (2019) [31] | Paraffin wax (PW-L: 44–48 °C PW-H: 64–66 °C) Low | Al2O3 | Melting and solidification | Numerical (3D) | Both paraffin thermal conductivities were improved by the NP |
| Abdulateef et al. (2021) [32] | Paraffin RT82 (78.15–82.15 °C) Low | Al2O3 | Melting | Experimental and numerical (2D) | The addition of NPs improved the system |
| Zaidan and Alhamdo (2018) [33] | Paraffin (63 °C) Low | Al2O3 | Melting and solidification | Experimental and numerical (3D) | 1% was the ideal amount of NPs |
| Farsani et al. (2017) [34] | Paraffin (RT 44HC) (45 °C) Low | Al2O3 | Melting | Numerical (2D) | Little to no improvement from NPs |
| Elbahjaoui and El Qarnia (2017) [35] | Paraffin wax (47 °C) Low | Al2O3 | Melting | Numerical (2D) | Increased NP, Reynolds number, Rayleigh number, and aspect ratio all decreased melting time |
| Elbahjaoui and El Qarnia (2017) [36] | n-Octadecane (28.2 °C) Low | Cu | Solidification | Numerical (2D) | NP, increased aspect ratio, and decreased HTF inlet temperature all improved performance |
| Nie et al. (2021) [37] | Paraffin RT35 (29–36 °C) Low | Cu | Melting | Numerical (2D) | Melting time shorter for top injection when R < 0.05, but longer with larger R. NP more effective for bottom injection |
| Hosseini et al. (2013) [38] | RT 50 (48 °C) Low | Cu | Melting | Numerical (3D) | Increased NP enhanced the system |
| Algarni et al. (2020) [39] | Paraffin wax (52 °C) Low | Cu | Melting and solidification | Experimental | NePCM enhanced efficiency by 32% |
| Nitsas and Koronaki (2020) [40] | RT50 (45–51 °C) Low | Cu and Al2O3 | Melting | Numerical (2D) | NPs enhanced melting |
| Aqib et al. (2020) [41] | Paraffin wax (55–58 °C) Low | Al2O3 and nonmetallic NPs, multiwall carbon nanotubes | Melting and solidification | Experimental | MWCNT worked better than Al2O3 |
| Temel and Çiftçi (2018) [42] | Paraffin wax (82 °C) Low | Al2O3, TiO2, MgO, ZnO, MWCNTs and Graphene nanoplatelet | Melting and solidification | Experimental | GNPs showed the best improvement |
| Murugan et al. (2018) [43] | Paraffin (18–23 °C) Low | MWCNT | Melting and solidification | Experimental | Melting improved up to 0.3 wt % MWCNT, solidification up to 0.9 wt % |
| Thalib et al. (2020) [44] | Paraffin (55 °C) Low | Graphene | Melting and solidification | Experimental | Graphene NPs greatly enhanced the system |
| Javadi et al. (2020) [45] | Paraffin (n-Octadecane) (28 °C) Low | Cu, CuO, Al2O3, TiO2, SiO2, multi-wall carbon nanotube, and graphene | Melting | Numerical (3D) | Cu at 0.2 concentration in blade shape were the best |
| Bashar and Siddiqui (2018) [46] | Paraffin wax, Polyfin (55 °C) Low | Silver, copper oxide, aluminum oxide, MWCNTs | Melting | Experimental | Silver and copper oxide performed best |
| Khatibi et al. (2021) [47] | RT82 (76.9–84.9 °C) Low | Al2O3, CuO, SiO2, ZnO | Solidification | Numerical (2D) | CuO at 0.04 volume fraction were ideal |
| Dastmalchi and Boyaghch (2020) [48] | RT25 (23–25 °C) Low | SiO2 | Melting and solidification | Numerical (2D) | Used properly, the system decreased the cost of the AC |
| Pasupathi (2020) [49] | Paraffin (63.74 °C) Low | SiO2, CeO2 | Melting | Experimental | 1% NP showed best improvement |
| Zhou et al. (2016) [50] | Octadecane Paraffin (27–29 °C) Low | Aluminum | Melting | Numerical (3D) and experimental | Thermal conductivities improved with Al % |
| Badakhsh et al. (2018) [51] | Paraffin (53–57 °C) Low | AlN-coated SiC | Melting and solidification | Experimental | The particles were successful at enhancing the system |
| Maher et al. (2021) [52] | Paraffin wax (≈52 °C) Low | SiC and Ag | Melting | Experimental | SiC enhanced at 15 wt % showed best results |
| Ghalambaz et al. (2017) [53] | Octadecane Paraffin (30 °C) Low | Hybrid NPs | Melting | Numerical (2D) | Large conductivity and low viscosity worked best, hybrid particles worked better than non-hybrids |
| Liang and Chen (2018) [54] | Eutectic hydrate salts (−11.9, −10.6, and −14.8 °C) Low | Carbon | Solidification | Experimental | A good balance for viscosity can increase efficiency of NePCM |
| Jamalabadi (2021) [55] | Th-29 (Mostly CaCl26H2O) (29 °C) Low | Cu | Melting | Numerical (2D) | NPs improved the system |
| Marcos et al. (2020) [56] | Polyethylene glycol (4 °C) Low | Ag | Melting and solidification | Experimental | Thermal conductivity was improved up to the highest tested percent (1.1 wt %) |
| Song et al. (2019) [57] | Polyethylene glycol (33.6 °C) Low | silver on 1D halloysite nanotube | Melting and solidification | Experimental | Nanostructures improved thermal conductivity |
| Qian et al. (2012) [58] | Polyethylene glycol/diatomite (60.51 °C) Low | Ag | Melting and solidification | Experimental | The Ag enhanced the PCM |
| Prabakaran et al. (2019) [59] | OM08 (8–9 °C) Low | Graphene | Solidification | Experimental | The graphene sped up solidification and improved thermal conductivity |
| Santhosh et al. (2020) [60] | Myristic acid (54.4 °C) Low | Fe2O3 | Melting and solidification | Experimental | NP increased melting and solidification times |
| Barreneche et al. (2019) [61] | Capric acid (32 °C) Palmitic acid (64 °C) Low | CuO | Melting | Experimental | CA was enhanced up to 1.5 wt % NPs, PA up to 3.0 wt % |
| Mayilvelnathan and Arasu (2019) [62] | Erythritol (120 °C) Middle | Graphene | Melting and solidification | Experimental | Increased NP increases thermal conductivity |
| Mayilvelnathan and Arasu (2020) [63] | Erythritol (120 °C) Middle | Graphene | Melting and solidification | Experimental | The NPs enhanced the system |
| Manickam et al. (2019) [64] | Erythritol (120 °C) Middle | TiO2 and CNTs | Melting and solidification | Experimental | This material would be good for solar applications |
| Mekrisuh et al. (2021) [65] | D-Mannitol (166–179 °C) Middle | Graphene nano-plates | Melting | Numerical (2D) | Optimizing geometry had better results than fins and nano-plates |
| Singh et al. (2021) [11] | Eutectic lithium nitrate and potassium chloride (150–200 °C) Middle | COOH-functionalized Graphene nanoplatelets (f-GNP) | Melting and solidification | Experimental and numerical (2D) | f-GNPs enhance PCM at 5% concentration |
| Chieruzzi et al. (2013) [66] | NaNO3-KNO3 (223–232 °C) Middle | SiO2, Al2O3, TiO2, and SiO2-Al2O3 | Melting and solidification | Experimental | 1.0 wt % SiO2-Al2O3 were the most effective |
| Chieruzzi et al. (2017) [67] | NaNO3-KNO3 (228.2 °C) Middle | silica (SiO2), alumina (Al2O3), and SiO2-Al2O3 | Melting and solidification | Experimental | Most effective were silica/alumina NPs 30 min at 200 rpm |
| Aslfattahi et al. (2019) [68] | NaNO3-KNO3 (225 °C) Middle | MgO | Melting | Experimental | MgO thermally enhanced the system |
| Saranprabhu and Rajan (2019) [69] | NaNO3-KNO3 (250 °C) Middle | MgO | Melting and solidification | Experimental | 0.25 wt % was the best concentration of MgO |
| Myers et al. (2016) [70] | KNO3, NaNO3, KNO3-NaNO3 Eutectic (334 °C, 306 °C, and 222 °C) Middle and High | CuO | Melting | Experimental | All showed improvement, but especially the eutectic mixture |
| Chieruzzi et al. (2015) [71] | KNO3 (334 °C) High | silica (SiO2), alumina (Al2O3), and SiO2-Al2O3 | Melting | Experimental | All improved but silica was significantly the best |
| Yaxuan et al. (2019) [72] | Eutectic mix of NaBr, KBr, LiBr, CaBr2 (310–314 °C) High | SiO2 | Melting | Experimental | Factors varied with diameter and concentration |
| Han et al. (2020) [73] | KCl:MgCl2:NaCl (399.7 °C) | Al2O3, CuO, and ZnO | Melting | Experimental | Al2O3 showed the best enhancement |
| Authors | PCM Melting Point (°C) | Nanoparticle Type/Enhancement Method(s) | Phase Change Process | Study Case | Result |
|---|---|---|---|---|---|
| Hosseinzadeh et al. (2021) [74] | Water (0 °C) Low | MoS2-TiO2 and branched fins | Solidification | Numerical (2D) | High NP concentration with branched fins were the best enhancement technique |
| Hajizadeh et al. (2020) [75] | RT35 (35 °C) Low | CuO and branched fins | Solidification | Numerical (2D) | NP improved all cases, case 2 worked best |
| Nakhachi et al. (2021) [76] | Lauric acid (43.4–48.1 °C) Low | CuO and stair fins | Melting | Numerical (2D) | NP and stair fins improved melting |
| Li et al. (2021) [77] | Paraffin (n-octadecane) Low | Copper oxide and fins | Melting | Numerical (2D) | Long, thin fins from both walls worked best |
| Ren et al. (2019) [78] | N-eicosane (35.85 °C) Low | Copper and triangle fins | Melting | Numerical (2D) | Triangle fins and NP improved system |
| Mahdi et al. (2019) [79] | RT82 (77–85 °C) Low | Al2O3 and copper fins | Simultaneous charging and discharging | Numerical (2D) | Fins and their geometry had greater effects than the NPs |
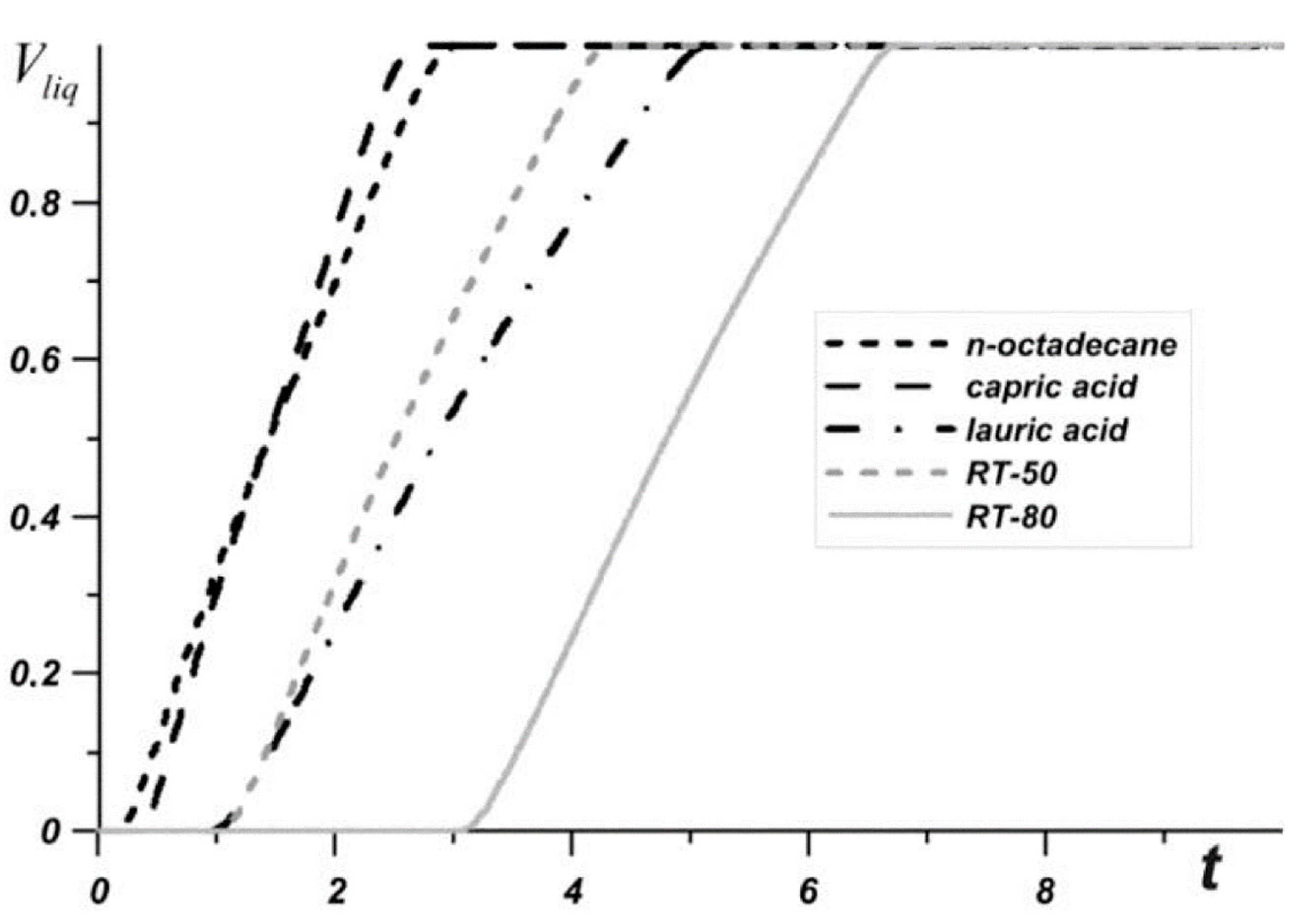
| Bondareva et al. (2020) [80] | n-Octadecane (28.05 °C), Capric acid (32 °C), Lauric acid (46 °C), RT-50 (49 °C), RT-80 (81 °C) Low | Al2O3 and fins | Melting | Numerical (2D) | NPs enhanced up to 2% concentration |
| Authors | PCM Melting Point (°C) | Nanoparticle Type/Enhancement Method(s) | Phase Change Process | Study Case | Result |
|---|---|---|---|---|---|
| Koukou et al. (2019) [81] | Paraffin (A44) (44 °C) Low | Graphite nano-platelets and finned heat pipes | Melting and solidification | Experimental | NPs reduced charging and discharging times |
| Mahdavi et al. (2020) [82] | Rubitherm 55 (51–57 °C) Low | Aluminum oxide, silver, copper, and copper oxide NPs and horizontal heat pipes | Melting and solidification | Numerical (2D) | More heat pipes and more NP decreased time, decreased storage capacity |
| Mahdavi et al. (2018) [83] | Rubitherm 55 (51–57 °C) Low | Cu, CuO, Al2O3, Ag, and heat pipes | Melting | Numerical (2D) | Enhancements improved melting, but type of NP was insignificant |
| Tiari et al. (2018) [84] | Potassium nitrate (335 °C) High | CuO and Al2O3, finned heat pipes | Melting | Numerical (2D) | Increased concentration of NP improved melting, type did not matter |
| Tiari et al. (2019) [85] | Potassium nitrate (335 °C) High | CuO and Al2O3 and finned heat pipes | Solidification | Numerical (2D) | NP improved the system up to 2% volume fraction, which was the same for either NP type |
| Ren et al. (2018) [86] | Li2CO3-K2CO3 (485.85 °C) High | Cu, metal foam, heat pipes | Melting | Numerical (2D) | All enhancements improved the system w/tradeoff of decreased storage |
| Authors | PCM Melting Point (°C) | Nanoparticle Type/Enhancement Method(s) | Phase Change Process | Study Case | Result |
|---|---|---|---|---|---|
| Yu et al. (2016) [87] | Acetamide (Not Reported) Low | Nano-AlN, carbon nanotubes and graphene and carbon graphite foam | N/A | Experimental and numerical (2D) | Dispersion techniques improved thermal conductivity |
| Senobar et al. (2020) [88] | RT44HC (41–44 °C) Low | Copper oxide NPs and copper foam | Melting and solidification | Experimental | Metal foam alone was the best enhancement |
| Buonomo et al. (2018) [89] | RT 58 Paraffin (48–62 °C) Low | Aluminum oxide NP, aluminum foam, heat pipes | Melting | Numerical (3D) | Metal foam enhanced more than NPs; their combination was best. |
| Ma et al. (2020) [90] | Paraffin (28–37 °C) Low | Cu, Al, Fe, and Ni NPs. Expanded graphite porous material | Melting | Experimental | Cu were the best and the EG prevented leakage |
| Kim et al. (2019) [91] | Erythritol (120 °C) Middle | Ag, Al, carbon nanotubes, and graphene NPs, with carbon foam | Melting and solidification | Experimental | All were effective, with graphene as the best enhancement |
| Yu et al. (2021) [92] | MgCl2-NaCl-KCl (383.5 °C) High | Expanded graphite and SiO2 NPs | Melting and solidification | Experimental | Both EG and NPs enhanced the system |
| Authors | PCM Melting Point (°C) | Enhancement Method(s) | Phase Change Process | Study Case | Result |
|---|---|---|---|---|---|
| Mahdi et al. (2020) [93] | RT-55 (51–57 °C) RT-60 (55–61 °C) RT-65 (58–67 °C) | Al2O3 NPs, metal foam (Al6061), and multiple PCMs | Solidification | Numerical (2D) | All showed enhancement, with multiple PCM and metal foam the best |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofani, K.; Tiari, S. Nano-Enhanced Phase Change Materials in Latent Heat Thermal Energy Storage Systems: A Review. Energies 2021, 14, 3821. https://doi.org/10.3390/en14133821
Tofani K, Tiari S. Nano-Enhanced Phase Change Materials in Latent Heat Thermal Energy Storage Systems: A Review. Energies. 2021; 14(13):3821. https://doi.org/10.3390/en14133821
Chicago/Turabian StyleTofani, Kassianne, and Saeed Tiari. 2021. "Nano-Enhanced Phase Change Materials in Latent Heat Thermal Energy Storage Systems: A Review" Energies 14, no. 13: 3821. https://doi.org/10.3390/en14133821
APA StyleTofani, K., & Tiari, S. (2021). Nano-Enhanced Phase Change Materials in Latent Heat Thermal Energy Storage Systems: A Review. Energies, 14(13), 3821. https://doi.org/10.3390/en14133821






