Modeling of Water Generation from Air Using Anhydrous Salts
Abstract
1. Introduction
1.1. Overview
Statement of the Problem
1.2. Outline of the Paper
2. Study Background and Literature Review
2.1. Overview of Water Scarcity and Alternative Sources of Water
2.2. Previous Studies
2.3. Significance of the Study
3. Anhydrous Salts and System Prototype
3.1. Anhydrous Salts
3.2. Atmospheric Water Generation System Prototype
4. Mathematical Modelling
- Model comparison of the salts;
- Model validation under different relative humidity modes;
- Sensitivity analysis on the computed model.
5. Results and Discussion
5.1. Model Validation Statistics for Relative Humidity
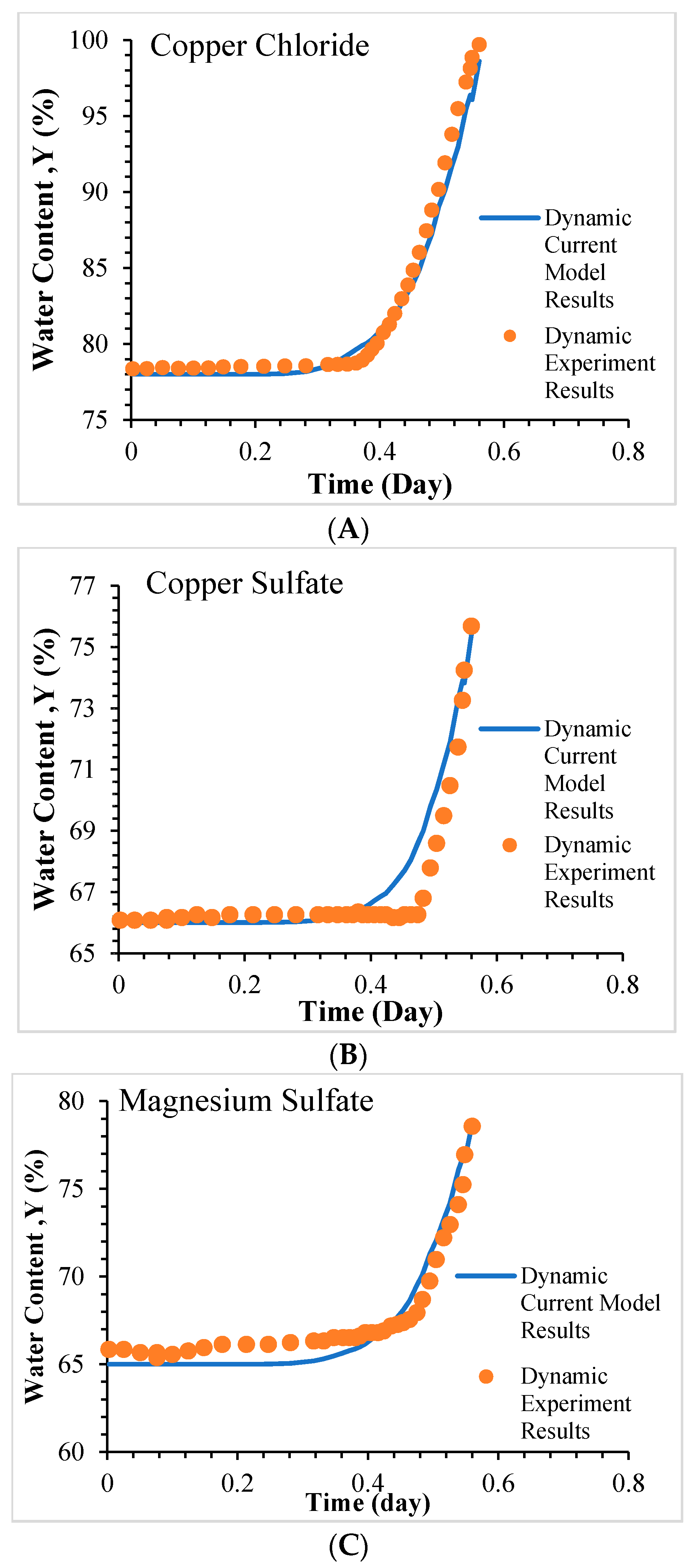
5.2. Model Validation for Dynamic Relative Humidity
5.3. Sensitivity Analysis
5.3.1. Sensitivity of the Porosity (ε)
5.3.2. Sensitivity of Thickness (xra)
5.3.3. Sensitivity of the Uniformity of the Stratified Structure (d)
5.4. Discussion
6. Conclusions and Recommendations
6.1. Conclusions
6.2. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AWG | Atmospheric Water Generators |
| AWH | Atmospheric Water Harvesting |
| CNT | Carbon Nanotubes |
| GHI | Global Horizontal Irradiance |
| GW | Giga Watt |
| KJ | Kilo Joule |
| MEIM | Ministry of Energy Industry and Mineral Resources |
| MOF | Metal-Organic Frameworks |
| REPDO | Renewable Energy Project Development Office |
| RH | Relative Humidity |
| TGA | Thermal Gravimetric Analysis |
List of Symbols
| Steady-state increment of moisture: equilibrium value (kg L/kg S) | |
| Pseudo diffusion coefficient of the vapor of diffusing component through the porous web material (m2 kg S/(kg L )) | |
| Steady-state moisture content, equilibrium value (kg L/kg S) | |
| complete concentration of the separated component (mg/L or mmol/L) | |
| concentrations of adsorbed components retained by oxycellulose, pulp, and so forth at times, and t, respectively (mg/L or mmol/L) | |
| equilibrium solution phase concentration (mg/L or mmol/L) | |
| one-dimensional diffusion flow of component molecules (mmol m/L s or mg m/L s) | |
| Half of sample thickness (m) | |
| Apparent density of paper mass, that is, grammage of the paper sample divided by the thickness of the sample (kg/m3) | |
| Increment of the moisture content (kg L/kg S) | |
| A | Outer surface of the pore sample given by its geometry (m2) |
| a | Parameter in (1) (kg L/kg S) |
| b | (=) parameter in (1) () |
| v | Condensation or moistening rate at the beginning of vapor condensation or moistening of web pore sample (kg L/(kg S day)) |
| Yi | Initial moisture content (kg L/kg S) |
| Δεr | Interval of pores (r min ≤ r ≤ r(ϕ)) filled in steady state by condensed water at a given ϕ(v/v) |
| εr | Interval of pores (0 ≤ r ≤ r(ϕ)) filled in steady state by condensed water at a given ϕ(v/v) |
| ρl | Density of condensed liquid (kg/m3) |
| ρs | Density of solid part of porous web material (kg/m3) |
| Vapor and saturated vapor concentration (kmol/ m3) | |
| Diffusion coefficient of the vapor of diffusing component through the porous web material (m2/day) | |
| Molar mass (kg/kmol) | |
| Current moisture content (kg L/kg S) | |
| (=ΔYe) parameter in (1) (kg L/kg S) | |
| Parameter characterizing a uniformity of stratified structure of porous web material (dimensionless) | |
| Proportionality coefficient (day (d)) | |
| Time of sample storage at given relative humidity (day) | |
| dx/dt | |
| Width of the surface layers of the porous web material filled with condensed liquid (m) | |
| Total porosity of pore sample (v/v) | |
| Relative humidity of air |
References
- Mekonnen, M.; Hoekstra, A. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Gleick, P.H. Water and Conflict: Fresh Water Resources and International Security. Int. Secur. 1993, 18, 79–112. [Google Scholar] [CrossRef]
- Wahlgren, R.V. Atmospheric water vapour processor designs for potable water production: A review. Water Res. 2001, 35, 1–22. [Google Scholar] [CrossRef]
- Beysens, D.; Milimouk, I.; Schweitzer, A. The Case for Alternative Fresh Water Sources. Pour Resour. Altern. Secher. 2000, 11, 1–16. [Google Scholar]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef]
- National Water Strategy Homepage. In Ministry of Enviroment Water and Agriculture. Available online: https://www.mewa.gov.sa/en/Ministry/Agencies/TheWaterAgency/Topics/Pages/Strategy.aspx (accessed on 5 January 2021).
- Schneider, S. Encyclopedia of Climate and Weather; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Park, K.C.; Chhatre, S.S.; Srinivasan, S.; Cohen, R.E.; McKinley, G.H. Optimal Design of Permeable Fiber Network Structures for Fog Harvesting. Langmuir 2013, 29, 13269–13277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Hedhili, M.; Yang, X.; Wang, P. Inkjet printing for direct micropatterning of a superhydrophobic surface: Toward biomimetic fog harvesting surfaces. J. Mater. Chem A 2014, 3, 2844–2852. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wu, J.; Hedhili, M.N.; Wang, P. A facile strategy for the fabrication of a bioinspired hydrophilic-superhydrophobic patterned surface for highly efficient fog-harvesting. J. Mater. Chem. A 2015, 3, 18963–18969. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional water collection on wetted spider silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Gim, S.; Cho, S.; Koratkar, N.; Oh, I. Graphene films: Wetting-transparent graphene films for hydrophobic water-harvesting surfaces. Adv. Mater. 2014, 26, 5070. [Google Scholar] [CrossRef]
- Olivier, J.; Rautenbach, C.J. The implementation of fog water collection systems in South Africa. Atmos. Res. 2002, 64, 227–238. [Google Scholar] [CrossRef]
- Estrela, M.; Valiente, J.; Corell, D.; Millán, M. Fog collection in the Western Mediterranean Basin (Valencia region, Spain). Atmos. Res. 2008, 87, 324–337. [Google Scholar] [CrossRef]
- Ji, J.G.; Wang, R.Z.; Li, L.X. New composite adsorbent for solar-driven fresh water production from the atmosphere. Desalination 2007, 212, 176–182. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting water from air: Using anhydrous salt with sunlight. Environ. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef] [PubMed]
- Brahimi, T. Using artificial intelligence to predict wind speed for energy application in Saudi Arabia. Energies 2019, 12, 4669. [Google Scholar] [CrossRef]
- 2019 Annual Report, Saline Water Conversion Corporation. Available online: https://www.swcc.gov.sa/Arabic/mediacenter/swccpublications/pages/default.aspx (accessed on 24 January 2021).
- AlYahya, S.; Irfan, M.A. Analysis from the new solar radiation Atlas for Saudi Arabia. Sol. Energy 2016, 130, 116–127. [Google Scholar] [CrossRef]
- Hepbasli, A.; Alsuhaibani, Z. A key review on present status and future directions of solar energy studies and applications in Saudi Arabia. Renew. Sustain. Energy Rev. 2011, 15, 5021–5050. [Google Scholar] [CrossRef]
- Invest Saudi. Energy and Water|Sectors & Opportunities|Invest Saudi Portal. 2021. Available online: https://investsaudi.sa/en/sectors-opportunities/energy-water. (accessed on 22 June 2021).
- Ejeian, M.; Entezari, A.; Wang, R.Z. Solar powered atmospheric water harvesting with enhanced LiCl/MgSO4/ACF composite. Appl. Therm. Eng. 2020, 176, 115396. [Google Scholar] [CrossRef]
- Energy Consumption for Water Use Cycles in Different Countries: A Review. Appl. Energy 2016, 178, 868–885. Available online: https://www.sciencedirect.com/science/article/pii/S0306261916308893 (accessed on 24 January 2021). [CrossRef]
- Gleick, P. Water in Crisis; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Chen, H.; Ran, T.; Gan, Y.; Zhou, J.; Zhang, Y.; Zhang, L.; Zhang, D.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Wikramanayake, E.D.; Ozkan, O.; Bahadur, V. Landfill gas-powered atmospheric water harvesting for oilfield operations in the United States. Energy 2017, 138, 647–658. [Google Scholar] [CrossRef]
- Water Harvest via Dewing Langmuir. Available online: https://pubs.acs.org/doi/10.1021/la3013987?mobileUi=0 (accessed on 24 January 2021).
- Solís-Chaves, J.S.; Rocha-Osorio, C.M.; Murari, A.L.L.; Lira, V.M.; Sguarezi Filho, A.J. Extracting potable water from humid air plus electric wind generation: A possible application for a Brazilian prototype. Renew. Energy 2018, 121, 102–115. [Google Scholar] [CrossRef]
- Bagheri, F. Performance Investigation of Atmospheric Water Harvesting Systems. Available online: https://www.researchgate.net/publication/326952559_Performance_Investigation_of_Atmospheric_Water_Harvesting_Systems (accessed on 24 January 2021).
- Tajeddini, F.; Eslami, M.; Etaati, N. Thermodynamic analysis and optimization of water harvesting from air using thermoelectric coolers. In Proceedings of the ISME2018, 26rd international conference on Mechanical, Semnan, Iran, 24–26 April 2018. [Google Scholar]
- Solar Resource Maps of Saudi Arabia. Available online: https://solargis.com/maps-and-gis-data/download/saudi-arabia (accessed on 24 January 2021).
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and expectation of atmospheric water harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Desalination and Sustainability—An Appraisal and Current Perspective. Available online: https://www.academia.edu/33100178/Desalination_and_sustainability_An_appraisal_and_current_perspective (accessed on 24 January 2021).
- Pinto, F.S.; Marques, R.C. Desalination projects economic feasibility: A standardization of cost determinants. Renew. Sustain. Energy Rev. 2017, 78, 904–915. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-Economic Assessment and Environmental Impacts of Desalination Technologies. Available online: https://www.sciencedirect.com/science/article/pii/S0011916410006296 (accessed on 24 January 2021).
- Beysens, D.; Milimouk, I.; Nikolayev, V.S.; Berkowicz, S.; Muselli, M.; Heusinkveld, B.; Jacobs, A.F.G. Comment on “The moisture from the air as water resource in arid region: Hopes, doubt and facts” by Kogan and Trahtman. J. Arid Environ. 2006, 67, 343–352. [Google Scholar] [CrossRef][Green Version]
- Pontious, K.; Weidner, B.; Guerin, N.; Dates, A.; Pierrakos, O.; Altaii, K. Design of an atmospheric water generator: Harvesting water out of thin air. In Proceedings of the 2016 IEEE Systems and Information Engineering Design Symposium (SIEDS), harlottesville, VA, USA, 29 April 2016; pp. 6–11. [Google Scholar] [CrossRef]
- Relative Humidity. Available online: http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/relhum.html (accessed on 24 January 2021).
- Bodamyalizade, D.; Alibaba, H.Z. International Conference on Contemporary Affairs in Architecture and Urbanism (ICCAUA-2018) Architectural Facade Design Proposal for Water Production via Air Content. Available online: https://www.researchgate.net/publication/325847932_International_Conference_on_Contemporary_Affairs_in_Architecture_and_Urbanism_ICCAUA-2018_Architectural_Facade_Design_Proposal_for_Water_Production_via_Air_Content (accessed on 24 January 2021).
- Bayside Fog Collectors Homepage. Available online: https://www.baysidefogcollectors.com/ (accessed on 24 January 2021).
- Domen, J.; Stringfellow, W.; Camarillo, M.; Gulati, S. Fog water as an alternative and sustainable water resource. Clean Technol. Environ. Policy 2013, 16, 235–249. [Google Scholar] [CrossRef]
- Scrivani, A.; Bardi, U. A Study of the Use of Solar Concentrating Plants for the Atmospheric Water Vapour Extraction from Ambient Air in the Middle East and Northern Africa Region. Desalination 2008, 220, 592–599. Available online: https://www.sciencedirect.com/science/article/pii/S0011916407006601 (accessed on 24 January 2021). [CrossRef]
- Edmund, A. Method for Gaining Water out of the Atmosphere. 2138689A. 1938. Available online: https://patents.google.com/patent/US2138689A/en (accessed on 21 June 2021).
- Water Adsorption in MOFs: Fundamentals and Applications. Available online: https://www.researchgate.net/publication/262811246_Water_adsorption_in_MOFs_Fundamentals_and_applications (accessed on 24 January 2021).
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. Available online: https://pubs.acs.org/doi/abs/10.1021/cr5002589 (accessed on 24 January 2021). [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. Available online: https://pubs.rsc.org/en/content/articlelanding/2016/cs/c6cs00362a#!divAbstract (accessed on 24 January 2021). [CrossRef] [PubMed]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water Transport in MgSO4·7H2O during Dehydration in View of Thermal Storage. J. Phys. Chem. C 2015, 119, 28711–28720. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.jpcc.5b08730 (accessed on 24 January 2021). [CrossRef]
- Alsaedi, M.K. Atmospheric Water Harvesting by an Anhydrate Salt and Its Release by a Photothermal Process towards Sustainable Potable Water Production in Arid Regions. Master’s Thesis, KAUST, Thuwal, Saudi Arabia, 2018. [Google Scholar]
- Cešek, B.; Milichovský, M.; Potucek, F. Kinetics of Vapour Diffusion and Condensation in Natural Porous Cellulosic Fibre Web. ISRN Mater. Sci. 2011, 2011, 794306. Available online: https://www.hindawi.com/journals/isrn/2011/794306/ (accessed on 24 January 2021). [CrossRef]








| Parameter | Value of the Parameters | Unit | ||
|---|---|---|---|---|
| Copper Chloride (CuCl2) | Copper Sulfate (CuSO4) | Magnesium Sulfate (MgSO4) | ||
| Ye | 0.9 | 0.099 | 0.009 | (kg L/kg S) |
| t | 1 | 1 | 1 | (day) |
| d | 3.4 | 4 | 3.2 | (dimensionless) |
| 15 | 35 | 35 | Relative Humidity in % | |
| 0.0015 | 0.0015 | 0.0015 | (m) | |
| 0.002720402 | 0.068666526 | 0.098730463 | m2 kg S/kg L dayd | |
| Parameter | Value of the Parameters | Unit | ||
|---|---|---|---|---|
| Copper Chloride (CuCl2) | Copper Sulfate (CuSO4) | Magnesium Sulfate (MgSO4) | ||
| Ye | 1 | 1 | 1 | (kg L/kg S) |
| t | 1 | 1 | 1 | (day) |
| d | 12 | 12.85 | 12 | (dimensionless) |
| 15 | 35 | 35 | Relative Humidity in % | |
| 0.0015 | 0.0015 | 0.0015 | (m) | |
| 0.002329022 | 0.002329022 | 0.002329022 | m2 kg S/kg L day | |
| Salt Type | Density (Ps) | Unit |
|---|---|---|
| Copper chloride (CuCl2) | 3386 | kg/m3 |
| Copper sulfate (CuSO4) | 3600 | kg/m3 |
| Magnesium sulfate (MgSO4) | 2660 | kg/m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibie, S.K.; El-Amin, M.F.; Sun, S. Modeling of Water Generation from Air Using Anhydrous Salts. Energies 2021, 14, 3822. https://doi.org/10.3390/en14133822
Sibie SK, El-Amin MF, Sun S. Modeling of Water Generation from Air Using Anhydrous Salts. Energies. 2021; 14(13):3822. https://doi.org/10.3390/en14133822
Chicago/Turabian StyleSibie, Shereen K., Mohamed F. El-Amin, and Shuyu Sun. 2021. "Modeling of Water Generation from Air Using Anhydrous Salts" Energies 14, no. 13: 3822. https://doi.org/10.3390/en14133822
APA StyleSibie, S. K., El-Amin, M. F., & Sun, S. (2021). Modeling of Water Generation from Air Using Anhydrous Salts. Energies, 14(13), 3822. https://doi.org/10.3390/en14133822








