Effects of Evaporator and Condenser in the Analysis of Adsorption Chillers
Abstract
:1. Introduction
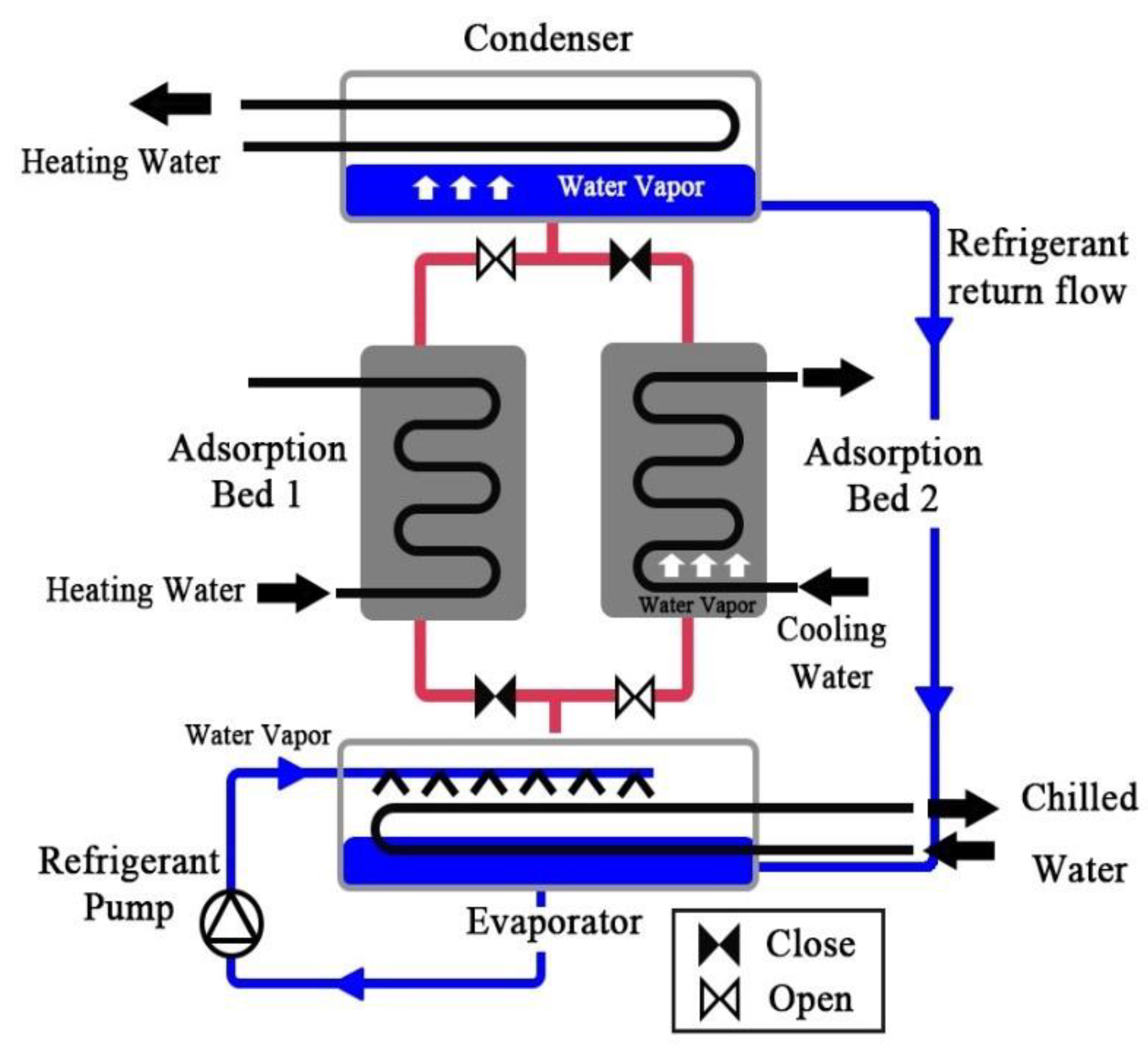
2. Numerical Method
2.1. Mathematical Model
- (1)
- The particles in the adsorption bed are all spherical with a uniform size and porosity.
- (2)
- Thermal equilibrium between the adsorbed and vapor phases is assumed.
- (3)
- A two-dimensional (2D) axisymmetric model is assumed.
- (4)
- Refrigerant vapor is an ideal gas and the adsorbed phase is liquid.
- (5)
- There is no heat loss through the chamber wall and the effect of radiation is negligible.
- (6)
- The thermo-physical properties of the thermal fluid, tube, fins, dry adsorbent, adsorbate liquid, and gas are constant, except for the density of the adsorbate gas.
2.2. Energy and Mass Balance Equations
2.2.1. Adsorption Bed
2.2.2. Evaporator
2.2.3. Condenser
2.2.4. Outlet temperature
2.2.5. Mass balance equation
2.3. Initial and Boundary Conditions
2.4. Performance Index
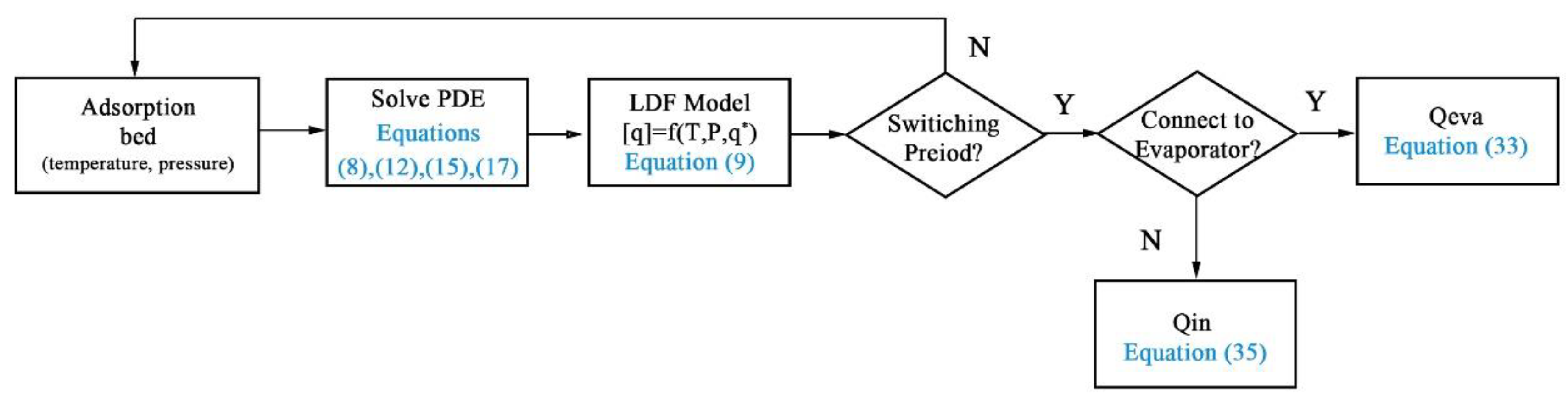
2.5. Numerical Procedure
3. Results and Discussion
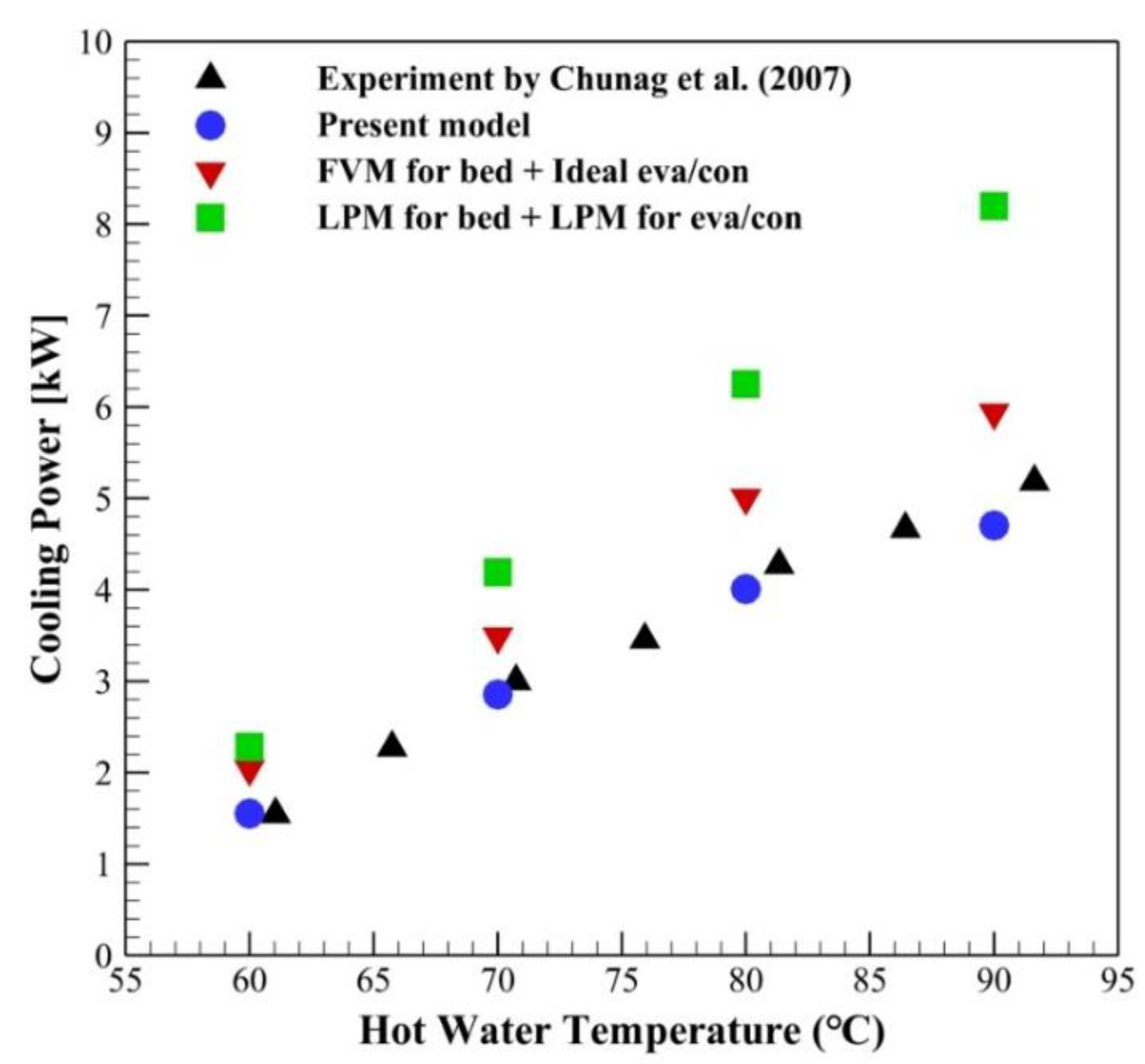
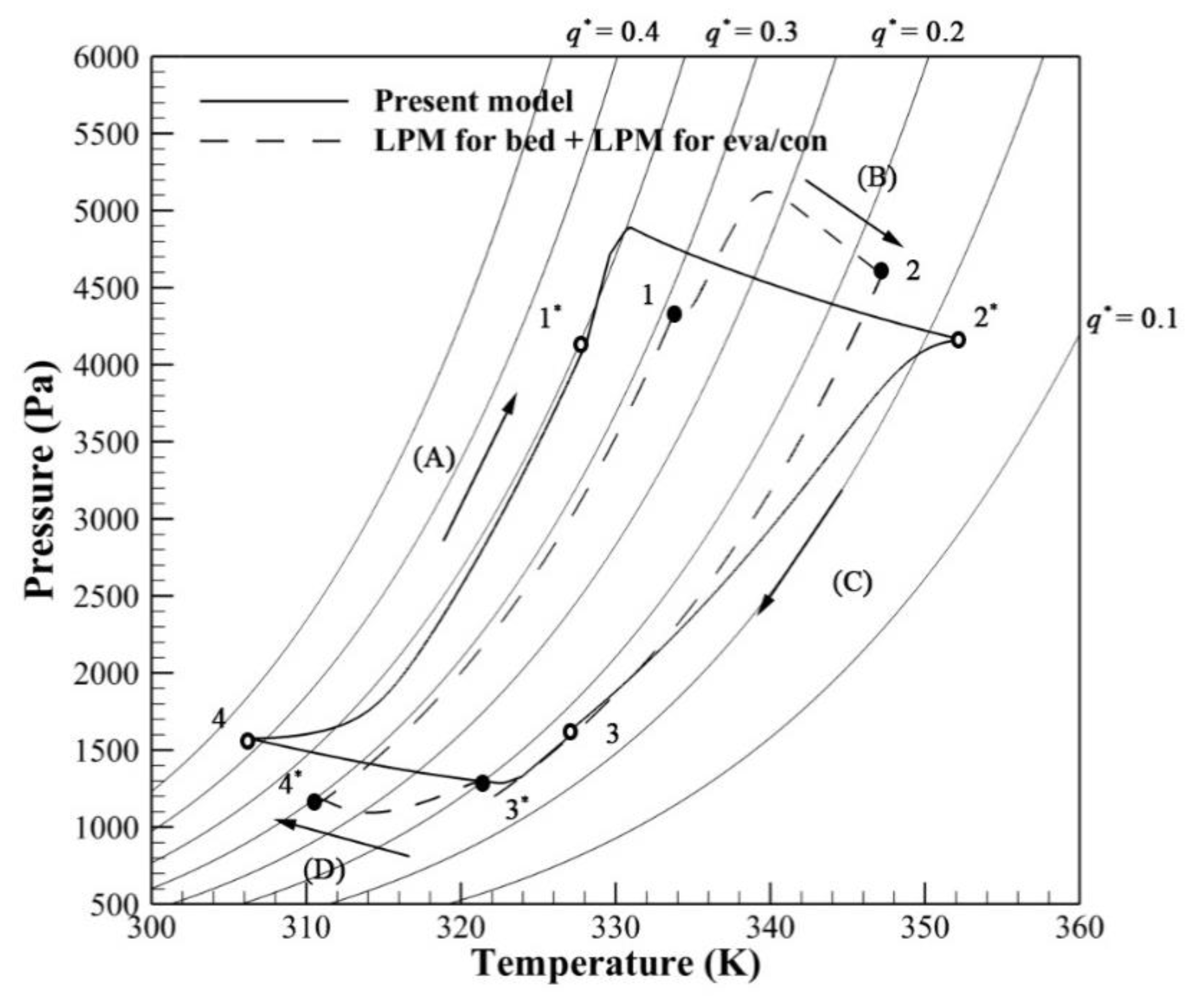
3.1. Validation
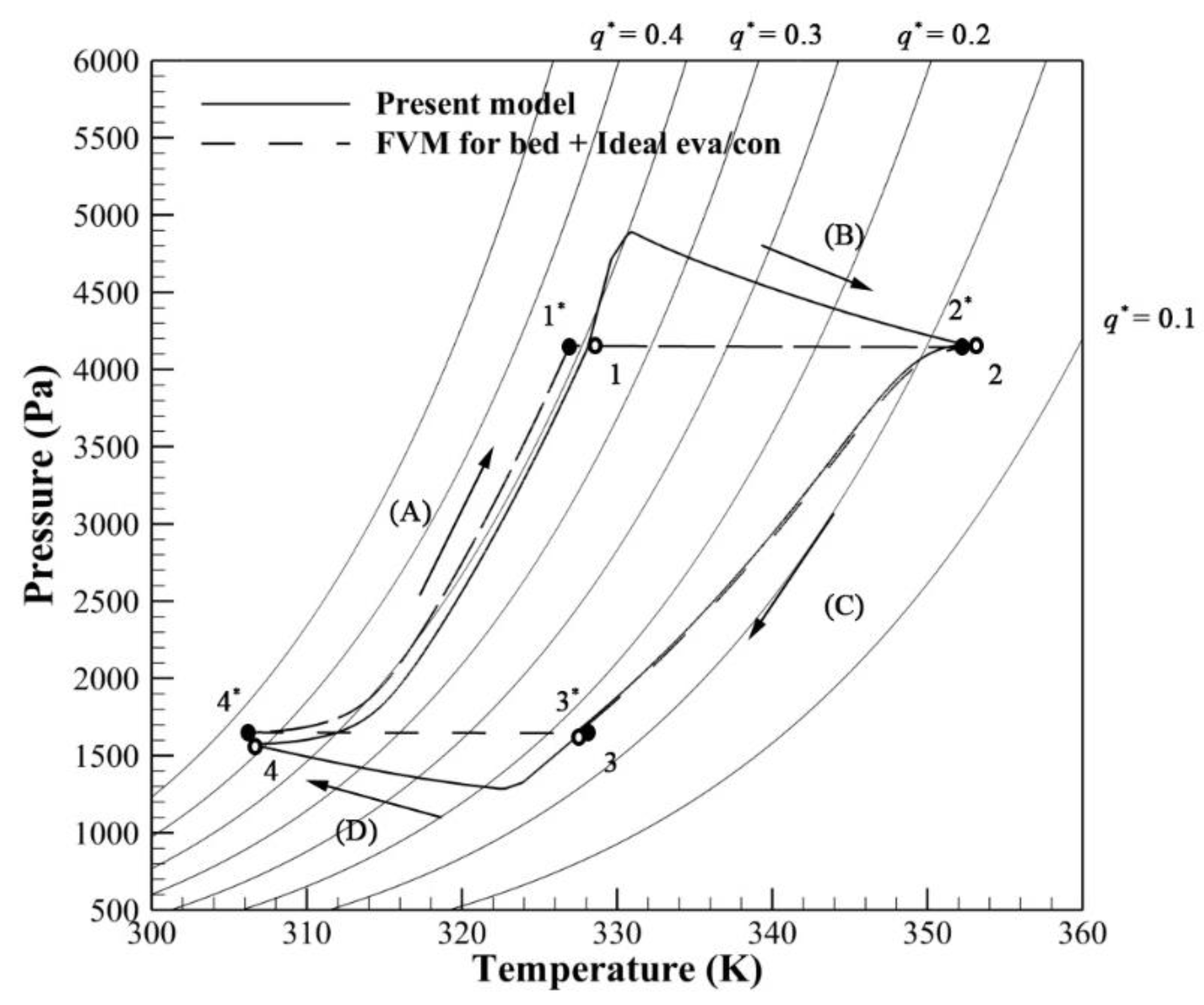
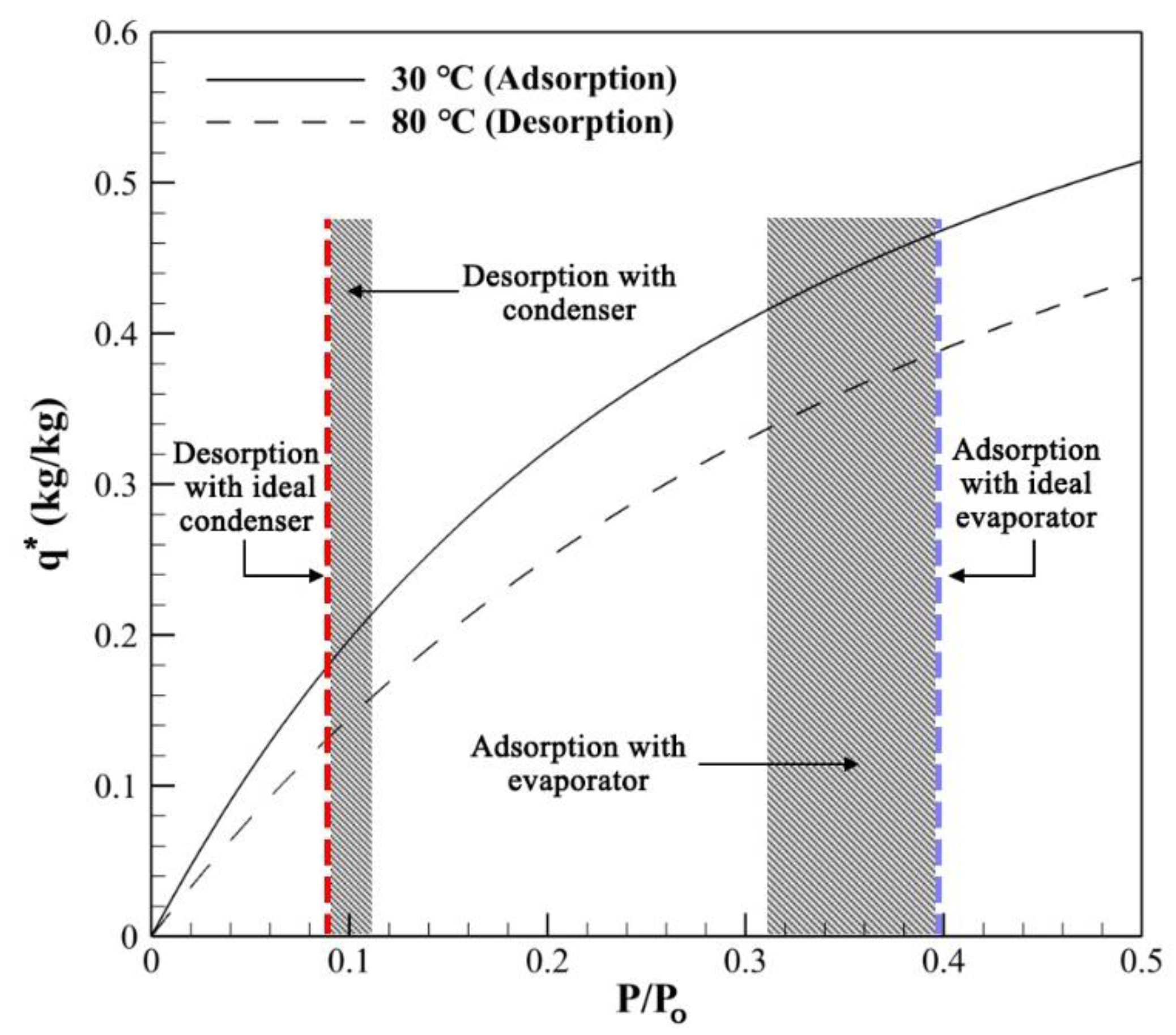
3.2. Effect of Evaporator/Condenser Model
3.3. Effect of Bed Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Freni, A.; Bonaccorsi, L.; Proverbio, E.; Maggio, G.; Restuccia, G. Zeolite synthesised on copper foam for adsorption chillers: A mathematical model. Micropor. Mesopor. Mater. 2009, 120, 402–409. [Google Scholar] [CrossRef]
- Askalany, A.A.; Saha, B.B.; Ahmed, M.S.; Ismail, I.M. Adsorption cooling system employing granular activated carbon-R134a pair for renewable energy applications. Int. J. Refrig. 2013, 36, 1037–1044. [Google Scholar] [CrossRef]
- Hong, S.W.; Ahn, S.H.; Chung, J.D.; Bae, K.J.; Cha, D.A.; Kwon, O.K. Characteristics of FAM-Z01 compared to silica gels in the performance of an adsorption bed. Appl. Therm. Eng. 2016, 104, 24–33. [Google Scholar] [CrossRef]
- Niazmand, H.; Talebian, H.; Mahdavikhah, M. Bed geometrical specifications effects on the performance of silica/water adsorption chillers. Int. J. Refrig. 2012, 35, 2261–2274. [Google Scholar] [CrossRef]
- Mahdavikhah, M.; Niazmand, H. Effects of plate finned heat exchanger parameters on the adsorption chiller performance. Appl. Therm. Eng. 2013, 50, 939–949. [Google Scholar] [CrossRef]
- Hong, S.W.; Kwon, O.K.; Chung, J.D. Application of an embossed plate heat exchanger to adsorption chiller. Int. J. Refrig. 2016, 65, 142–153. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Alam, K.C.A.; Saha, B.B.; Hamamoto, Y.; Akisawa, A.; Kashiwagi, T. Parametric study of a two-stage adsorption chiller using re-heat—The effect of overall thermal conductance and adsorbent mass on system performance. Int. J. Therm. Sci. 2006, 45, 511–519. [Google Scholar] [CrossRef]
- Uyun, A.S.; Akisawa, A.; Miyazaki, T.; Ueda, Y.; Kashiwagi, T. Numerical analysis of an advanced three-bed mass recovery adsorption refrigeration cycle. Appl. Therm. Eng. 2009, 29, 2876–2884. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akisawa, A.; Saha, B.B. The performance analysis of a novel dual evaporator type three-bed adsorption chiller. Int. J. Refrig. 2010, 33, 276–285. [Google Scholar] [CrossRef]
- Duong, X.Q.; Cao, N.V.; Hong, S.W.; Ahn, S.H.; Chung, J.D. Numerical study on the combined heat and mass recovery adsorption cooling cycle. Energy Technol. 2018, 6, 296–305. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Saha, B.B.; Chakraborty, A. Study on a waste heat-driven adsorption cooling cum desalination cycle. Int. J. Refrig. 2012, 35, 685–693. [Google Scholar] [CrossRef]
- Zajaczkowski, B. Optimizing performance of a three-bed adsorption chiller using new cycle time allocation and mass recovery. Appl. Therm. Eng. 2016, 100, 744–752. [Google Scholar] [CrossRef]
- Hong, S.W.; Ahn, S.H.; Kwon, O.K.; Chung, J.D. Optimization of a fin-tube type adsorption chiller by design of experiment. Int. J. Refrig. 2015, 49, 49–56. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y. Theoretical research of a silica gel–water adsorption chiller in a micro combined cooling, heating and power (CCHP) system. Appl. Energy 2009, 86, 958–967. [Google Scholar] [CrossRef]
- Schicktanz, M.; Nunez, T. Modelling of an adsorption chiller for dynamic system simulation. Int. J. Refrig. 2009, 32, 588–595. [Google Scholar] [CrossRef]
- Hong, S.W.; Ahn, S.H.; Kwon, O.K.; Chung, J.D. Validity of intra-particle models of mass transfer kinetics in the analysis of a fin-tube type adsorption bed. J. Mech. Sci. Technol. 2014, 28, 1985–1993. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: Hoboken, NJ, USA, 1960. [Google Scholar]
- Lee, H.; Thodos, G. Generalized treatment of self-diffusivity for the gaseous and liquid states of fluids. Ind. Eng. Chem. Fundam. 1983, 22, 17–26. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Process; Wiley: Hoboken, NJ, USA, 1984. [Google Scholar]
- Saha, B.B.; Chakraborty, A.; Koyama, S.; Aristov, Y.I. A new generation cooling device employing CaCl2-in-silica gel–water system. Int. J. Heat. Mass. Transf. 2009, 52, 516–524. [Google Scholar] [CrossRef]
- Miyazaki, T.; Akisawa, A.; Saha, B.B.; El-Sharkawy, I.I.; Chakraborty, A. A new cycle time allocation for enhancing the performance of two-bed adsorption chillers. Int. J. Refrig. 2009, 32, 846–853. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, S. Experimental investigation of contact resistance in adsorber of solar adsorption refrigeration. Sol. Energy 2002, 73, 177–185. [Google Scholar] [CrossRef]
- Chang, W.S.; Wang, C.C.; Shieh, C.C. Experimental study of a solid adsorption cooling system using flat-tube heat exchangers as adsorption bed. Appl. Therm. Eng. 2007, 27, 2195–2199. [Google Scholar] [CrossRef]
- Duong, X.Q.; Cao, N.V.; Lee, W.S.; Chung, J.D. Module integration in an adsorption cooling system. Appl. Therm. Eng. 2019, 155, 508–514. [Google Scholar] [CrossRef]
- Rogala, Z. Adsorption chiller using flat-tube adsorbers—Performance assessment and optimization. Appl. Therm. Eng. 2017, 121, 431–442. [Google Scholar] [CrossRef]
- Mitra, S.; Muttakin, M.; Thu, K.; Saha, B.B. Study on the influence of adsorbent particle size and heat exchanger aspect ratio on dynamic adsorption characteristics. Appl. Therm. Eng. 2018, 133, 764–773. [Google Scholar] [CrossRef]
- Kowsari, M.M.; Niazmand, H.; Tokarev, M.M. Bed configuration effects on the finned flat-tube adsorption heat exchanger performance: Numerical modeling and experimental validation. Appl. Energy 2018, 213, 540–554. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.K.; Mahmoud, S.; Elsayed, A. Effects of contact resistance and metal additives in finned-tube adsorbent beds on the performance of silica gel/water adsorption chiller. Appl. Therm. Eng. 2013, 53, 278–284. [Google Scholar] [CrossRef]
- Girnik, I.S.; Aristov, Y.I. Making adsorptive chillers more fast and efficient: The effect of bi-dispersed adsorbent bed. Appl. Therm. Eng. 2016, 106, 254–256. [Google Scholar] [CrossRef]



| Parameter | Values |
|---|---|
| Fin pitch | 1.68 mm |
| Fin thickness | 0.06 mm |
| Fin height | 6.82 mm |
| Inner diameter of tube | 9.6 mm |
| Outer diameter of tube | 8.8 mm |
| Fluid velocity | 1 m/s |
| Heating temperature | 80 ℃ |
| Cooling temperature | 30 ℃ |
| Cycle time | 900 s |
| Density | 700 kg/m3 |
| Specific heat | 900 J/kgK |
| Thermal conductivity | 0.2 W/mK |
| Heat of adsorption | 2760 kJ/kg |
| Total porosity | 0.6352 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.S.; Park, M.Y.; Duong, X.Q.; Cao, N.V.; Chung, J.D. Effects of Evaporator and Condenser in the Analysis of Adsorption Chillers. Energies 2020, 13, 1901. https://doi.org/10.3390/en13081901
Lee WS, Park MY, Duong XQ, Cao NV, Chung JD. Effects of Evaporator and Condenser in the Analysis of Adsorption Chillers. Energies. 2020; 13(8):1901. https://doi.org/10.3390/en13081901
Chicago/Turabian StyleLee, Woo Su, Moon Yong Park, Xuan Quang Duong, Ngoc Vi Cao, and Jae Dong Chung. 2020. "Effects of Evaporator and Condenser in the Analysis of Adsorption Chillers" Energies 13, no. 8: 1901. https://doi.org/10.3390/en13081901
APA StyleLee, W. S., Park, M. Y., Duong, X. Q., Cao, N. V., & Chung, J. D. (2020). Effects of Evaporator and Condenser in the Analysis of Adsorption Chillers. Energies, 13(8), 1901. https://doi.org/10.3390/en13081901





