Theoretical Investigation of the Temperature Limits of an Actively Cooled High Concentration Photovoltaic System
Abstract
1. Introduction
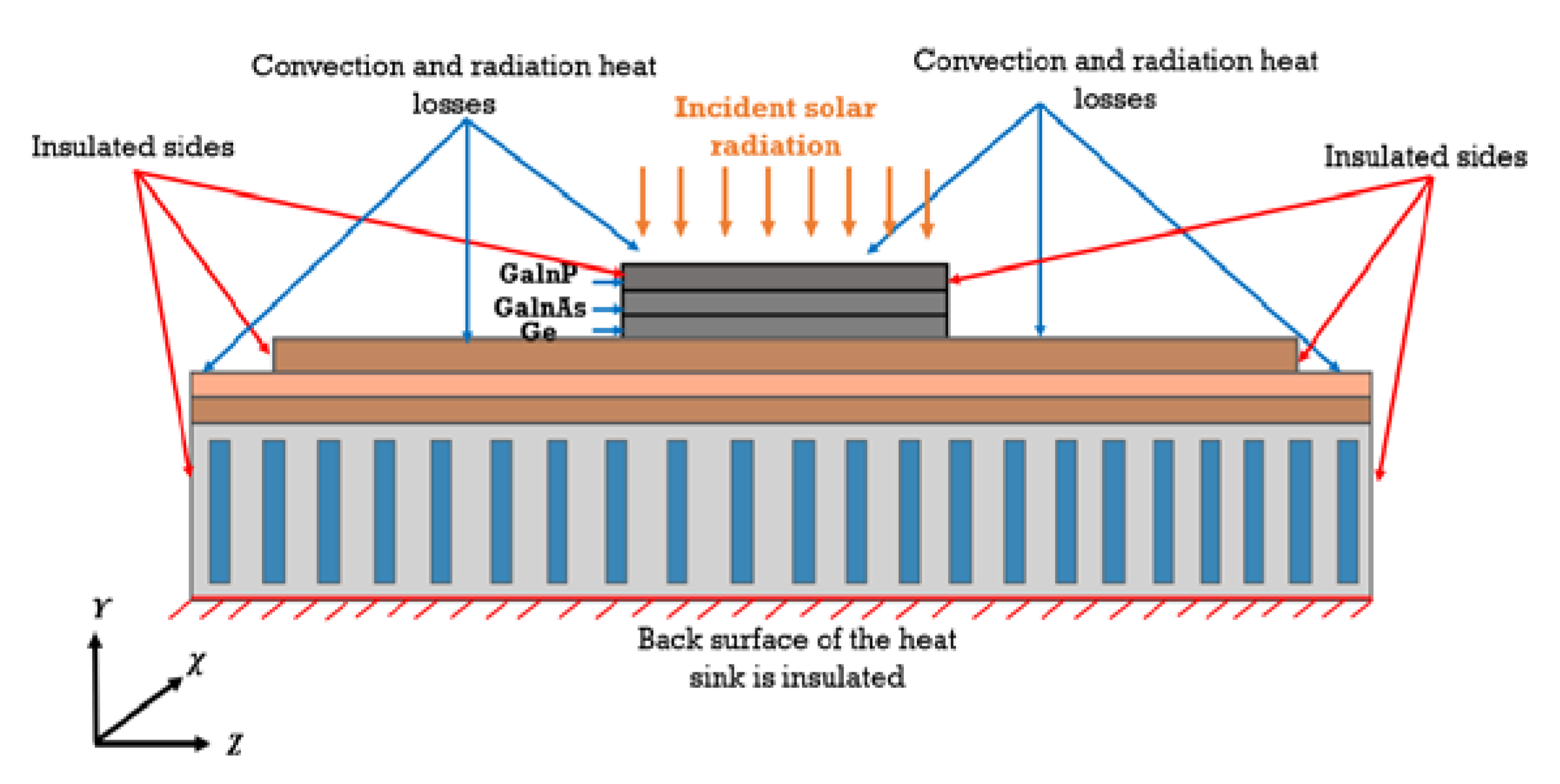
2. Mathematical Modeling
- The MJ solar cell is subjected to concentration ratios varying from 500× to 2000×.
- The germanium layer is the heat source of the system.
- The sides of the module and its back surface are insulated.
- The ambient temperature is fixed at 25 °C.
- The flow inside the channel is steady, incompressible, and laminar.
- The effects of the body force and viscous dissipation are ignored.
- The inlet temperature of the fluid is fixed at 25 °C and the outlet pressure is atmospheric.
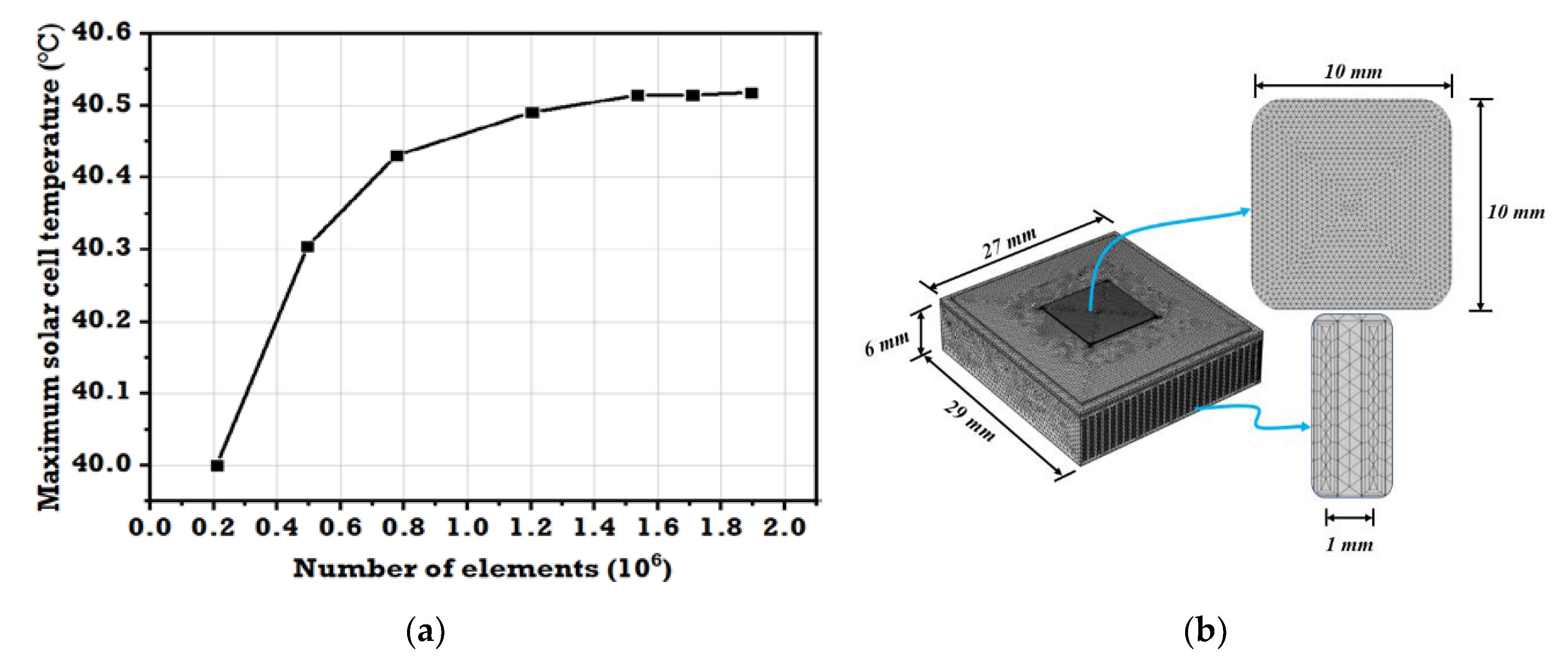
2.1. Grid Independence Study
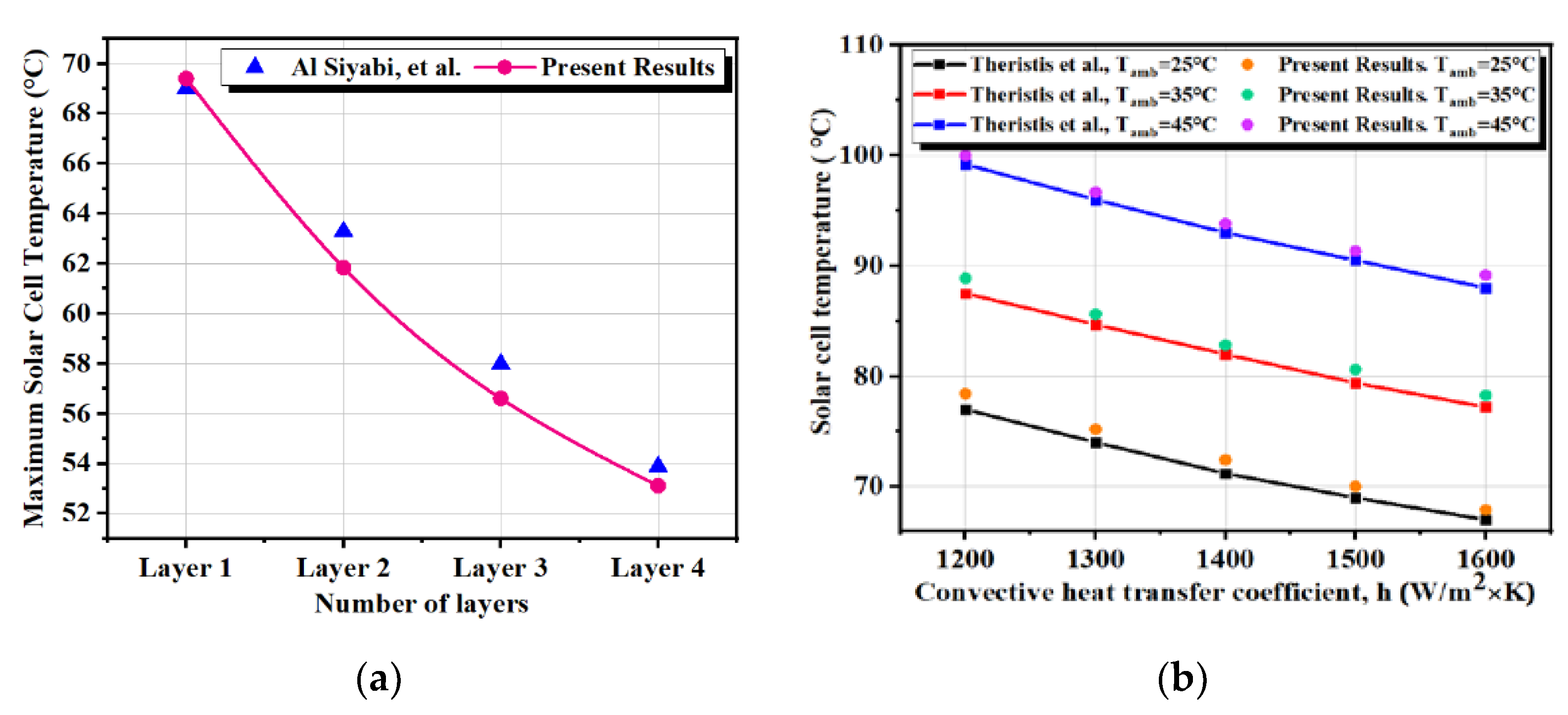
2.2. Validation Study
3. Results and Discussion
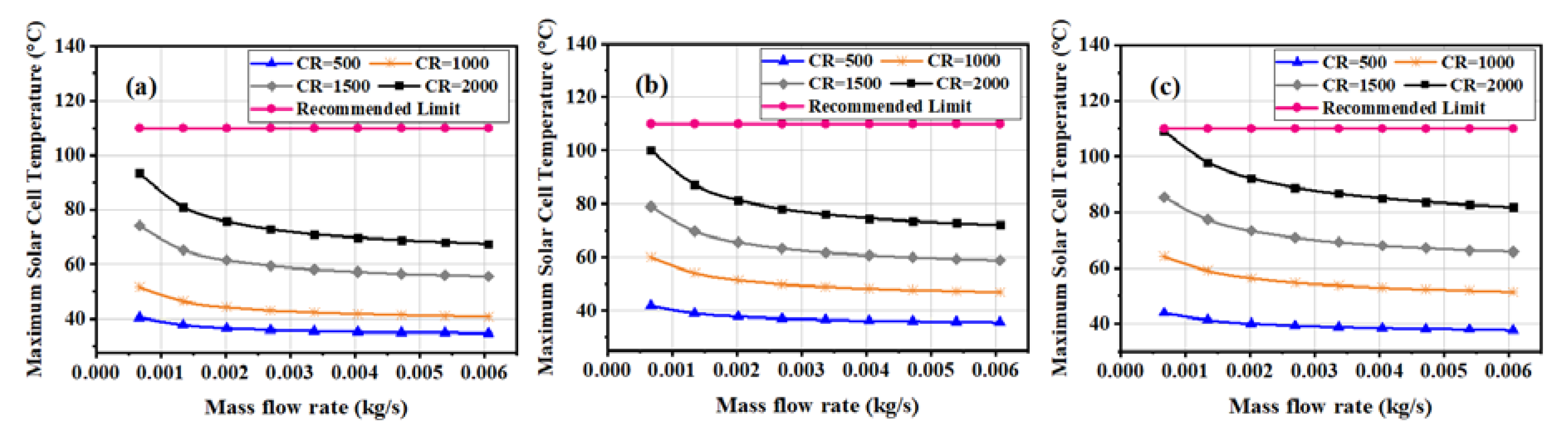
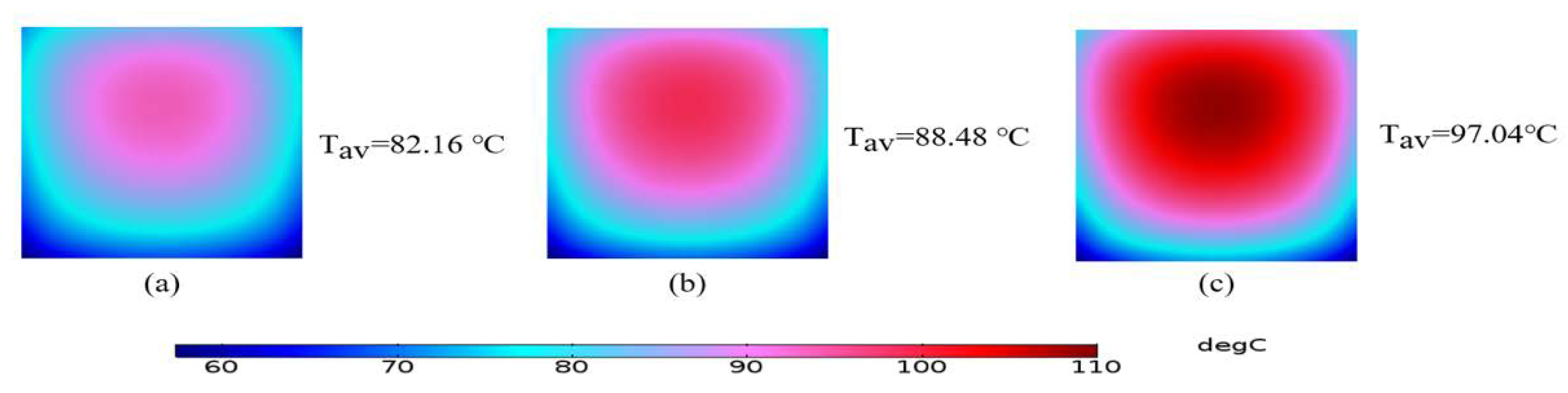
3.1. Maximum Solar Cell Temperature and Temperature Distribution over the Surface of the Solar Cell
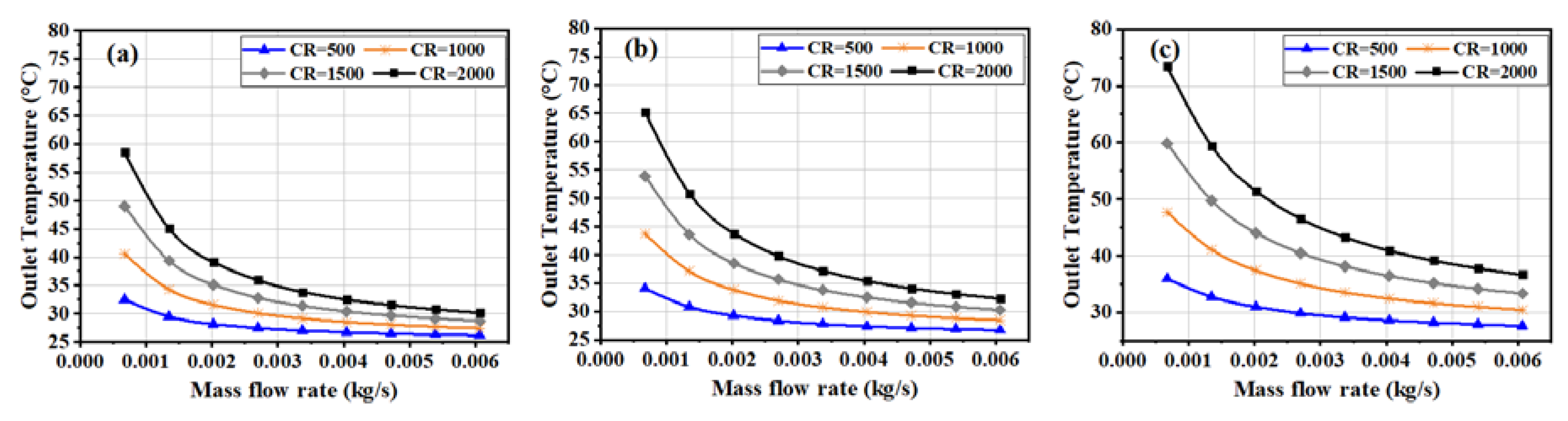
3.2. Outlet Fluid Temperature
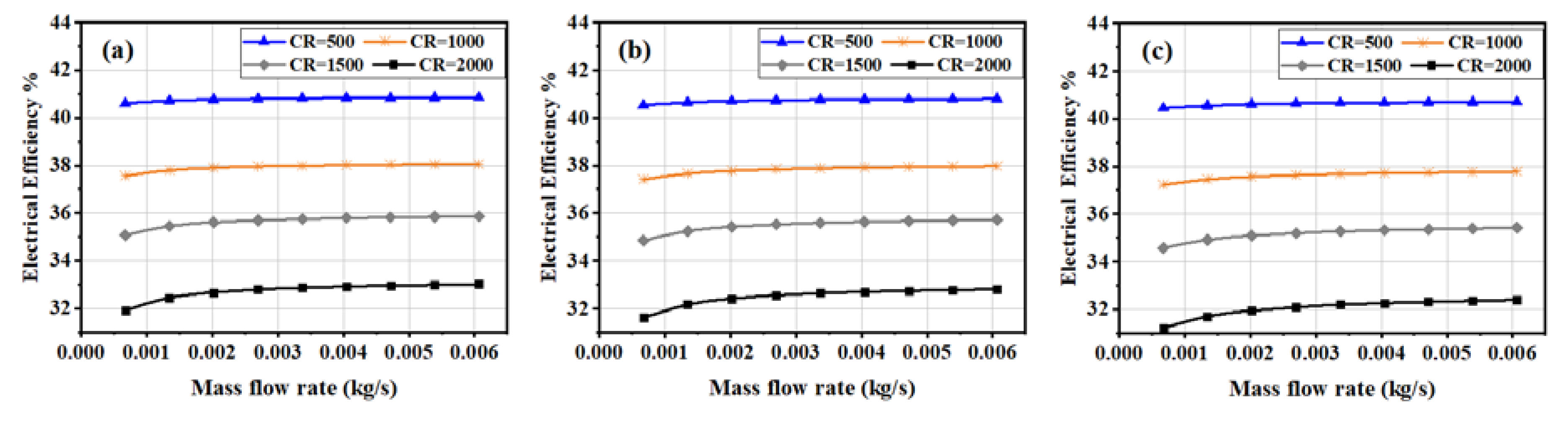
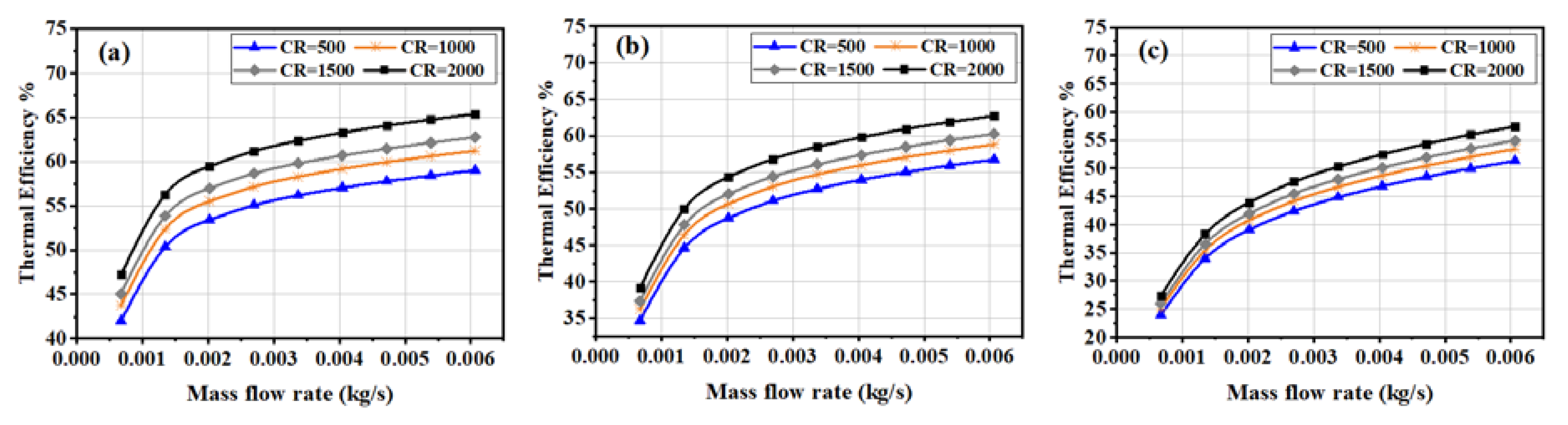
3.3. Electrical and Thermal Efficiencies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiesenfarth, M.; Philipps, S.P.; Bett, A.W.; Horowitz, K.; Kurtz, S. Current Status of Concentrated Photovoltaic (CPV); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2015.
- NREL. Best Research-Cell Efficiency Chart; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2019; Volume 1.
- Valera, A.; Fernández, E.F.; Rodrigo, P.M.; Almonacid, F. Feasibility of flat-plate heat-sinks using microscale solar cells up to 10,000 suns concentrations. Sol. Energy 2019, 181, 361–371. [Google Scholar] [CrossRef]
- Araki, K.; Uozumi, H.; Yamaguchi, M. A Simple Passive Cooling Structure and Its Heat Analysis for 500× Concentrator PV Module. In Proceedings of the Twenty-Ninth IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 19–24 May 2002; pp. 1568–1571. [Google Scholar]
- Aldossary, A.; Mahmoud, S.; Al-Dadah, R. Technical feasibility study of passive and active cooling for concentrator PV in harsh environment. Appl. Therm. Eng. 2016, 100, 490–500. [Google Scholar] [CrossRef]
- Siyabi, I.A.; Khanna, S.; Sundaram, S.; Mallick, T. Experimental and numerical thermal analysis of multi-layered microchannel heat sink for concentrating photovoltaic application. Energies 2019, 12, 122. [Google Scholar] [CrossRef]
- Abo-Zahhad, E.M.; Ookawara, S.; Radwan, A.; El-Shazly, A.H.; Elkady, M.F. Numerical analyses of hybrid jet impingement/microchannel cooling device for thermal management of high concentrator triple-junction solar cell. Appl. Energy 2019, 253, 113538. [Google Scholar] [CrossRef]
- Abo-Zahhad, E.M.; Ookawara, S.; Radwan, A.; El-Shazly, A.H.; El-Kady, M.F.; Esmail, M.F.C. Performance, limits, and thermal stress analysis of high concentrator multijunction solar cell under passive cooling conditions. Appl. Therm. Eng. 2020, 164, 114497. [Google Scholar] [CrossRef]
- Sung, S.K.; Suh, S.H.; Kim, D.W. Characteristics of cooling water fouling in a heat exchange system. J. Mech. Sci. Technol. 2008, 22, 1568–1575. [Google Scholar] [CrossRef]
- Theristis, M.; O’Donovan, T.S. An Integrated Thermal Electrical Model for Single Cell Photovoltaic Receivers Under Concentration. In Proceedings of the International Heat Transfer Conference, Kyoto, Japan, 10–15 August 2014. [Google Scholar]
- Zhou, J.; Yi, Q.; Wang, Y.; Ye, Z. Temperature distribution of photovoltaic module based on finite element simulation. Sol. Energy 2015, 111, 97–103. [Google Scholar] [CrossRef]
- Siyabi, I.A.; Shanks, K.; Mallick, T.; Sundaram, S. Thermal Analysis of a Multi-Layer Microchannel Heat Sink for Cooling Concentrator Photovoltaic (CPV) Cells. In Proceedings of the AIP Conference Proceedings, Bydgoszcz, Poland, 9–11 May 2018; Volume 1881. [Google Scholar]
- Azure Space Solar Power GmbH. AZUR Space Solar Cell Assembly 10 × 10 mm2; Azure Space Solar Power GmbH: Heilbronn, Germany, 2014. [Google Scholar]
- Yazdanifard, F.; Ameri, M.; Ebrahimnia-Bajestan, E. Performance of nanofluid-based photovoltaic/thermal systems: A review. Renew. Sustain. Energy Rev. 2017, 76, 323–352. [Google Scholar] [CrossRef]
- Loikits Syltherm 800. Available online: http://www.loikitsdistribution.com/files/syltherm-800-technical-data-sheet.pdf (accessed on 1 January 2019).
- COMSOL Multi-Physics. Available online: https://www.comsol.com (accessed on 5 January 2019).
- Theristis, M.; O’Donovan, T.S. Electrical-thermal analysis of III-V triple-junction solar cells under variable spectra and ambient temperatures. Sol. Energy 2015, 118, 533–546. [Google Scholar] [CrossRef]
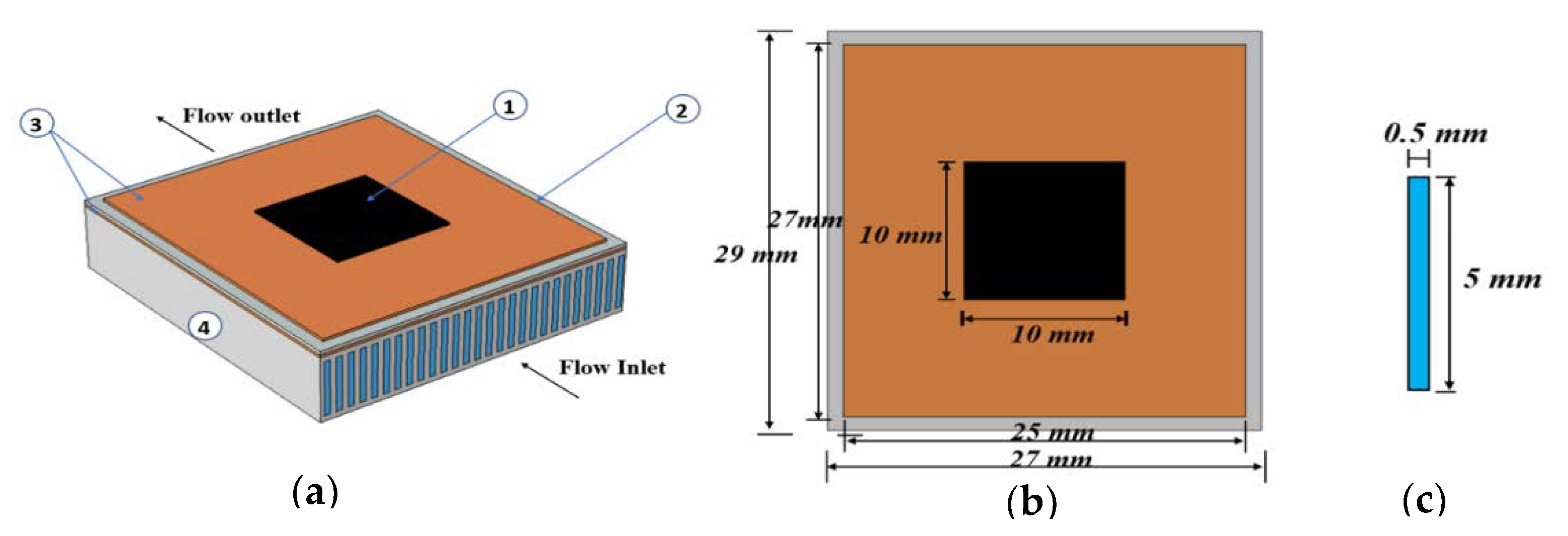
| Number | Layer | Length (mm) | Width (mm) | Thickness (mm) |
|---|---|---|---|---|
| 1 | GalnP | 10 | 10 | 0.07 |
| 1 | GalnAs | 10 | 10 | 0.07 |
| 1 | Ge | 10 | 10 | 0.07 |
| 2 | Copper I | 27 | 25 | 0.25 |
| 3 | Ceramic | 29 | 27 | 0.32 |
| 2 | Copper II | 29 | 27 | 0.25 |
| 4 | Aluminum | 29 | 27 | 6 |
| Layer | Specific Heat, | Thermal Conductivity, | Density, | Emissivity, |
|---|---|---|---|---|
| GalnP | 370 | 73 | 4470 | 0.9 |
| GalnAs | 550 | 65 | 5316 | |
| Ge | 320 | 60 | 5323 | |
| Copper | 385 | 400 | 8700 | 0.05 |
| Ceramic | 900 | 27 | 3900 | 0.75 |
| Aluminum | 900 | 160 | 2700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Shanks, K.; Sundaram, S.; Mallick, T.K. Theoretical Investigation of the Temperature Limits of an Actively Cooled High Concentration Photovoltaic System. Energies 2020, 13, 1902. https://doi.org/10.3390/en13081902
Ahmed A, Shanks K, Sundaram S, Mallick TK. Theoretical Investigation of the Temperature Limits of an Actively Cooled High Concentration Photovoltaic System. Energies. 2020; 13(8):1902. https://doi.org/10.3390/en13081902
Chicago/Turabian StyleAhmed, Asmaa, Katie Shanks, Senthilarasu Sundaram, and Tapas Kumar Mallick. 2020. "Theoretical Investigation of the Temperature Limits of an Actively Cooled High Concentration Photovoltaic System" Energies 13, no. 8: 1902. https://doi.org/10.3390/en13081902
APA StyleAhmed, A., Shanks, K., Sundaram, S., & Mallick, T. K. (2020). Theoretical Investigation of the Temperature Limits of an Actively Cooled High Concentration Photovoltaic System. Energies, 13(8), 1902. https://doi.org/10.3390/en13081902









