High-Rate Layered Cathode of Lithium-Ion Batteries through Regulating Three-Dimensional Agglomerated Structure
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of LiNi1/3Co1/3Mn1/3O2
2.2. Structural Characterization
2.3. Electrochemical Measurements
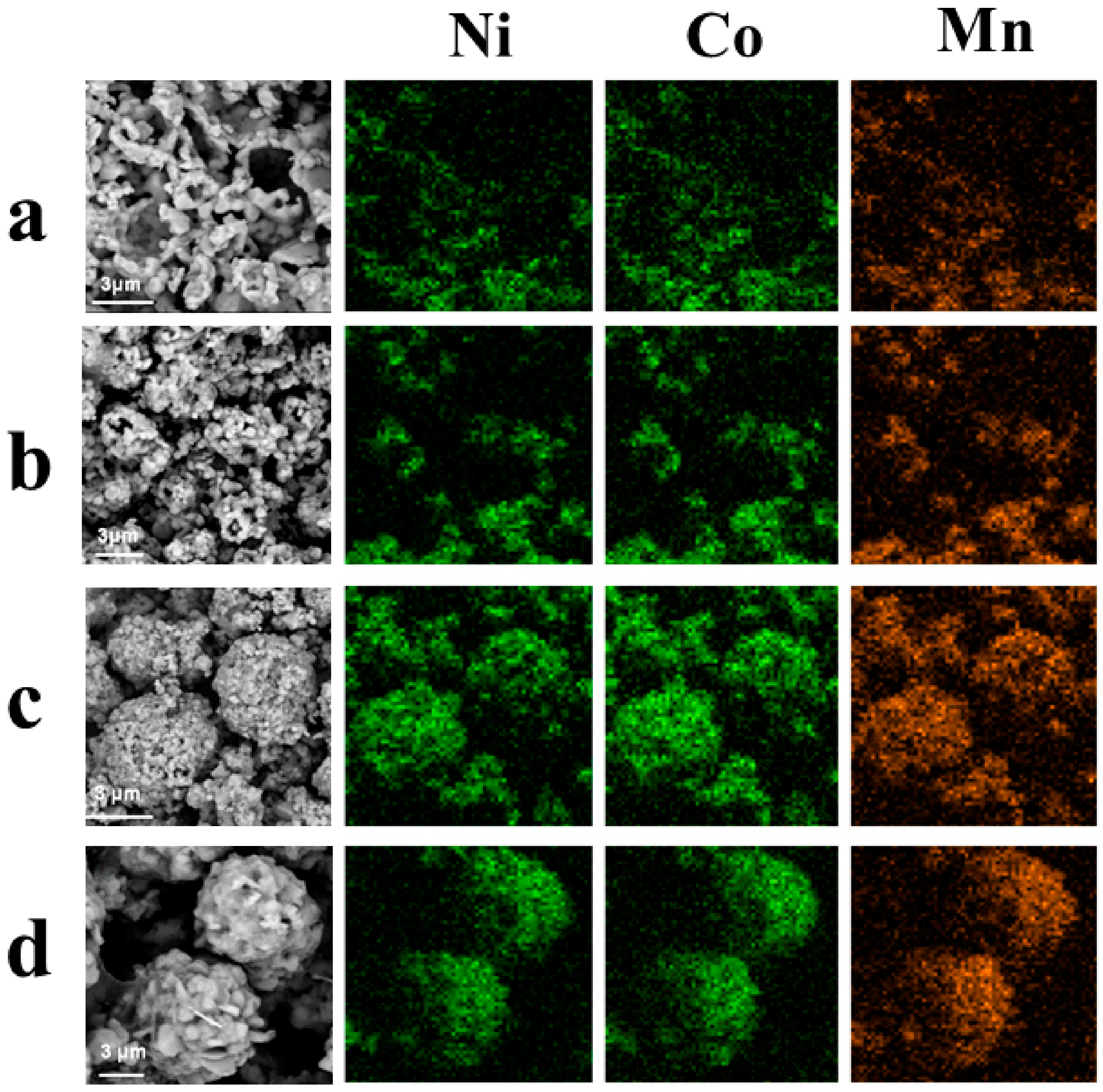
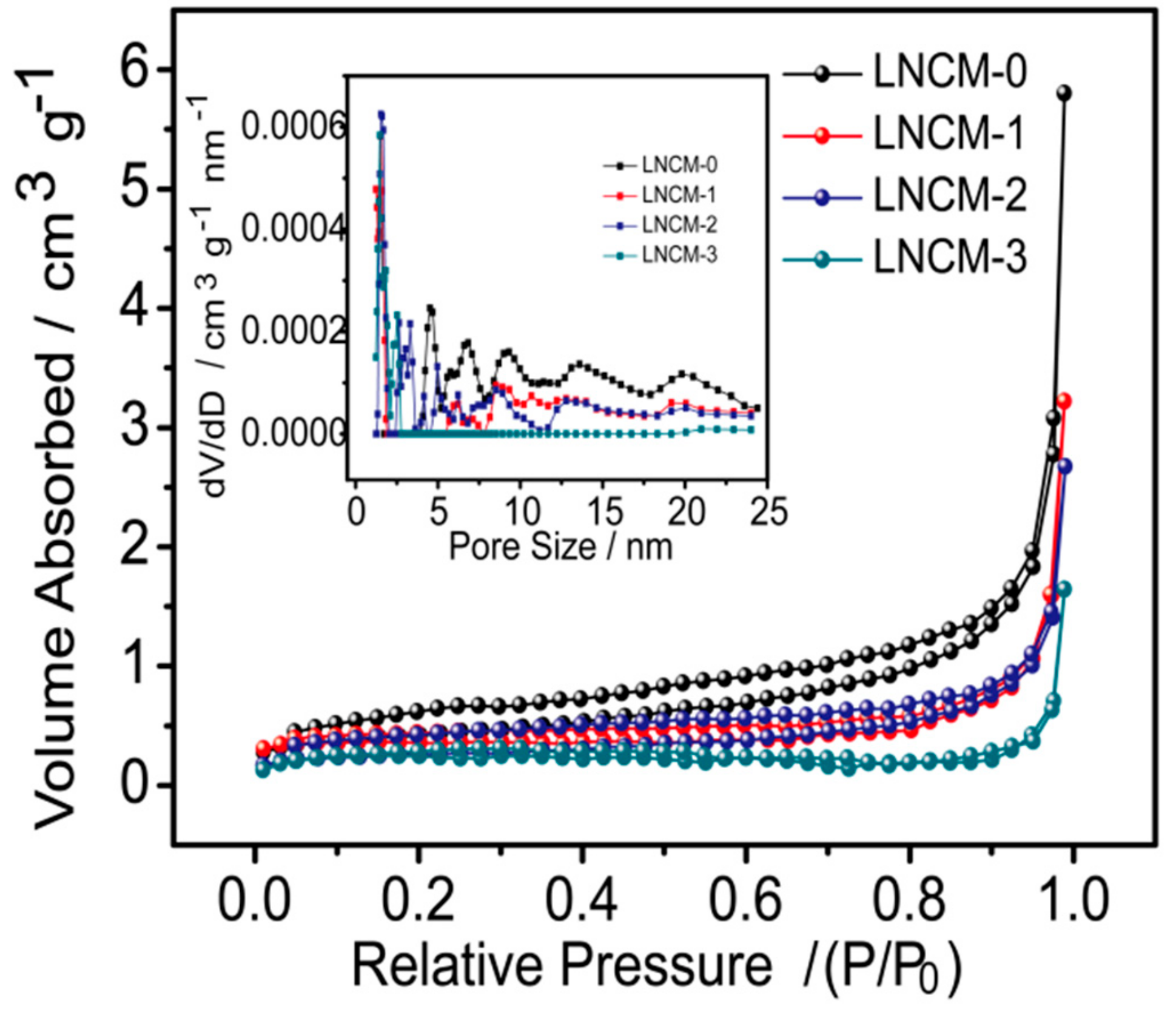
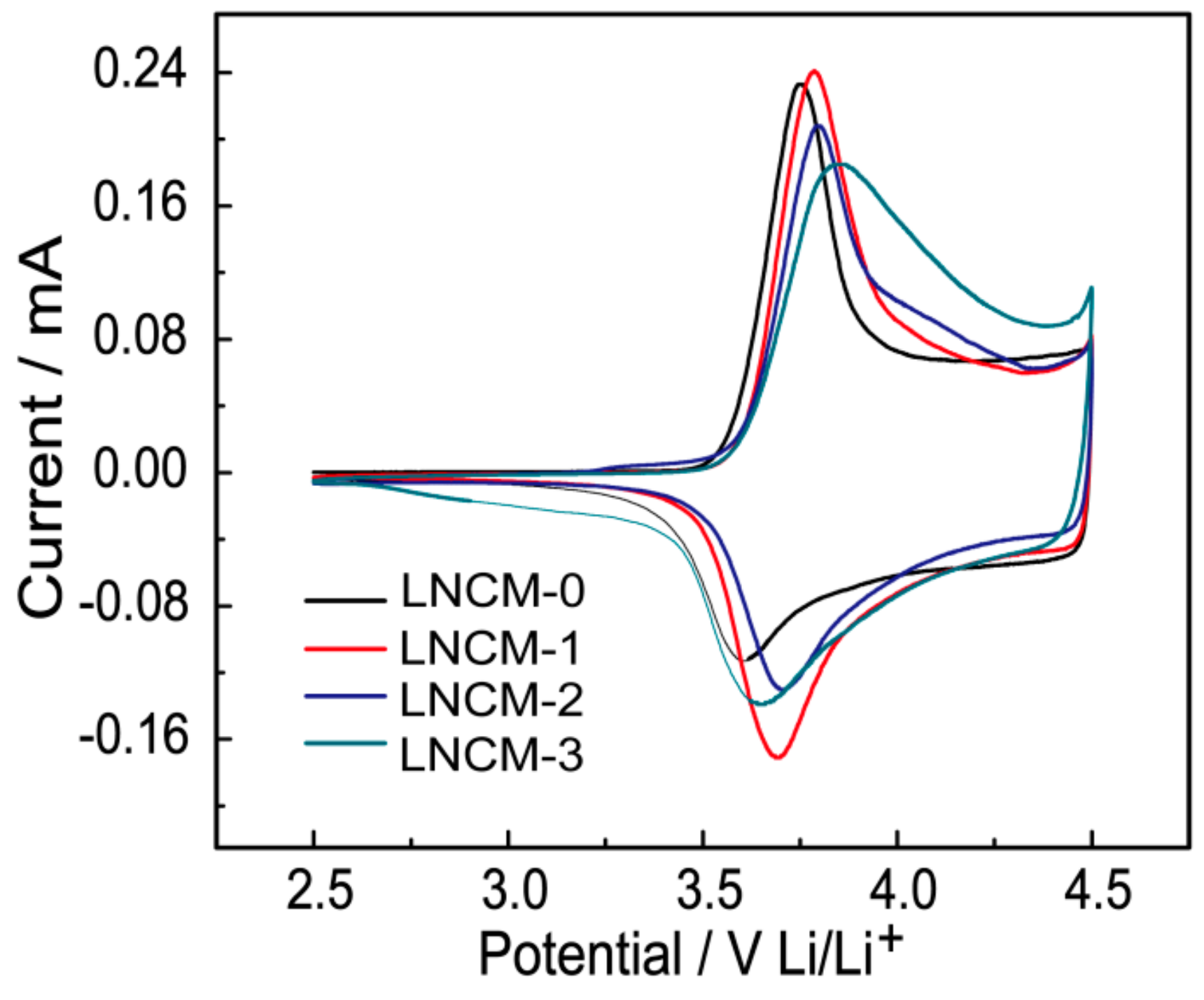
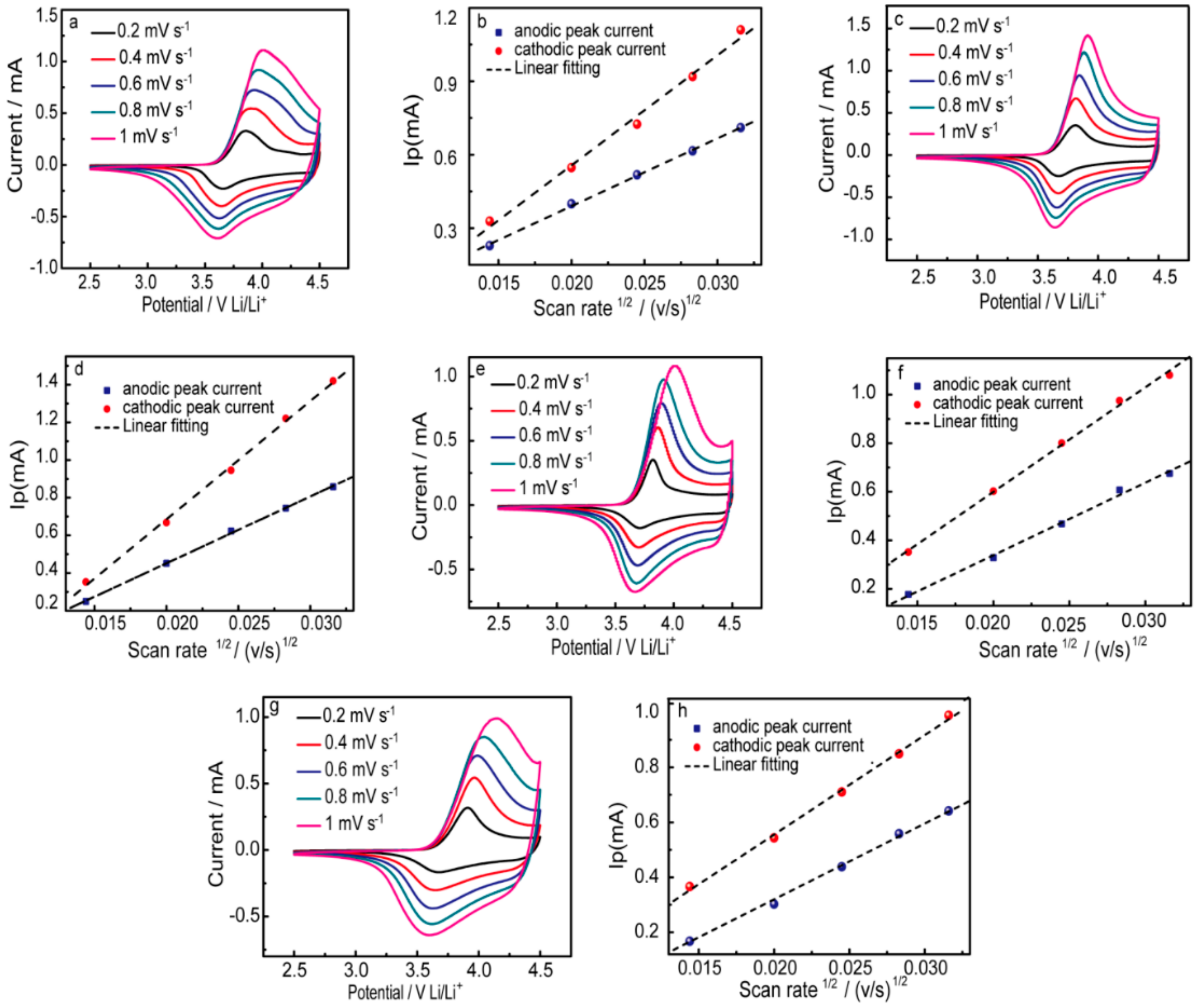
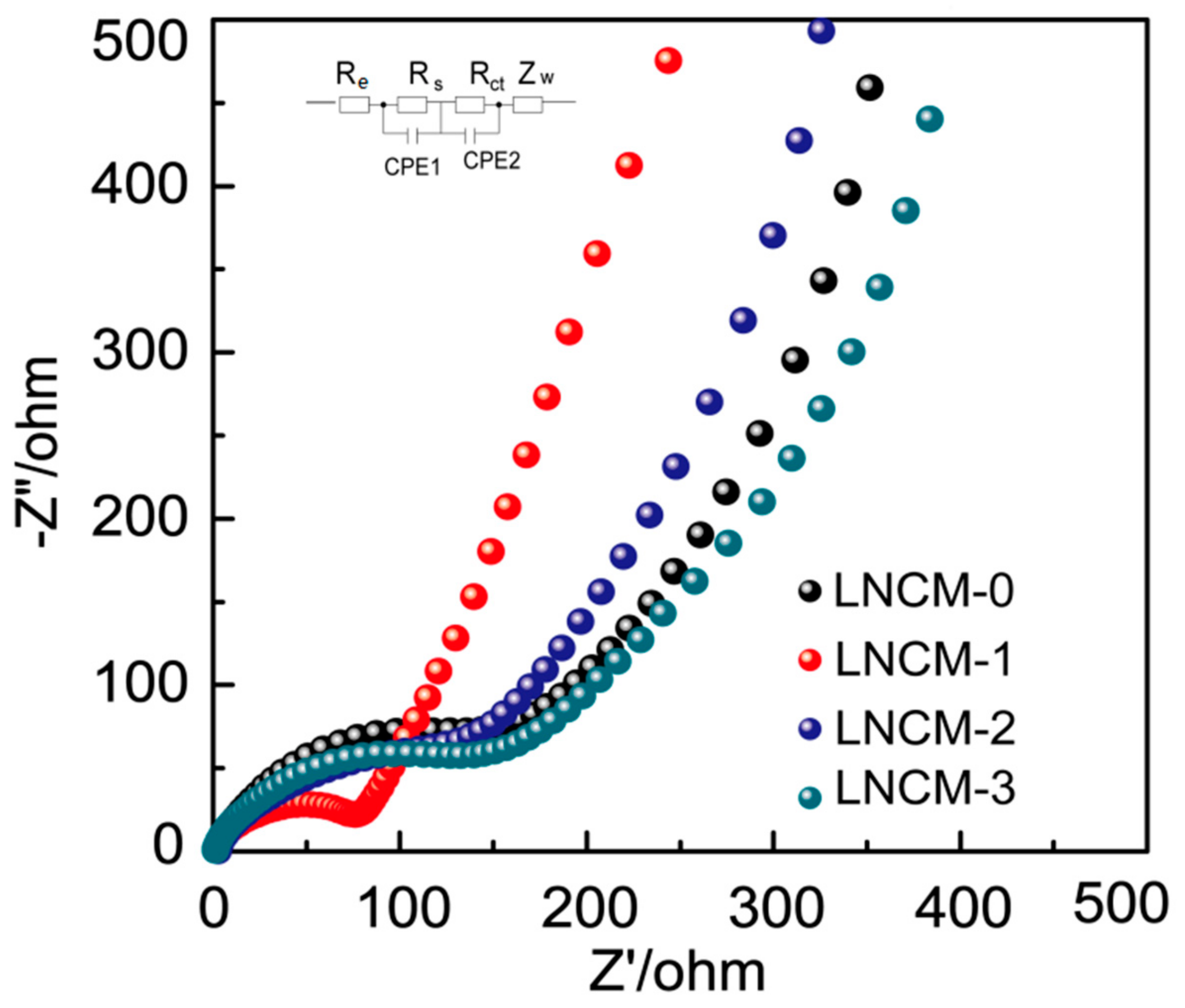
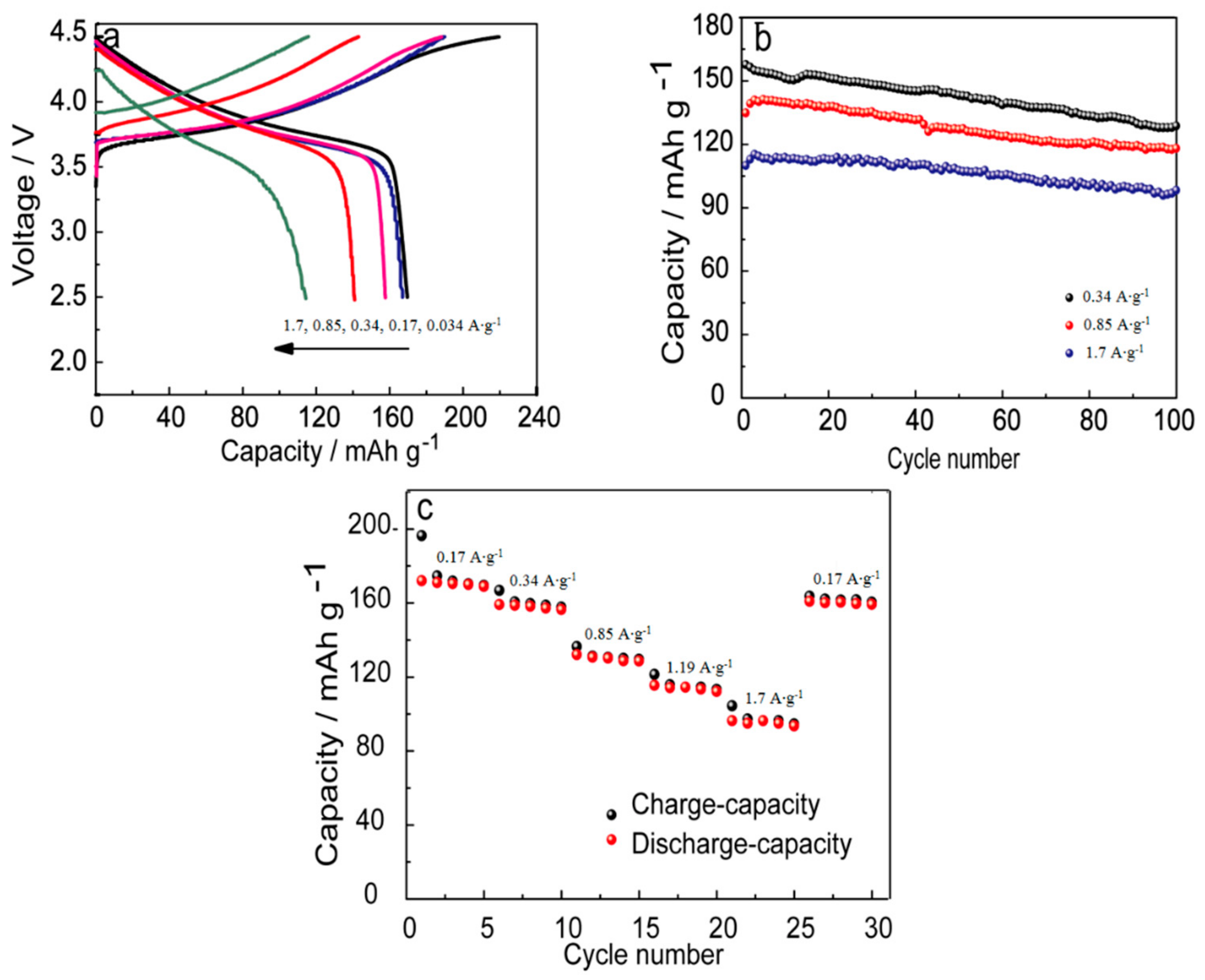
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guo, Y.-G.; Chen, J. Special topic on electrochemical power sources. Sci. China Chem. 2017, 60, 1481–1482. [Google Scholar] [CrossRef][Green Version]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Bock, R.; Onsrud, M.; Karoliussen, H.; Pollet, B.G.; Seland, F.; Burheim, O.S. Thermal Gradients with Sintered Solid State Electrolytes in Lithium-Ion Batteries. Energies 2020, 13, 253. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Li[NixCo1−2xMnx]O2 Cathode Materials for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A200–A203. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Lu, Z.; Dahn, J.R. Structure and Electrochemistry of Li[NixCo1-2xMnx]O2 (0 ≤ x ≤ 1/2). J. Electrochem. Soc. 2002, 149, A1332–A1336. [Google Scholar] [CrossRef]
- Zhang, N.; Zaker, N.; Li, H.; Liu, A.; Inglis, J.; Jing, L.; Li, J.; Li, Y.; Botton, G.A.; Dahn, J.R. Cobalt-Free Nickel-Rich Positive Electrode Materials with a Core–Shell Structure. Chem. Mater. 2019, 31, 10150–10160. [Google Scholar] [CrossRef]
- Li, H.; Liu, A.; Zhang, N.; Wang, Y.; Yin, S.; Wu, H.; Dahn, J.R. An Unavoidable Challenge for Ni-Rich Positive Electrode Materials for Lithium-Ion Batteries. Chem. Mater. 2019, 31, 7574–7583. [Google Scholar] [CrossRef]
- 1Beletskii, E.V.; Alekseeva, E.V.; Spiridonova, D.Y.V.; Yankin, A.N.; Levin, O.V. Overcharge Cycling Effect on the Surface Layers and Crystalline Structure of LiFePO4 Cathodes of Li-Ion Batteries. Energies 2019, 12, 4652. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Yao, S.; Qiu, X. High capacity lithium-manganese-nickel-oxide composite cathodes with low irreversible capacity loss and good cycle life for lithium ion batteries. Sci. China Chem. 2016, 59, 1479–1485. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Kim, T.; Kim, H.; Lee, E.; Jeong, M.; Son, S.; Ryou, J.-H.; Yoon, W.-S. New Insight into Ni-Rich Layered Structure for Next-Generation Li Rechargeable Batteries. Adv. Energy Mater. 2018, 8, 1701788. [Google Scholar] [CrossRef]
- Ahn, Y.-K.; Jo, Y.N.; Cho, W.; Yu, J.-S.; Kim, K.J. Mechanism of Capacity Fading in the LiNi0.8Co0.1Mn0.1O2 Cathode Material for Lithium-Ion Batteries. Energies 2019, 12, 1638. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. A First-Principles Approach to Studying the Thermal Stability of Oxide Cathode Materials. Chem. Mater. 2007, 19, 543–552. [Google Scholar] [CrossRef]
- Yudha, C.S.; Muzayanha, S.U.; Widiyandari, H.; Iskandar, F.; Sutopo, W.; Purwanto, A. Synthesis of LiNi0.85Co0.14Al0.01O2 Cathode Material and its Performance in an NCA/Graphite Full-Battery. Energies 2019, 12, 1886. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Yao, W.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.; Cheng, H.; Yan, Z. Synergistically Enhanced Electrochemical Performance of Ni-rich Cathode Materials for Lithium-ion Batteries by K and Ti Co-modification. J. Phys. Chem. C 2020, 124, 2346–2356. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Shi, J.-L.; Liang, J.-Y.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.-J. Structurally modulated Li-rich cathode materials through cooperative cation doping and anion hybridization. Sci. China Chem. 2017, 60, 1554–1560. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Heubner, C.; Nickol, A.; Seeba, J.; Reuber, S.; Junker, N.; Wolter, M.; Schneider, M.; Michaelis, A. Understanding thickness and porosity effects on the electrochemical performance of LiNi0.6Co0.2Mn0.2O2-based cathodes for high energy Li-ion batteries. J. Power Sources 2019, 419, 119–126. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Wang, D.; He, X.; Liu, S.; Gao, Y.; AlZahrani, A.; Kim, S.H.; Chen, L.-Q.; Wang, D. Electrokinetic Phenomena Enhanced Lithium-Ion Transport in Leaky Film for Stable Lithium Metal Anodes. Adv. Energy Mater. 2019, 9, 1900704. [Google Scholar] [CrossRef]
- Qi, R.; Shi, J.-L.; Zhang, X.-D.; Zeng, X.-X.; Yin, Y.-X.; Xu, J.; Chen, L.; Fu, W.-G.; Guo, Y.-G.; Wan, L.-J. Improving the stability of LiNi0.80Co0.15Al0.05O2 by AlPO4 nanocoating for lithium-ion batteries. Sci. China Chem. 2017, 60, 1230–1235. [Google Scholar] [CrossRef]
- Shi, J.-L.; Xiao, D.-D.; Ge, M.; Yu, X.; Chu, Y.; Huang, X.; Zhang, X.-D.; Yin, Y.-X.; Yang, X.-Q.; Guo, Y.-G.; et al. High-Capacity Cathode Material with High Voltage for Li-Ion Batteries. Adv. Mater. 2018, 30, 1705575. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, Y.; Tong, W.; Cai, Y.; Wang, X.; Guo, Y.; Jia, D.; Zong, J. Synthesis of hollow peanut-like hierarchical mesoporous LiNi1/3Co1/3Mn1/3O2 cathode materials with exceptional cycle performance for lithium-ion batteries by a simple self-template solid-state method. J. Alloys Compd. 2018, 743, 707–715. [Google Scholar] [CrossRef]
- Fu, F.; Xu, G.-L.; Wang, Q.; Deng, Y.-P.; Li, X.; Li, J.-T.; Huang, L.; Sun, S.-G. Synthesis of single crystalline hexagonal nanobricks of LiNi1/3Co1/3Mn1/3O2 with high percentage of exposed {010} active facets as high rate performance cathode material for lithium-ion battery. J. Mater. Chem. A 2013, 1, 3860–3864. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Chang, Z.; Yang, Y.; Wu, Y. Nanoporous LiNi1/3Co1/3Mn1/3O2 as an ultra-fast charge cathode material for aqueous rechargeable lithium batteries. Chem. Commun. 2013, 49, 9209–9211. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Y.; Khakoo, U.; Chen, D.; Yu, G. Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathodes with tunable rate capability. Nano Energy 2015, 17, 36–42. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, X.; Yang, Y.; Li, L.; Li, G.; Guo, Z.; Feng, C. Self-Assembled LiNi1/3Co1/3Mn1/3O2 Nanosheet Cathode with High Electrochemical Performance. ACS Appl. Mater. Interfaces 2017, 9, 39560–39568. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, L.; Liu, A.; Ma, X.; Li, J.; Wang, J.; Dahn, J.R. The reactivity of charged positive Li1-n[NixMnyCoz]O2 electrodes with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Sources 2018, 390, 78–86. [Google Scholar] [CrossRef]
- Accardo, G.; Spiridigliozzi, L.; Cioffi, R.; Ferone, C.; Di Bartolomeo, E.; Yoon, S.P.; Dell’Agli, G. Gadolinium-doped ceria nanopowders synthesized by urea-based homogeneous co-precipitation (UBHP). Mater. Chem. Phys. 2017, 187, 149–155. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Spiridigliozzi, L.; Pansini, M.; Accardo, G.; Yoon, S.P.; Frattini, D. Effect of the carbonate environment on morphology and sintering behaviour of variously co-doped (Ca, Sr, Er, Pr) Samarium-doped Ceria in co-precipitation/hydrothermal synthesis. Ceram. Int. 2018, 44, 17935–17944. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Accardo, G.; Frattini, D.; Dell’Agli, G. Entropy-Stabilized Oxides owning Fluorite Structure obtained by Hydrothermal Treatment. Materials 2020, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Bortolotti, M.; Dell’Agli, G. New Insights in the Hydrothermal Synthesis of Rare-Earth Carbonates. Materials 2019, 12, 2062. [Google Scholar] [CrossRef]
- Wei, G.-Z.; Lu, X.; Ke, F.-S.; Huang, L.; Li, J.-T.; Wang, Z.-X.; Zhou, Z.-Y.; Sun, S.-G. Crystal Habit-Tuned Nanoplate Material of Li[Li1/3–2x/3NixMn2/3–x/3]O2 for High-Rate Performance Lithium-Ion Batteries. Adv. Mater. 2010, 22, 4364–4367. [Google Scholar] [CrossRef]
- Hwang, B.J.; Tsai, Y.W.; Carlier, D.; Ceder, G. A Combined Computational/Experimental Study on LiNi1/3Co1/3Mn1/3O2. Chem. Mater. 2003, 15, 3676–3682. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, D.; Tang, Y.; Huang, Y.; Pang, W.; Guo, Z.; Zhou, Z. In Situ Chelating Synthesis of Hierarchical LiNi1/3Co1/3Mn1/3O2 Polyhedron Assemblies with Ultralong Cycle Life for Li-Ion Batteries. Small 2018, 14, 1704354. [Google Scholar] [CrossRef]
- Li, Z.; Du, F.; Bie, X.; Zhang, D.; Cai, Y.; Cui, X.; Wang, C.; Chen, G.; Wei, Y. Electrochemical Kinetics of the Li[Li0.23Co0.3Mn0.47]O2 Cathode Material Studied by GITT and EIS. J. Phys. Chem. C 2010, 114, 22751–22757. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. Uniform LiNi1/3Co1/3Mn1/3O2 hollow microspheres: Designed synthesis, topotactical structural transformation and their enhanced electrochemical performance. Nano Energy 2013, 2, 1249–1260. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chung, H.-T. Role of transition metals in layered Li[Ni,Co,Mn]O2 under electrochemical operation. Electrochim. Acta 2004, 49, 3573–3580. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Chao, D.; Baikie, T.; Bai, L.; Chen, S.; Zhao, Y.; Sum, T.C.; Lin, J.; Shen, Z. Hierarchical Porous LiNi1/3Co1/3Mn1/3O2 Nano-/Micro Spherical Cathode Material: Minimized Cation Mixing and Improved Li+ Mobility for Enhanced Electrochemical Performance. Sci. Rep. 2016, 6, 25771. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-P.; Sheng, H.; Deng, Q.; Ma, Q.; Liu, J.; Wu, X.-W.; Liu, J.-J.; Wu, Y.-P. High-Rate Layered Cathode of Lithium-Ion Batteries through Regulating Three-Dimensional Agglomerated Structure. Energies 2020, 13, 1602. https://doi.org/10.3390/en13071602
Hu J-P, Sheng H, Deng Q, Ma Q, Liu J, Wu X-W, Liu J-J, Wu Y-P. High-Rate Layered Cathode of Lithium-Ion Batteries through Regulating Three-Dimensional Agglomerated Structure. Energies. 2020; 13(7):1602. https://doi.org/10.3390/en13071602
Chicago/Turabian StyleHu, Jun-Ping, Hang Sheng, Qi Deng, Qiang Ma, Jun Liu, Xiong-Wei Wu, Jun-Jie Liu, and Yu-Ping Wu. 2020. "High-Rate Layered Cathode of Lithium-Ion Batteries through Regulating Three-Dimensional Agglomerated Structure" Energies 13, no. 7: 1602. https://doi.org/10.3390/en13071602
APA StyleHu, J.-P., Sheng, H., Deng, Q., Ma, Q., Liu, J., Wu, X.-W., Liu, J.-J., & Wu, Y.-P. (2020). High-Rate Layered Cathode of Lithium-Ion Batteries through Regulating Three-Dimensional Agglomerated Structure. Energies, 13(7), 1602. https://doi.org/10.3390/en13071602





