Aldehydes-Aided Lignin-First Deconstruction Strategy for Facilitating Lignin Monomers and Fermentable Glucose Production from Poplar Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin-first Deconstruction of Poplar Wood
2.3. The production of Lignin Monomers via Hydrogenolysis
2.4. Enzymatic Hydrolysis of the Cellulose-rich Residues
2.5. Characterization of the Lignin Samples
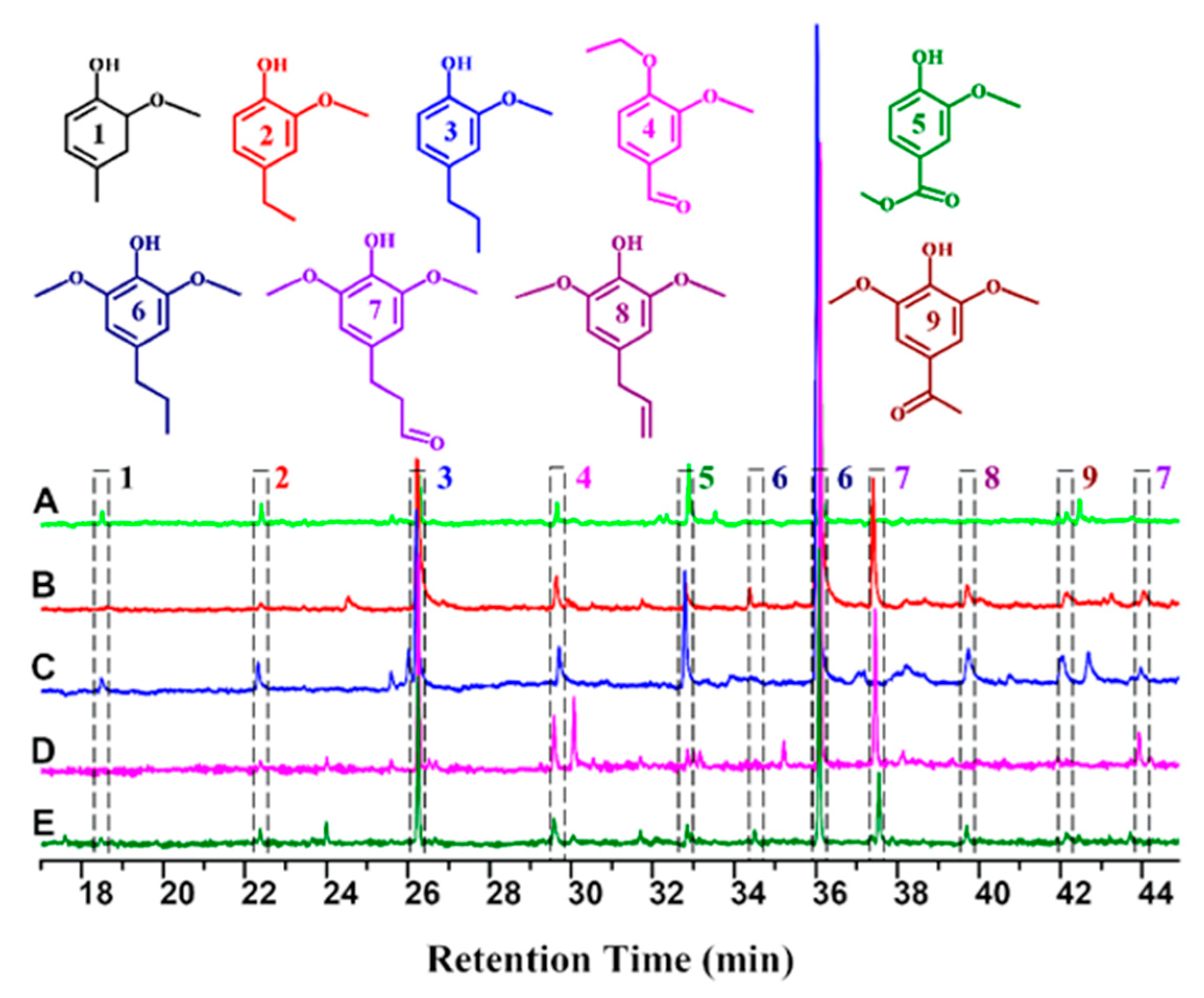
2.6. Analysis of Lignin Monomers
3. Results and Discussion
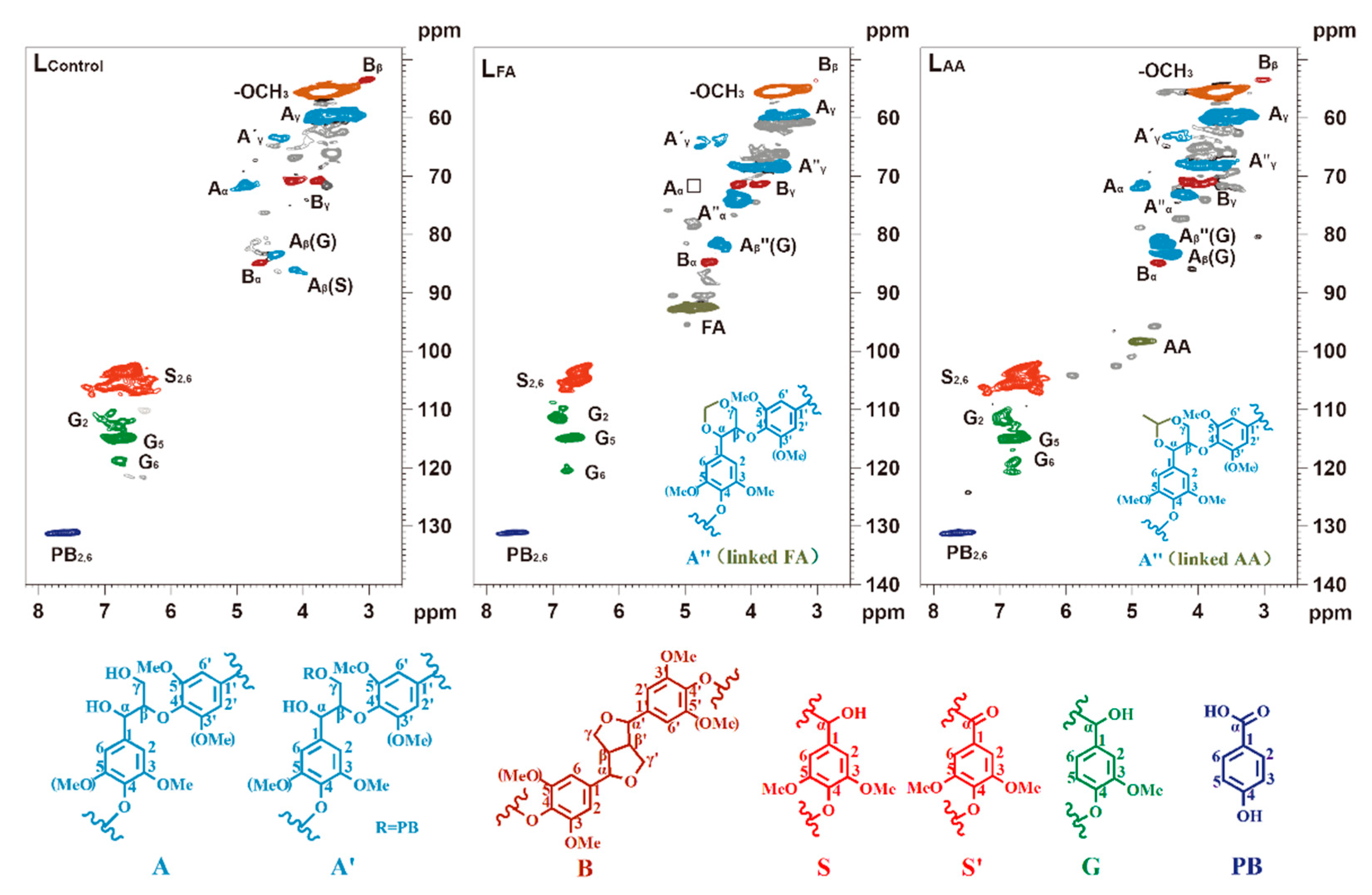
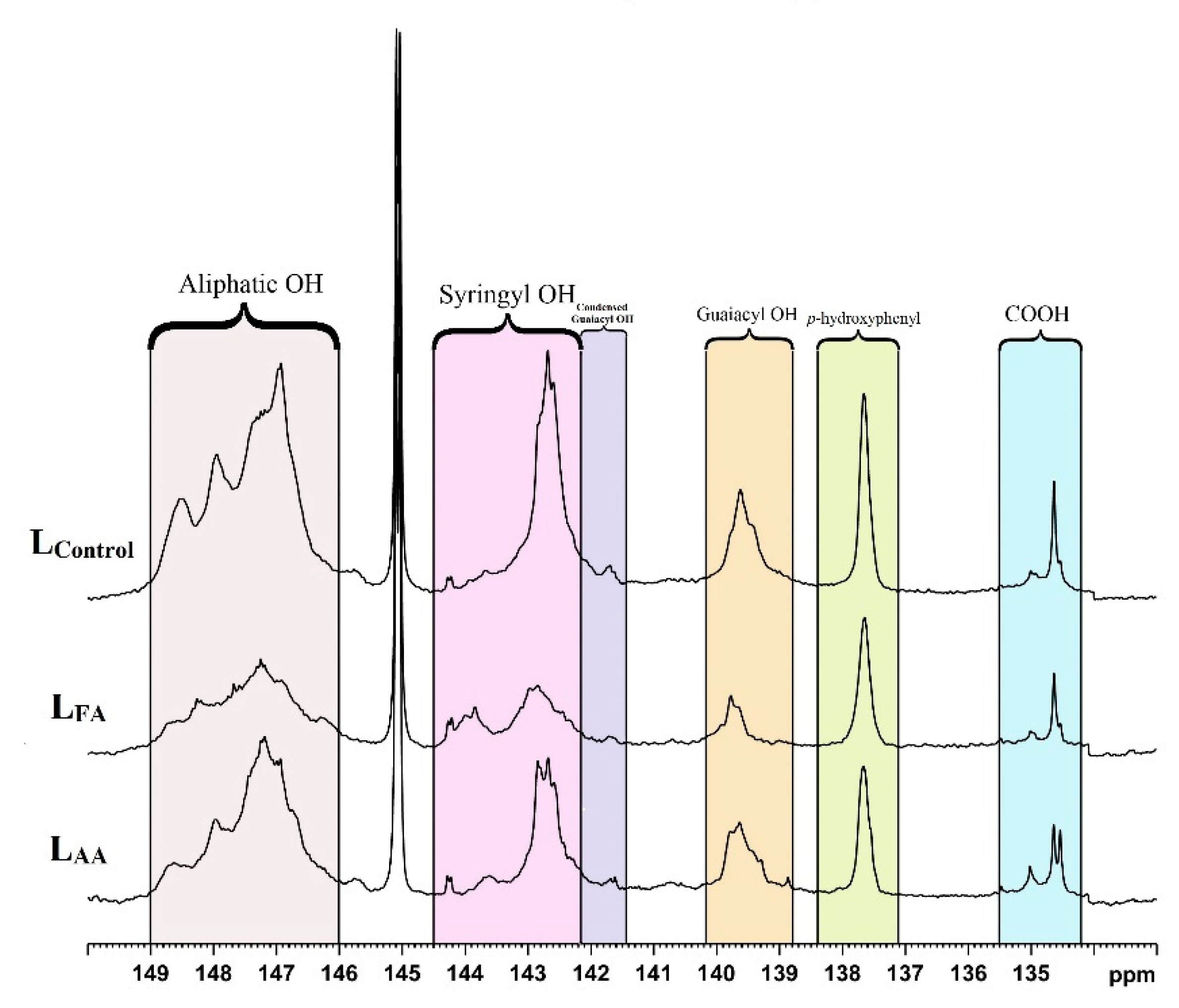
3.1. Structural Characteristics of the Control and Stabilized Lignin
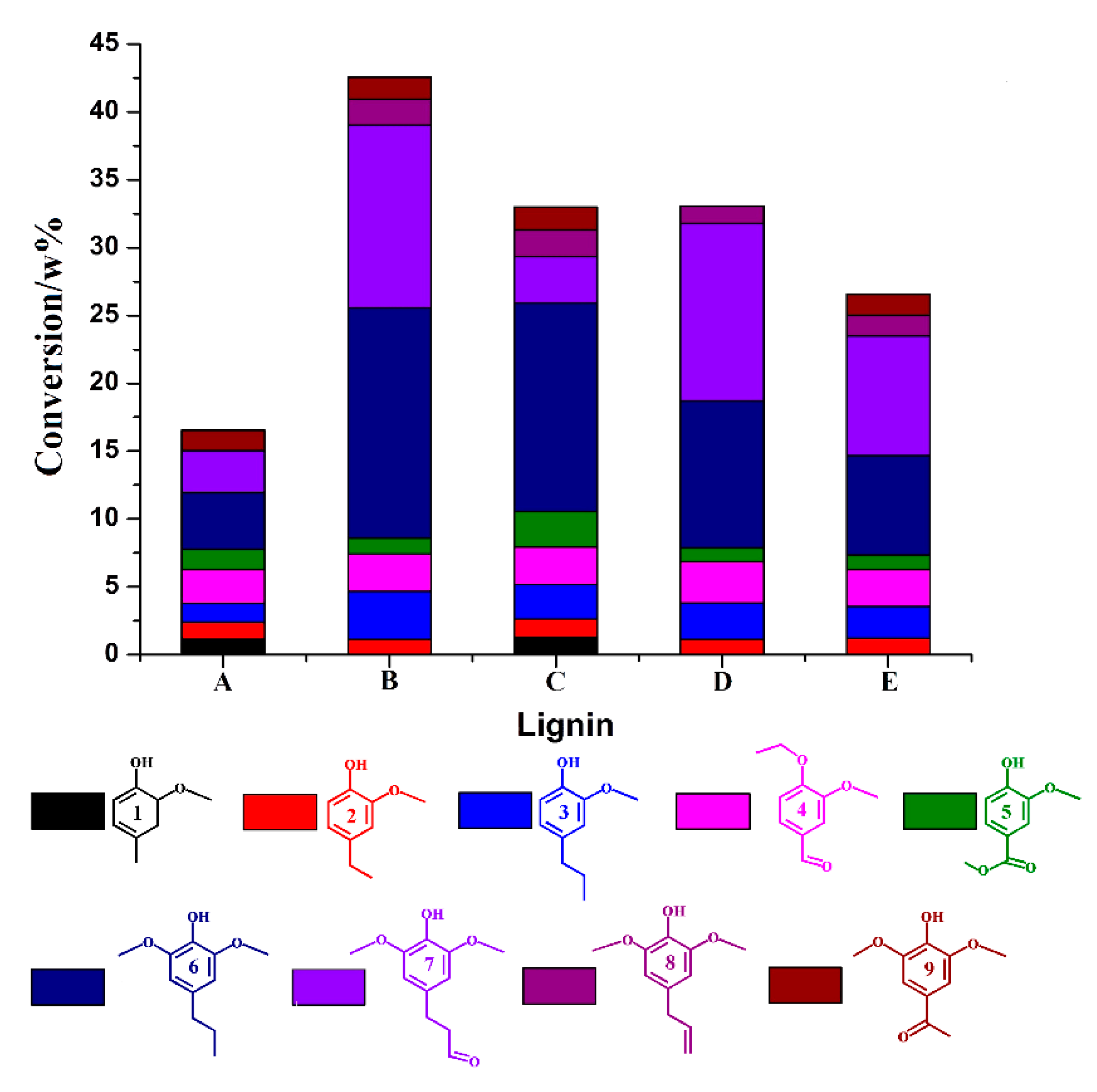
3.2. Hydrogenolysis of the Extracted Lignin Feedstock
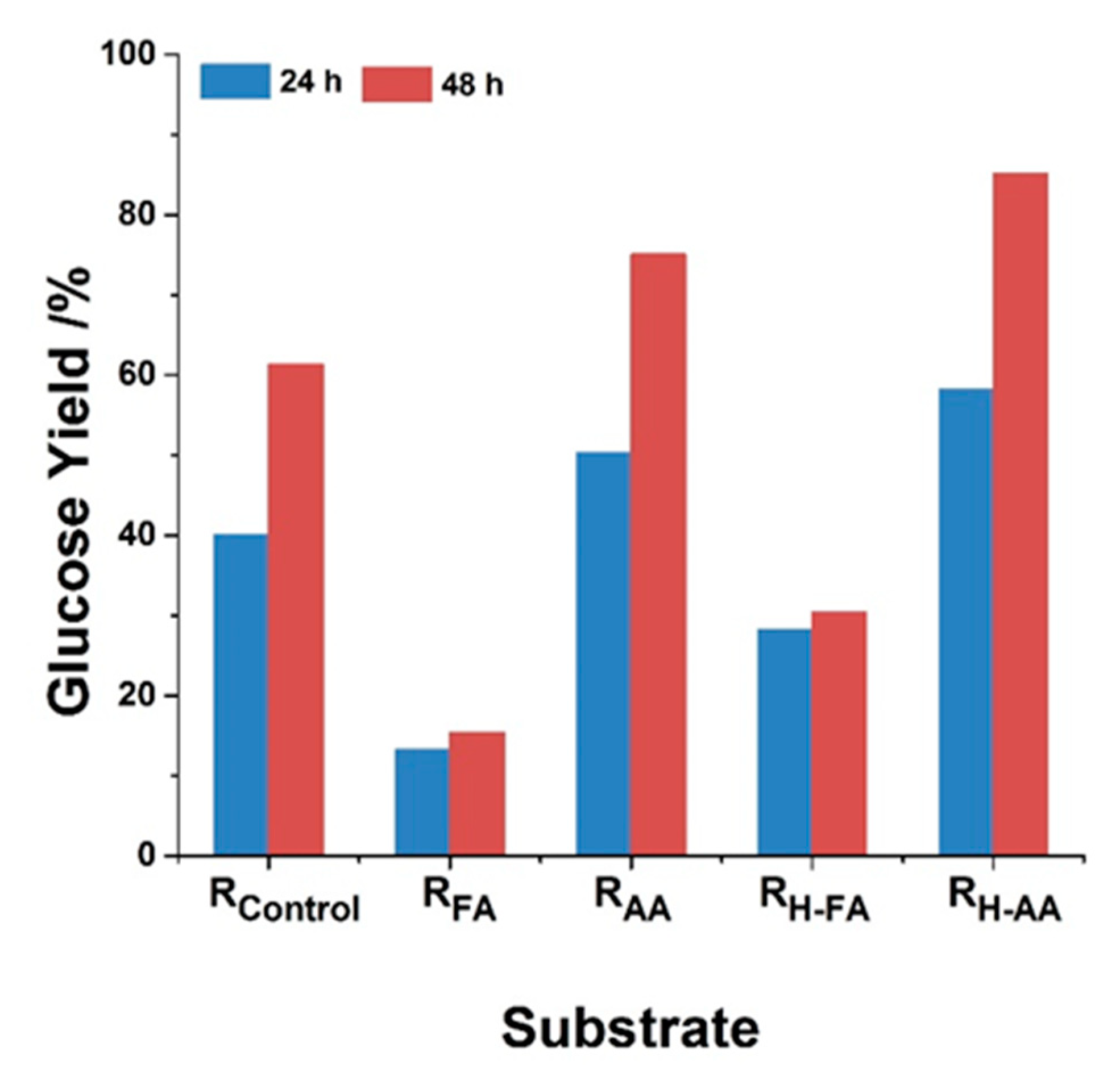
3.3. Enzymatic Hydrolysis of the Substrates after Lignin-first Deconstruction Strategy
3.4. A Proposed Biorefinery Scheme Focused on the Production of Lignin Monomer and Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sagues, W.J.; Bao, H.X.; Nemenyi, J.L.; Tong, Z. Lignin-First Approach to Biorefining: Utilizing Fenton’s Reagent and Supercritical Ethanol for the Production of Phenolics and Sugars. ACS Sustain. Chem. Eng. 2018, 6, 4958–4965. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Park, J.H. Advancement in technologies for the depolymerization of lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Bosch, S.V.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Renders, T.; Bosch, S.V.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: the advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Jeuck, B.; Du, J.; Yong, Q.; Chang, H.M.; Jameel, H.; Phillips, R. Novel process for the coproduction of xylo-oligosaccharides, fermentable sugars, and lignosulfonates from hardwood. Bioresour. Technol. 2016, 219, 600–607. [Google Scholar] [CrossRef]
- Jin, Y.C.; Jameel, H.; Chang, H.M.; Phillips, R. Green liquor pretreatment of mixed hardwood for ethanol production in a repurposed kraft pulp mill. J. Wood Chem. Technol. 2010, 30, 86–104. [Google Scholar] [CrossRef]
- Li, Y.D.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.X.; Luterbacher, J.S. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Wang, J.H.; Zhou, H.Z.; Zhang, Y.; Yang, Y.Y.; Liu, X.H.; Ye, J.W.; Chen, D.Y.; Wang, S.R. Relationship of thermal degradation behavior and chemical structure of lignin isolated from palm kernel shell under different process severities. Fuel Process. Technol. 2018, 181, 142–156. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Lin, F.; Zhang, H.; Xiao, R. Structural elucidation of industrial bioethanol residual lignin from corn stalk: A potential source of vinyl phenolics. Fuel Process. Technol. 2018, 169, 50–57. [Google Scholar] [CrossRef]
- Cordella, M.; Torri, C.; Adamiano, A.; Fabbri, D.; Barontini, F.; Cozzani, V. Bio-oils from biomass slow pyrolysis: a chemical and toxicological screening. J. Hazard. Mater. 2012, 231, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, X.; Yin, J.; Jin, Z.; Lu, Q.; Zheng, Z.; Dong, C. Fast pyrolysis product distribution of biopretreated corn stalk by methanogen. Bioresour. Technol. 2014, 169, 812–815. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Z.; Lu, Q.; Zhang, Y. Selective production of 4-vinylphenol by fast pyrolysis of herbaceous biomass. Ind. Eng. Chem. Res. 2013, 52, 12771–12776. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Boerjan, W. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.N.; Yuan, T.Q.; Xu, F.; Sun, R.C. Fractionation of bamboo culms by autohydrolysis, organosolv delign.ification and extended delignification: understanding the fundamental chemistry of the lignin during the integrated process. Bioresour. Technol. 2013, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: a review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Engl. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Lan, W.; Luterbacher, J.S. Preventing Lignin Condensation to Facilitate Aromatic Monomer Production. CHIMIA Int. J. Chem. 2019, 73, 591–598. [Google Scholar] [CrossRef]
- Shuai, L.; Saha, B. Towards high-yield lignin monomer production. Green Chem. 2017, 19, 3752–3758. [Google Scholar] [CrossRef]
- Bosch, S.V.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; Meester, B.D.; Huijgen, W.; Dehaen, W.; Courtin, C. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Bian, H.; Yelle, D.J.; Vuorinen, T.; Fu, S.; Pan, X.J.; et al. Rapid and near-complete dissolution of wood lignin at ≤80 degrees C by a recyclable acid hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef]
- Shuai, L.; Sitison, J.; Sadula, S.; Ding, J.; Thies, M.C.; Saha, B. Selective C–C bond cleavage of methylene-linked lignin models and kraft lignin. ACS Catal. 2018, 8, 6507–6512. [Google Scholar] [CrossRef]
- Avelino, F.; Silva, K.T.; Filho, M.S.M.; Mazzetto, S.E.; Lomonaco, D. Microwave-assisted organosolv extraction of coconut shell lignin by Brønsted and Lewis acids catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Lou, R.; Ma, R.; Lin, K.T.; Ahamed, A.; Zhang, X. Facile extraction of wheat straw by deep eutectic solvent (des) to produce lignin nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Lan, W.; Amiri, M.T.; Hunston, C.M.; Luterbacher, J. Protection Group Effects during α, γ-Diol Lignin Stabilization Promote High-Selectivity Monomer Production. Angew. Chem. 2018, 57, 1356–1360. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the g-valerolactone sugar platform. Energy Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, B.; Shen, X.J.; Li, H.Y.; Wu, Y.Y.; Wen, J.L.; Liu, Q.Y.; Sun, R.C. Assessment of structural characteristics of regenerated cellulolytic enzyme lignin based on a mild DMSO/[Emim]OAc dissolution system from triploid of Populus tomentosa Carr. RSC Adv. 2017, 7, 3376–3387. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Hashmi, S.F.; Meriö-Talvio, H.; Hakonen, K.J.; Ruuttunen, K.; Sixta, H. Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process. Technol. 2017, 168, 74–83. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Jääskeläinen, A.; Sun, Y.; Argyropoulos, D.; Tamminen, T.; Hortling, B. The effect of isolation method on the chemical structure of residual lignin. Wood Sci. Technol. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Xiao, L.P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.J.; Xiao, L.; Sun, R.C.; Fang, Y.; Song, G. Catalytic hydrogenolysis of lignins into phenolic compounds over carbon nanotube supported molybdenum oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Koelewijn, S.F.; Renders, T.; Van den Bossche, G.; Vangeel, T.; Schutyser, W.; Sels, B.F. Catalytic Strategies Towards Lignin-Derived Chemicals. Top. Curr. Chem. 2018, 376, 36. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic depolymerisation of isolated lignins to fine chemicals using a Pt/alumina catalyst: part 1—impact of the lignin structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- Zijlstra, D.S.; Analbers, C.A.; Korte, J.D.; Wilbers, E.; Deuss, P.J. Efficient mild organosolv lignin extraction in a flow-through setup yielding lignin with high β-O-4 content. Polymers 2019, 11, 1913. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (nmr) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Structural characterization of lignin from triploid of Populus tomentosa Carr. J. Agric. Food Chem. 2011, 59, 6605–6615. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, B.; Wu, Y.Y.; Wen, J.L.; Liu, C.F.; Yuan, T.Q.; Sun, R.C. Structural variations of lignin macromolecule from different growth years of Triploid of Populus tomentosa Carr. Int. J. Biol. Macromol. 2017, 101, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Van den Bosch, S.; Renders, T.; De Boe, T.; Koelewijn, S.F.; Dewaele, A.; Sels, B.F. Influence of bio-based solvents on the catalytic reductive fractionation of birch wood. Green Chem. 2015, 17, 5035–5045. [Google Scholar] [CrossRef]
- Sun, Z.H.; Barta, K. Cleave and couple: toward fully sustainable catalytic conversion of lignocellulose to value added building blocks and fuels. Chem. Commun. 2018, 54, 7725–7745. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Enhanced enzymatic digestibility of bamboo by a combined system of multiple steam explosion and alkaline treatments. Appl. Energy 2014, 136, 519–526. [Google Scholar] [CrossRef]

| Entry | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Lignin (L40–60, LBM) | L40–60 a | LBM a | |||||
| LControlb | LFAb | LAAb | LBM-Controlb | LBM-FAb | LBM-AAb | ||
| Yieldc/% | 65.7 | 68.0 | 85.1 | 78.0 | 82.5 | 86.5 | |
| Characteristicsd | Mw (g/mol) | 1540 | 6580 | 4490 | 3160 | 6440 | 4840 |
| Mn (g/mol) | 750 | 2590 | 2100 | 1740 | 2540 | 2160 | |
| Mw/Mn | 2.05 | 2.54 | 2.14 | 1.82 | 2.54 | 2.24 | |
| Samples | PB2,6 | S/G | β-β | β-O-4 | |
|---|---|---|---|---|---|
| β-O-4′b | β-O-4”c | ||||
| LControl | 14.51 | 11.55 | 4.50 | 11.18 | Tr |
| LFA | 13.99 | 2.49 | 16.46 | Tra | 89.73 |
| LAA | 11.52 | 5.62 | 5.46 | 9.98 | 30.26 |
| LBM-Control | 13.19 | 11.11 | 3.96 | 10.88 | Tr |
| LBM-FA | 10.53 | 2.79 | 0.96 | 4.52 | 76.49 |
| LBM-AA | 9.36 | 4.81 | 3.34 | 1.66 | 58.82 |
| Samples | Aliphatic OH | Syringyl OH | Guaiacyl OH | H-OH (PB and/or H) | Carboxylic group | |
|---|---|---|---|---|---|---|
| Ca | NCb | |||||
| LControl | 2.60 | 1.15 | 0.14 | 0.49 | 0.40 | 0.19 |
| LFA | 1.14 | 0.61 | 0.05 | 0.20 | 0.30 | 0.14 |
| LAA | 1.75 | 0.78 | 0.10 | 0.41 | 0.30 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Ma, C.-Y.; Min, D.-Y.; Liu, C.-F.; Sun, S.-N.; Cao, X.-F.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Aldehydes-Aided Lignin-First Deconstruction Strategy for Facilitating Lignin Monomers and Fermentable Glucose Production from Poplar Wood. Energies 2020, 13, 1113. https://doi.org/10.3390/en13051113
Chen T-Y, Ma C-Y, Min D-Y, Liu C-F, Sun S-N, Cao X-F, Wen J-L, Yuan T-Q, Sun R-C. Aldehydes-Aided Lignin-First Deconstruction Strategy for Facilitating Lignin Monomers and Fermentable Glucose Production from Poplar Wood. Energies. 2020; 13(5):1113. https://doi.org/10.3390/en13051113
Chicago/Turabian StyleChen, Tian-Ying, Cheng-Ye Ma, Dou-Yong Min, Chuan-Fu Liu, Shao-Ni Sun, Xue-Fei Cao, Jia-Long Wen, Tong-Qi Yuan, and Run-Cang Sun. 2020. "Aldehydes-Aided Lignin-First Deconstruction Strategy for Facilitating Lignin Monomers and Fermentable Glucose Production from Poplar Wood" Energies 13, no. 5: 1113. https://doi.org/10.3390/en13051113
APA StyleChen, T.-Y., Ma, C.-Y., Min, D.-Y., Liu, C.-F., Sun, S.-N., Cao, X.-F., Wen, J.-L., Yuan, T.-Q., & Sun, R.-C. (2020). Aldehydes-Aided Lignin-First Deconstruction Strategy for Facilitating Lignin Monomers and Fermentable Glucose Production from Poplar Wood. Energies, 13(5), 1113. https://doi.org/10.3390/en13051113









