Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells
Abstract
1. Introduction
2. Experiment Details
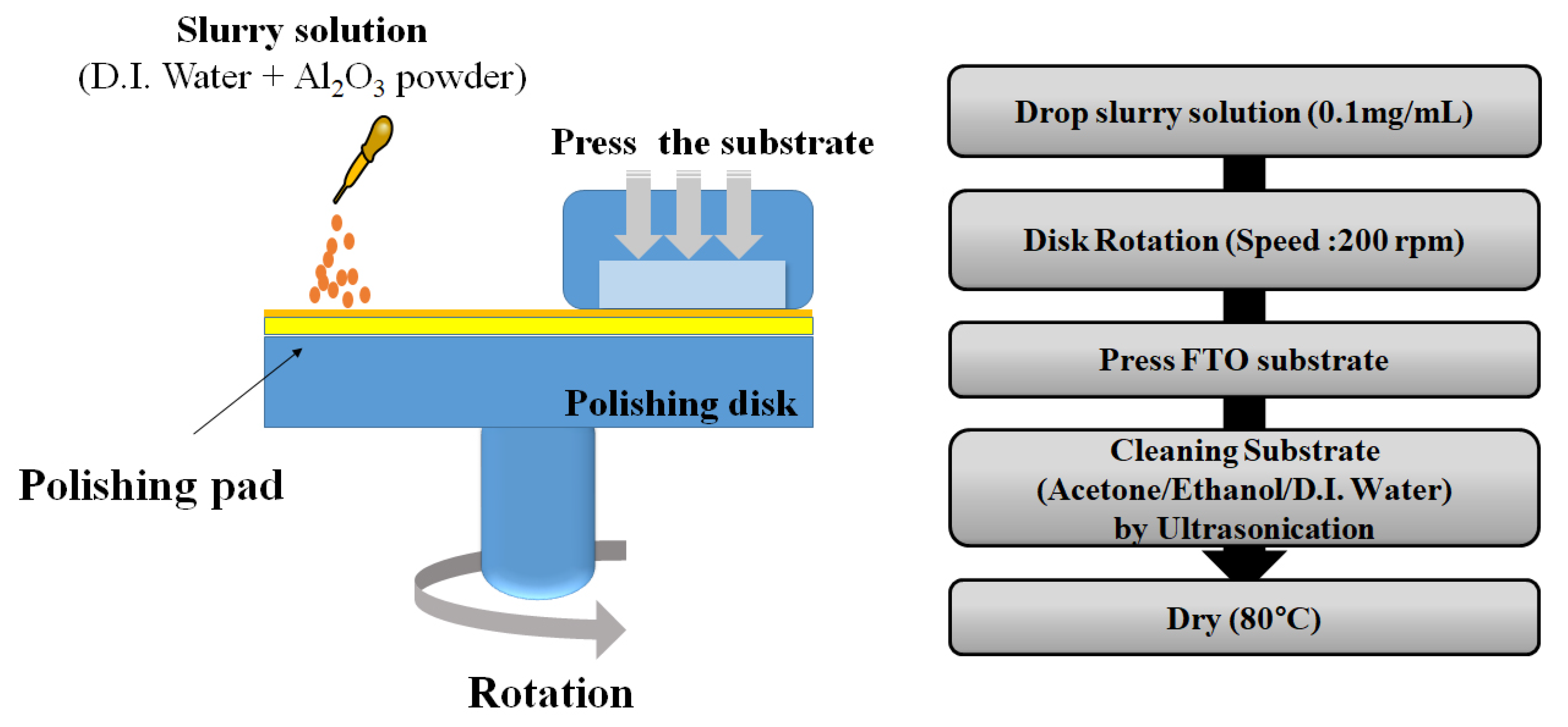
2.1. Surface Treatment
2.2. Fabrication of Perovskite Solar Cells
2.3. Measurements
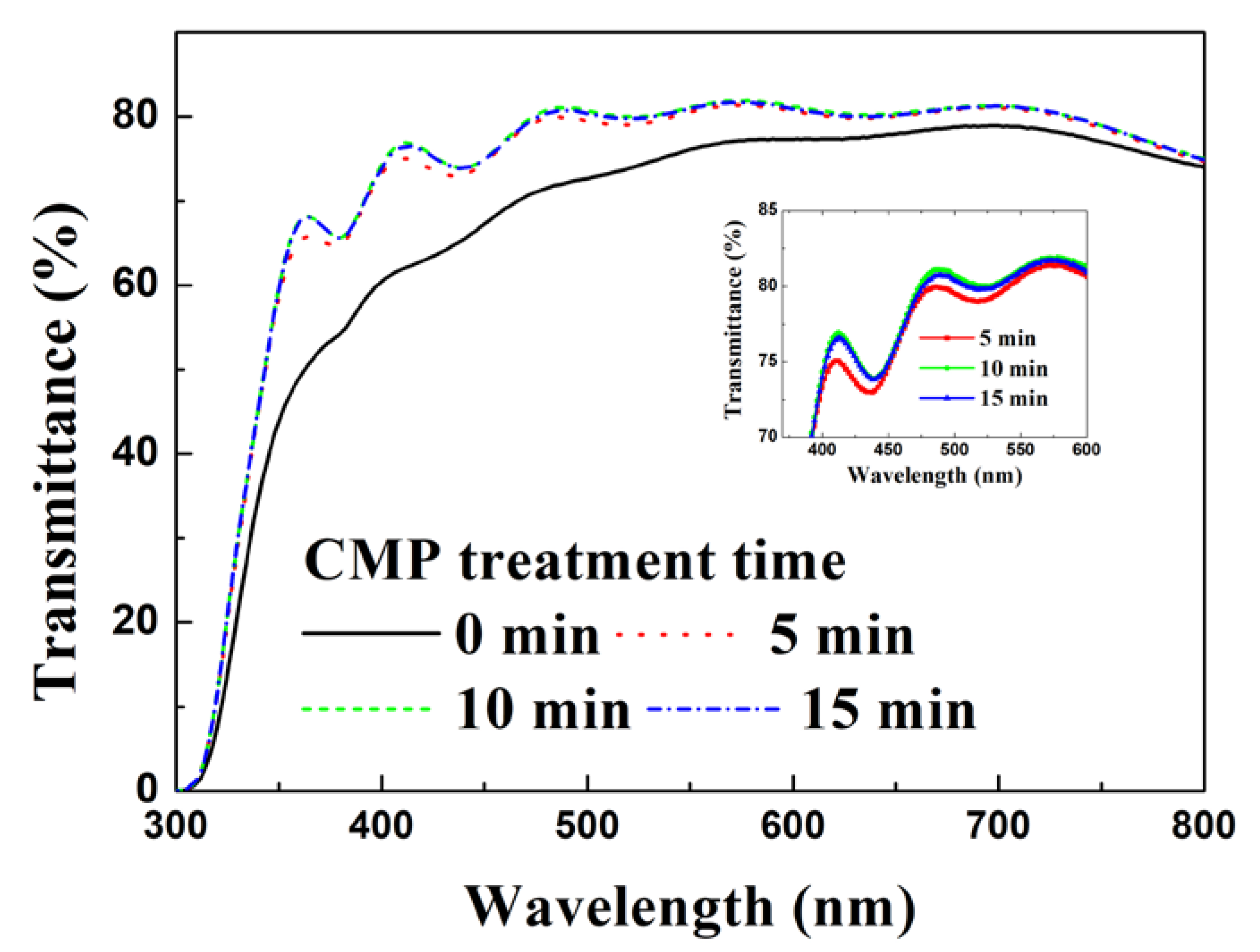
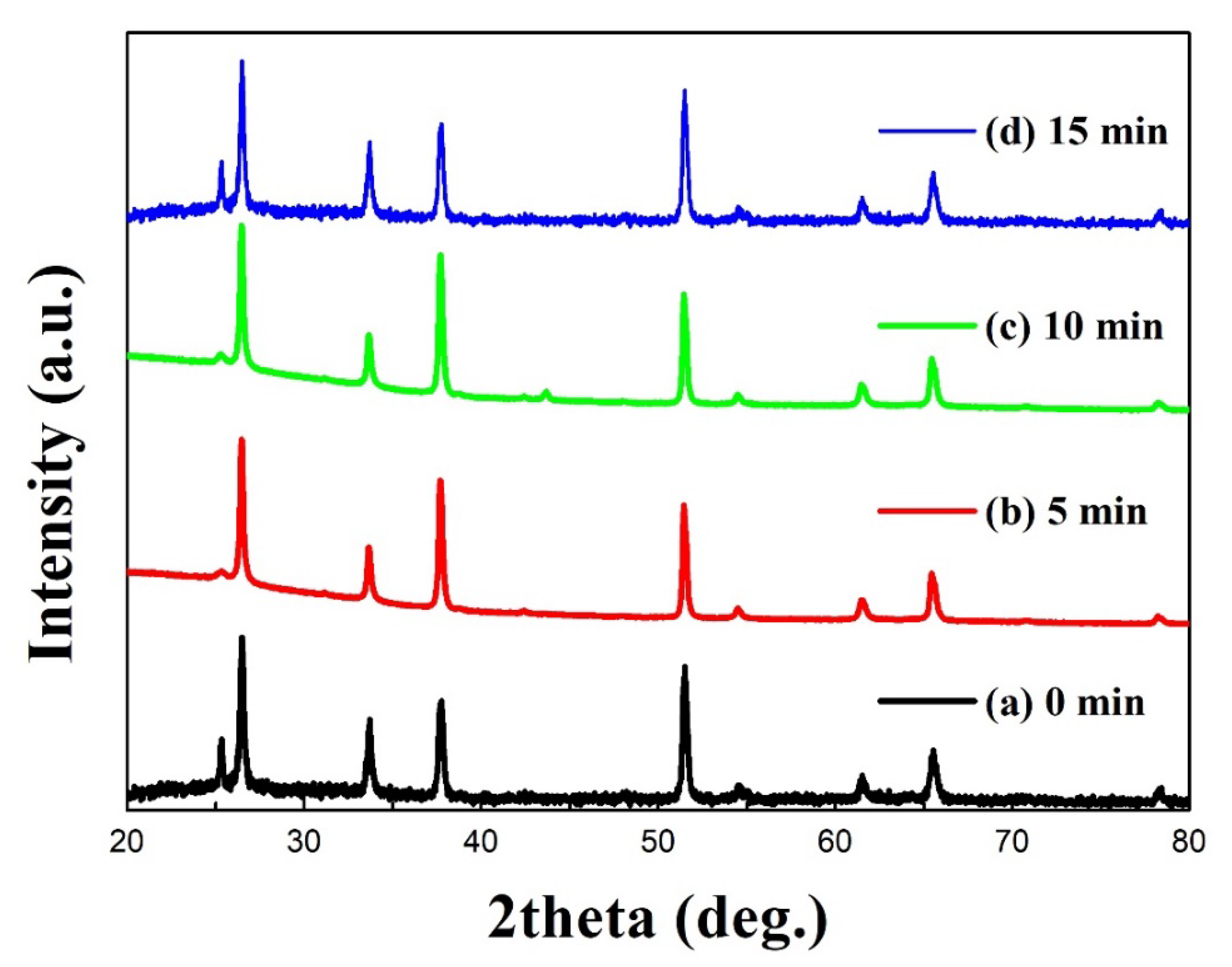
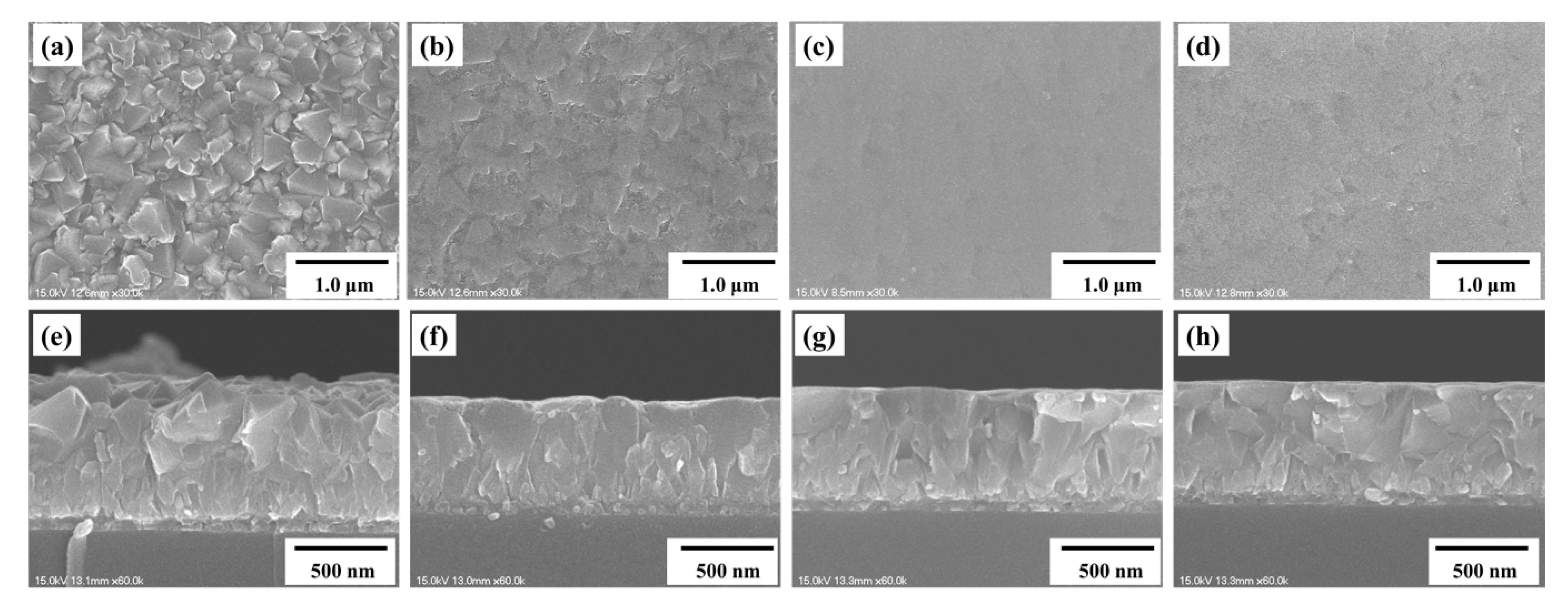
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Assadi, M.K.; Bakhodaa, S.; Saidur, R.; Hanaei, H. Recent progress in perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Bhattacharya, S.; John, S. Beyond 30% Conversion Efficiency in Silicon Solar Cells: A Numerical Demonstration. Sci. Rep. 2019, 9, 12482. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Balaji, S.; Panella, R.A.; Ydstie, B.E. Silicon solar cell production. Comput. Chem. Eng. 2011, 35, 1439–1453. [Google Scholar] [CrossRef]
- Noh, J.H.; Jeon, N.J.; Choi, Y.C.; Nazeeruddin, K.; Grätzel, M.; Seok, S.I. Nanostructured TiO2/CH3NH3PbI3 heterojunction solar cells employing spiro-OMeTAD/Co-complex as hole-transporting material. J. Mater. Chem. A 2013, 1, 11842–11847. [Google Scholar] [CrossRef]
- Wali, Q.; Elumalai, N.K.; Iqbal, Y.; Uddin, A.; Jose, R. Tandem perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 84, 89–110. [Google Scholar] [CrossRef]
- Yu, X.; Yu, X.; Zhang, J.; Zhang, D.; Ni, J.; Cai, H.; Zhang, D.; Zhao, Y. Efficient inverted polymer solar cells based on surface modified FTO transparent electrodes. Sol. Energy Mater. Sol. Cells 2015, 136, 142–147. [Google Scholar] [CrossRef]
- Murakami, T.N.; Miyadera, T.; Funaki, T.; Cojocaru, L.; Kazaoui, S.; Chikamatsu, M.; Segawa, H. Adjustment of Conduction Band Edge of Compact TiO2 Layer in Perovskite Solar Cells Through TiCl4 Treatment. ACS Appl. Mater. Interfaces 2017, 9, 36708–36714. [Google Scholar] [CrossRef]
- Kim, B.; So, C.I.; Ko, S.G.; Ri, J.H.; Ryu, G.I.; Sonu, G.S. Effects of TiCl4 post-treatment on the performance of hole transport material-free, screen printable mesoscopic perovskite solar cells with carbon electrode. Thin Solid Film. 2019, 692, 137627. [Google Scholar] [CrossRef]
- Adli, H.K.; Harada, T.; Nakanishi, S.; Ikeda, S. Effects of TiCl4 treatment on the structural and electrochemical properties of a porous TiO2 layer in CH3NH3PbI3perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 26898–26905. [Google Scholar] [CrossRef]
- Cojocaru, L.; Uchida, S.; Sanehira, Y.; Nakazaki, J.; Kubo, T.; Segawa, H. Surface Treatment of the Compact TiO2 Layer for Efficient Planar Heterojunction Perovskite Solar Cells. Chem. Lett. 2015, 44, 674–676. [Google Scholar] [CrossRef]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef]
- Yang, J.H.; Bark, C.H.; Kim, K.H.; Choi, H.W. Characteristics of the Dye-Sensitized Solar Cells Using TiO2 Nanotubes Treated with TiCl4 . Materials 2014, 7, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lu, X. Chemical mechanical polishing: Theory and experiment. Friction 2013, 1, 306–326. [Google Scholar] [CrossRef]
- Nagaoka, S.; Ryu, N.; Yamanouchi, A.; Shirosaki, T.; Horikawa, M.; Sakurai, H.; Takafuji, M.; Ihara, H. Chemical mechanical polishing of transparent conductive layers using spherical cationic polymer microbeads. Thin Solid Film. 2015, 576, 31–37. [Google Scholar] [CrossRef]
- Zhu, H.; Tessaroto, L.A.; Sabia, R.; Greenhut, V.A.; Smith, M.; Niesz, D.E. Chemical mechanical polishing (CMP) anisotropy in sapphire. Appl. Surf. Sci. 2004, 236, 120–130. [Google Scholar] [CrossRef]
- Zantye, P.B.; Kumar, A.; Sikder, A. Chemical mechanical planarization for microelectronics applications. Mater. Sci. Eng. R Rep. 2004, 45, 89–220. [Google Scholar] [CrossRef]
- Li, X.; Dar, M.I.; Yi, C.; Luo, J.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.; Grätzel, M. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef]
- Tathavadekar, M.C.; Agarkar, S.A.; Game, O.S.; Bansode, U.P.; Kulkarni, S.A.; Mhaisalkar, S.G.; Ogale, S.B. Enhancing efficiency of perovskite solar cell via surface microstructuring: Superior grain growth and light harvesting effect. Sol. Energy 2015, 112, 12–19. [Google Scholar] [CrossRef]
- Choi, H.; Nahm, C.; Kim, J.; Moon, J.; Nam, S.; Jung, D.-R.; Park, B. The effect of TiCl4 -treated TiO2 compact layer on the performance of dye-sensitized solar cell. Curr. Appl. Phys. 2012, 12, 737–741. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Choi, G.-W.; Lee, W.-S. Evaluation of electrical and optical properties of indium tin oxide thin film using chemical mechanical polishing technique. Microelectron. Eng. 2007, 84, 2896–2900. [Google Scholar] [CrossRef]
- Hu, X.; Song, Z.; Liu, W.; Qin, F.; Zhang, Z.; Wang, H. Chemical mechanical polishing of stainless steel foil as flexible substrate. Appl. Surf. Sci. 2012, 258, 5798–5802. [Google Scholar] [CrossRef]
- Choi, G.-W.; Lee, K.-Y.; Kim, N.-H.; Park, J.-S.; Seo, Y.-J.; Lee, W.-S. CMP characteristics and optical property of ITO thin film by using silica slurry with a variety of process parameters. Microelectron. Eng. 2007, 83, 2213–2217. [Google Scholar] [CrossRef]
- Choi, G.-W.; Kim, N.-H.; Seo, Y.-J.; Lee, W.-S. Behaviour of electrical and optical properties of indium tin oxide transparent electrode after CMP process. Electron. Lett. 2006, 42, 487. [Google Scholar] [CrossRef]
- Dai, X.; Shi, C.; Zhang, Y.; Wu, N. Hydrolysis preparation of the compact TiO2 layer using metastable TiCl 4 isopropanol/water solution for inorganic–organic hybrid heterojunction perovskite solar cells. J. Semicond. 2015, 36, 74003. [Google Scholar] [CrossRef]
- Lee, H.; Bark, C.W.; Choi, H.W. Fabrication and characterization of perovskite solar cells with ZnGa2O4 mixed TiO2 photoelectrode. Jpn. J. Appl. Phys. 2019, 58, SDDE15. [Google Scholar] [CrossRef]


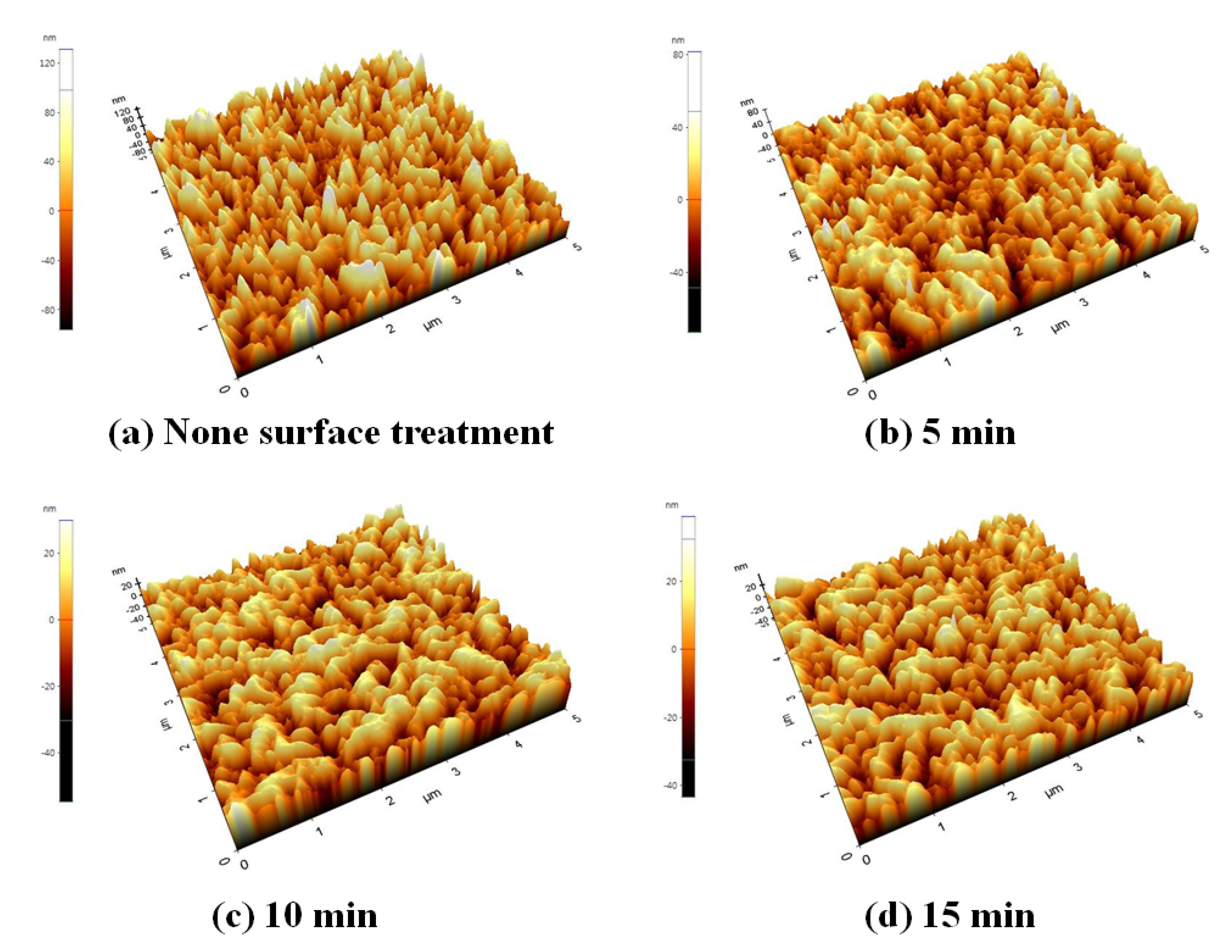

| Treatment Time | Ra (nm) | RMS (nm) | |
|---|---|---|---|
| None surface treatment | - | 26.620 | 32.996 |
| After CMP treatment | 5 min | 12.917 | 16.043 |
| 10 min | 9.320 | 11.379 | |
| 15 min | 9.132 | 11.177 |
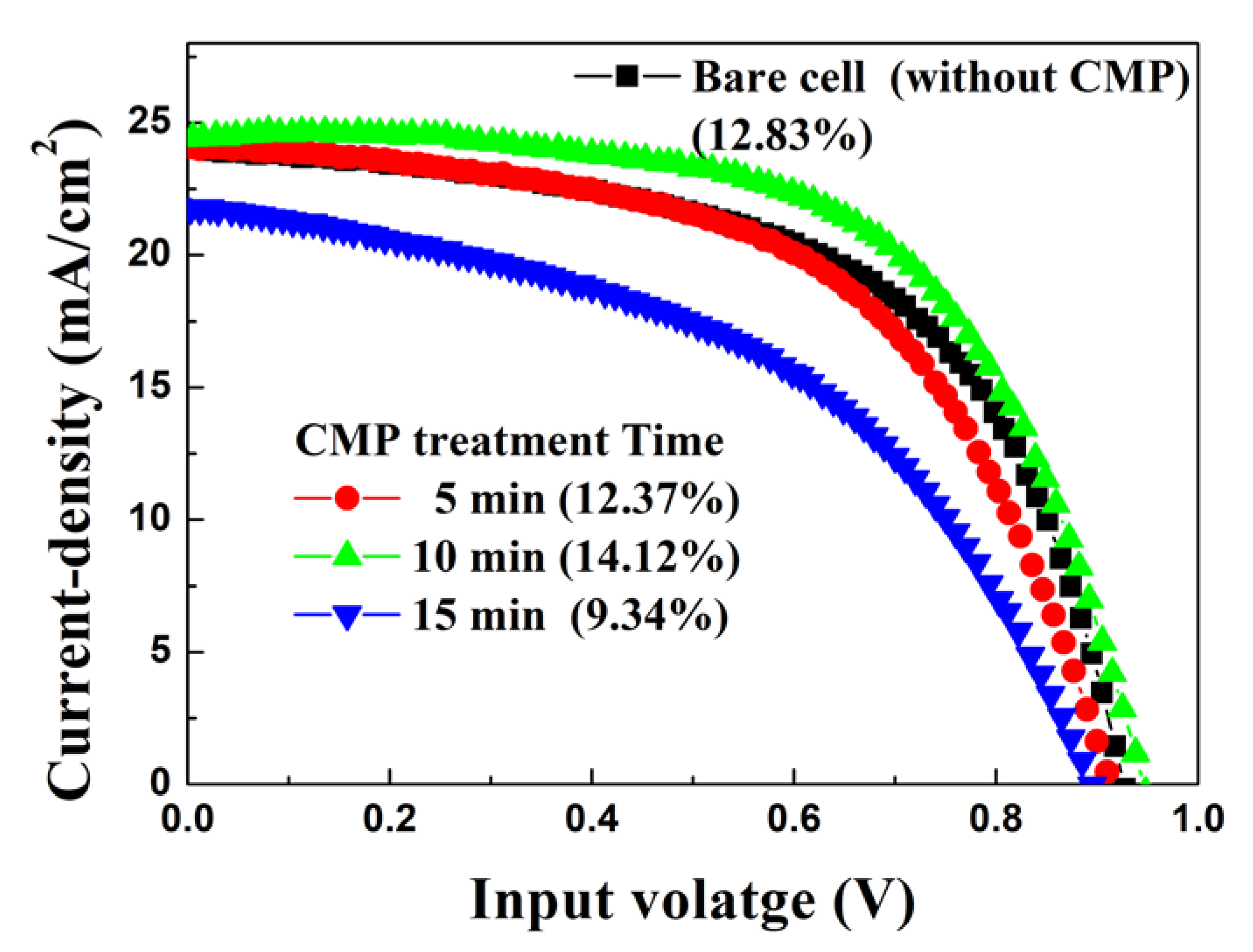
| VOC a (V) | ISC b (mA) | JSC c (mA/cm2) | F. F. d (%) | PCE e (%) | ||
|---|---|---|---|---|---|---|
| Bare cell (Before CMP) | 0.899 (0.024) | 1.291 (0.050) | 24.365 (0.947) | 52.582 (3.879) | 11.551 (1.351) | |
| After CMP treatment | 5 min | 0.947 (0.020) | 1.264 (0.065) | 23.833 (1.256) | 56.005 (2.476) | 12.648 (0.999) |
| 10 min | 0.931 (0.015) | 1.306 (0.033) | 24.621 (0.657) | 58.270 (3.081) | 13.387 (1.173) | |
| 15 min | 0.901 (0.015) | 1.206 (0.082) | 22.742 (6.269) | 47.751 (6.269) | 9.840 (1.939) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Bark, C.W. Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells. Energies 2020, 13, 585. https://doi.org/10.3390/en13030585
Kim S, Bark CW. Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells. Energies. 2020; 13(3):585. https://doi.org/10.3390/en13030585
Chicago/Turabian StyleKim, Sangmo, and Chung Wung Bark. 2020. "Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells" Energies 13, no. 3: 585. https://doi.org/10.3390/en13030585
APA StyleKim, S., & Bark, C. W. (2020). Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells. Energies, 13(3), 585. https://doi.org/10.3390/en13030585






