Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides
Abstract
1. Introduction
2. Experimental Part
3. Results
3.1. Structural and Elemental Characterization of the Pristine Compounds
3.2. H2 Sorption Properties
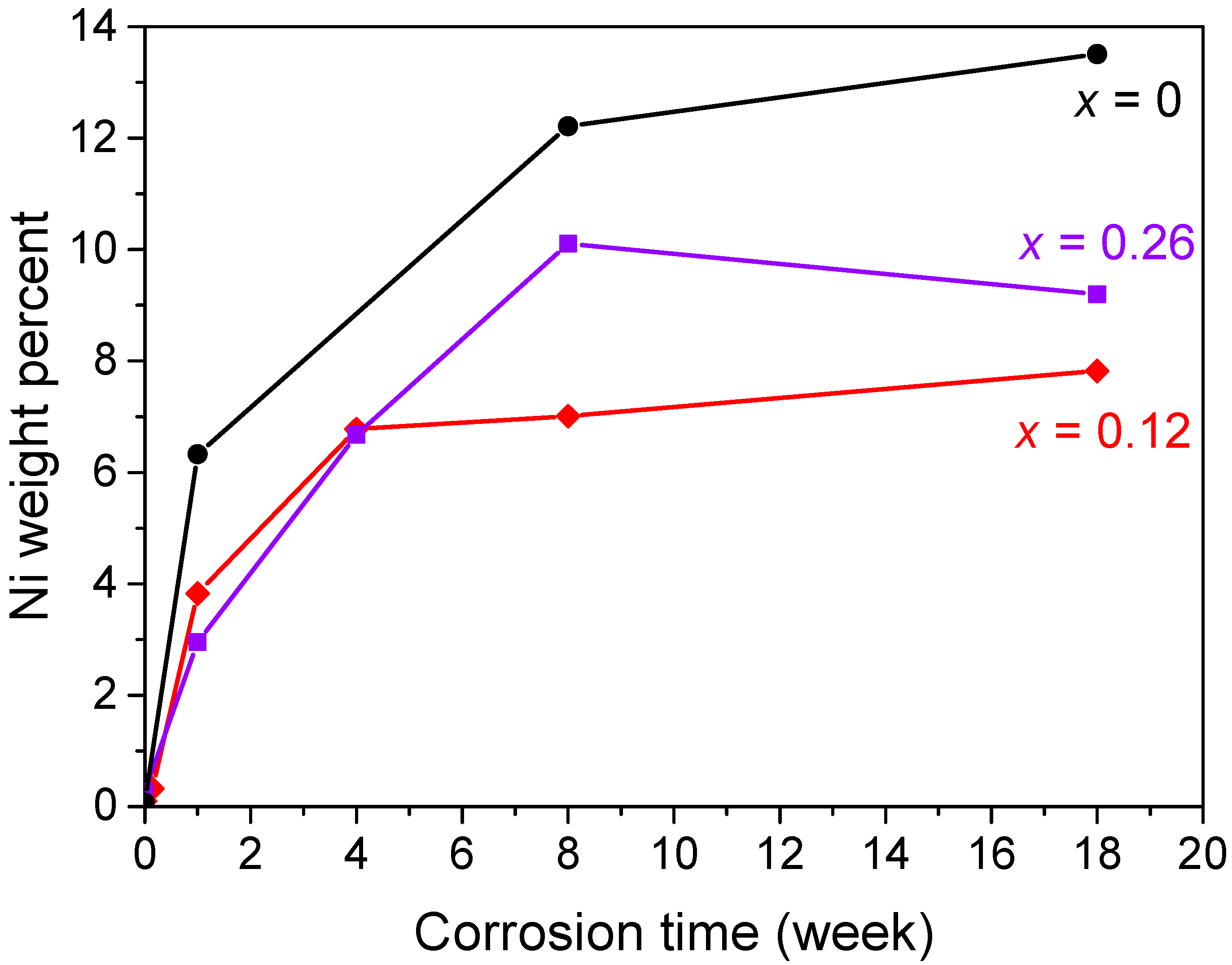
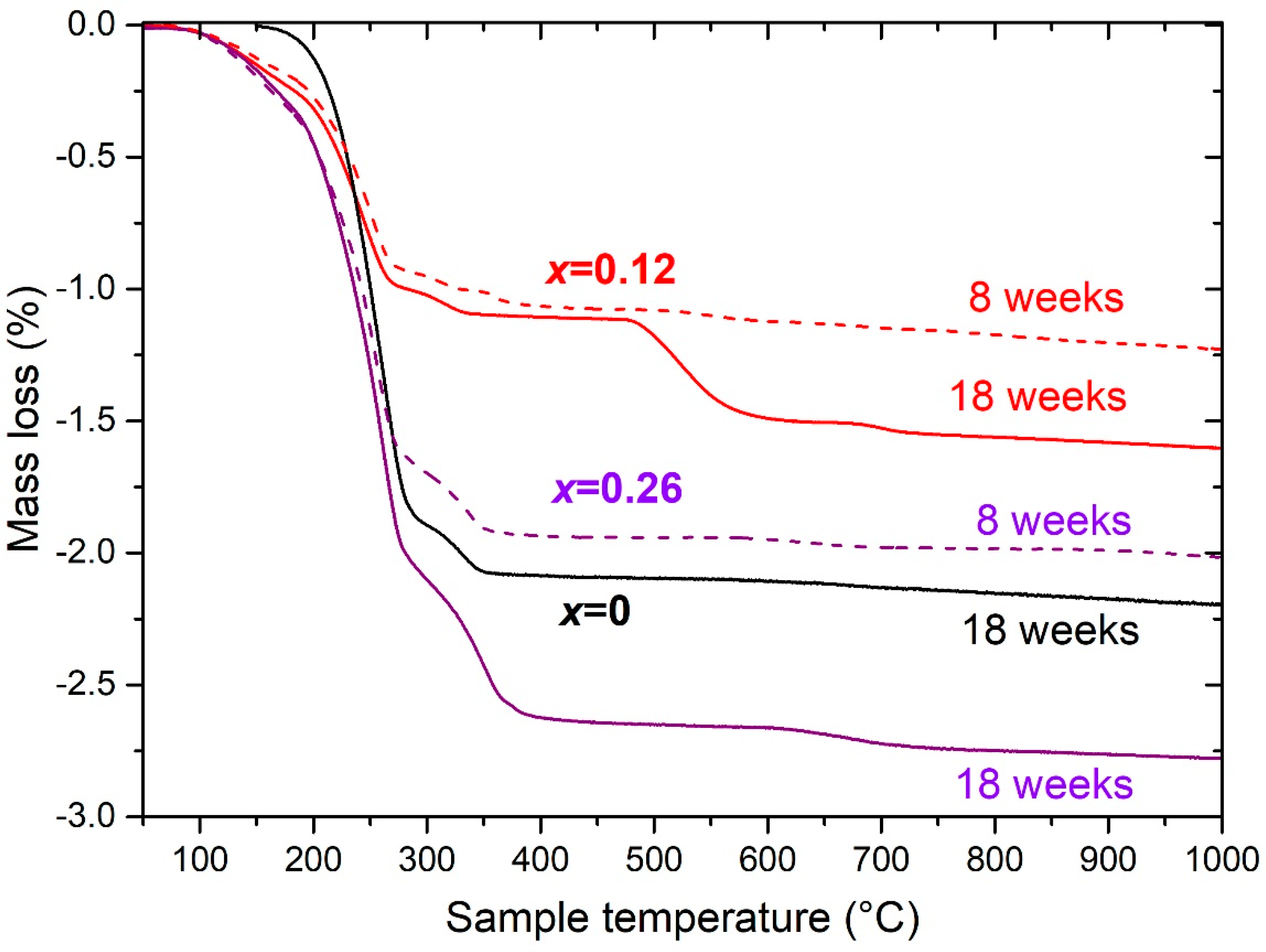
3.3. Calendar Corrosion
4. Discussion
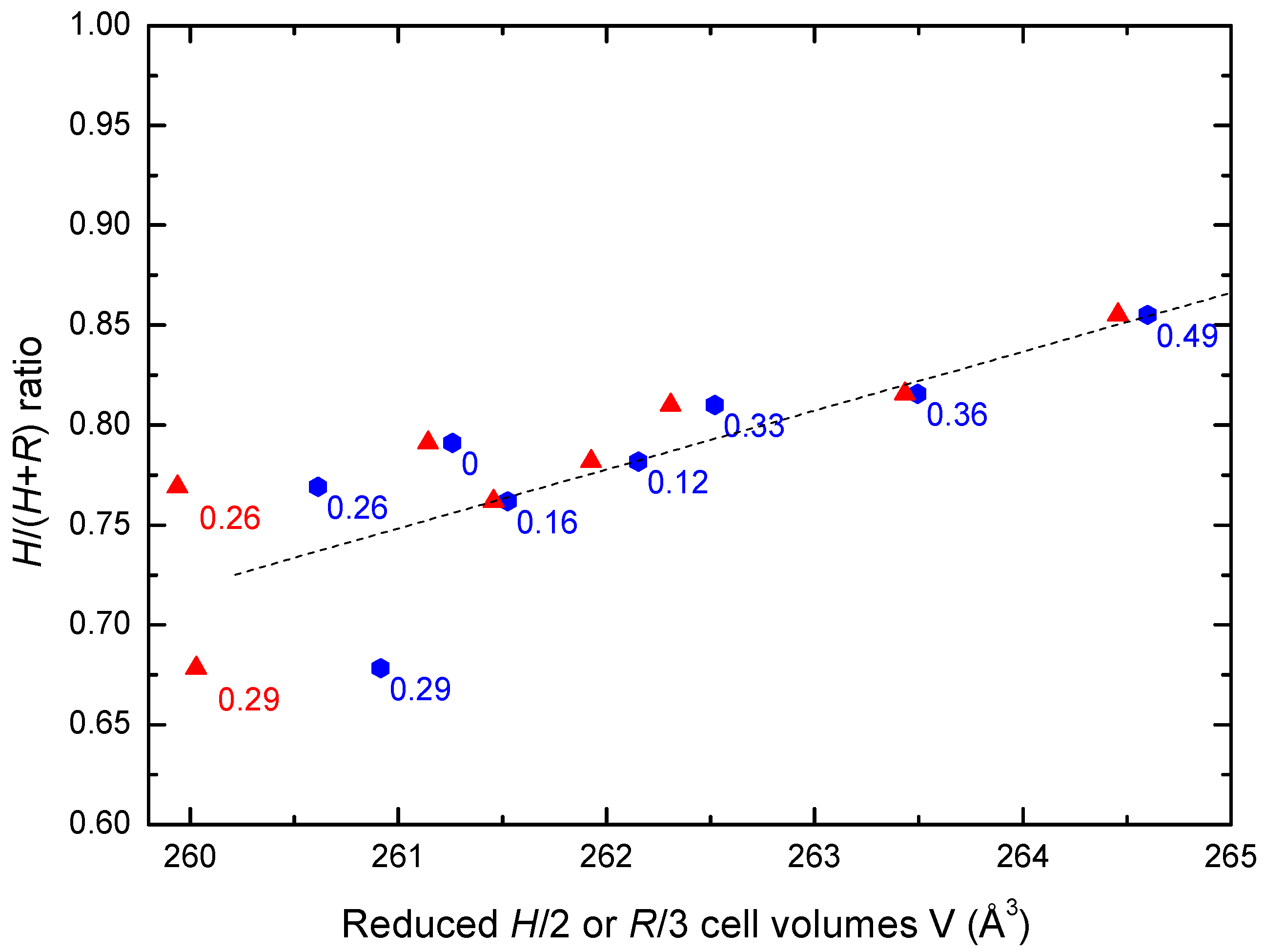
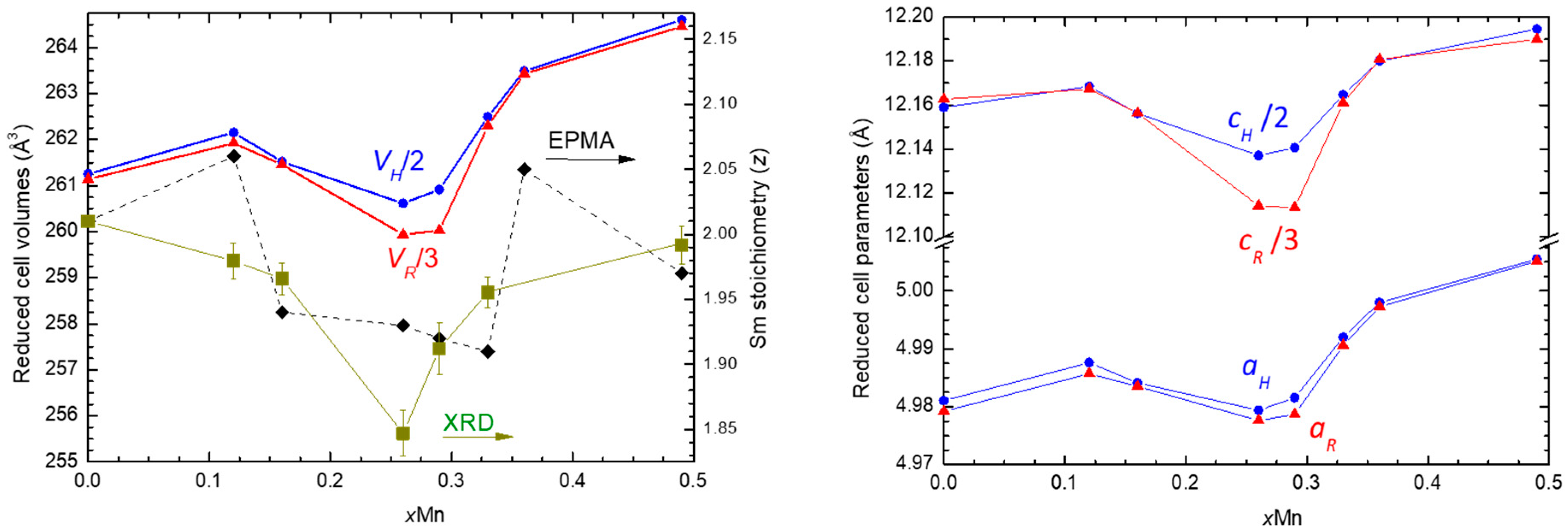
4.1. Evolution of the Structural Properties
4.2. Hydrogen Sorption Properties
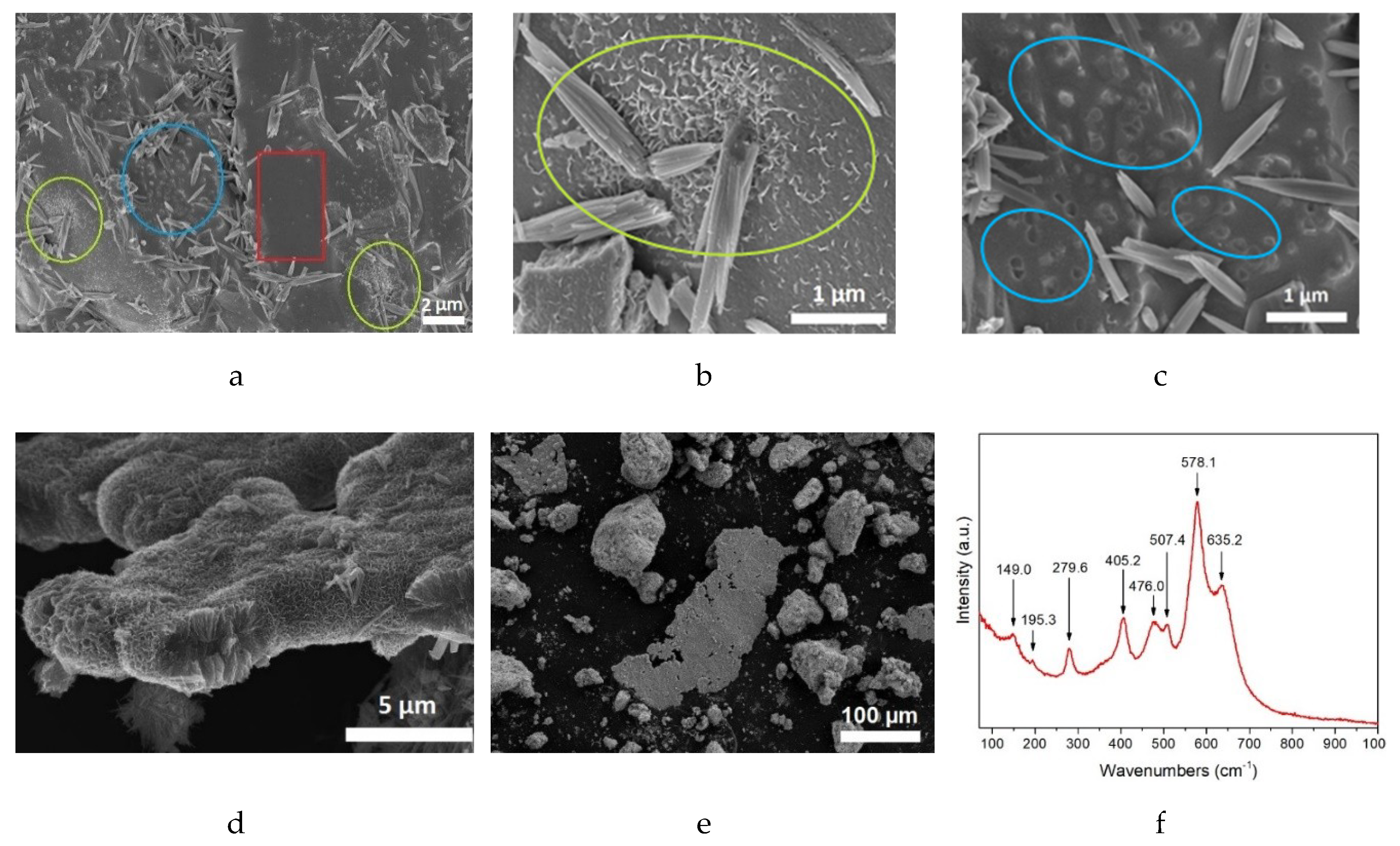
4.3. Calendar Corrosion Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. J. Alloys Compd. 1999, 293–295, 877–888. [Google Scholar] [CrossRef]
- Sandrock, G. Hydrides for Energy Storage; Pergamon: Oxford, UK, 1978. [Google Scholar]
- Cuevas, F.; Joubert, J.-M.; Latroche, M.; Percheron-Guégan, A. Intermetallic compounds as negative electrodes of Ni/MH batteries. Appl. Phys. A 2001, 72, 225–238. [Google Scholar] [CrossRef]
- Sartori, S.; Cuevas, F.; Latroche, M. Metal hydrides used as negative electrode materials for Li-ion batteries. Appl. Phys. A 2016, 122, 135. [Google Scholar] [CrossRef]
- Latroche, M.; Blanchard, D.; Cuevas, F.; El Kharbachi, A.; Hauback, B.C.; Jensen, T.R.; de Jongh, P.E.; Kim, S.; Nazer, N.S.; Ngene, P.; et al. Full-cell hydride-based solid-state Li batteries for energy storage. Spec. Issue Hydrog.-Based Energy Storage 2019, 44, 7875–7887. [Google Scholar] [CrossRef]
- Graetz, J. New approaches to hydrogen storage. Chem. Soc. Rev. 2009, 38, 73–82. [Google Scholar] [CrossRef]
- Zhou, L. Progress and problems in hydrogen storage methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Notten, P.H.L. Rechargeable nickel-metal hydride batteries: A successfull new concept. In Interstitial Intermetallic Alloys; Grandjean, F., Long, G.J., Buschow, K.H.J., Eds.; NATO ASI Series; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1995; Volume 281, pp. 150–194. [Google Scholar]
- Notten, P.H.L.; Latroche, M. Secondary Batteries: Nickel Batteries Metal Hydride Alloys. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 4, pp. 502–521. [Google Scholar]
- Møller, K.; Jensen, T.; Akiba, E.; Hai-Wen, L. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Yartys, V.; Noreus, D.; Latroche, M. Metal hydrides as negative electrode materials for Ni–MH batteries. Appl. Phys. A 2016, 122, 43. [Google Scholar] [CrossRef]
- Toshiba autonomous hydrogen energy for Kyushu hotel, JR East. Fuel Cells Bull. 2016, 2016, 6. [CrossRef]
- Kohno, T.; Yoshida, H.; Kanda, M. Hydrogen storage properties of La(Ni0.9M0.1)3 alloys. J. Alloys Compd. 2004, 363, 249–252. [Google Scholar] [CrossRef]
- Tliha, M.; Khaldi, C.; Boussami, S.; Fenineche, N.; El-Kedim, O.; Mathlouthi, H.; Lamloumi, J. Kinetic and thermodynamic studies of hydrogen storage alloys as negative electrode materials for Ni/MH batteries: A review. J. Solid State Electrochem. 2014, 18, 577–593. [Google Scholar] [CrossRef]
- Khan, Y. The crystal structure of R5Co19. Acta Crystallogr. B 1974, 30, 1533–1537. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Latroche, M.; Percheron-Guégan, A. Metallic hydrides II: Materials for electrochemical storage. MRS Bull. 2002, 27, 694–698. [Google Scholar] [CrossRef]
- Aoki, K.; Masumoto, T. Hydrogen-induced amorphisation of intermetallics. J. Alloys Compd. 1995, 231, 20–28. [Google Scholar] [CrossRef]
- Koch, J.; Young, K.; Nei, J.; Hu, C.; Reichman, B. Performance Comparison between AB5 and Superlattice Metal Hydride Alloys in Sealed Cells. Batteries 2017, 3, 35. [Google Scholar] [CrossRef]
- Kohno, T.; Yoshida, H.; Kawashima, F.; Inaba, T.; Sakai, I.; Yamamoto, M.; Kanda, M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J. Alloys Compd. 2000, 311, L5–L7. [Google Scholar] [CrossRef]
- Gal, L.; Charbonnier, V.; Zhang, J.; Goubault, L.; Bernard, P.; Latroche, M. Optimization of the La substitution by Mg in the La2Ni7 hydride-forming system for use as negative electrode in Ni-MH battery. Int. J. Hydrog. Energy 2015, 40, 17017–17020. [Google Scholar] [CrossRef]
- Lemort, L.; Latroche, M.; Knosp, B.; Bernard, P. Elaboration and Characterization of New Pseudo-Binary Hydride-Forming Phases Pr(1.5)Mg(0.5)Ni(7) and Pr(3.75)Mg(1.25)Ni(19): A Comparison to the Binary Pr(2)Ni(7) and Pr(5)Ni(19) Ones. J. Phys. Chem. C 2011, 115, 19437–19444. [Google Scholar] [CrossRef]
- Ferey, A.; Cuevas, F.; Latroche, M.; Knosp, B.; Bernard, P. Elaboration and characterization of magnesium-substituted La5Ni19 hydride forming alloys as active materials for negative electrode in Ni-MH battery. Electrochim. Acta 2009, 54, 1710–1714. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Fullprof: A program for Rietveld refinement and pattern matching analysis. Phys. B 1993, 192, 55–69. [Google Scholar]
- Bruker. Bruker AXS (2008): TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Buschow, K.H.J.; Van der Goot, A.S. Crystal structure or rare earth nickel compounds of the type R2Ni7. J. -Common Met. 1970, 22, 419–428. [Google Scholar] [CrossRef]
- Serin, V.; Zhang, J.; Magén, C.; Serra, R.; Hÿtch, M.; Lemort, L.; Latroche, M.; Ibarra, M.R.; Knosp, B.; Bernard, P. Identification of the atomic scale structure of the La0.65Nd0.15Mg0.20Ni3.5 alloy synthesized by spark plasma sintering. Intermetallics 2013, 32, 103–108. [Google Scholar] [CrossRef]
- Madern, N.; Monnier, J.; Cachet-Vivier, C.; Zhang, J.; Bastide, S.; Paul-Boncour, V.; Latroche, M. Anisotropic Nanoporous Nickel Obtained through the Chemical Dealloying of Y2Ni7 for the Comprehension of Anode Surface Chemistry of Ni-MH Batteries. ChemElectroChem 2019, 6, 5022–5031. [Google Scholar] [CrossRef]
- Fueglein, E.; Walter, D. Thermal analysis of lanthanum hydroxide. J. Therm. Anal. Calorim. 2012, 110, 199–202. [Google Scholar] [CrossRef]
- Charbonnier, V.; Madern, N.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Latroche, M. Thermodynamic and corrosion study of Sm1−xMgxNiy (y = 3.5 or 3.8) compounds forming reversible hydrides. Int. J. Hydrog. Energy 2020, 45, 11686–11694. [Google Scholar] [CrossRef]
- Charbonnier, V.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Joiret, S.; Puga, B.; Goubault, L.; Bernard, P.; Latroche, M. Relationship between H2 sorption properties and aqueous corrosion mechanisms in A2Ni7 hydride forming alloys (A = Y, Gd or Sm). J. Power Sources 2016, 326, 146–155. [Google Scholar] [CrossRef]
- Charbonnier, V.; Madern, N.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Latroche, M. Relationship between H2 sorption, electrochemical cycling and aqueous corrosion properties in A5Ni19 hydride-forming alloys (A = Gd, Sm). J. Power Sources 2018, 397, 280–287. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z Phys. Chem. Neue Folge. 1921, 5, 17–26. [Google Scholar]
- Maurel, F.; Knosp, B.; Backhaus-Ricoult, M. Characterization of corrosion products of AB5-type hydrogen storage alloys for nickel-metal hydride batteries. J. Electrochem. Soc. 2000, 147, 78–86. [Google Scholar] [CrossRef]
- Monnier, J.; Chen, H.; Joiret, S.; Bourgon, J.; Latroche, M. Identification of a new pseudo-binary hydroxide during calendar corrosion of (La, Mg)(2)Ni-7-type hydrogen storage alloys for Nickel-Metal Hydride batteries. J. Power Sources 2014, 266, 162–169. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.-P. Raman Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.P.; Becerra, M.E.; Giraldo, O. Structural and Electrical Studies for Birnessite-Type Materials Synthesized by Solid-State Reactions. Nanomaterials 2019, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Cygan, R.T.; Post, J.E.; Heaney, P.J.; Kubicki, J.D. Molecular models of birnessite and related hydrated layered minerals. Am. Mineral. 2012, 97, 1505–1514. [Google Scholar] [CrossRef]
- Bodak, O.I.; Pecharskiy, V.K.; Kalychak, Y.M.; Kharchenko, O.I.; Mokra, I.R.; Muratova, L.A.; Berezyuk, D.A.; Shevchuk, M.M. Some ternary systems containing rare earth metals. Fazovye Ravnovesiya Met. Splavakh 1981, 57–63. [Google Scholar]
- Berezovets’, V.; Denys, R.; Zavaliy, I.Y. Crystal structure and thermodynamic properties of YNi2.67Mn0.33H4 hydride 2008. No 49. Part 1. P. 121–127У. Ser. Khim. 2008, 49, 121–127. [Google Scholar]
- Latroche, M.; Paul-Boncour, V.; Percheron-Guegan, A.; Achard, J.C. Deviations from the C15 type structure in nickel binary intermetallic RNi2 compounds (R = yttrium, cerium). Eur. J. Solid State Inorg. Chem. 1991, 28, 597–600. [Google Scholar]
- Van Essen, R.M.; Buschow, K.H.J. Composition and Hydrogen Absorption of C14 Type Zr-Mn Compounds. Mater. Res. Bull. 1980, 15, 1149–1155. [Google Scholar] [CrossRef]
- Flandorfer, H.; Rogl, P.; Groebner, J.; Stamou, A.; Hassiotis, N.; Saccone, A.; Maccio, D.; Ferro, R.; Wouters, R.; Delaey, L.; et al. Experimental investigation and thermodynamic calculation of the ternary system Mn-Y-Zr. Z. Met. 1997, 88, 529–538. [Google Scholar]
- Latroche, M.; Joubert, J.-M.; Percheron-Guegan, A.; Notten, P.H.L. Crystal Structure of Nonstoichiometric Copper-Substituted La(Ni1-zCuz)x Compounds Studied by Neutron and Synchrotron Anomalous Powder Diffraction. J. Solid State Chem. 1999, 146, 313–321. [Google Scholar] [CrossRef]
- Cuevas, F.; Latroche, M.; Hirscher, M.; Percheron-Guegan, A. Formation and structure of highly over-stoichiometric LaNi5+x, (x>=1) alloys obtained by manifold non-equilibrium methods. J. Alloys Compd. 2001, 323–324, 4–7. [Google Scholar] [CrossRef]
- Berezovets’, V.V.; Denys, R.V.; Ryabov, O.B.; Zavalii, I.Y. Hydrides of substituted derivatives based on the YNi3 compound. Mater. Sci. 2007, 43, 499–507. [Google Scholar] [CrossRef]
- Denys, R.V.; Riabov, B.; Yartys, V.A.; Delaplane, R.G.; Sato, M. Hydrogen storage properties and structure of La1−xMgx(Ni1−yMny)3 intermetallics and their hydrides. J. Alloys Compd. 2007, 446–447, 166–172. [Google Scholar] [CrossRef]
- Denys, R.V.; Riabov, A.B.; Yartys, V.A.; Sato, M.; Delaplane, R.G. Mg substitution effect on the hydrogenation behaviour, thermodynamic and structural properties of the La2Ni7-H(/D)2 system. J. Solid State Chem. 2008, 181, 812–821. [Google Scholar] [CrossRef]
- Im, D.; Manthiram, A.; Coffey, B. Manganese III Chemistry in KOH Solutions in the Presence of Bi- or Ba-Containing Compounds and Its Implications on the Rechargeability of g-MnO2 in Alkaline Cells. J. Electrochem. Soc. 2003, 150, A1651–A1659. [Google Scholar] [CrossRef]
- Boytsova, O.V.; Shekunova, T.O.; Baranchikov, A.E. Nanocrystalline manganese dioxide synthesis by microwave-hydrothermal treatment. Russ. J. Inorg. Chem. 2015, 60, 546–551. [Google Scholar] [CrossRef]
- Le Bihan, S.; Figlarz, M. Évolution thermique d’un hydroxyde de nickel lamellaire mal organisé avec des molécules d’eau en insertion entre les feuillets: Comparaison avec l’hydroxyde de nickel cristallisé Ni(oH)2. Thermochim. Acta 1973, 6, 319–326. [Google Scholar] [CrossRef]
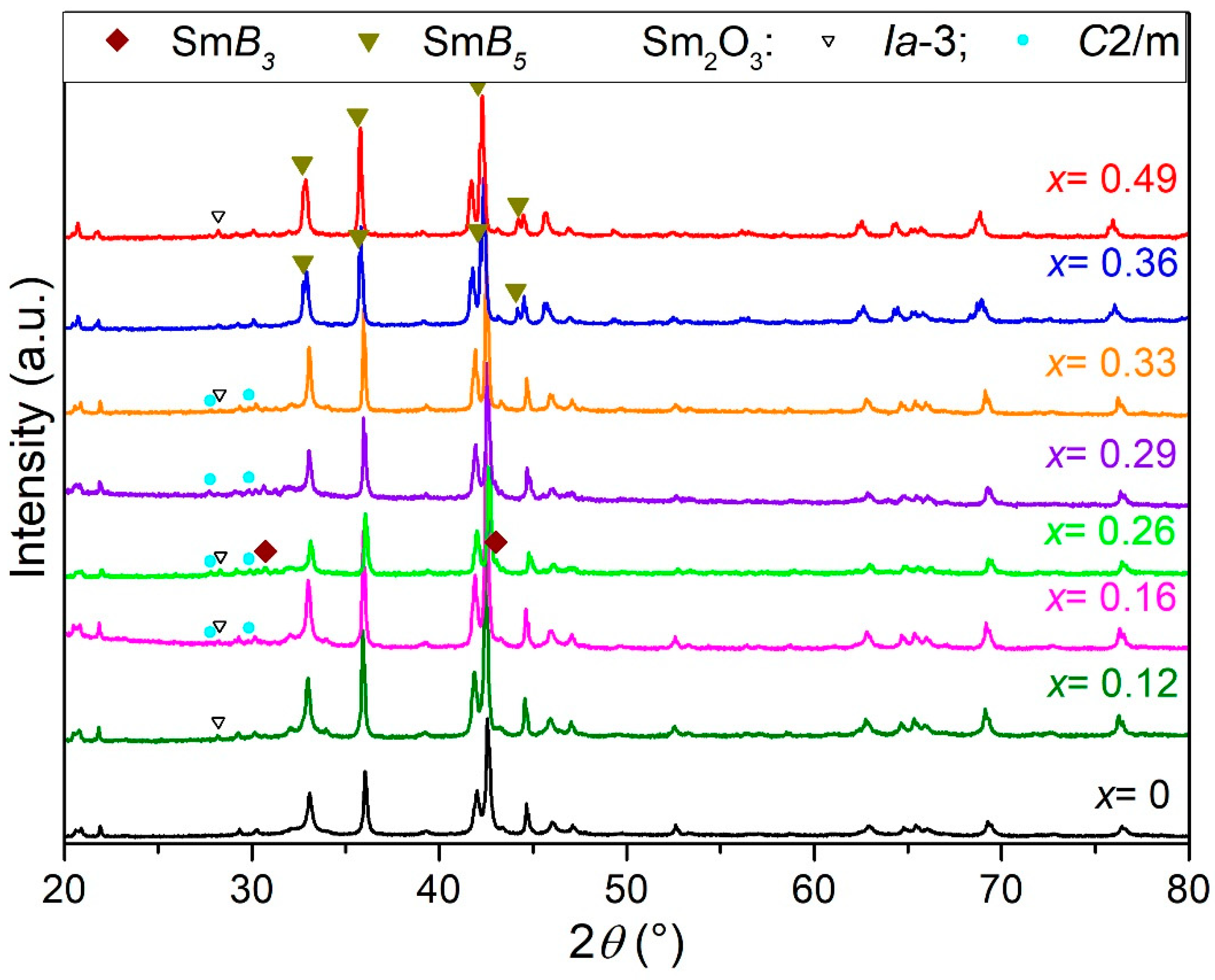

| SmzMnxNi7−x (EPMA)*(z from XRD) | x | Rwp | Phase | a | b | c | RB | Wt % (±1%) | |
|---|---|---|---|---|---|---|---|---|---|
| Sm2.01Ni7 (z = 2.01(1)) | 0 | 20.1 | 2:7 (H) | 4.9811(3) | 4.9811(3) | 24.318(3) | 8.7 | 79.1 | 79.1 |
| 2:7 (R) | 4.9792(4) | 4.9792(4) | 36.488(5) | 15.5 | 20.9 | ||||
| Sm2.06Mn0.12Ni6.88 (z = 1.98(1)) | 0.12 | 2.9 | 2:7 (H) | 4.9876(3) | 4.9876(3) | 24.337(3) | 1.7 | 77.7 | 78.2 |
| 2:7 (R) | 4.9857(2) | 4.9857(2) | 36.502(2) | 3.6 | 21.7 | ||||
| Sm2O3 (Ia) | 10.939(4) | 10.939(4) | 10.939(4) | 2.2 | 0.6 | ||||
| Sm1.94Mn0.16Ni6.84 (z = 1.97(1)) | 0.16 | 2.9 | 2:7 (H) | 4.9842(2) | 4.9842(2) | 24.312(2) | 2.5 | 73.2 | 76.2 |
| 2:7 (R) | 4.9835(2) | 4.9835(2) | 36.469(3) | 3.9 | 22.9 | ||||
| Sm2O3 (C2/m) | 14.13(2) | 3.641(3) | 8.893(8) | 1.8 | 3.1 | ||||
| Sm2O3 (Ia) | 10.931(4) | 10.931(4) | 10.931(4) | 2.4 | 0.8 | ||||
| Sm1.93Mn0.26Ni6.74 (z = 1.85(2)) | 0.26 | 20.4 | 2:7 (H) | 4.9794(3) | 4.9794(3) | 24.274(2) | 8.8 | 66.4 | 76.9 |
| 2:7 (R) | 4.9776(7) | 4.9776(7) | 36.342(7) | 14.4 | 19.9 | ||||
| AB5 | 4.955(2) | 4.955(2) | 3.969(2) | 6.1 | 5.8 | ||||
| Sm2O3 (C2/m) | 14.159(5) | 3.632(2) | 8.869(4) | 8.9 | 6.4 | ||||
| Sm2O3 (Ia) | 10.932(2) | 10.932(2) | 10.932(2) | 12.1 | 1.5 | ||||
| Sm1.92Mn0.29Ni6.71 (z = 1.91(2)) | 0.29 | 4.6 | 2:7(H) | 4.9816(3) | 4.9816(3) | 24.281(2) | 3.3 | 60.1 | 67.8 |
| 2:7 (R) | 4.9787(6) | 4.9787(6) | 36.340(6) | 3.8 | 28.5 | ||||
| AB5 | 4.963(2) | 4.963(2) | 3.969(3) | 1.2 | 5.9 | ||||
| Sm2O3 (C2/m) | 14.180(4) | 3.6226(8) | 8.855(2) | 1.5 | 5.5 | ||||
| Sm1.91Mn0.33Ni6.67 (z = 1.96(1)) | 0.33 | 2.9 | 2:7 (H) | 4.9920(1) | 4.9920(1) | 24.329(1) | 3.2 | 78.3 | 81.0 |
| 2:7 (R) | 4.9906(3) | 4.9906(3) | 36.483(3) | 4.8 | 18.4 | ||||
| Sm2O3 (C2/m) | 14.15(2) | 3.640(3) | 8.888(9) | 1.0 | 2.8 | ||||
| Sm2O3 (Ia) | 10.941(7) | 10.941(7) | 10.941(7) | 1.9 | 0.5 | ||||
| Sm2.05Mn0.36Ni6.64 | 0.36 | 17.5 | 2:7 (H) | 4.9980(2) | 4.9980(2) | 24.360(2) | 9.8 | 62.0 | 81.6 |
| 2:7 (R) | 4.9973(4) | 4.9973(4) | 36.542(4) | 14.6 | 14.0 | ||||
| AB3 | 5.0114(2) | 5.0114(2) | 24.549(2) | 9.4 | 24.0 | ||||
| Sm1.97Mn0.49Ni6.51 (z = 1.99(1)) | 0.49 | 2.0 | 2:7 (H) | 5.0055(2) | 5.0055(2) | 24.389 (1) | 1.3 | 57.8 | 85.5 |
| 2:7 (R) | 5.0051(6) | 5.0051(6) | 36.570(6) | 2.0 | 9.8 | ||||
| AB3 | 5.0144(2) | 5.0144(2) | 24.528(2) | 1.6 | 31.3 | ||||
| Sm2O3 (Ia) | 10.938(2) | 10.938(2) | 10.938(2) | 1.8 | 1.1 |
| Sm2MnxNi7−x | Peq abs. (MPa) | Total Capacity (10 MPa) | Desorption Capacity (%) | ||
|---|---|---|---|---|---|
| 1st Peq | 2nd Peq | (H/f.u.) | (wt.%) | ||
| 0 | 0.011 | 6.5 | 8.5 | 1.19 | 24 |
| 0.12 | 0.027 | 6.0 | 9.0 | 1.25 | 41 |
| 0.29 * | 0.1 | 0.39 | 10.5 | 1.47 | 86 |
| 0.33 | 0.041 | 0.21 | 9.8 | 1.41 | 96 |
| Sm2MnxNi7−x | Corrosion Time (weeks) | TGA Mass Loss [150–450 °C] (±0.3 wt %) | Ni Calculated from TGA Loss (±0.3 wt %) | Metallic Ni Measured from Magnetism (±0.2 wt %) | Ni(OH)2 from the Difference between TGA and Magnetism (±0.5 wt %) | Sm(OH)3 from the Difference between TGA and Magnetism (±0.5 wt %) |
|---|---|---|---|---|---|---|
| 0 | 18 | 15.6 | 15.9 | 13.5 | 0.8 | 13.8 |
| 0.12 | 8 | 6.4 | 6.2 | 7 | 0 | 7 |
| 0.12 | 18 | 8.4 | 8.2 | 7.8 | 0.1 | 8.0 |
| 0.26 | 8 | 12.2 | 12.4 | 10.1 | 1.2 | 9.6 |
| 0.26 | 18 | 17.6 | 17.9 | 9.2 | 3.3 | 10.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madern, N.; Charbonnier, V.; Monnier, J.; Zhang, J.; Paul-Boncour, V.; Latroche, M. Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides. Energies 2020, 13, 3470. https://doi.org/10.3390/en13133470
Madern N, Charbonnier V, Monnier J, Zhang J, Paul-Boncour V, Latroche M. Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides. Energies. 2020; 13(13):3470. https://doi.org/10.3390/en13133470
Chicago/Turabian StyleMadern, Nicolas, Véronique Charbonnier, Judith Monnier, Junxian Zhang, Valérie Paul-Boncour, and Michel Latroche. 2020. "Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides" Energies 13, no. 13: 3470. https://doi.org/10.3390/en13133470
APA StyleMadern, N., Charbonnier, V., Monnier, J., Zhang, J., Paul-Boncour, V., & Latroche, M. (2020). Investigation of H Sorption and Corrosion Properties of Sm2MnxNi7−x (0 ≤ x < 0.5) Intermetallic Compounds Forming Reversible Hydrides. Energies, 13(13), 3470. https://doi.org/10.3390/en13133470






