Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effects of the Operational Parameters of AS on the Removal Efficiency of Ammonia Nitrogen
- [NH3] = molecular ammonia concentration (mol L−1).
- [NH3 + NH4+] = total ammonia nitrogen (TAN, mol L−1).
- [H+] = hydrogen ion concentration (mol L−1).
- Ka = acid ionization constant (dimensionless).
- T = temperature (K) [37].
2.2. Experimental Setup
- M = cumulative methane production [L per gram of tCOD added, L gtCODadded−1].
- P = methane potential [L per gram of tCOD added, L gtCODadded−1].
- Rm = maximum production rate of methane [L per g of tCOD added and per day, L gtCODadded−1 d−1].
- λ = lag phase period or the minimum time required to produce biogas [d].
- t = time for digestion [d].
2.3. Physico-Chemical Characteristics of Inoculum and SW
2.4. Analytical Methods
- Total solids (TS), on oven-dried biomass at 70 °C (until weight stabilization).
- Total volatile solids (VS), on calcinated dried matter.
- pH, by a portable pH-meter from XS Instruments.
3. Results and Discussions
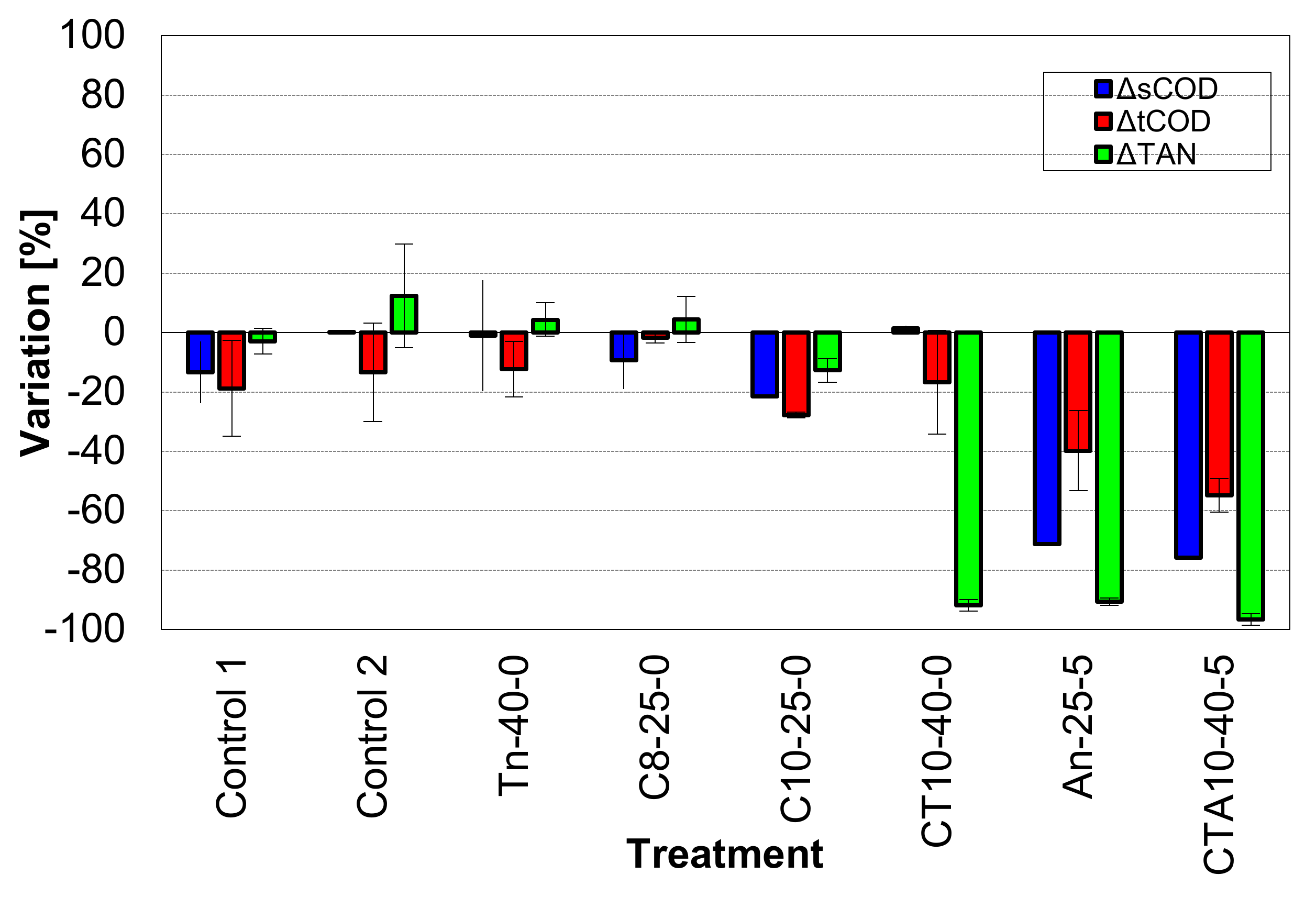
3.1. Effect of SW Pretreatments on TAN and COD Removal Efficiencies
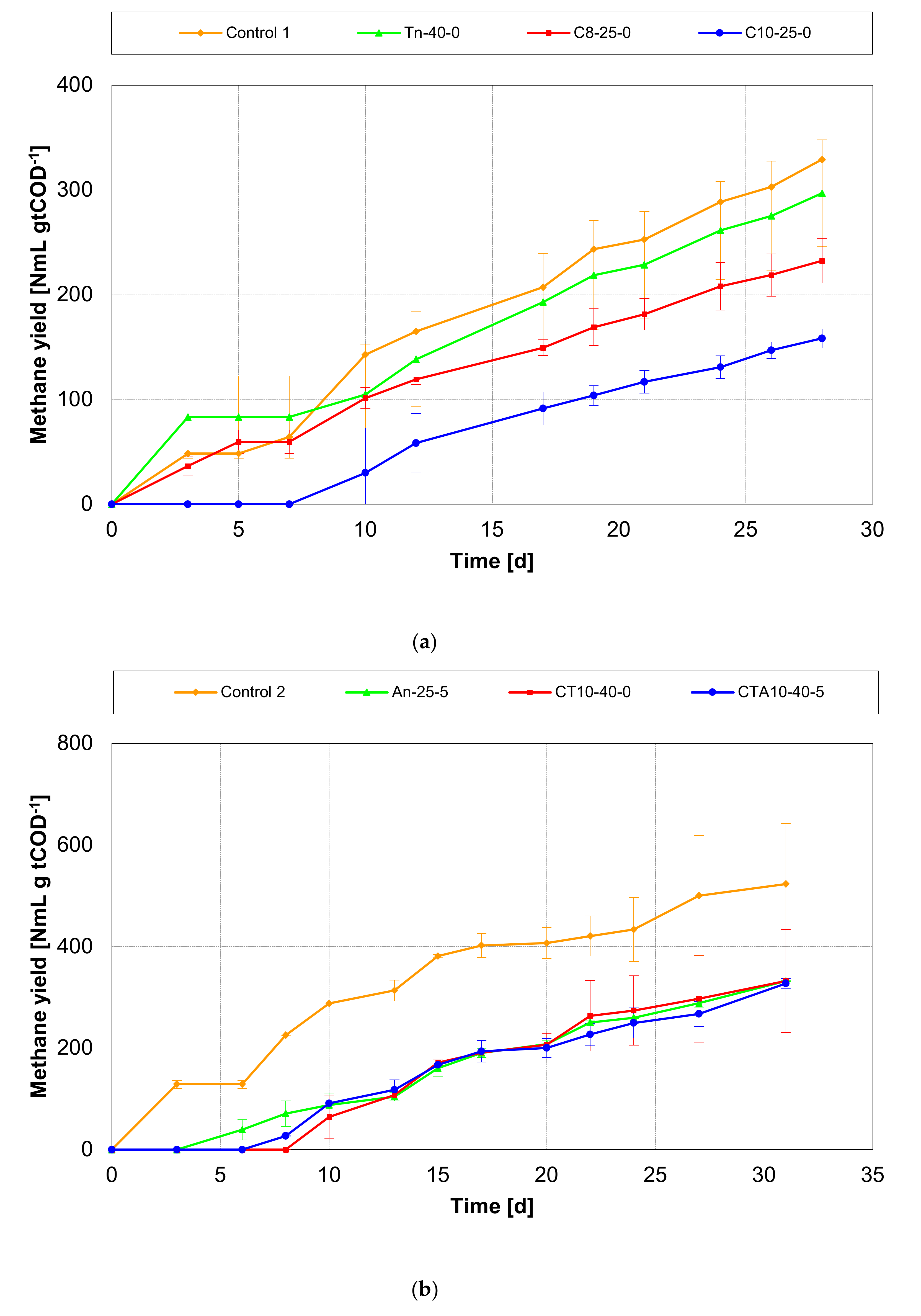
3.2. Effect of Pretreatments on Methane Yields of AD
3.2.1. Run 1 (BMP Tests at the Same TAN Concentration)
3.2.2. Run 2 (BMP Tests with Low TAN Concentration)
3.3. Comparisons of BMP Values with Literature Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Viancelli, A.; Kunz, A.; Steinmetz, R.L.R.; Kich, J.D.; Souza, C.K.; Canal, C.W.; Coldebella, A.; Esteves, P.A.; Barardi, C.R.M. Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 2013, 90, 1539–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jahng, D. Enhanced anaerobic digestion of piggery wastewater by ammonia stripping: Effects of alkali types. J. Hazard. Mater. 2010, 182, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2017, 621, 1664–1682. [Google Scholar] [CrossRef]
- Kim, J.-H.; Chen, M.; Kishida, N.; Sudo, R. Integrated real-time control strategy for nitrogen removal in swine wastewater treatment using sequencing batch reactors. Water Res. 2004, 38, 3340–3348. [Google Scholar] [CrossRef]
- Safavi, S.M.; Unnthorsson, R. Enhanced methane production from pig slurry with pulsed electric field pre-treatment. Environ. Technol. 2017, 3330, 1–11. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to recover nutrients from waste streams: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 385–427. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Mo, X.; Cheng, G.; Du, D. Pretreatment of piggery wastewater by a stable constructed microbial consortium for improving the methane production. Water Sci. Technol. 2015, 71, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Deng, L.; Zheng, D.; Wang, L.; Liu, Y. Separation of swine wastewater into different concentration fractions and its contribution to combined anaerobic-aerobic process. J. Environ. Manag. 2016, 168, 87–93. [Google Scholar] [CrossRef]
- Trias, M.; Hu, Z.; Mortula, M.M.; Gordon, R.J.; Gagnon, G.A. Impact of Seasonal Variation on Treatment of Swine Wastewater. Environ. Technol. 2004, 25, 775–781. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Quintanar, A.I.; Cook, K.L.; Lovanh, N.C.; Lane, B. Seasonal Variation in Heat Fluxes, Predicted Emissions of Malodorants, and Wastewater Quality of an Anaerobic Swine Waste Lagoon. Water Air Soil Pollut. 2012, 223, 3611–3618. [Google Scholar] [CrossRef]
- Craggs, R.A.; Park, J.A.; Heubeck, S.A. Methane emissions from anaerobic ponds on a piggery and a dairy farm in New Zealand. Aust. J. Exp. Agric. 2008, 48, 142–146. [Google Scholar] [CrossRef]
- Zema, D.A.; Andiloro, S.; Bombino, G.; Tamburino, V.; Sidari, R.; Caridi, A. Depuration in aerated ponds of citrus processing wastewater with a high concentration of essential oils. Environ. Technol. 2012, 33, 1255–1260. [Google Scholar] [CrossRef]
- Andiloro, S.; Bombino, G.; Tamburino, V.; Zema, D.A.; Zimbone, S.M. Aerated lagooning of agro-industrial wastewater: Depuration performance and energy requirements. J. Agric. Eng. 2013, 44, 827–832. [Google Scholar] [CrossRef]
- Zema, D.A.; Andiloro, S.; Bombino, G.; Caridi, A.; Sidari, R.; Tamburino, V. Comparing Different Schemes of Agricultural Wastewater Lagooning: Depuration Performance and Microbiological Characteristics. Water Air Soil Pollut. 2016, 227, 439. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, J.W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199–200, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Noike, T.; Goo, I.S.; Matsumoto, H.; Miyahara, T. Development of a new type of anaerobic digestion process equipped with the function of nitrogen removal. Water Sci. Technol. 2004, 49, 173–179. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Demirer, G.N.; Frear, C.; Chen, S. Anaerobic digestion of dairy manure with enhanced ammonia removal. J. Environ. Manag. 2008, 86, 193–200. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; He, L.; Zhang, Z.; Lei, Z.; Liu, R.; Zheng, W. Enhanced biogasification from ammonia-rich swine manure pretreated by ammonia fermentation and air stripping. Int. Biodeterior. Biodegrad. 2019, 140, 84–89. [Google Scholar] [CrossRef]
- Maurer, M.; Pronk, W.; Larsen, T.A. Treatment processes for source-separated urine. Water Res. 2006, 40, 3151–3166. [Google Scholar] [CrossRef] [PubMed]
- Macura, B.; Johannesdottir, S.L.; Piniewski, M.; Haddaway, N.R.; Kvarnström, E. Effectiveness of ecotechnologies for recovery of nitrogen and phosphorus from anaerobic digestate and effectiveness of the recovery products as fertilisers: A systematic review protocol. Environ. Evid. 2019, 8, 29. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Søtoft, L.F. Ammonium Fertilizers Production from Manure: A Critical Review. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for Recovering Nutrients from Wastewater: A Critical Review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Binti, N.; Wahab, A.; Fikri, K.; Tamrin, B.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Zeng, Z.; Zheng, P.; Shi, C.; Zhang, M.; Li, Y.; Zhang, W.; Shan, S. A challenge in anaerobic digestion of swine wastewater: Recalcitrance and enhanced-degradation of dietary fibres. Biodegradation 2019, 30, 389–400. [Google Scholar] [CrossRef]
- Córdoba, V.; Fernández, M.; Santalla, E. The effect of different inoculums on anaerobic digestion of swine wastewater. J. Environ. Chem. Eng. 2016, 4, 115–122. [Google Scholar] [CrossRef]
- Deng, L.; Yang, H.; Liu, G.; Zheng, D.; Chen, Z.; Liu, Y.; Pu, X.; Song, L.; Wang, Z.; Lei, Y. Kinetics of temperature effects and its significance to the heating strategy for anaerobic digestion of swine wastewater. Appl. Energy 2014, 134, 349–355. [Google Scholar] [CrossRef]
- Belmonte, M.; Hsieh, C.-F.; Figueroa, C.; Campos, J.L.; Vidal, G. Effect of free ammonia nitrogen on the methanogenic activity of swine wastewater. Electron. J. Biotechnol. 2011, 14, 2. [Google Scholar] [CrossRef]
- Jiang, M.; Westerholm, M.; Qiao, W.; Wandera, S.M.; Dong, R. High rate anaerobic digestion of swine wastewater in an anaerobic membrane bioreactor. Energy 2020, 193, 116783. [Google Scholar] [CrossRef]
- Ahn, J.H.; Do, T.H.; Kim, S.D.; Hwang, S. The effect of calcium on the anaerobic digestion treating swine wastewater. Biochem. Eng. J. 2006, 30, 33–38. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of pig slurry characteristics on ammonia stripping efficiencies and quality of the recovered ammonium-sulfate solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Zhou, T.; Li, Z.; Xiang, S.; Xu, F.; Ruan, R.; Liu, Y. Evaluation of ammonia recovery from swine wastewater via a innovative spraying technology. Bioresour. Technol. 2019, 272, 235–240. [Google Scholar] [CrossRef]
- Liao, P.H.; Chen, A.; Lo, K.V. Removal of nitrogen from swine manure wastewaters by ammonia stripping. Bioresour. Technol. 1995, 54, 17–20. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L. Wastewater Engineering, Treatment and Resourse Recovery; McGraw-Hill Education, Ed.; Metcalf & Eddy Inc: New York, NY, USA, 2003. [Google Scholar]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical review of abatement of ammonia from wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Kamel, G. Characterization and biological treatment of pre-treated landfill leachate. Ecol. Eng. 2016, 94, 268–274. [Google Scholar] [CrossRef]
- Gustin, S.; Marinsek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process. Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Quan, X.; Wang, F.; Zhao, Q.; Zhao, T.; Xiang, J. Air stripping of ammonia in a water-sparged aerocyclone reactor. J. Hazard. Mater. 2009, 170, 983–988. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.P.; Catalán, E.; Folino, A.; Sánchez, A.; Komilis, D. Effect of three pretreatment techniques on the chemical composition and on the methane yields of Opuntia ficus-indica (prickly pear) biomass. Waste Manag. Res. 2018, 36, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Paone, E.; Komilis, D.; Calabrò, P.S.; Fazzino, F.; Folino, A.; Paone, E.; Komilis, D. Semi-Continuous Anaerobic Digestion of Orange Peel Waste: Effect of Activated Carbon Addition and Alkaline Pretreatment on the Process. Sustainability 2019, 11, 3386. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.S.; Pontoni, L.; Porqueddu, I.; Greco, R.; Pirozzi, F.; Malpei, F. Effect of the concentration of essential oil on orange peel waste biomethanization: Preliminary batch results. Waste Manag. 2016, 48, 440–447. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Ghatak, M.D.; Mahanta, P. Comparison of kinetic models for biogas production rate from dust. Int. J. Res. Eng. Technol. 2014, 3, 248–254. [Google Scholar]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals. Public Work. 1964, 95, 91–94. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Ho, L.; Ho, G. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: Use of pH reduction, zeolite, biomass and humic acid. Water Res. 2012, 46, 4339–4350. [Google Scholar] [CrossRef] [Green Version]
- Angelidaki, I.; Ahring, B.K. Anaerobic thermophilic digestion of manure at different ammonia loads: Effect of temperature. Water Res. 1994, 28, 727–731. [Google Scholar] [CrossRef]
- Gallert, C.; Winter, J. Mesophilic and thermophilic anaerobic digestion of source-sorted organic wastes: Effect of ammonia on glucose degradation and methane production. Appl. Microbiol. Biotechnol. 1997, 48, 405–410. [Google Scholar] [CrossRef]
- Ortner, M.; Leitzinger, K.; Skupien, S.; Bochmann, G.; Fuchs, W. Efficient anaerobic mono-digestion of N-rich slaughterhouse waste: Influence of ammonia, temperature and trace elements. Bioresour. Technol. 2014, 174, 222–232. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clescer, L.S., Eds.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Parkin, G.F.; Owen, W.F. Fundamentals of Anaerobic Digestion of Wastewater Sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Kugelman, I.J.; Chin, K.K. Toxicity, Synergism, and Antagonism in Anaerobic Waste Treatment Processes. In Anaerobic Biological Treatment Processes; Advances in Chemistry Series, 105; American Chemical Society: Washington, DC, USA; pp. 55–90.
- Sambusiti, C.; Monlau, F.; Ficara, E.; Carrère, H.; Malpei, F. A comparison of different pre-treatments to increase methane production from two agricultural substrates. Appl. Energy 2013, 104, 62–70. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Calabrò, P.S.P.S.; Greco, R.; Evangelou, A.; Komilis, D. Anaerobic digestion of tomato processing waste: Effect of alkaline pretreatment. J. Environ. Manag. 2015, 163, 49–52. [Google Scholar] [CrossRef]
- Bullock, C.M.; Bicho, P.A.; Zhang, Y.; Saddler, J.N. A solid chemical oxygen demand (COD) method for determining biomass in waste waters. Water Res. 1996, 30, 1280–1284. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Paone, E.; Komilis, D. Strategies for the sustainable management of orange peel waste through anaerobic digestion. J. Environ. Manag. 2018, 212, 462–468. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Kim, S.H.; Shin, K.S.; Kim, C.H. Effects of substrate to inoculum ratio on the biochemical methane potential of piggery slaughterhouse wastes. Asian-Australas. J. Anim. Sci. 2014, 27, 600–607. [Google Scholar] [CrossRef]
- Jackson-Moss, C.A.; Duncan, J.R.; Cooper, D.R. The effect of calcium on anaerobic digestion. Biotechnol. Lett. 1989, 11, 219–224. [Google Scholar] [CrossRef]
- Rivard, C.J.; Grohmann, K. Degradation of furfural (2-furaldehyde) to methane and carbon dioxide by an anaerobic consortium. Appl. Biochem. Biotechnol. 1991, 28–29, 285–295. [Google Scholar] [CrossRef]
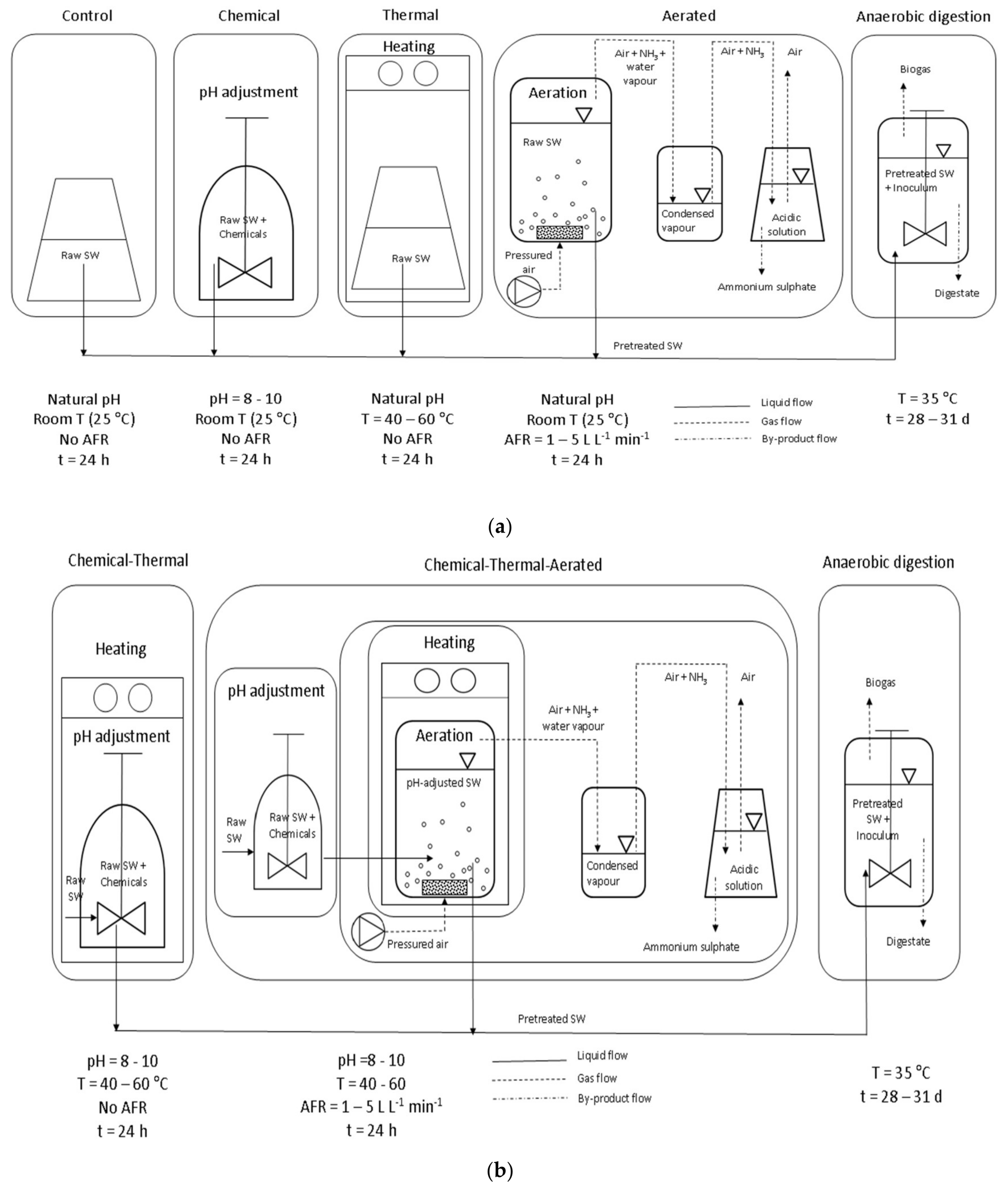


| Test | pH | Temperature [°C] | Air Flow Rate [Lair LSW−1 min−1] |
|---|---|---|---|
| Control | Natural | Room | 0 |
| Tn-40-0 | Natural | 40 | 0 |
| C8-25-0 | 8 | Room | 0 |
| C10-25-0 | 10 | ||
| An-25-5 | Natural | Room | 5 |
| CT10-40-0 | 10 | 40 | 0 |
| CTA10-40-5 | 10 | 40 | 5 |
| Parameter | Run 1 | Run 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blank | c1 | Tn-40-0 | C8-25-0 | C10-25-0 | Blank | c2 | An-25-5 | CT10-40-0 | CTA10-40-5 | ||
| Vinoculum | mL | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| Vsubstrate | mL | - | 66 | 62 | 61 | 73 | - | 76 | 130 | 80 | 110 |
| F/M | gVS gVS−1 | - | 0.16 | 0.16 | 0.17 | 0.23 | - | 0.06 | 0.10 | 0.17 | 0.34 |
| TAN | mg L−1 | - | 352 | 354 | 351 | 353 | - | 346 | 50 | 31 | 24 |
| sCOD | g | - | 0.391 | 0.417 | 0.380 | 0.398 | - | 0.206 | 0.115 | 0.230 | 0.125 |
| tCOD | g | - | 0.441 | 0.447 | 0.499 | 0.442 | - | 0.554 | 0.747 | 0.586 | 0.726 |
| tCOD/TAN | g g−1 | - | 5.22 | 5.27 | 5.92 | 5.22 | - | 6.69 | 49.87 | 77.48 | 109.66 |
| Ca | g L−1 | - | - | - | 0.65 | 1.96 | - | - | - | 2.28 | 4.37 |
| TSmix | [%] | 1.41 | 1.67 | 1.67 | 1.71 | 2.02 | 2.32 | 2.58 | 2.16 | 2.78 | 2.82 |
| Description | TS [%] | VS [%TS] | pH | sCOD [g L−1] | tCOD [g L−1] | TAN [gN L−1] |
|---|---|---|---|---|---|---|
| i1 | 2.26 ± 0.18 | 70.12 ± 0.17 | 8.13 ± 0.02 | - | - | - |
| i2 | 3.87 ± 0.10 | 67.83 ± 0.47 | 8.16 ± 0.03 | - | - | - |
| c1 | 1.02 ± 0.01 | 65.16 ± 2.65 | 7.04 ± 0.03 | 6675 ± 601 | 8063 ± 880 | 1280 ± 42 |
| c2 | 0.48 ± 0.00 | 55.33 ± 0.57 | 7.61 ± 0.01 | 2708 ± 18 | 8593 ± 1798 | 970 ± 0 |
| Treatment | pHi | pHf | sCOD [mg L−1] | ΔsCOD [%] | tCOD [mg L−1] | ΔtCOD [%] | TAN [mg L−1] | ΔTAN [%] | MR [%] | Average Conversion of Added COD to Methane [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| c1 | 7.03 ± 0.03 | 7.16 ± 0.01 | 5925 ± 177 | −13.40 ± 10.37 | 6675 ± 601 | −18.78 ± 16.10 | 1280 ± 14 | −2.89 ± 4.29 | −2.96 ± 0.05 | 94 |
| Tn-40-0 | 7.08 ± 0.04 | 7.50 ± 0.28 | 6725 ± 672 | −0.98 ± 18.72 | 7215 ± 21 | −12.29 ± 9.32 | 1370 ± 28 | 4.39 ± 5.61 | −2.56 ± 0.10 | 85 |
| C8-25-0 | 8.03 ± 0.03 | 8.14 ± 0.01 | 6225 ± 106 | −9.27 ± 9.71 | 8173 ± 746 | −1.77 ± 1.77 | 1380 ± 57 | 4.51 ± 7.74 | −3.18 ± 0.03 | 66 |
| C10-25-0 | 10.07 ± 0.04 | 10.19 ± 0.27 | 5450 ± 495 | −21.48 ± 0.06 | 6051 ± 576 | −27.77 ± 1.01 | 1160 ± 14 | −12.77 ± 3.95 | −3.82 ± 0.2 | 45 |
| c2 | 7.61 ± 0.01 | 7.95 ± 0.02 | 2713 ± 25 | 0.18 ± 0.26 | 7295 ± 134 | −13.37 ± 16.56 | 1090 ± 170 | 12.37 | 0.00 ± 0.00 | 149 |
| An-25-5 | 7.98 ± 0.59 | 8.92 ± 0.01 | 883 ± 18 | −71.32 ± 0.39 | 5748 ± 81 | −39.74 ± 13.44 | 115 ± 2 | −90.60 | −12.01 ± 10.27 | 95 |
| CT10-40-0 | 8.86 ± 0.91 | 9.91 ± 0.64 | 2875 ± 7 | 1.48 ± 0.91 | 7330 ± 0 | −16.66 ± 17.44 | 95 ± 36 | −91.86 | −4.44 ± 0.00 | 95 |
| CTA10-40-5 | 9.96 ± 0.01 | 9.30 ± 0.03 | 1135 ± 21 | −75.69 ± 0.30 | 6603 ± 562 | −54.84 ± 5.65 | 60 ± 27 | −96.64 | −42.00 ± 0.35 | 93 |
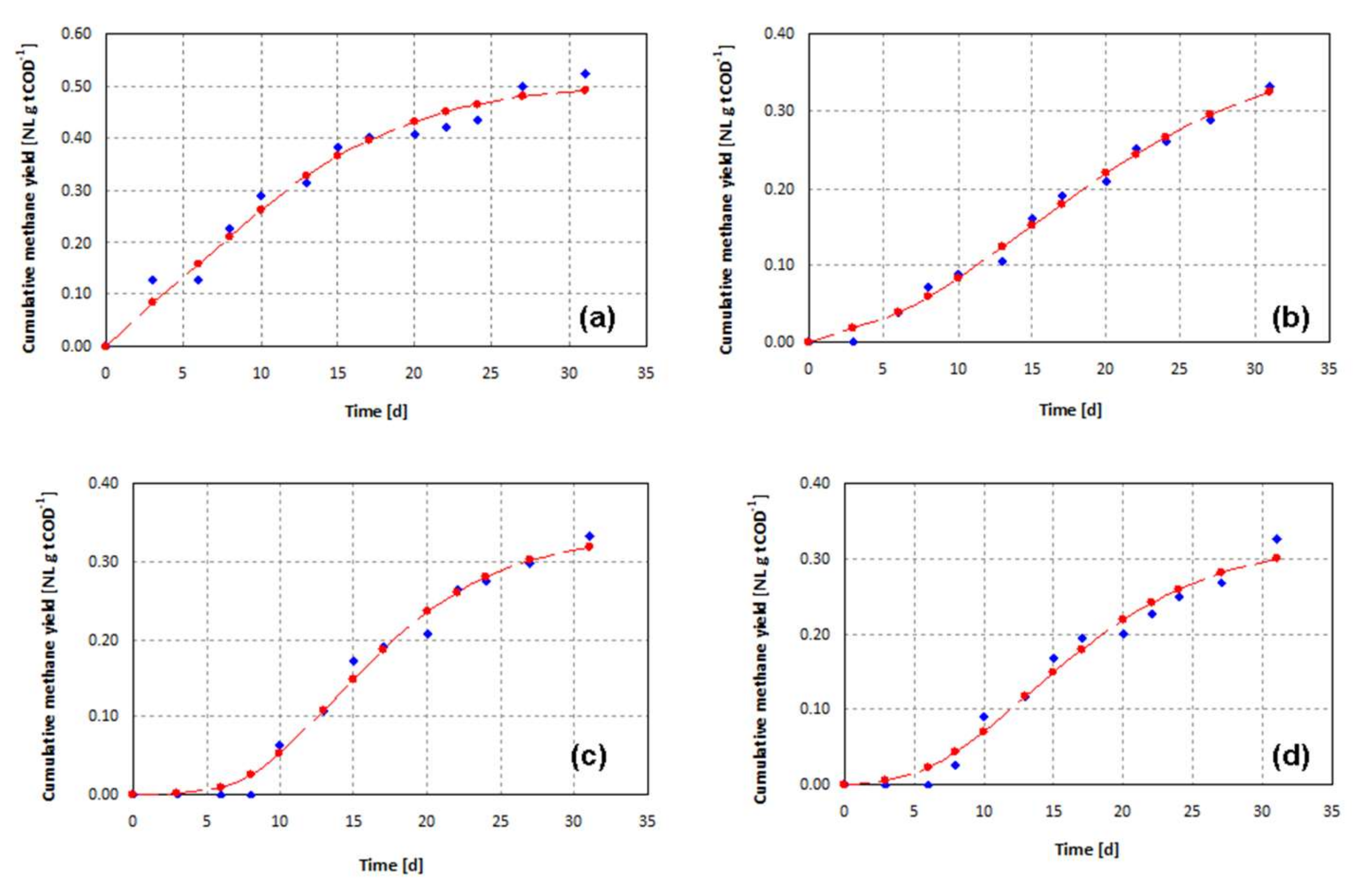
| BMP Test | t50 | Parameters of Gompertz Equation (5) | ||
|---|---|---|---|---|
| [d] | P | Λ | Rm | |
| c1 | 12.0 | 0.390 | 1.2 | 0.014 |
| Tn-40-0 | 12.9 | 0.769 | 0.0 | 0.011 |
| C8-25-0 | 11.7 | 0.297 | 0.0 | 0.009 |
| C10-25-0 | 15.2 | 0.174 | 7.0 | 0.009 |
| c2 | 9.2 | 0.510 | 0.0 | 0.026 |
| An-25-5 | 15.4 | 0.399 | 4.4 | 0.014 |
| CT10-40-0 | 14.8 | 0.339 | 7.7 | 0.020 |
| CTA10-40-5 | 14.7 | 0.330 | 5.8 | 0.016 |
| Reference | Sample (Adjusted pH) | Pre-Treatment Conditions | AD Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali type | T [°C]–t [h]–Q [L L−1 min−1] | Ammonia Variation [%] | pH, i | T [°C]–Time [d] | TAN [g L−1] | NH4+–N [g L−1] | NH3 [g L−1] | pH,f | BMP [mL gCODadded−1] | Efficiency [%] | ||
| [36] | Raw SW | - | - | - | 7.7 | 35–80 | 3.24 * | n.a. | 0.16 * | 8.3 | 38.4 | - |
| AS-SW (-) | - | 80–4 0.05 * | −65 | 8.5 | 2.40 * | 0.68 * | 8.0 | 20.5 | −46.6 * | |||
| AS-SW (9.5) | Ca(OH)2 | −69 | 8.8 | 2.15 * | 0.89 * | 7.5 | 10.5 | −72.6 * | ||||
| AS-SW (11.5) | −98.8 | 9.9 | 1.18 * | 1.06 * | 7.9 | 17.6 | −54.2 * | |||||
| [2] ** | Raw SW | - | - | - | 8.34 ± 0.10 | 37–20 | n.a. | n.a. | 0.877 ± 0.068 | 54.0 ± 14.5 | - | |
| AS-SW (9.5) | NaOH | 37–24–1.0 * | −49.3 * | 8.20 ± 0.09 | 0.290 ± 0.035 | 182.3 ± 15.7 | 238 | |||||
| AS-SW (10.0) | −70.5 * | 8.20 ± 0.13 | 0.216 ± 0.030 | 165.7 ± 11.1 | 207 | |||||||
| AS-SW (9.5) | KOH | −40.4 * | 8.30 ± 0.08 | 0.347 ± 0.062 | 155.3 ± 20.2 | 188 | ||||||
| AS-SW (10) | −71.3 * | 8.49 ± 0.15 | 0.419 ± 0.044 | 69.3 ± 13.9 | 28 | |||||||
| AS-SW (9.5) | CaO | −30.5 * | 8.06 ± 0.10 | 0.258 ± 0.049 | 262.3 ± 12.0 | 386 | ||||||
| AS-SW (10) | −49.1 * | 8.00 ± 0.12 | 0.185 ± 0.039 | 258.9 ± 17.3 | 379 | |||||||
| [16] | Raw SW | - | - | - | n.a. | 37–20 | n.a. | 4.801 | n.a. | 3.5 * | - | |
| AS-SW (7.2) | NaOH | 37–48–1.0 | −28.0 | 3.272 | 35 * | 900 | ||||||
| AS-SW (9.0) | −47.0 | 2.314 | 90 * | 2471 | ||||||||
| AS-SW (10.0) | −80.0 | 0.838 | 182 * | 5100 | ||||||||
| AS-SW (11.0) | −88.1 | 0.465 | 142 * | 3957 | ||||||||
| [16] | Raw SW | - | 37–48–0.0 | - | n.a. | 37–20 | n.a. | 4.495 | n.a. | 10 * | - | |
| AS-SW(9.0) | NaOH | 37–48–1.0 | −46.0 | 2.314 | 75 * | 650 | ||||||
| 37–48–2.0 | −62.2 | 1.702 | 97 * | 870 | ||||||||
| 37–48–4.0 | −77.9 | 0.997 | 155 * | 1450 | ||||||||
| 37–48–10.0 | −92.0 | 0.359 | 122 * | 1120 | ||||||||
| [16] ** | Raw SW | - | - | - | 8.0 ± 0.2 | 37−20 | n.a. | 6.30 ± 0.045 | n.a. | 49.2 ± 16.6 | - | |
| AS-SW (9.5) | NaOH | 37–24–4.0 | n.a. | 2.93 ± 0.054 | 170.3 ± 26.0 | 246 | ||||||
| AS-SW (10.0) | 1.85 ± 0.072 | 132.6 ± 8.6 | 170 | |||||||||
| AS-SW (11.0) | 0.86 ± 0.061 | 78.9 ± 17.9 | 60 | |||||||||
| This study | c1 | - | - | −2.89 | 7.9 ± 0.03 | n.a. | 0.352 | n.a. | n.a. | 7.47 ± 0.01 | 329 | - |
| Tn-40-0 | - | 40–24–0 | +4.39 | 8.03 ± 0.01 | 0.354 | 7.48 ± 0.01 | 297 ± 51 | −9.8 | ||||
| C8-25-0 (8) | Ca(OH)2 | 25–24–0 | +4.51 | 8.13 ± 0.02 | 0.351 | 7.50 ± 0.00 | 232 ± 21 | −29.4 | ||||
| C10-25-0 (10) | 25–24–0 | −12.77 | 8.93 ± 0.01 | 0.353 | 7.80 ± 0.01 | 159 ± 9 | −51.9 | |||||
| c2 | - | 25–24–0 | +12.37 | 8.02 ± 0.02 | n.a. | 0.346 | n.a. | n.a. | 7.75 ± 0.03 | 523 ± 120 | - | |
| An-25-5 | - | 25–24–5 | −90.60 | 8.42 ± 0.02 | 0.050 | 7.66 ± 0.01 | 332 ± 1 | −36.5 | ||||
| CT10-40-0 (10) | Ca(OH)2 | 40–24–0 | −91.86 | 8.65 ± 0.01 | 0.031 | 7.72 ± 0.04 | 332 ± 102 | −36.5 | ||||
| CTA10-40-5 (10) | 40–24–5 | −96.64 | 8.22 ± 0.02 | 0.024 | 7.66 ± 0.00 | 327 ± 10 | −37.4 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folino, A.; Calabrò, P.S.; Zema, D.A. Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater. Energies 2020, 13, 3413. https://doi.org/10.3390/en13133413
Folino A, Calabrò PS, Zema DA. Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater. Energies. 2020; 13(13):3413. https://doi.org/10.3390/en13133413
Chicago/Turabian StyleFolino, Adele, Paolo Salvatore Calabrò, and Demetrio Antonio Zema. 2020. "Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater" Energies 13, no. 13: 3413. https://doi.org/10.3390/en13133413
APA StyleFolino, A., Calabrò, P. S., & Zema, D. A. (2020). Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater. Energies, 13(13), 3413. https://doi.org/10.3390/en13133413







