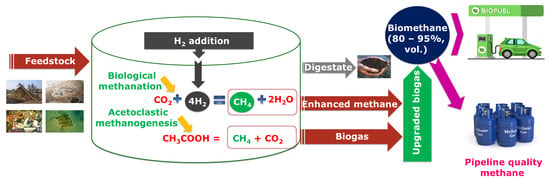
Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| APHA CM | American Public Health Association Cattle Manure |
| CFD | Computational Fluid Dynamics |
| MFC | Microbial Fuel Cells |
| P2G | Power to Gas |
| PLC | Programmable Logic Control |
| R&D | Research and Development |
| S:I | Substrate to Inoculum ratio |
| STP | Standard Temperature and Pressure |
| TAN | Total Ammonium Nitrogen |
| TS | Total Solids |
| VFA | Volatile Fatty Acids |
| VS | Volatile Solids |
References
- Weschenfelder, F.; Leite, G.N.P.; da Costa, A.C.A.; de Castro Vilela, O.; Ribeiro, C.M.; Ochoa, A.A.V.; Araújo, A.M. A review on the complementarity between grid-connected solar and wind power systems. J. Clean. Prod. 2020, 257, 120617. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Liu, W.; Wen, F.; Xue, Y. Power-to-gas technology in energy systems: Current status and prospects of potential operation strategies. J. Mod. Power Syst. Clean Energy 2017, 5, 439–450. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- McCarty, P.L.; Smith, D.P. Anaerobic wastewater treatment. Environ. Sci. Technol. 1986, 20, 1200–1206. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Hattori, S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef]
- Mulat, D.G.; Mosbæk, F.; Ward, A.J.; Polag, D.; Greule, M.; Keppler, F.; Nielsen, J.L.; Feilberg, A. Exogenous addition of H 2 for an in situ biogas upgrading through biological reduction of carbon dioxide into methane. Waste Manag. 2017, 68, 146–156. [Google Scholar] [CrossRef]
- Pauss, A.; Andre, G.; Perrier, M.; Guiot, S.R. Liquid-to-gas mass transfer in anaerobic processes: Inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl. Environ. Microbiol. 1990, 56, 1636–1644. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-situ biogas upgrading in thermophilic granular UASB reactor: Key factors affecting the hydrogen mass transfer rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of recent progress towards in-situ biogas upgradation techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Singh, K.S. Understanding the mixing pattern in an anaerobic expanded granular sludge bed reactor: Effect of liquid recirculation. J. Environ. Eng. 2009, 136, 576–584. [Google Scholar] [CrossRef]
- Oyama, S.; Lee, D.; Hacarlioglu, P.; Saraf, R. Theory of hydrogen permeability in nonporous silica membranes. J. Membr. Sci. 2004, 244, 45–53. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-type injection system as a potential H2 mass transfer technology for full-scale in situ biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Díaz, I.; Pérez, C.; Alfaro, N.; Fdz-Polanco, F. A feasibility study on the bioconversion of CO2 and H2 to biomethane by gas sparging through polymeric membranes. Bioresour. Technol. 2015, 185, 246–253. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Kim, H.W.; Marcus, A.K.; Shin, J.H.; Rittmann, B.E. Advanced control for photoautotrophic growth and CO2-utilization efficiency using a membrane carbonation photobioreactor (MCPBR). Environ. Sci. Technol. 2011, 45, 5032–5038. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Zhang, L.; Kuroki, A.; Tong, Y.W. A Mini-Review on In situ Biogas Upgrading Technologies via Enhanced Hydrogenotrophic Methanogenesis to Improve the Quality of Biogas from Anaerobic Digesters. Front. Energy Res. 2020. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Johri, B.N.; Das, S.K. Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 1. Microbial Diversity in Normal & Extreme Environments; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Hupfauf, S.; Winkler, A.; Wagner, A.O.; Podmirseg, S.M.; Insam, H. Biomethanation at 45 °C offers high process efficiency and supports hygienisation. Bioresour. Technol. 2020, 300, 122671. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Sarker, S. By-products of fish-oil refinery as potential substrates for biogas production in Norway: A preliminary study. Results Eng. 2020, 6, 100137. [Google Scholar] [CrossRef]
- APHA; WPCF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1995. [Google Scholar]
- Bergland, W.H.; Dinamarca, C.; Toradzadegan, M.; Nordgård, A.S.R.; Bakke, I.; Bakke, R. High rate manure supernatant digestion. Water Res. 2015, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Varel, V.; Isaacson, H.; Bryant, M. Thermophilic methane production from cattle waste. Appl. Environ. Microbiol. 1977, 33, 298–307. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B. Regulating feeding and increasing methane yield from co-digestion of C 5 molasses and cattle manure. Energy Convers. Manag. 2014, 84, 7–12. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- De Bok, F.; Plugge, C.; Stams, A. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef]
- Ong, H.; Greenfield, P.; Pullammanappallil, P. Effect of mixing on biomethanation of cattle-manure slurry. Environ. Technol. 2002, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J. Biomethanation and its potential. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 494. [Google Scholar]
- Velmurugan, B. Anaerobic digestion of vegetable wastes for biogas production in a fed-batch reactor. Int. J. Emerg. Sci. 2011, 1, 478. [Google Scholar]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H 2 additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef] [PubMed]

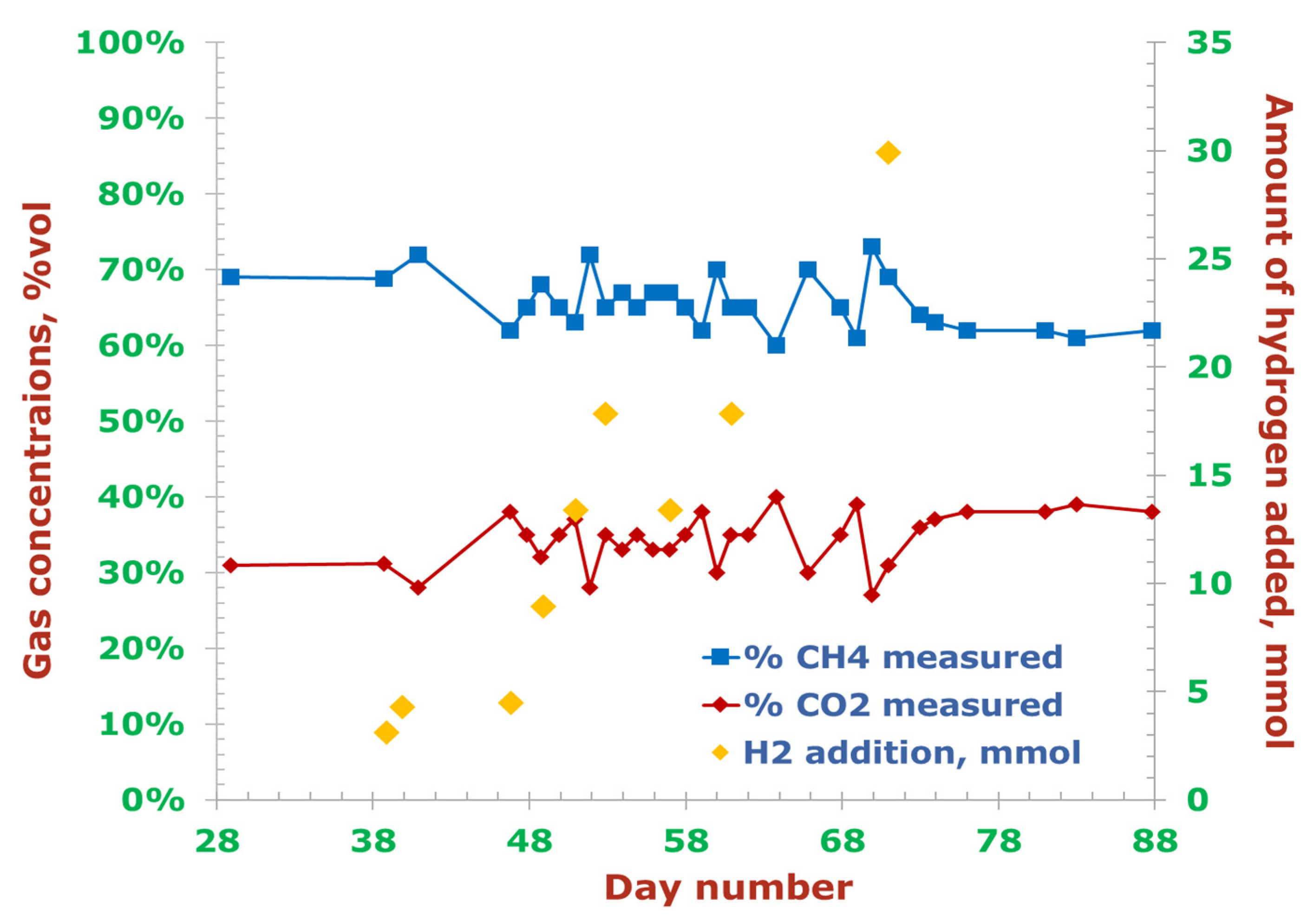
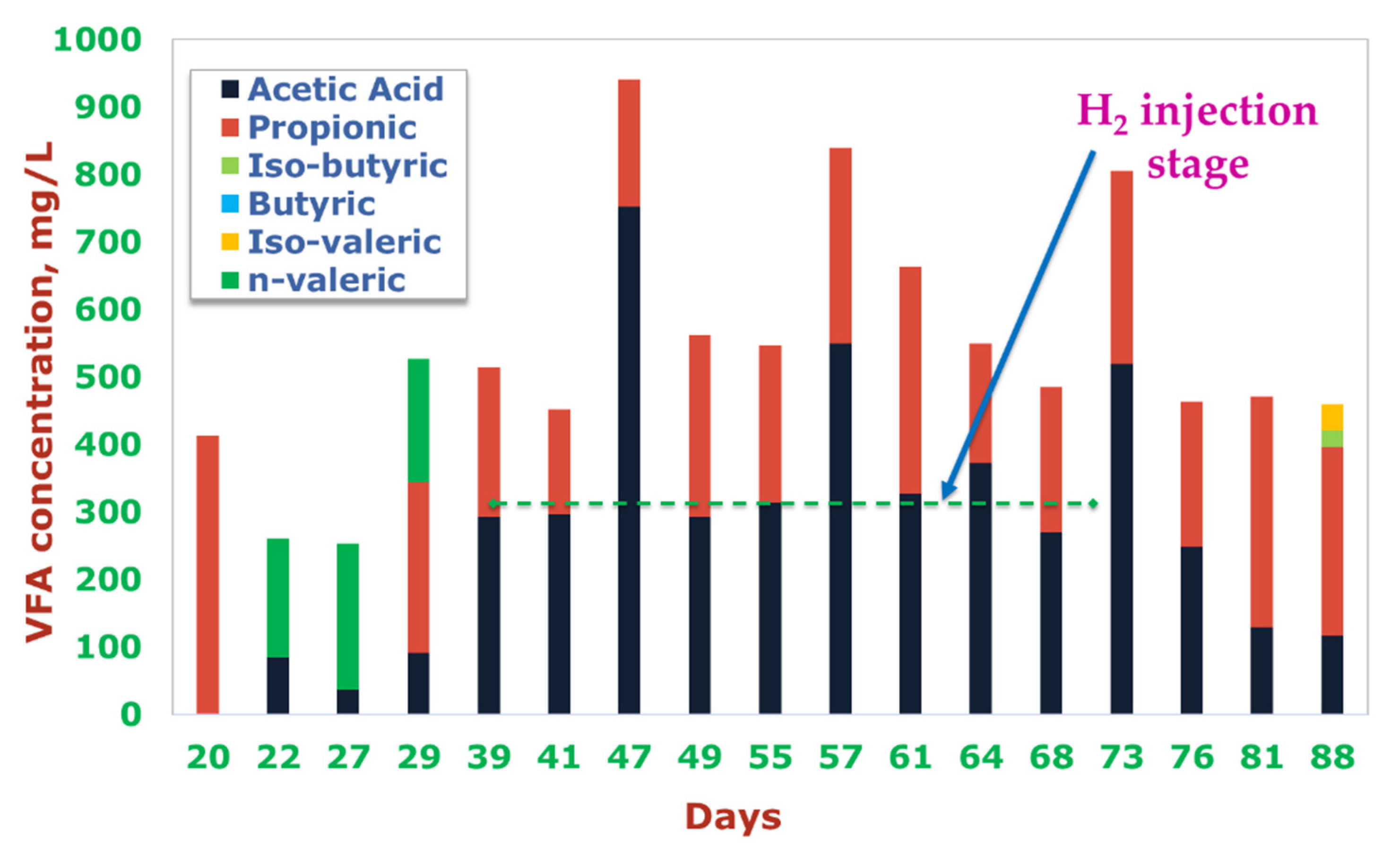
| Day | 39 | 40 | 47 | 49 | 51 | 53 | 57 | 61 | 71 |
| CM input, g/d | 64.1 | 71 | 69.3 | 70.5 | 61 | 71.9 | 69.5 | 64.3 | 72.5 |
| S:I | 2.32 | 2.61 | 3.19 | 3.48 | 3.73 | 4.03 | 4.6 | 5.08 | 6.29 |
| Properties | Unit | Inoculum before Exp. | Substrate before Exp. |
|---|---|---|---|
| TS | wt% | 0.83 ± 0.03 | 12.11 ± 0.07 |
| VS | wt% | 0.39 ± 0.04 | 9.54 ± 0.04 |
| Carbohydrates | wt% | 0.0 | 5.3 |
| Proteins | wt% | <0.30 | 1.56 |
| Lipids | wt% | 2.18 | 0.93 |
| pH | pH | 7.38 | 7.41 |
| TAN | mg/L | 584 | 1590 |
| Total VFA | mg/L | 52.95 ± 1.3 | 5518.23 ± 19.1 |
| Acetic acid | mg/L | n.d. | 4263.85 ± 17.2 |
| Propionic acid | mg/L | n.d. | 694.18 ± 0.6 |
| Iso-butyric acid | mg/L | n.d. | 118.00 ± 0.1 |
| n-butyric acid | mg/L | n.d. | 240.38 ± 0.4 |
| Iso-valeric acid | mg/L | n.d. | 201.82 ± 1.2 |
| n-valeric acid | mg/L | n.d. | n.d. |
| H2 Injection Batch | Day | H2 Injection Amount, mL | H2 Injection Amount, mmol | H2:CO2 | CH4 Content in Biogas | Biogas Amount, mL/d |
|---|---|---|---|---|---|---|
| Batch 1 | 39 | 70.0 | 3.12 | 0.40 | 69% | 525 |
| Batch 2 | 40 | 95.8 | 4.27 | 0.76 | 72% | 591 |
| 41 | - | - | 0.76 | 72% | 590 | |
| Batch 3 | 47 | 100.0 | 4.46 | 0.27 | 62% | |
| 48 | - | - | 0.27 | 65% | 666 | |
| Batch 4 | 49 | 200.0 | 8.92 | 0.62 | 68% | |
| 50 | - | - | 0.62 | 65% | 778 | |
| Batch 5 | 51 | 300.0 | 13.38 | 0.71 | 63% | |
| 52 | - | - | 0.71 | 72% | 686 | |
| Batch 6 | 53 | 400.0 | 17.85 | 0.97 | 65% | |
| 54 | - | - | 0.97 | 67% | 690 | |
| Batch 7 | 57 | 300.0 | 13.38 | 1.11 | 67% | |
| 58 | - | - | 1.11 | 65% | 493 | |
| Batch 8 | 61 | 400.0 | 17.85 | 1.78 | 65% | |
| 62 | - | - | 1.78 | 65% | 391 | |
| Batch 9 | 71 | 670.0 | 29.89 | 2.86 | 69% | |
| 72 | - | - | 2.86 | 64% | 325 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, S.; Wijnsma, S.N.; Lien, K.M. Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion. Energies 2020, 13, 3542. https://doi.org/10.3390/en13143542
Sarker S, Wijnsma SN, Lien KM. Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion. Energies. 2020; 13(14):3542. https://doi.org/10.3390/en13143542
Chicago/Turabian StyleSarker, Shiplu, Sander N. Wijnsma, and Kristian M. Lien. 2020. "Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion" Energies 13, no. 14: 3542. https://doi.org/10.3390/en13143542
APA StyleSarker, S., Wijnsma, S. N., & Lien, K. M. (2020). Lessons Learned from an Experimental Campaign on Promoting Energy Content of Renewable Biogas by Injecting H2 during Anaerobic Digestion. Energies, 13(14), 3542. https://doi.org/10.3390/en13143542