A Numerical Study for Performance Prediction of a Metal Hydride Thermal Energy Conversion System Elaborating the Superadiabatic Condition
Abstract
:1. Introduction
2. Mathematical Model
2.1. Thermodynamic Characteristics of a Metal Hydride
2.2. Thermodynamic Cycle of a Metal Hydride Heat Pump (MHHP)
2.3. A Metal Hydride Thermal Conversion System with Superadiabatic Thermal Energy Conversion Waves (TECWs)
2.4. Heat Transfer Model for Parametric Study
3. Numerical Calculation Results and Discussion
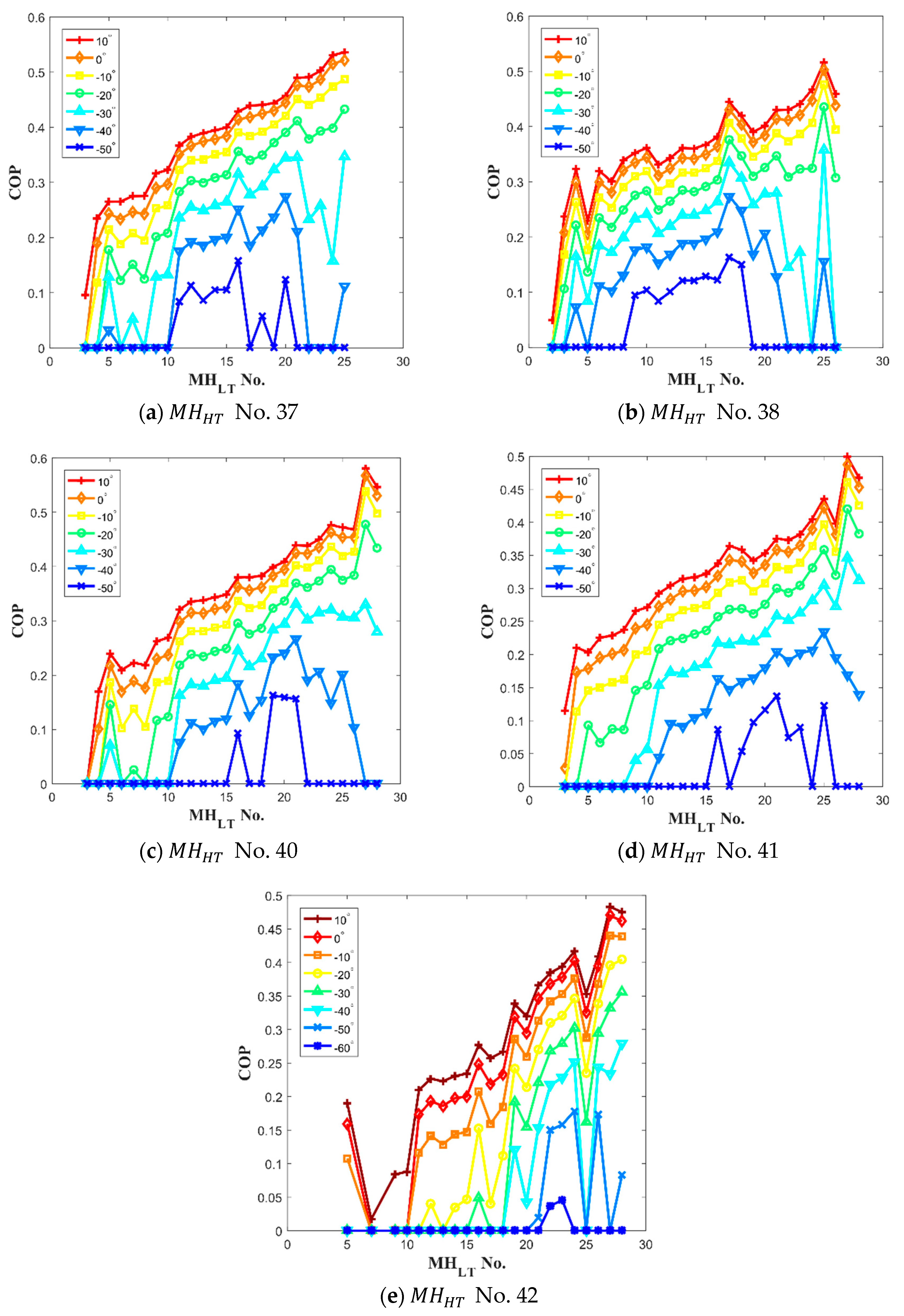
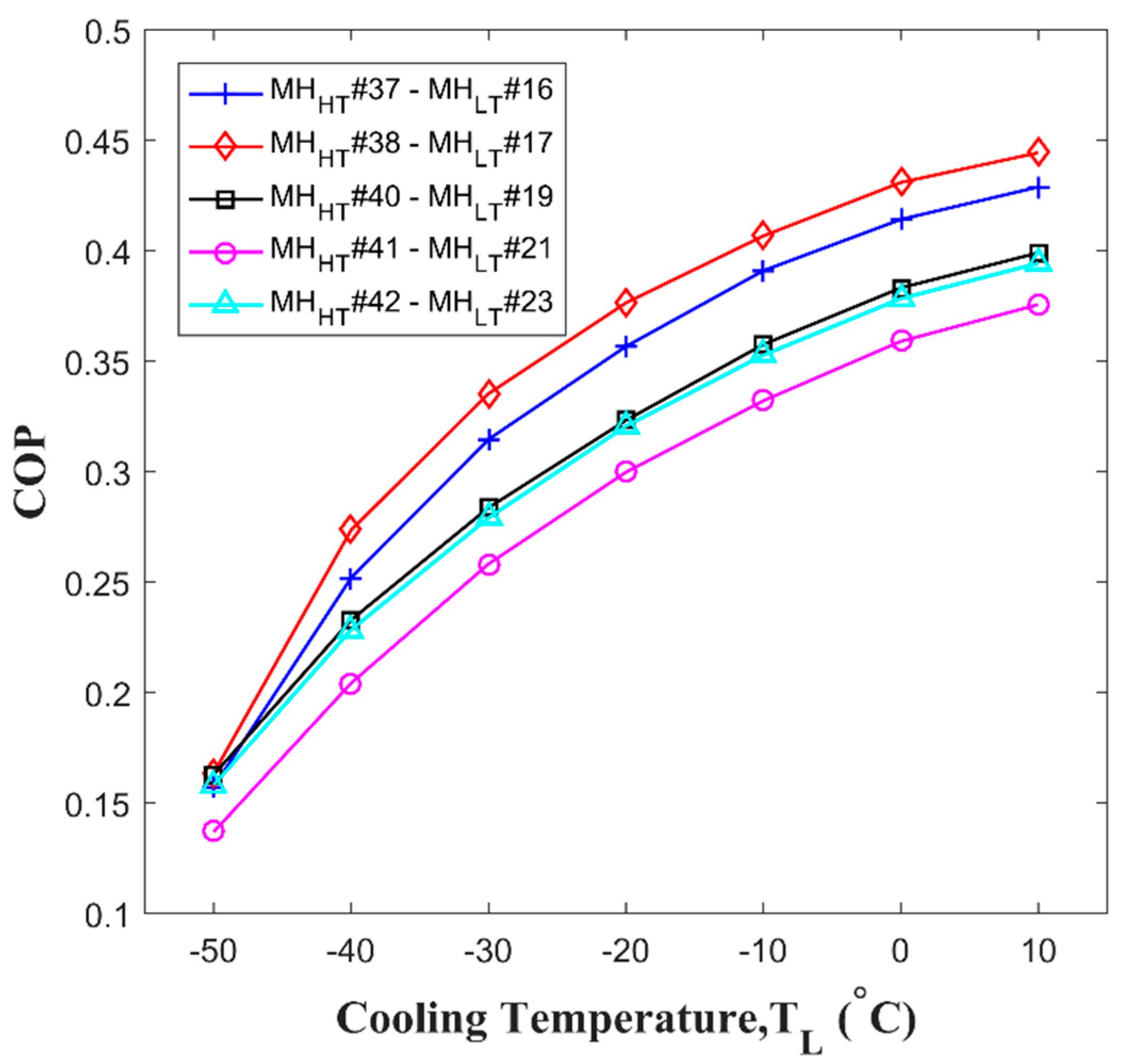
3.1. Selecting the Hydride Pair
3.2. Design and Operation Parameters of the Thermal Conversion System
4. Conclusions
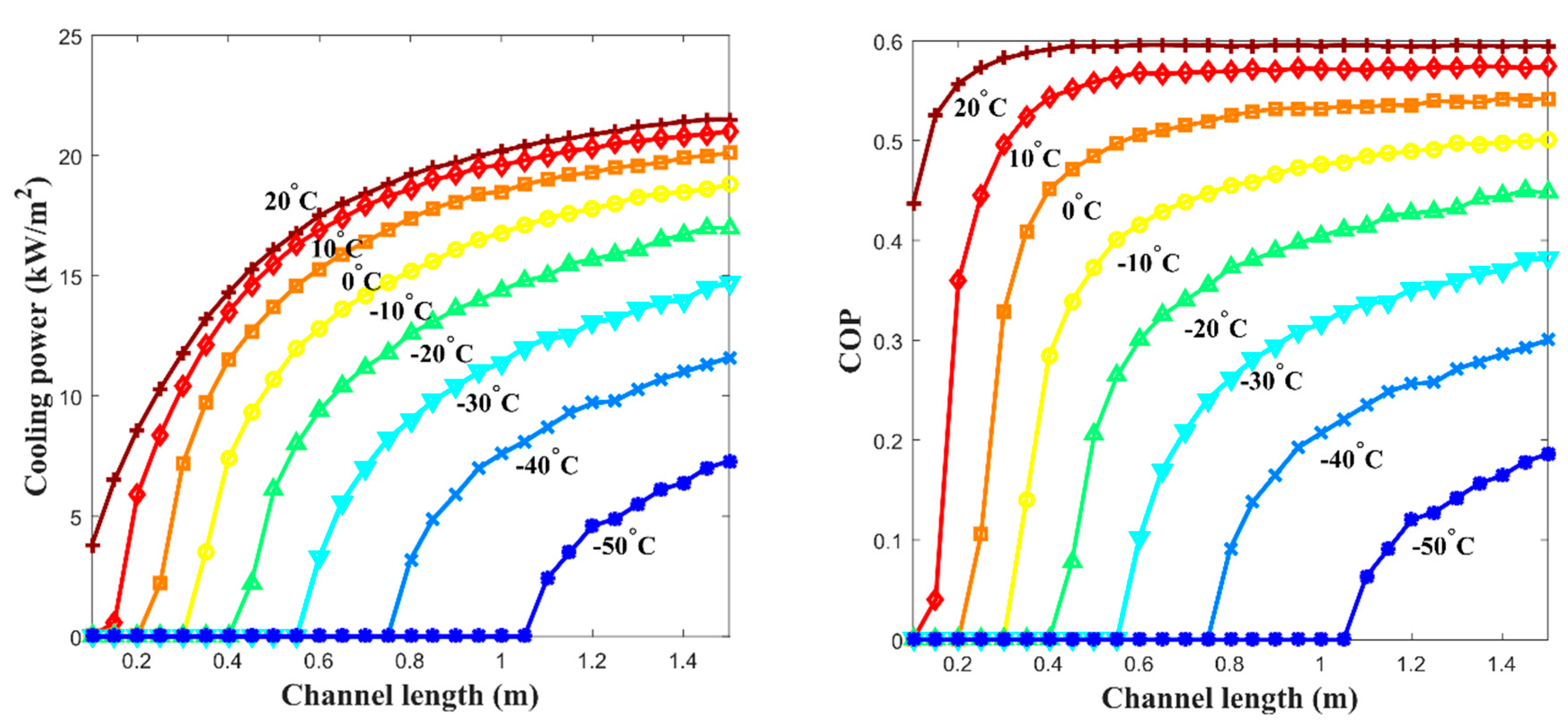
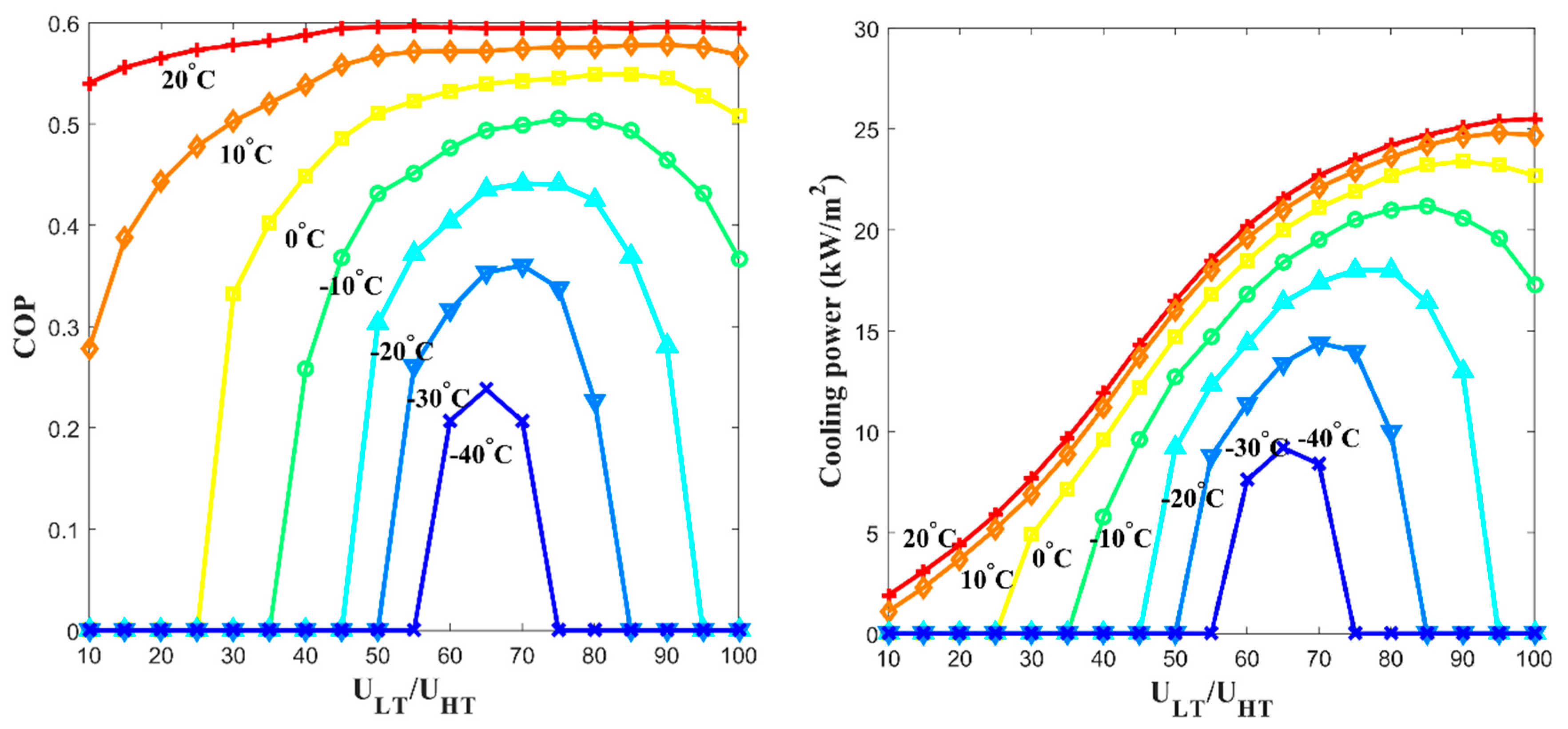
- The specific cooling power; 16.4 kW/m2 at −20 °C, 9.2 kW/m2 at −40 °C was achieved by parametric study results when the channel length is 1 m, the free thermal wave velocity at the high-temperature channel is 10 cm/min, and the free thermal wave velocity at the low-temperature channel is 6.5 cm/min. If the cross-sectional area of the channel is 0.04 m2 (=20 cm × 20 cm), each calculated power is 656 W and 368 W. It was shown that the proposed system’s amount of specific cooling power could be beneficial in an actual application.
- In the case of 10 °C and −20 °C of the cooling effect () from the ambient mean temperature at 30 °C, each of the proposed system was 0.59 and 0.57, which is similar to the ideal of the selected pair. It seemed that the heat exchange between the arrayed MHHP units and the propagation of the thermal wave contributes to the improvement of the metal hydride thermal conversion performance.
Author Contributions
Funding
Conflicts of Interest
References
- Sarma, K.M.; Bankobeza, G.M.; Mulumba, M.A. The Montreal Protocol on Substances that Deplete the Ozone Layer United; Ozone Secretariat: Nairobi, Kenya, 1990. [Google Scholar]
- Yang, L.; Jiang, W.; Ji, W.; Mahian, O.; Bazri, S.; Sadri, R.; Badruddin, I.A.; Wongwises, S. A review of heating/cooling processes using nanomaterials suspended in refrigerants and lubricants. Int. J. Heat Mass Transf. 2020, 153, 119611. [Google Scholar] [CrossRef]
- Goetzler, W.; Zogg, R.; Burgos, J.; Hiraiwa, H.; Young, J. Energy Savings Potential and RD & D Opportunities for Commercial Building HVAC Systems. 2017. Available online: https://www.energy.gov/sites/prod/files/2017/12/f46/bto-DOE-Comm-HVAC-Report-12-21-17.pdf (accessed on 5 May 2020).
- Sheft, I.; Gruen, D.M.; Lamich, G.J. HYCSOS Chemical Heat Pump and Energy Conversion System Based on Metal. Hydrides; Argonne National Lab: Lemont, IL, USA, 1979.
- Lototskyy, M.; Sekhar, B.S.; Muthukumar, P.; Linkov, V.; Pollet, B.G. Niche applications of metal hydrides and related thermal management issues. J. Alloy. Compd. 2015, 645, S117–S122. [Google Scholar] [CrossRef]
- Kim, K. Propagation of Waves of Metal-Hydride Thermal Conversion in Blown-Through Porous Media. J. Eng. Phys. 1998, 71, 1–11. [Google Scholar] [CrossRef]
- Fateev, G.A.; Silenkov, M.A.; Kim, K. Experimental Investigation of The Propagation of Heat Waves of Energy Conversion in Blown-through Porous Media. J. Eng. Phys. 2000, 73, 1069–1081. [Google Scholar]
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials. In Thermodynamic Properties of Metal Hydrides. Thermodynamics—Interaction Studies—Solids, Liquids and Gases; InTech: London, UK, 2011. [Google Scholar]
- Johnson, J.R.; Reilly, J.J.; Reidinger, F.; Corliss, L.M.; Hastings, J.M. On the Existence of F.C.C. TiCr1.8H5.3. J. Less Common Met. 1982, 88, 107–114. [Google Scholar] [CrossRef]
- Vogt, T.; Reilly, J.J.; Johnson, J.R.; Adzic, G.D.; McBreen, J. Crystal Structure of NonstoichiometricLa(Ni,Sn)5+x Alloys and Their Properties as Metal Hydride Electrodes. Electrochem. Solid State Lett. 1999, 2, 111–114. [Google Scholar] [CrossRef]
- Klyamkin, S.N.; Verbetsky, V.N.; Karih, A.A. Thermodynamic particularities of some CeNi5-based metal hydride systems with high dissociation pressure. J. Alloy. Compd. 1995, 231, 479–482. [Google Scholar] [CrossRef]
- Libowitz, G.G.; Maeland, A.J. Hydride Formation by B.C.C. Solid Solution Alloys. Mater. Sci. Forum 1988, 31, 177–196. [Google Scholar] [CrossRef]
- Liu, J.; Huston, E.L. RNi5 Hydrogen Storage Compounds (R = Rare Earth). J. Less Common Met. 1983, 90, 11–20. [Google Scholar] [CrossRef]
- Gao, X.; Song, D.; Zhang, Y.; Zhou, Z.; Zhang, W.; Wang, M.; Shen, P. Electrochemical and surface properties of the Zr(V0.2Mn0.2Ni0.6)2.4 alloy electrode. J. Alloy. Compd. 1995, 299, 268–273. [Google Scholar] [CrossRef]
- Uchida, H.; Tada, M.; Huang, Y.C. The Influence of Cerium, Praseodymium, Neodymium and Samarium on Hydrogen Absorption in LaNi5 Alloys. J. Less Common Met. 1982, 88, 81–87. [Google Scholar] [CrossRef]
- Noh, H.; Clewley, J.D.; Flanagan, T.B.; Craft, A.P. Hydrogen-induced phase separation in Pd-Rh alloys. J. Alloy. Compd. 1996, 240, 7–16. [Google Scholar] [CrossRef]
- Mishima, R.; Miyamura, H.; Sakai, T.; Kuriyama, N.; Ishikawa, H.; Uehara, I. Hydrogen storage alloys rapidly solidified by the melt-spinning method and their characteristics as metal hydride electrodes. J. Alloy. Compd. 1993, 192, 176–178. [Google Scholar] [CrossRef]
- Ron, M. A Hydride Heat Pump as a Bus Air Conditioner. J. Less Common Met. 1984, 104, 259–278. [Google Scholar] [CrossRef]
- Tsugita, Y.; Okajima, Y.; Sakai, T.; Miyamura, H.; Kuriyama, N.; Ishikawa, H.; Uehara, I. Hydrogen storage alloy powder produced by reduction-diffusion process and their electrode properties. J. Alloy. Compd. 1993, 192, 167–169. [Google Scholar]
- Sandrock, G.D. Development of Low Cost Nickel-Rare Earth Hydrides for Hydrogen Storage. In Hydrogen Energy System; Vezeroglu, T.N., Seifritz, W., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 1625–1656. [Google Scholar]
- Lundin, C.E.; Lynch, F.E. Modification of Hydriding Properties of AB5 Type Hexagonal Alloys through Manganese Substitution. Int. Conf. Altern. Energy Sources 1978, 8, 3803–3805. [Google Scholar]
- Matsumoto, T.; Matsushita, A. Hydrides in the PrNi5-H2 System. J. Less Common Met. 1987, 132, 115–121. [Google Scholar] [CrossRef]
- Latroche, M.; Percheron-Guegan, A.; Chabre, Y.; Bouet, J.; Pannetier, J.; Ressouche, E. Intrinsic behavior analysis of substituted LaNi5-type electrodes by means of in-situ neutron diffraction. J. Alloy. Compd. 1995, 231, 537–545. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Nash, P. The Ni-Pr (Nickel-Praesodymium) system. Bull. Alloy. Phase Diagr. 1989, 10, 253–257. [Google Scholar] [CrossRef]
- Sakai, T.; Yoshinaga, H.; Miyamura, H.; Kuriyama, N.; Ishikawa, H. Rechargeable hydrogen batteries using rare-earth-based hydrogen storage alloys. J. Alloy. Compd. 1992, 180, 37–54. [Google Scholar] [CrossRef]
- Gamo, T.; Moriwaki, Y.; Yanagihara, N.; Iwaki, T. Life properties of ti-mn alloy hydrides and their hydrogen purification effect. J. Less Common Met. 1983, 89, 495–504. [Google Scholar] [CrossRef]
- Bershadsky, E.; Klyuch, A.; Ron, M. Hydrogen Absorption and Desorption Kinetics of TiFe0.8Ni0.2. Int. J. Hydrogen Energy 1995, 20, 29–33. [Google Scholar] [CrossRef]
- Ivey, D.G.; Northwood, D.O. Hydriding Properties of Zr(FexCr1−x)2 Intermetallic Compounds. Int. J. Hydrogen Energy 1986, 11, 583–591. [Google Scholar] [CrossRef]
- Nobile, A.; Walters, R.T.; Mosley, W.C. Effects of radiolytic tritium decay on the thermodynamic behavior of LaNi4.25Al0.75 tritides. J. Less Common Met. 1991, 172, 1352–1362. [Google Scholar] [CrossRef]
- Ortman, M.S.; Heung, L.K.; Nobile, A.; Rabun, R.L. Tritium processing at the savannah river site: Present and Future. J. Vac. Sci. Technol. 1990, 8, 2881–2889. [Google Scholar] [CrossRef]
- Percheron-Guegan, A.; Lartigue, C.; Achard, J.C. Correlations between the structural Properties, the Stability and the Hydrogen Content of Substituted LaNi5 Compounds. J. Less Common Met. 1985, 109, 287–309. [Google Scholar] [CrossRef]
- Huston, E.L.; Sandrock, G.D. Engineering properties of metal hydrides. J. Less Common Met. 1980, 74, 435–443. [Google Scholar] [CrossRef]
- Osumi, Y.; Suzuki, H.; Kato, A.; Oguro, K.; Sugioka, T.; Fujita, T. Hydrogen storage properties of Ti1+xCr2−yMny alloys. J. Less Common Met. 1983, 89, 257–262. [Google Scholar] [CrossRef]
- Bernauer, O.; Ziegler, K. Hydrogen storage alloy. J. Res. Phi. Chem. Chem. 1984, 4, 891. [Google Scholar]
- Perevesenzew, A.; Lanzel, E.; Elder, O.J.; Tuscher, E.; Weinzierl, P. Thermodynamics and Kinetics of Hydrogen Absorption in the Intermetallic Compounds Zr(Cr1−xVx)2. J. Less Common Met. 1988, 143, 39–47. [Google Scholar] [CrossRef]
- Sandrock, G.D.; Goodell, P.D. Cyclic life of metal hydrides with impure hydrogen: Overview and engineering considerations. J. Less Common Met. 1984, 104, 159–173. [Google Scholar] [CrossRef]
- Lasocka, M. Binary Alloy. Phase Diagrams; ASM International: Novelty, OH, USA, 1990; Volume 3. [Google Scholar]
- Willems, J.J.G.; Buschow, K.H.J. From permanent magnets to rechargeable hydride electrodes. J. Less Common Met. 1987, 129, 13–30. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E. Binary Alloy. Phase Diagrams, 2nd ed.; ASM International: Novelty, OH, USA, 1990; Volume 2. [Google Scholar]
- Osumi, Y.; Suzuki, H.; Kato, A.; Nakane, M.; Miyake, Y. Absorption-desorption characteristics of hydrogen for mischmetal-nickel-cobalt alloys. J. Soc. Chem. Ind. Jpn. 1979, 6, 722–726. [Google Scholar]
- Karakaya, I.; Thompson, W.T. Binary Alloy. Phase Diagrams, 2nd ed.; ASM International: Novelty, OH, USA, 1990; Volume 1. [Google Scholar]
- Park, J.-M.; Lee, J.-Y. Thermodynamic Properties of the Zr0.8Ti0.2(MnxCr1−x)Fe-H2 System. J. Less Common Met. 1991, 167, 245–253. [Google Scholar]
- Eisenberg, F.G.; Goodell, P.D. Cycling response of reversible hydriding alloys in hydrogen containing carbon monoxide. J. Less Common Met. 1983, 89, 55–62. [Google Scholar] [CrossRef]
- Buschow, K.H.; van Mal, H.H. Phase relations and hydrogen absorption in the lanthanum-nickel system. J. Less Common Met. 1972, 29, 203–210. [Google Scholar] [CrossRef]
- Groll, M.; Isselhorst, A.; Wierse, M. Metal hydride devices for environmentally clean energy technology. Int. J. Hydrogen Energy 1994, 19, 507–515. [Google Scholar] [CrossRef]
- Lewis, D. Metal. Hydride Heat-Pump Development at Studsvik, the Heat-Upgrading Experiment, GE; Studsvik Energiteknik: Nyköping, Sweden, 1984. [Google Scholar]
- Nakamura, K.; Hoshi, T. Supply and recovery of hydrogen isotopes in high vacuum systems using ZrNi hydride getter pumps. J. Vac. Sci. Technol. 1985, 3, 34–38. [Google Scholar] [CrossRef]
- Bawa, M.S.; Ziem, a.E.A. Long Term Testing and Stability of CaNi5 Alloy for a Hydrogen Storage Application. Int. J. Hydrogen Energy 1982, 7, 775–781. [Google Scholar] [CrossRef]
- Akiba, E.; Hayakowa, H.; Ishido, Y.; Nomura, K. Mg-Zn-Ni hydrogen storage alloys. J. Less Common Met. 1991, 172, 1071–1075. [Google Scholar] [CrossRef]
- Luo, S.; Luo, W.; Clewley, J.D.; Flanagan, T.B.; Bowman, J.R.C. Thermodynamic and Degredation Studies of LaNi4.8Sn0.2-H using Isotherms and Calorimetry. J. Alloy. Compd. 1995, 231, 473–478. [Google Scholar] [CrossRef]
- Rapkin, E.; Steele, G.; Schavey, R. Modern tritium handling in the synthesis laboratory. Am. Lab. 1995, 27, 31–38. [Google Scholar]
- Willers, E.; Groll, M. Evaluation of metal hydride machines for heat pumping and cooling applications. Int. J. Refrig. 1999, 22, 47–58. [Google Scholar] [CrossRef]
- Warren, D.E.; Faughnan, K.A.; Fellows, R.A.; Godden, J.W.; Seck, B.M. Aircraft thermal detection utilizing metal hydrides. J. Less Common Met. 1984, 104, 375–383. [Google Scholar] [CrossRef]
- Marmaro, R.W.; Lynch, F.E. Investigation of Long Term Stability, in Metal. Hydrides; Hydrogen Consultants, Inc.: Littleton, CO, USA, 1991. [Google Scholar]
- Beck, R.L.; Mueller, W.A. Zirconium Hydrides and Hafnium Hydrides. In Metal Hydrides; Mueller, W.M., Blackledge, J.P., Libowitz, G.G., Eds.; Academic Press: New York, NY, USA, 1968; pp. 241–335. [Google Scholar]
- Ally, M.R.; Rebello, W.J.; Rosso, M.J., Jr. Metal hydride chemical heat pumps for industrial use. In Proceedings of the Sixth Annual Industrial Energy Technology Conference, Houston, TX, USA, 15–18 April 1984; Volume II, pp. 686–693. [Google Scholar]
- Domschke, T.; Nietsch, T.; Schütt, E. The temperature-dependence of hydrogen sorption in metal hydrides. Int. J. Hydrogen Energy 1991, 16, 255–263. [Google Scholar] [CrossRef]
- Meng, X.; Wu, Z.; Bao, Z.; Yang, F.; Zhang, Z. Performance simulation and experimental confirmation of a mini-channel metal hydrides reactor. Int. J. Hydrogen Energy 2013, 38, 15242–15253. [Google Scholar] [CrossRef]
- Weckerle, C.; Bürger, I.; Linder, M. Novel reactor design for metal hydride cooling systems. Int. J. Hydrogen Energy 2017, 42, 8063–8074. [Google Scholar] [CrossRef]
- Silin, N.; Melnichuk, M. A case study of a hydride container performance applying non dimensional parameters. Int. J. Hydrogen Energy 2014, 39, 21060–21067. [Google Scholar] [CrossRef]
- Mellouli, S.; Askri, F.; Dhaou, H.; Jemni, A.; Nasrallah, S.B. A study of the thermal behavior of a deformable metal-hydride bed. Int. J. Hydrogen Energy 2016, 41, 1711–1724. [Google Scholar] [CrossRef]
- Fateev, G.A.; Rabinovich, O.S. Metal hydride heat conversion on the basis of superadiabatic combustion waves in porous media. Int. J. Hydrogen Energy 1997, 22, 915–924. [Google Scholar] [CrossRef]
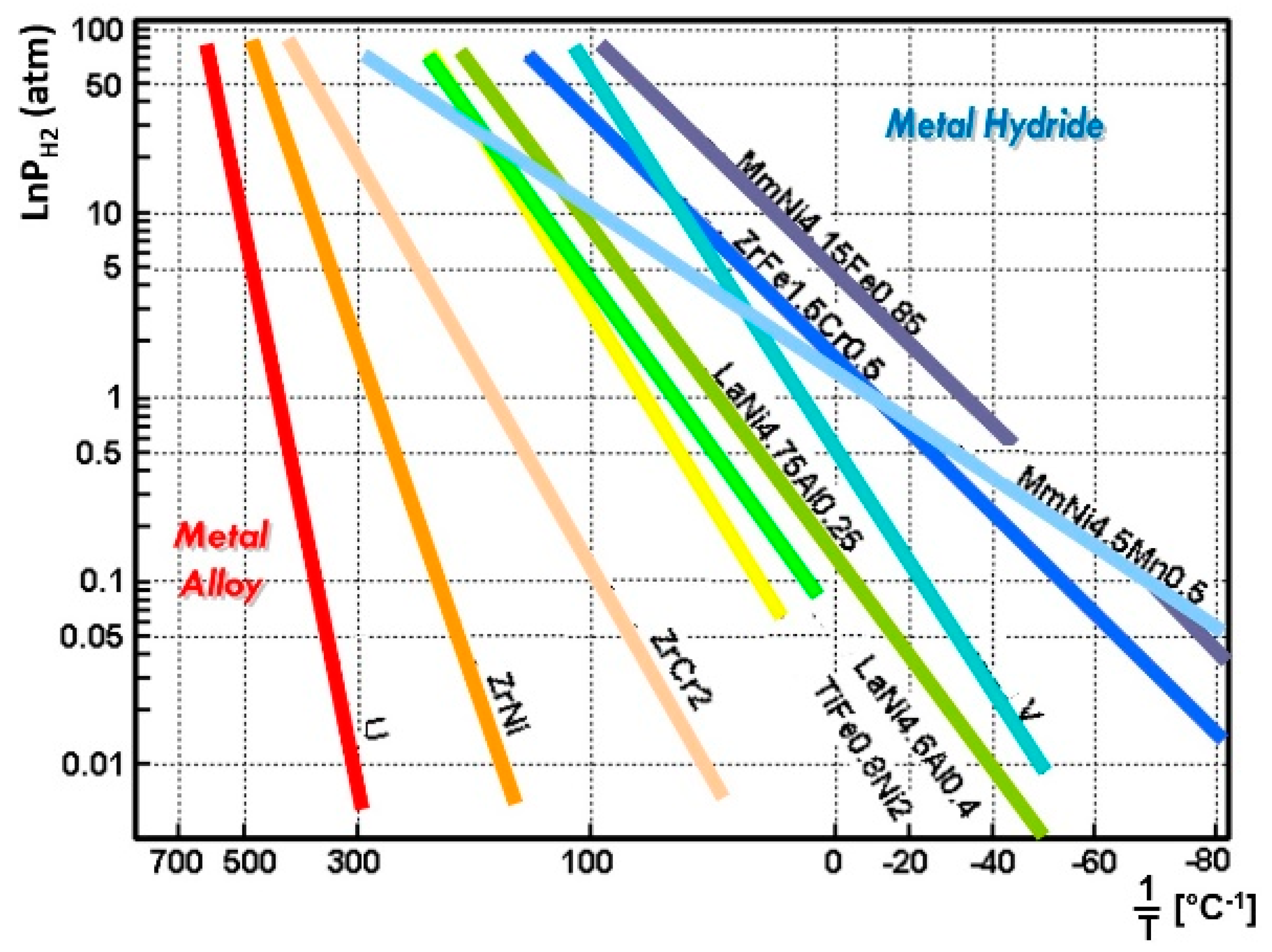

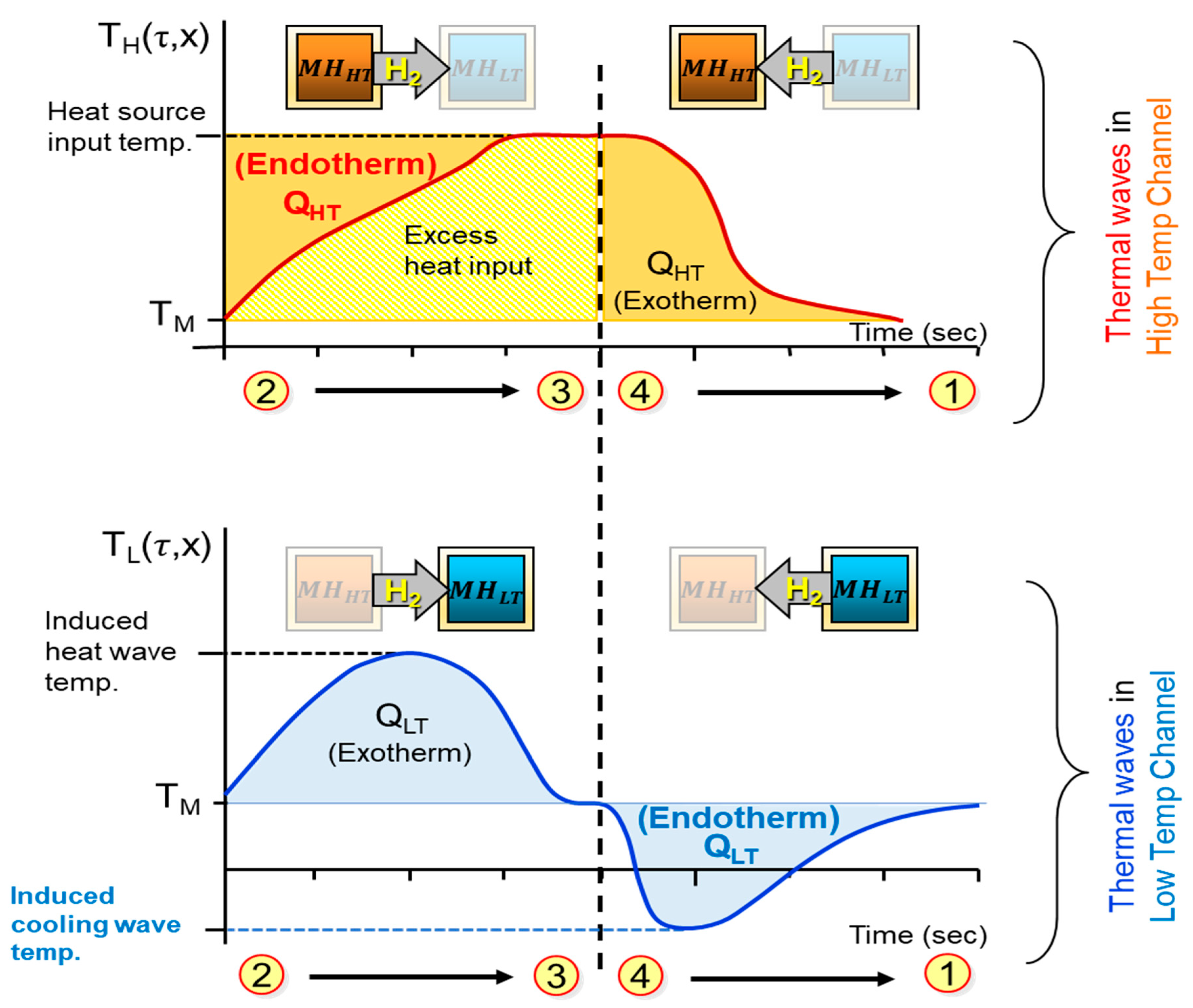
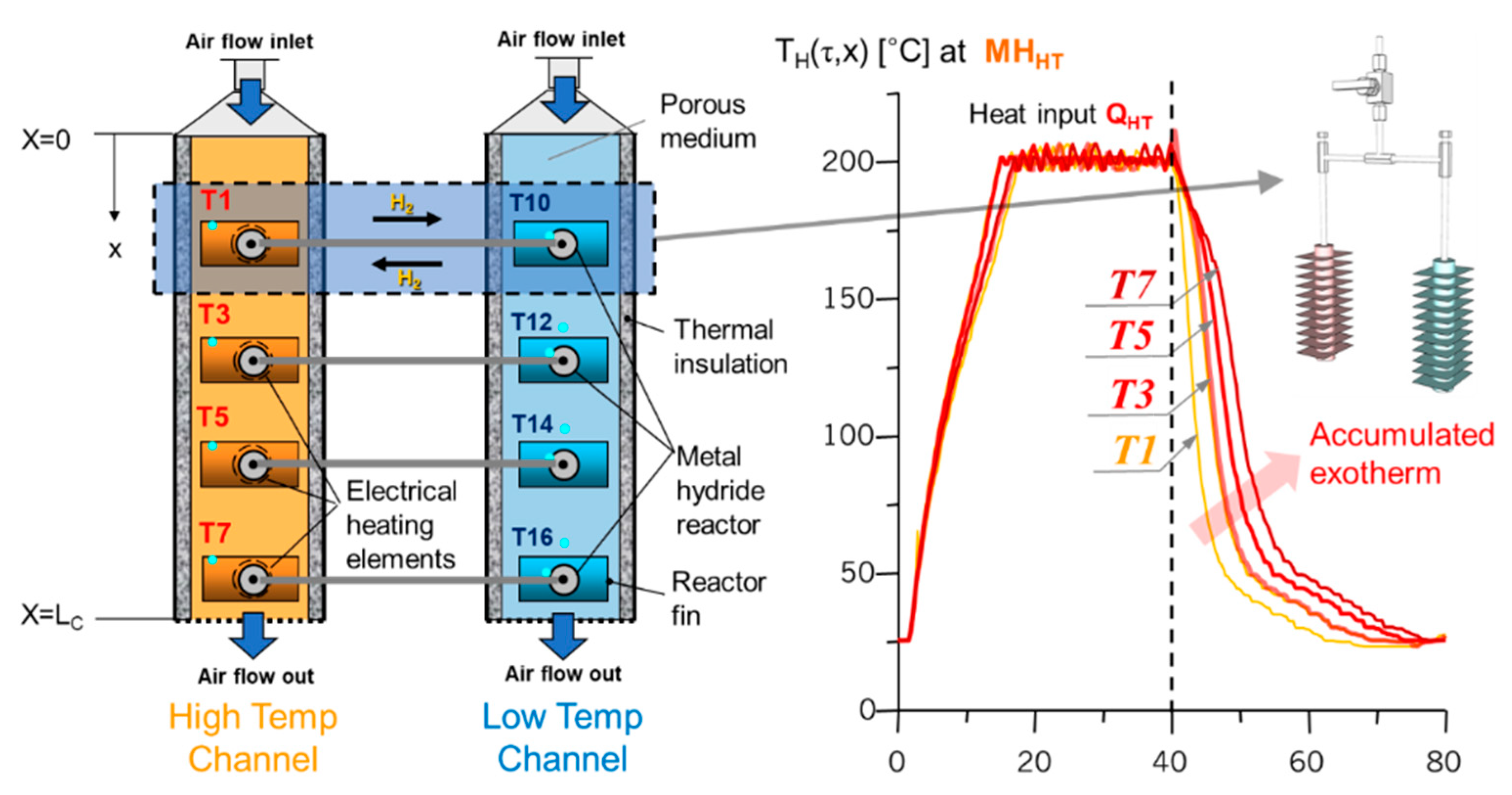
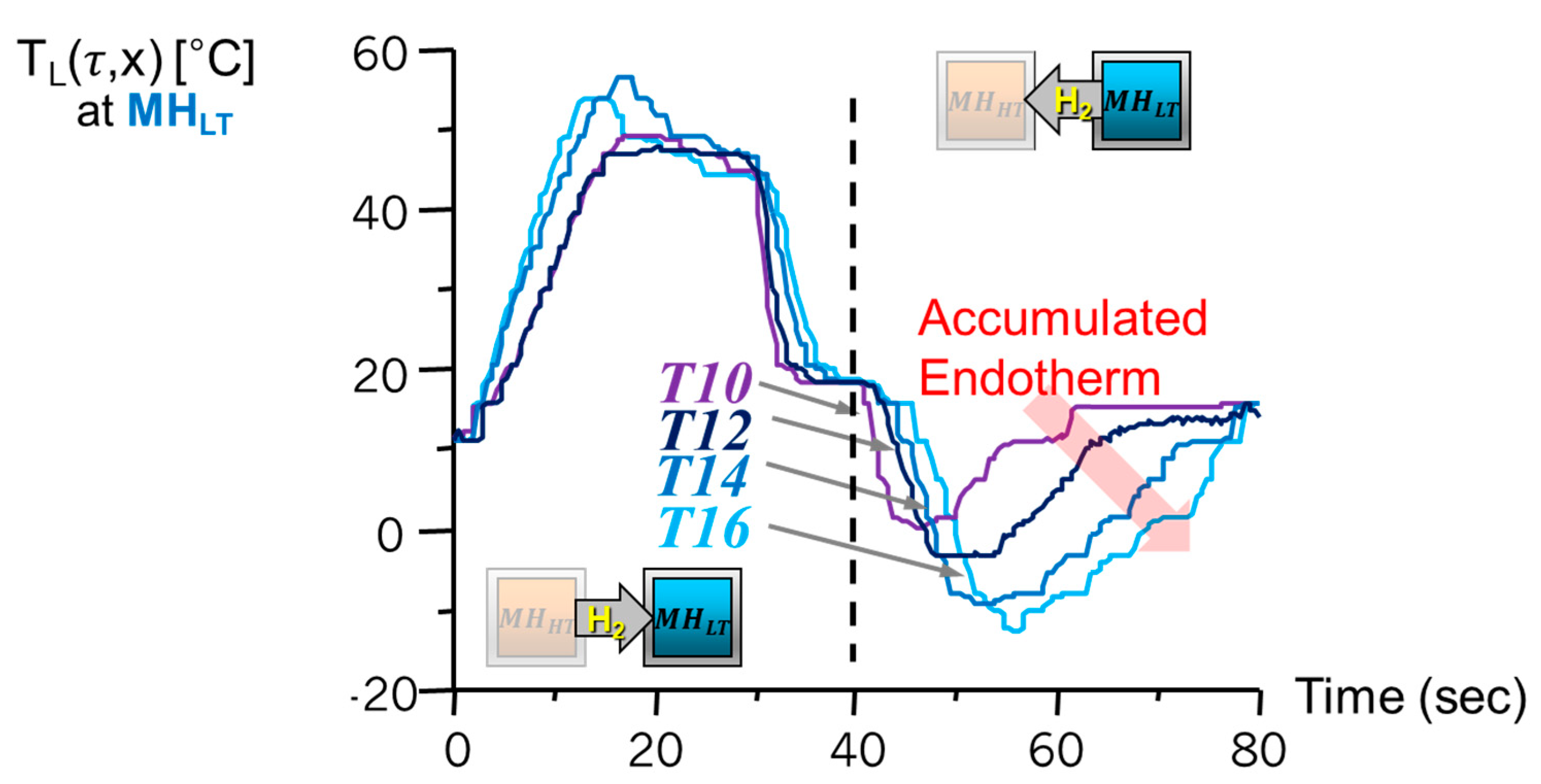
| No. | Nominal Composition | (kJ/mol·H2) | (kJ/mol H2·K) | No. | Nominal Composition | (kJ/mol·H2) | (kJ/mol H2·K) |
|---|---|---|---|---|---|---|---|
| 1 | TiCr1.8 [9] | 20.2 | 0.111 | 26 | LaNi4.7Al0.3 [10] | 34.0 | 0.1068 |
| 2 | CeNi5 [11] | 22.2 | 0.111 | 27 | (V0.9Ti0.1)0.95Fe0.05 [12] | 43.20 | 0.1396 |
| 3 | MmNi5 [13] | 21.1 | 0.097 | 28 | Zr(V0.2Mn0.2Ni0.6)2.4 [14] | 39.9 | 0.1257 |
| 4 | NdNi5 [15] | 27.8 | 0.116 | 29 | Pd0.9Rh0.1 [16] | 34.2 | 0.102 |
| 5 | MmNi4.5Mn0.5 [9] | 17.6 | 0.067 | 30 | LaNi4.6Al0.4 [17] | 36.4 | 0.1092 |
| 6 | MmNi4.15Fe0.85 [18] | 25.3 | 0.105 | 31 | MmNi4.2Co0.2Mn0.3Al0.3 [19] | 36.5 | 0.1087 |
| 7 | MmNi3.5Cu1.5 [20] | 23.4 | 0.097 | 32 | LaNi4.6Mn0.4 [21] | 39.4 | 0.117 |
| 8 | PrNi5 [22] | 27.6 | 0.113 | 33 | LaNi4.5Al0.5 [23] | 38.49 | 0.11129 |
| 9 | Pr2Ni7 [24] | 27.8 | 0.111 | 34 | MmNi3.5Co0.7Al0.8 [25] | 39.8 | 0.115 |
| 10 | TiMn1.5 [26] | 28.7 | 0.114 | 35 | TiFe0.8Ni0.2 [27] | 41.2 | 0.119 |
| 11 | ZrFe1.5Cr0.5 [28] | 25.61 | 0.0975 | 36 | LaNi4.25Al0.75 [29] | 44.1 | 0.117 |
| 12 | Ca0.7Mm0.3Ni5 [20] | 26.6 | 0.1 | 37 | Pd [30] | 41.0 | 0.0976 |
| 13 | TiFe [27] | 28.1 | 0.106 | 38 | LaNi4Al [31] | 47.7 | 0.11883 |
| 14 | MmNi4.5Al0.5 [32] | 28 | 0.105 | 39 | TiCo [33] | 54 | 0.135 |
| 15 | TiV0.62Mn1.5 [34] | 28.6 | 0.107 | 40 | ZrCr2 [35] | 45.2 | 0.103 |
| 16 | TiFe0.9Mn0.1 [36] | 29.5 | 0.107 | 41 | ZrMn2 [37] | 53.2 | 0.121 |
| 17 | SmCo5 [38] | 34.95 | 0.129 | 42 | GdFe3 [39] | 50.4 | 0.105 |
| 18 | MmNi3Co2 [40] | 32.7 | 0.12 | 43 | Pd0.7Ag0.3 [41] | 50.0 | 0.101 |
| 19 | Zr0.8Ti0.2MnFe [42] | 29.6 | 0.101 | 44 | Mg2Ni [43] | 64.5 | 0.122 |
| 20 | LaNi5 [44] | 30.8 | 0.108 | 45 | Mg [45] | 74.5 | 0.135 |
| 21 | LaNi4.9Al0.1 [46] | 32.64 | 0.11046 | 46 | ZrNi [47] | 76.85 | 0.136 |
| 22 | CaNi5 [48] | 31.9 | 0.101 | 47 | Mg51Zn20 [49] | 84.0 | 0.157 |
| 23 | LaNi4.8Sn0.2 [50] | 32.8 | 0.105 | 48 | U [51] | 127 | 0.180 |
| 24 | LaNi4.75Al0.25 [52] | 34.73 | 0.11046 | 49 | Ti [53] | 164 | 0.179 |
| 25 | V [54] | 40.1 | 0.1407 | 50 | Zr [55] | 217 | 0.188 |
| Parameter | Value | Unit |
|---|---|---|
| Length of Channel (Lc) | 50 | cm |
| Ambient mean temperature (TM) | 20 | °C |
| Input heat temperature at HT Channel (TH) | 320 | °C |
| Time duration of heat input at HT Channel () | 7.5 | min |
| Free Thermal Wave Velocity at HT Channel () | 5 | cm/min |
| Free Thermal Wave Velocity at LT Channel () | 3 | cm/min |
| The Adiabatic Temp. of the HT Channel () | 40 | °C |
| The Adiabatic Temp. of the LT Channel () | 20 | °C |
| The Thermal Conductivity of the Porous Medium () | 1 | W/m∙°C |
| The volumetric heat transfer coefficient between metal hydride alloy and porous media at HT Channel () | 10,000 | W/m∙°C |
| The volumetric heat transfer coefficient between metal hydride alloy and porous media at LT Channel() | 10,000 | W/m∙°C |
| The Density of the Porous Medium () | 1500 | kg/m3 |
| The Specific Heat of the Porous Medium () | 1000 | W/m3∙°C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, S.; Kang, S.; Kim, K.-J. A Numerical Study for Performance Prediction of a Metal Hydride Thermal Energy Conversion System Elaborating the Superadiabatic Condition. Energies 2020, 13, 3095. https://doi.org/10.3390/en13123095
Ham S, Kang S, Kim K-J. A Numerical Study for Performance Prediction of a Metal Hydride Thermal Energy Conversion System Elaborating the Superadiabatic Condition. Energies. 2020; 13(12):3095. https://doi.org/10.3390/en13123095
Chicago/Turabian StyleHam, Suyun, Sanggoo Kang, and Kyu-Jung Kim. 2020. "A Numerical Study for Performance Prediction of a Metal Hydride Thermal Energy Conversion System Elaborating the Superadiabatic Condition" Energies 13, no. 12: 3095. https://doi.org/10.3390/en13123095
APA StyleHam, S., Kang, S., & Kim, K.-J. (2020). A Numerical Study for Performance Prediction of a Metal Hydride Thermal Energy Conversion System Elaborating the Superadiabatic Condition. Energies, 13(12), 3095. https://doi.org/10.3390/en13123095






