Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding
Abstract
1. Introduction
1.1. Recovery Mechanisms of Low-Salt/Sulfate-Modified Water
1.1.1. Wettability Alteration
1.1.2. Oil–Brine Interfacial Interactions (Interfacial Viscoelasticity)
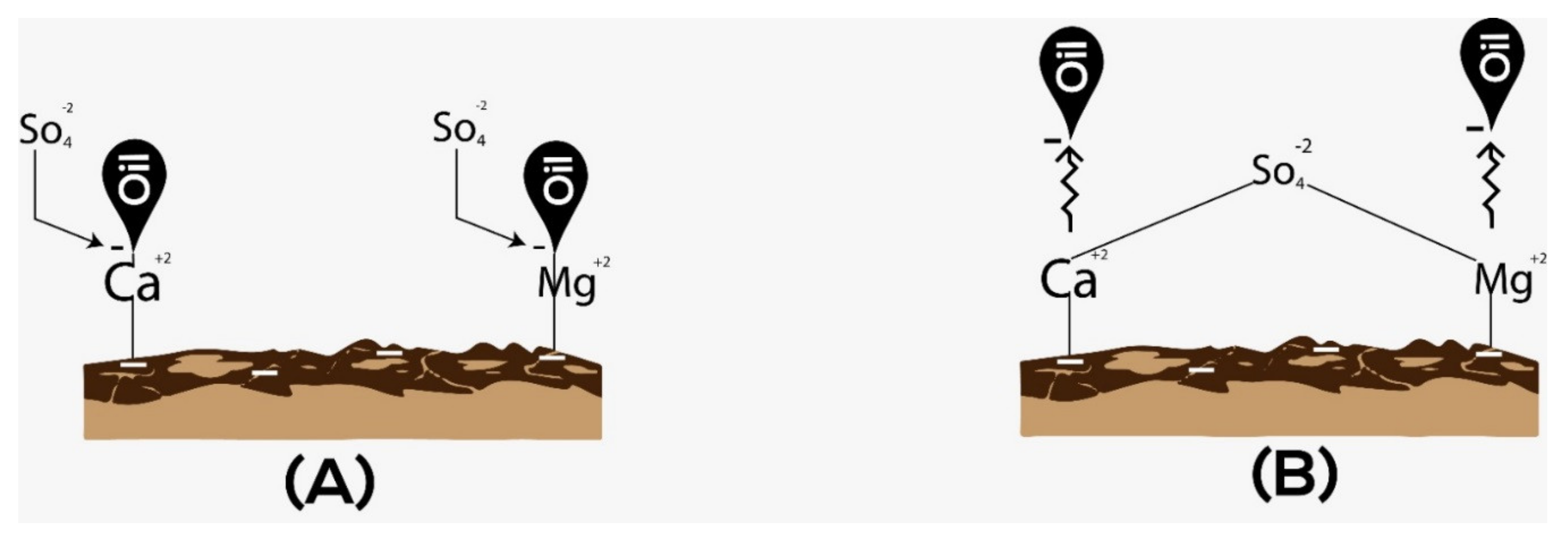
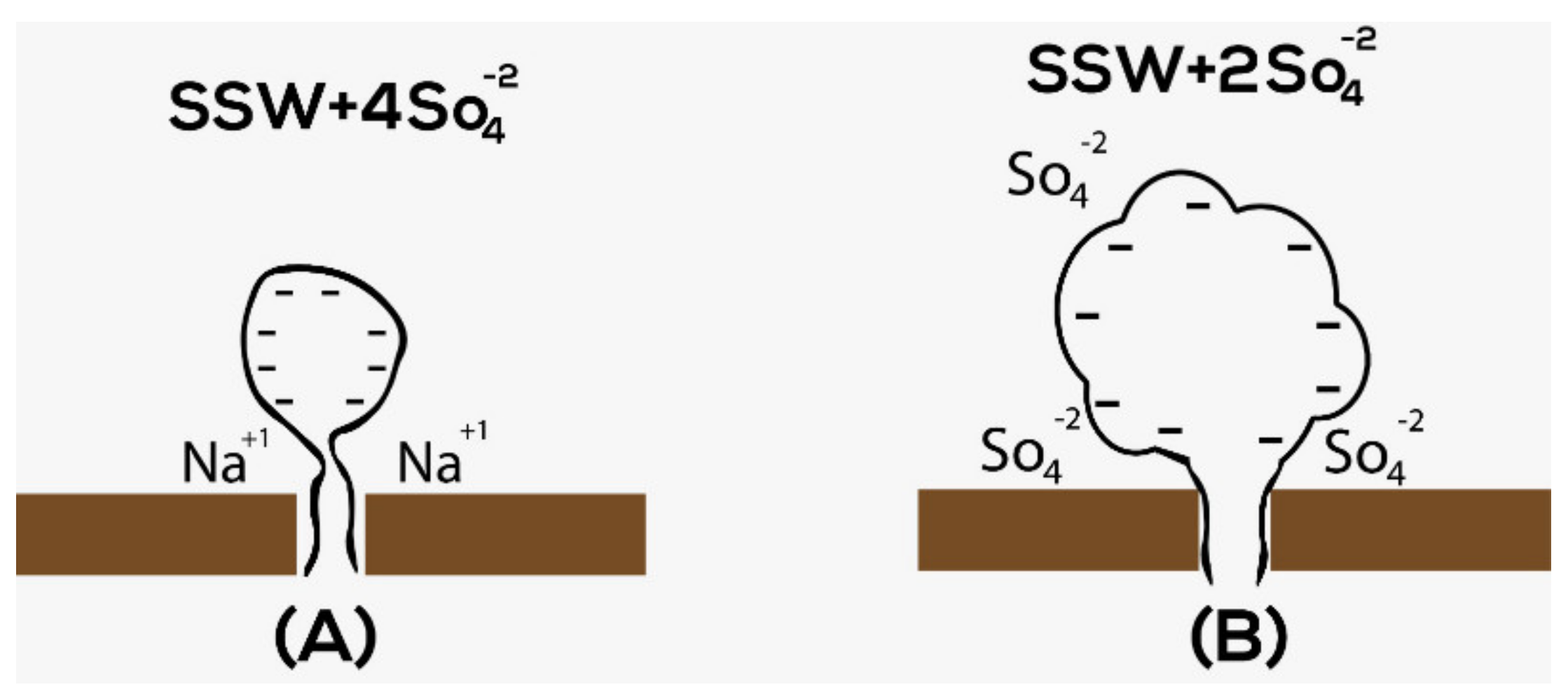
1.2. Sulfate as the Effective Potential Determining Ions (PDI)
1.3. Hybrid EOR Processes
2. Overall Methodology and Approach
- Optimization of Formation and Injection Brines: Two types of formation brine and four types of injection brine were generated. The primary approach was to prepare brines, focusing on the role of increasing the sulfate and varying the TDS of the synthetic seawater (SSW) to correlate with the impact of salinity on oil recovery.
- Polymer Diluted Solutions at Target Concentration: Low-concentration (750 ppm) polymer solutions were prepared to achieve a viscosity ratio of 2 between the oil and polymer. Polymer solutions were prepared to investigate the synergies and benefits of the combined EOR processes.
- Contact Angle Measurements: Contact angle measurements were performed to confirm the wettability alteration of Bentheimer core plugs from a water-wet to oil-wet condition. Two approaches were adopted to cross-validate the results.
- Mechanical degradation of polymer solutions: This step allowed us to understand and define any possible degradation prior to the core face and to draw better conclusions on polymer performance.
- Fluid–fluid Interactions: Interfacial tension and interfacial viscoelastic response measurements were performed to investigate the ionic interaction between oil polar compounds and active ions in brine. The results of fluid–fluid interactions were incorporated to determine the possible impact on oil recovery.
- Oil Recovery through Hybrid EOR Process: Different brines were flooded through oil-wet core plugs, as the secondary mode, followed by tertiary-mode polymer flooding. The purpose was to investigate the synergies of the promising hybrid EOR process.
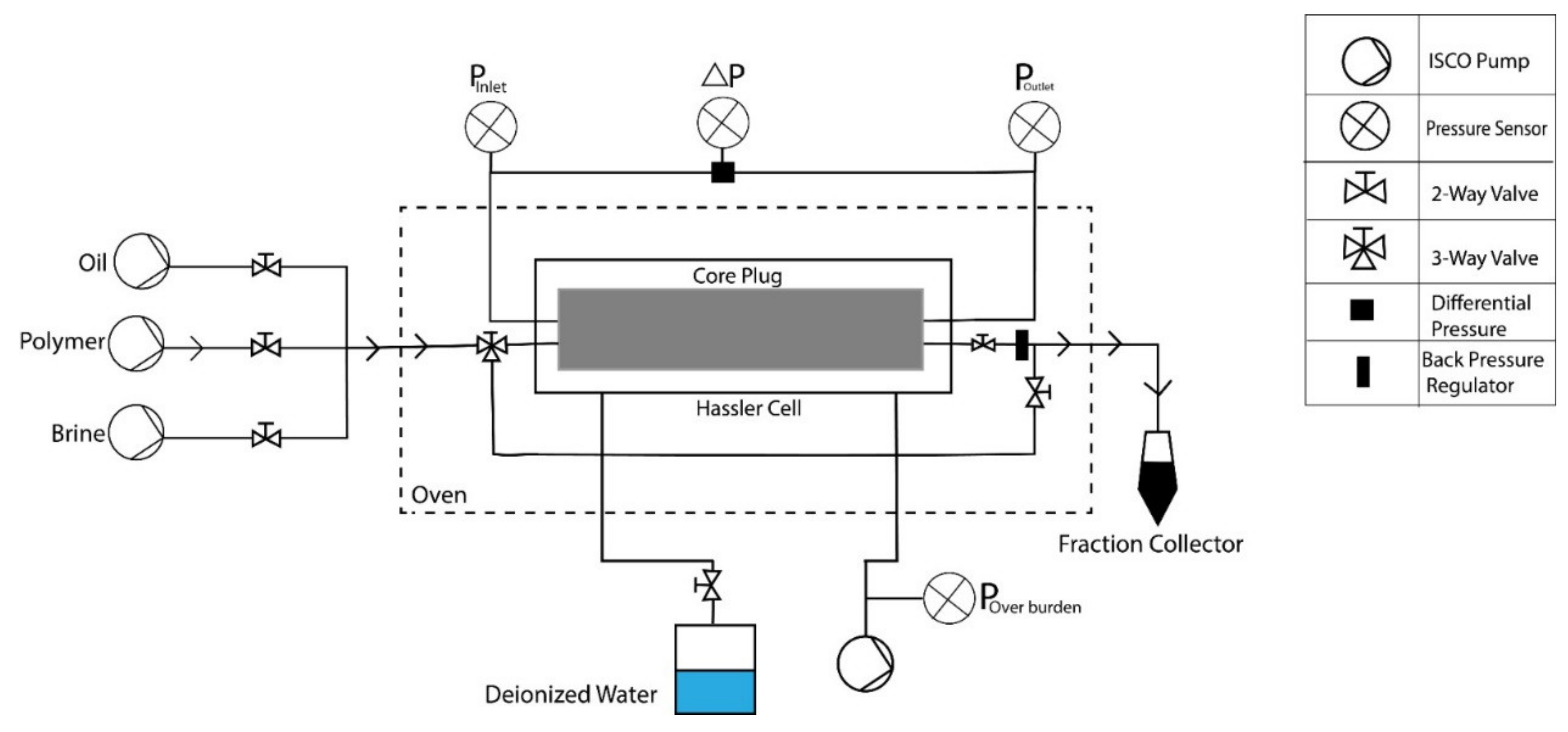
3. Materials and Methods
3.1. Fluids and Chemicals
3.1.1. Brine Composition
- Group 1 is used to evaluate the different formation brines using SSW and doubled TDS of synthetic seawater (2*SSW TDS).
- Group 2 is prepared to optimize a modified water based on the divalent ions, specifically with sulfates.
3.1.2. Polymer Solutions
3.1.3. Dead Oil
3.2. Porous Media
- Water Saturation: Vacuum saturation is used for initial water saturation at 100% Brine.
- Permeability to Water (Brine): SSW brine is injected at three injection rates (0.5, 1.0 and 0.5 mL/min).
- Oil saturation to initial water saturation (Swi): Oil saturation of core plugs is performed using a porous plate with a maximum injection pressure of 8.0 bar.
- Core Ageing for Wettability Alteration: All cores are aged at 45 °C (core flooding experiments temperature) for six weeks prior to each core flood experiment, in order to establish attachment of polar components on the core. The wettability alteration of Berea and Bentheimer sandstone core plugs through a two- or three-week aging process has been established by many researchers [22,42,78,79].
- Two-phase flooding experiments: The workflow/sequence adopted for the core flooding experiments is listed in Table 6. Bump rate injection is performed to avoid capillary end effects before tertiary mode flooding.
3.2.1. Contact Angle Measurements
3.2.2. Mechanical Degradation of Polymer-Before Entering the Plug Face
3.3. Fluid–Fluid Interaction
3.3.1. Interfacial Tension (IFT) Measurements
3.3.2. Interfacial Viscoelastic Response (IFV)
3.4. Rheological Measurements
4. Results and Discussion
4.1. Wettability Alteration
4.2. Fluid–Fluid Interaction
4.2.1. Impact of Sulfates on IFT
- Doubling the amount of sulfates in SSW resulted in a doubled value of IFT (compared to SSW).
- Quadrupling the sulfates in SSW resulted in a decrease in the IFT response.
4.2.2. Interfacial Viscoelastic Response (IFV)
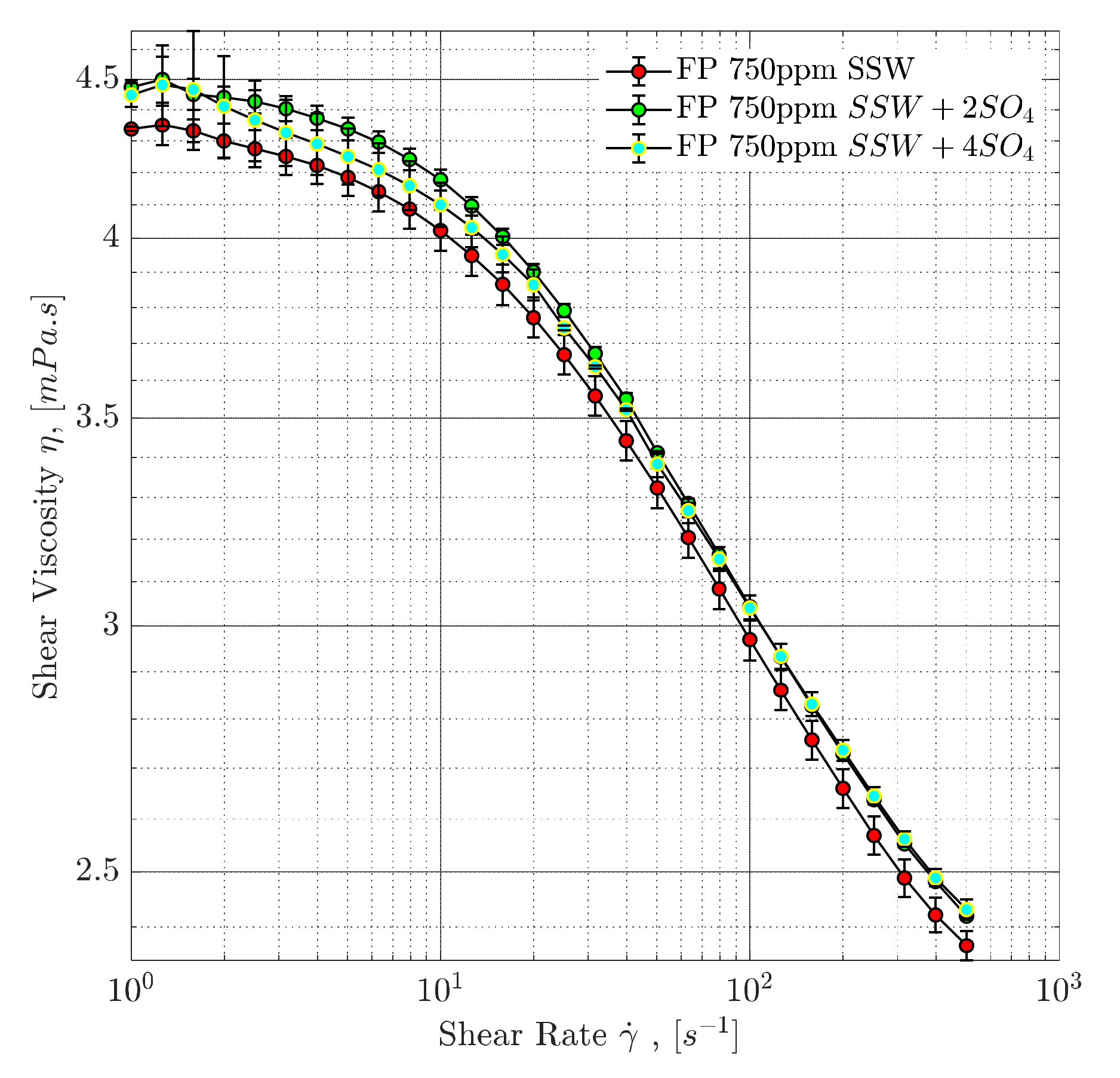
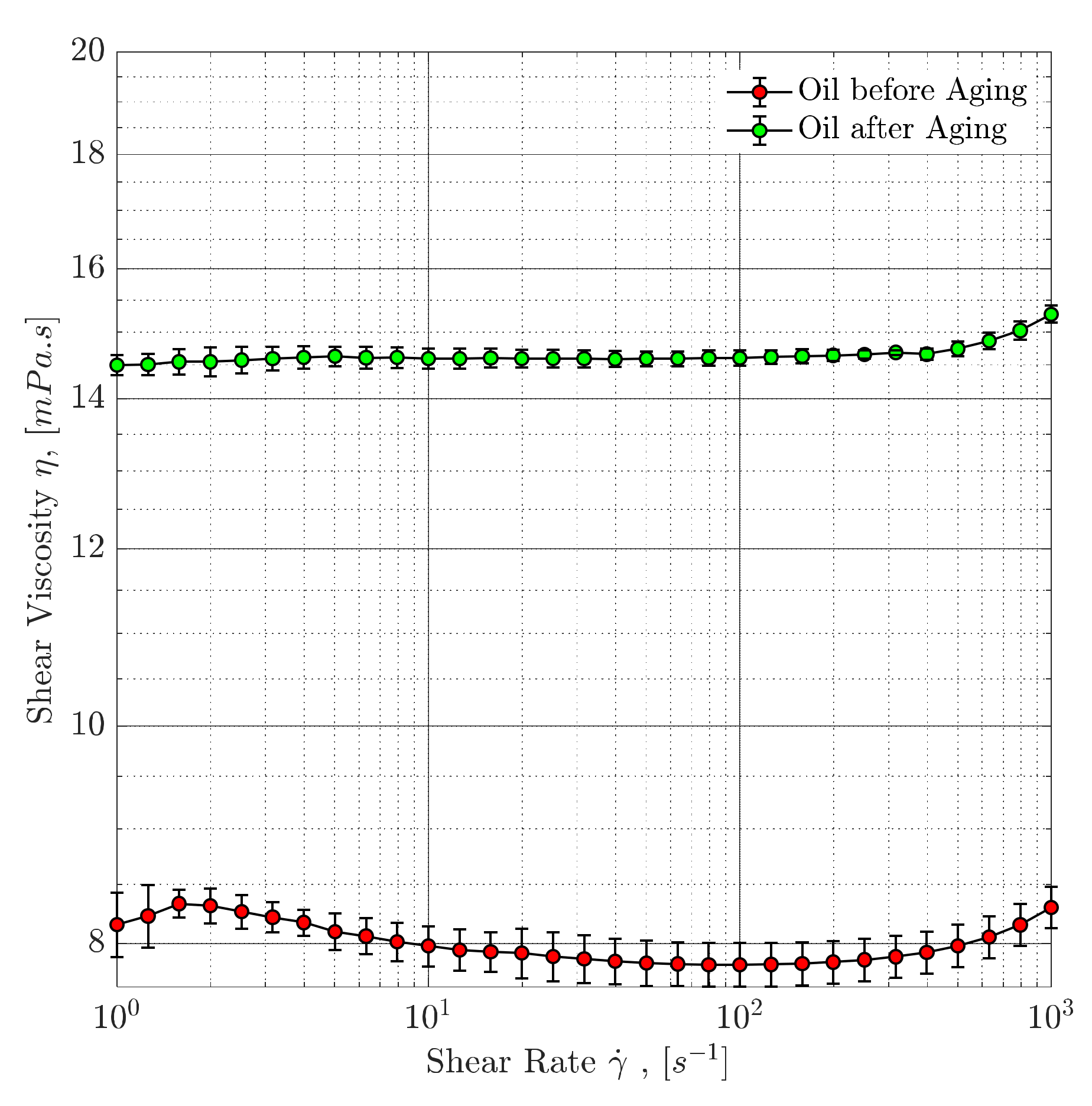
4.3. Rheological Evaluation
4.3.1. Steady-Shear Rheology (Shear Viscosity)
4.3.2. Polymer Degradation and Stability Analysis
- Mixing polymer solutions in sulfate-modified water may not be an excellent or a promising approach.
- Mixing polymer solutions in SSW can result in mechanically stable polymer solutions, subsequently affecting economics (utility factor).
4.4. Core Flood Experiments and Oil Recovery Observations
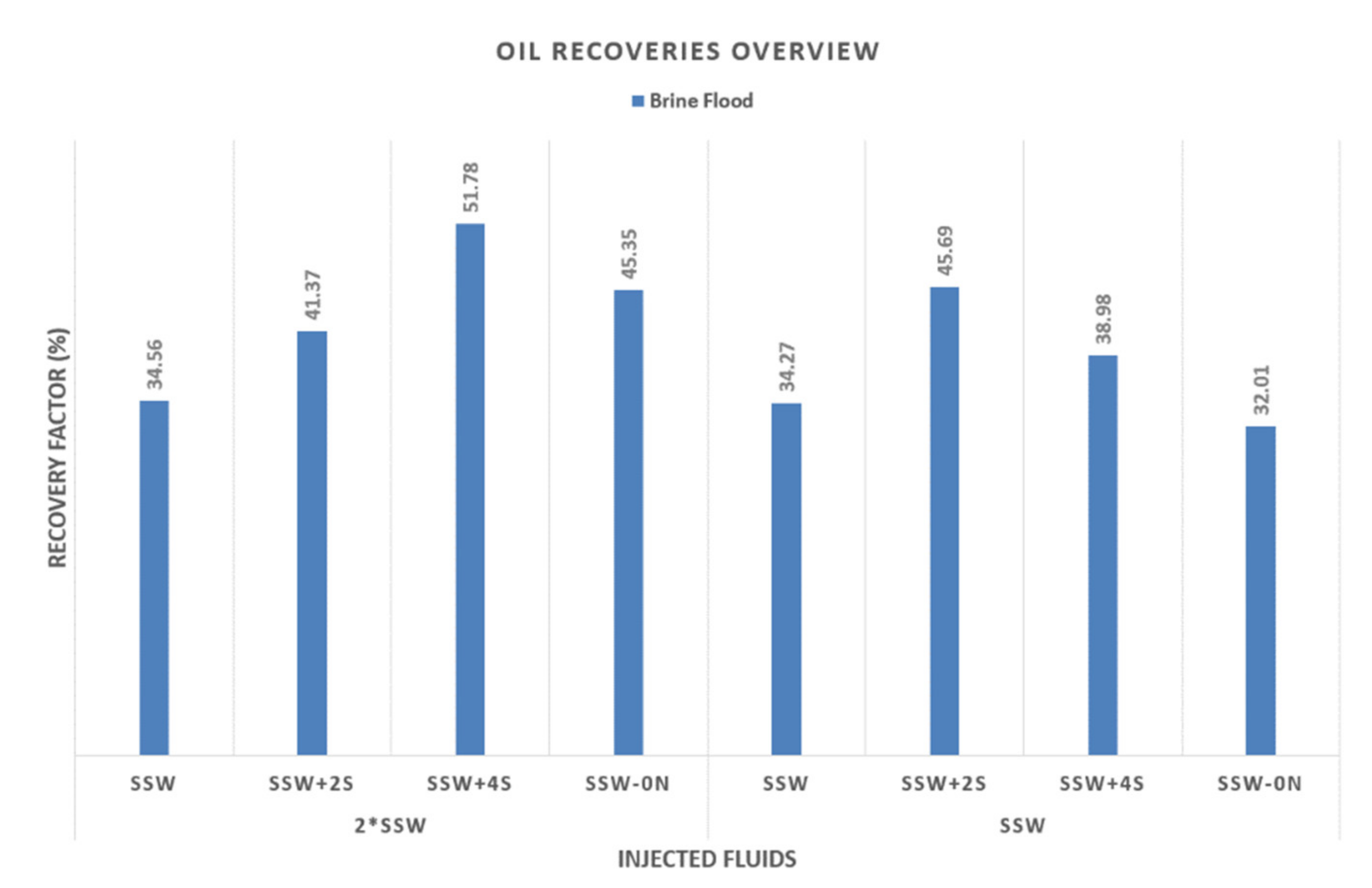
4.4.1. SSW as Formation Brine (Secondary-mode Brine Flooding)
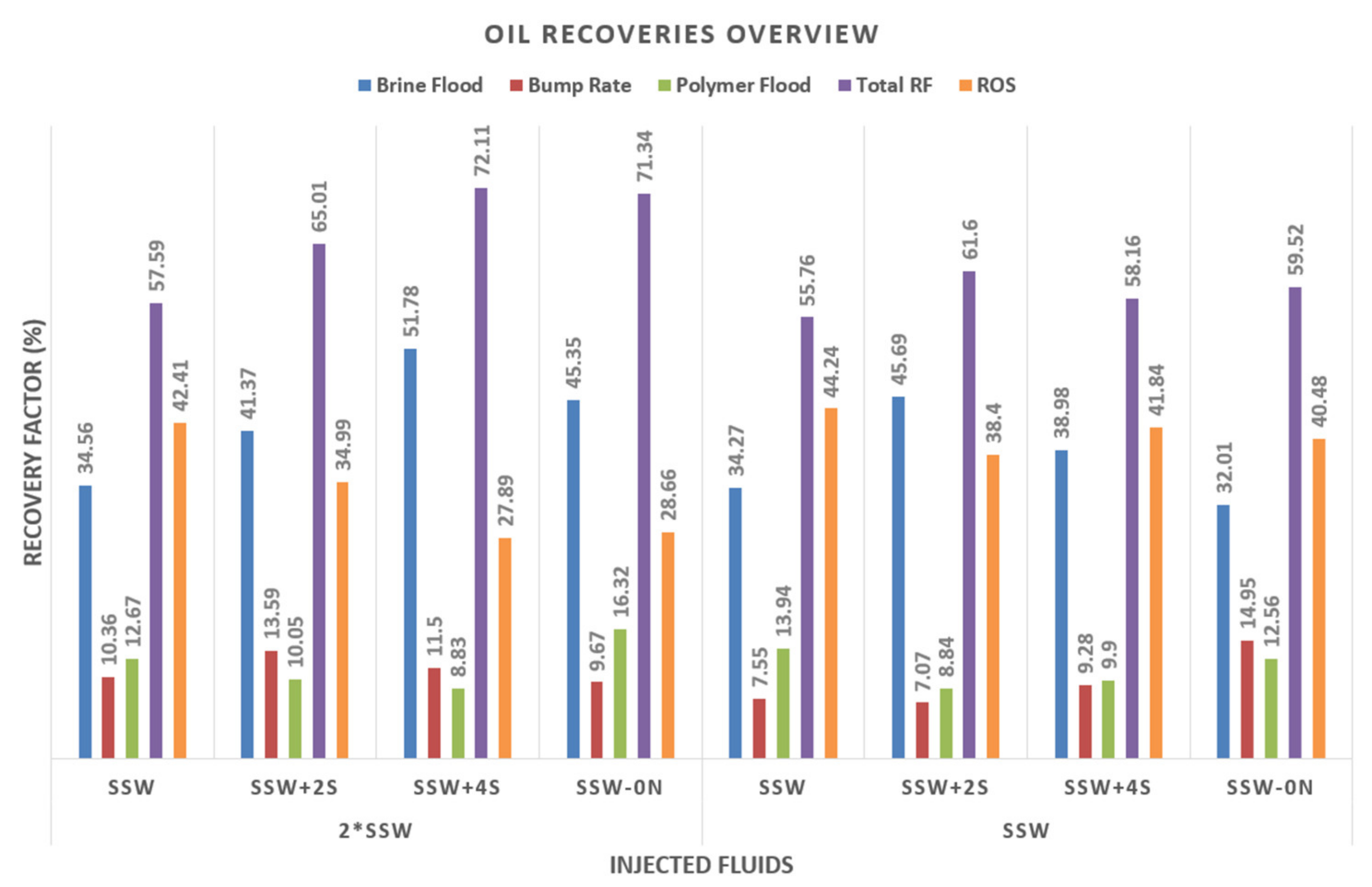
- Secondary-mode SSW injection (T2 core) resulted in the lowest RF at around 34%. A similar result was obtained under the same flooding conditions in the previous work [10].
- Injection of chemically modified brines disturbed the ionic equilibrium system inside the core plug. However, the aging of core plugs resulted in the attachment of polar compounds on the rock surface (previously confirmed in this work).
- Core plug T7 had in the lowest remaining oil saturation (54.31%) after secondary-mode sulfate-modified water injection (SSW as formation brine), as shown in Figure 12. This response is assumed to correlate with improved fluid–fluid interfacial interaction (previously discussed). SSW+2SO4−2 had the highest fluid–fluid interfacial interaction (IFT and IFV measurements), as also reported by Tetteh and Barati [83]. During core flooding, the wettability alteration process is initiated through modified brine, resulting in detachment of the oil from the rock formation. In parallel, in the oleic phase, contact with the injected brine forms an ionic interfacial layer. Presumably, this ionic interfacial layer helps with the continuous flow of oil. This further restricts the oil drop’s reattachment on the rock surface, hence the plugging and entrapment of the oleic phase.
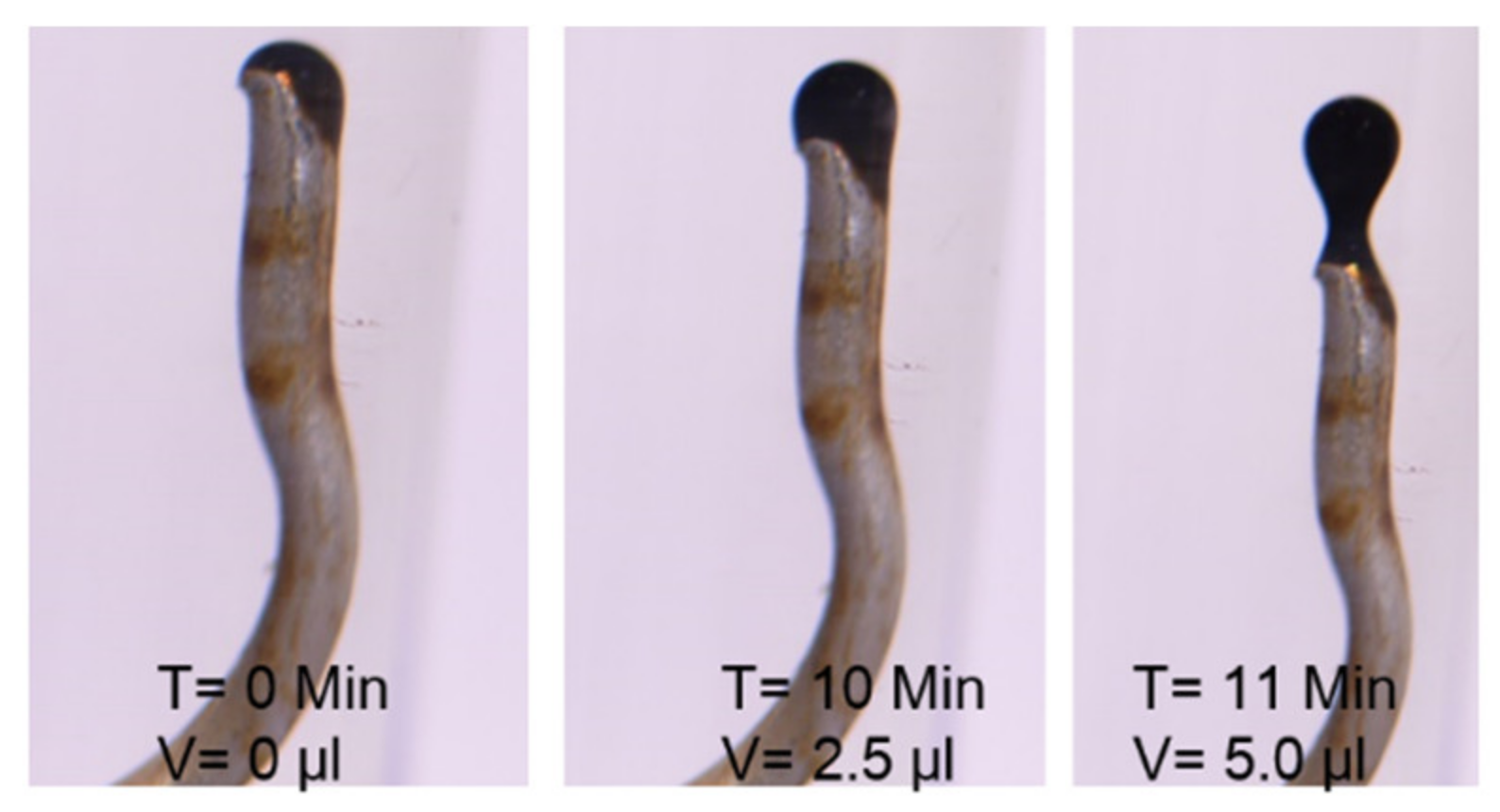
- Flooding with SSW+4SO4−2 produced the second-highest oil recovery of 38.98%. This significantly lowered the remaining oil saturation compared to SSW brine and SSW-Na injection, which may be associated with improved fluid–fluid interaction. It is believed that the excessive spiked amount of sulfates (4SO4−2) helped to decrease IFT to 2.06 mN/m. Related to the IFT and oil-drop snap-off evaluations, the smallest drop-size detachment was observed, and the improved oil–brine interface reaction helped produce microscale water-in-oil emulsions (or microscale soap formation).
- The oil recovery results are in line with the results obtained by Mohamed and Alvarado [22]. Maximum oil recovery was obtained by increasing the sulfates in SSW by three and five times. According to the authors, three and five times more sulfate results in the highest interfacial viscoelasticity.
- It can be seen from experiments Nr. 1 through 4 of Table 9 (Figure 12) that the improved fluid–fluid properties have a dominant role compared to microscale water-in-oil emulsions for higher oil production. Moreover, the amount of sulfate added to designed modified water is a critical parameter that requires attention. Injecting SSW and SSW-0NaCl did not lead to additional oil production due to a lack of active sulfate ions. It is assumed that neither of the brines could disturb the established rock–oil–brine (ROB) ionic equilibrium and hence could not improve oil recovery (weaker fluid–fluid interaction).
4.4.2. Double SSW as Formation Brine (Secondary-mode Brine Flooding)
- Focusing on the second group of experiments (Nr. 5 through 8 from Table 9), SSW injection produced the lowest recovery of 34.56%, leaving a significant amount of remaining oil saturation (65.44%) in the core.
- Injecting SSW+4SO4−2 resulted in the highest RF of 51.78% and the lowest remaining oil saturation of 48.22%, as shown in Figure 12. This behavior is in contrast with injecting SSW+2SO4−2 (Nr. 1 through 4 from Table 9), which produced the highest recovery with SSW as formation brine. The same result can be reasonably expected for remaining oil saturation (ROS) with 2*SSW formation brine. However, this did not happen and instead, SSW+4SO4−2 produced the highest recovery. This is associated with the relationship between the chemical composition of injection and formation brines. As the formation brine composition is doubled to 2*SSW, the sulfate amount must be quadrupled in the injection brine. This process is necessary to compensate for the ionic reaction based on fluid–fluid interaction. Therefore SSW+4SO4−2 produced the highest recovery with 2*SSW formation brine.
- The second-lowest ROS (54.65%) was achieved by injecting SSW-0NaCl brine, as shown in Figure 12. This result is in line with the results reported by Zhang et al. [13]. The higher recovery is believed to be connected to the role of the PDI and non-PDI ions in the system. 2*SSW formation brine was saturated with PDI (Ca+2 and Mg+2 ions) while the injection brine had no non-PDI (Na+1). An additional supporting argument for higher recovery is the reduced TDS. TDS of SSW-0NaCl were less than half that of SSW. Therefore, in this case, the low concentrations of salts could also play a role. It can be claimed that the combined scenario of PDI and non-PDI ions and low salinity played a crucial role in lowering the ROS. However, the ROS obtained in the T1 core plug indicates otherwise, suggesting that the role of low concentrations of salts was negligible. Finally, the activity of PDI and non-PDI ions support the lowered ROS in the T3 core flood.
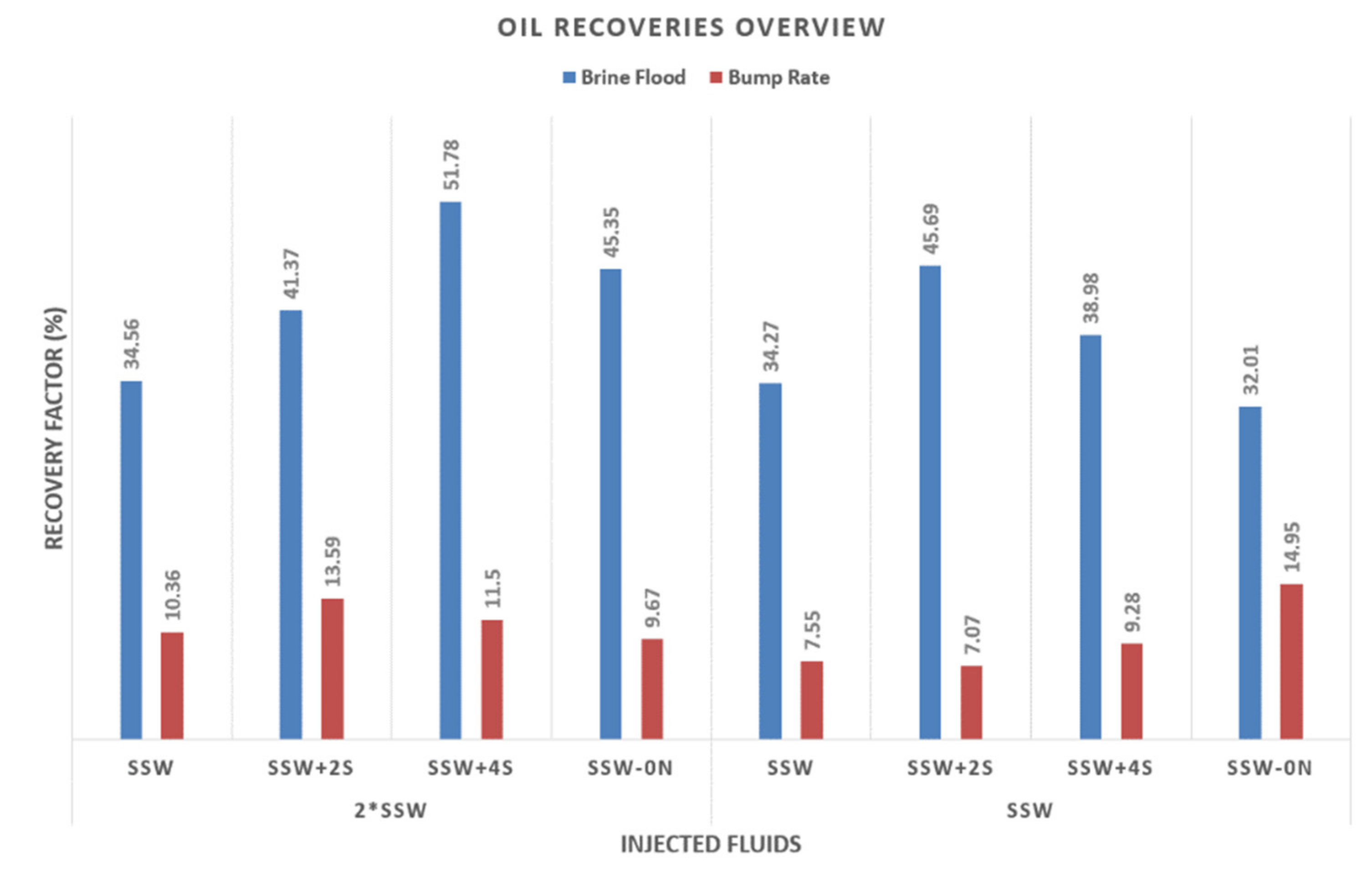
4.4.3. Bump Rate: Remaining Oil Saturation
- The RF bump rate results in Table 9 and Figure 13 indicate the oil produced through the bump rate. Bump-rate injection is performed at a higher rate (more than doubled) than brine flooding, as described earlier. This higher injection rate could cause a higher-pressure differential along the core and cause oil to be trapped due to capillary end effects.
- Looking at the Figure 13 for oil recovery, the T4 and T1 plugs produced the maximum oil. There is no specific trend regarding the relationship between the bump rate and RF based on the chemical composition of the previously injected brine. The RF obtained for the bump rate provides significant insights into the production of trapped oil due to capillary end effects. A higher recovery (14.95%) from T1 is expected because of the highest ROS (67.98%) after secondary-mode brine flooding. Interestingly, however, T4 also produced significant oil (13.59%). Comparing the RF of the T4 and T5 experiments (secondary mode), the difference becomes significant (10%). It is assumed that some oil is released due to improved fluid–fluid interaction in T4 and could not be recovered due to the strong capillary end effects. However, the bump rate helped to release this trapped oil due to the excessive pressure drop.
4.4.4. Remaining Oil Saturation after Tertiary-mode Polymer Flooding
- Polymer flooding in the tertiary recovery mode also resulted in a significant reduction in ROS, as shown in Table 9 and Figure 14. As previously mentioned, the objective is to lower ROS due to viscosity support from tertiary-mode flooding. Therefore, the viscosity of the polymer is defined as half that of the oil viscosity so the viscosity value lies between that of the brine and oil. It can be argued that this oil recovery is only due to mobility control of the polymer flood. However, Shaker Shiran and Skauge [65] found that hybrid EOR (combined EOR techniques) flooding results in higher recovery compare to a single EOR technique.
- Looking at the recovery numbers, polymer flooding after SSW-0NaCl significantly decreased ROS in the T3 core (with 2*SSW as formation brine). The combined effect of (potential determining ions, PDI) ions in the formation fluid and removal of non-PDI ions in the injected fluid released oil from the core surface, but this oil could not be produced even with the bump rate. The changes in viscosity due to the polymer flood made it possible for this detached oil to flow. Therefore, this contributed to lower the ROS to 16.32%. Interestingly enough, polymer flooding after SSW flooding contributed to significant recovery (12.67% and 13.4%), but the reason for this is the higher amount of ROS (in both T2 and T6 plugs). Moreover, comparing the final ROS from T2 and T6, the data depict the highest values among all scenarios. It is likely that polymer flooding produced higher recoveries due to the higher ROS of T2 and T6 plugs. Subsequently, polymer flooding after sulfate-modified water flooding reduced ROS by 9% to 11%, which is significant.
4.4.5. Final Recovery and Remaining Oil Saturation
- Depleted Na+1 in injection brine and a spiked amount of Ca+2 and Mg+2 ions in formation brine also produced significant oil recovery (71.34%). This result is in agreement with the data reported by Zhang et al. [53].
- According to the final ROS data of the T3 and T5 experiments, the flooding process of T5 (SSW+4SO4−2) is commercially economical. The main reason for this is the brine processing cost of SSW-0NaCl for T3. Na+1 removal from SSW can cost much more compared to the addition of a small amount of sulfate in the injection brine to design the modified water. Economically and technically, the spiked amount of sulfate is a feasible process on a commercial scale.
- The highest ROS was seen after the injection of SSW in both groups of experiments, as shown in Table 9. As previously described, this is because the injection and formation brines shown in Table 4 have the same hardness value. The modification of SSW to design modified water established a contrast in hardness between injected and formation brine. This process further manipulates the ionic equilibrium in the reservoir system. As seen in Table 4 and Table 9, the lowest ROS values were observed when the hardness contrast between injection and formation brine is significant. To summarize, the combined EOR process worked efficiently, contributing to significant oil recovery.
- Maximum total oil recovery was observed for the hybrid EOR process of SSW+4SO4−2 and SSW+2SO4−2 injection in the secondary mode and polymer flooding in the tertiary mode (SSW as formation brine). Similarly, in the case of 2*SSW formation brine, the highest total recovery was achieved through a hybrid EOR process comprising injecting SSW+4SO4−2 and SSW-Na in the secondary mode followed by polymer flooding in the tertiary mode. However, SSW-Na preparation is not commercially economical, which further indicates that sulfate-modified brine should be injected in the secondary mode. To sum up, hybrid EOR processes worked efficiently, contributing to significant oil recovery, as found by Shaker Shiran and Skauge [65] and Tahir et al. [7,8,10].
4.4.6. Modified Water Injection in the Post-Tertiary Mode
4.4.7. Oil Recovery Profile versus PV Injected
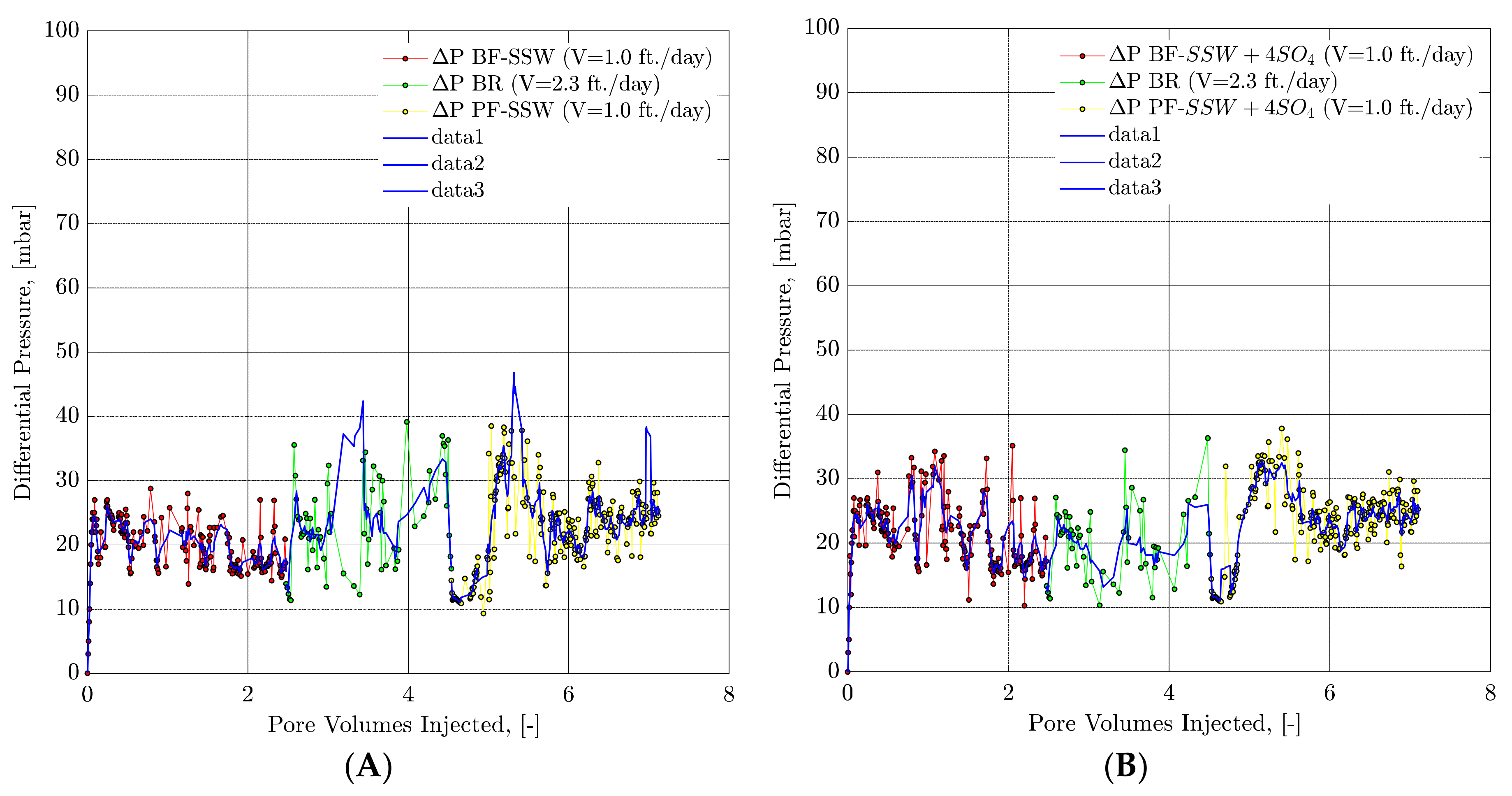
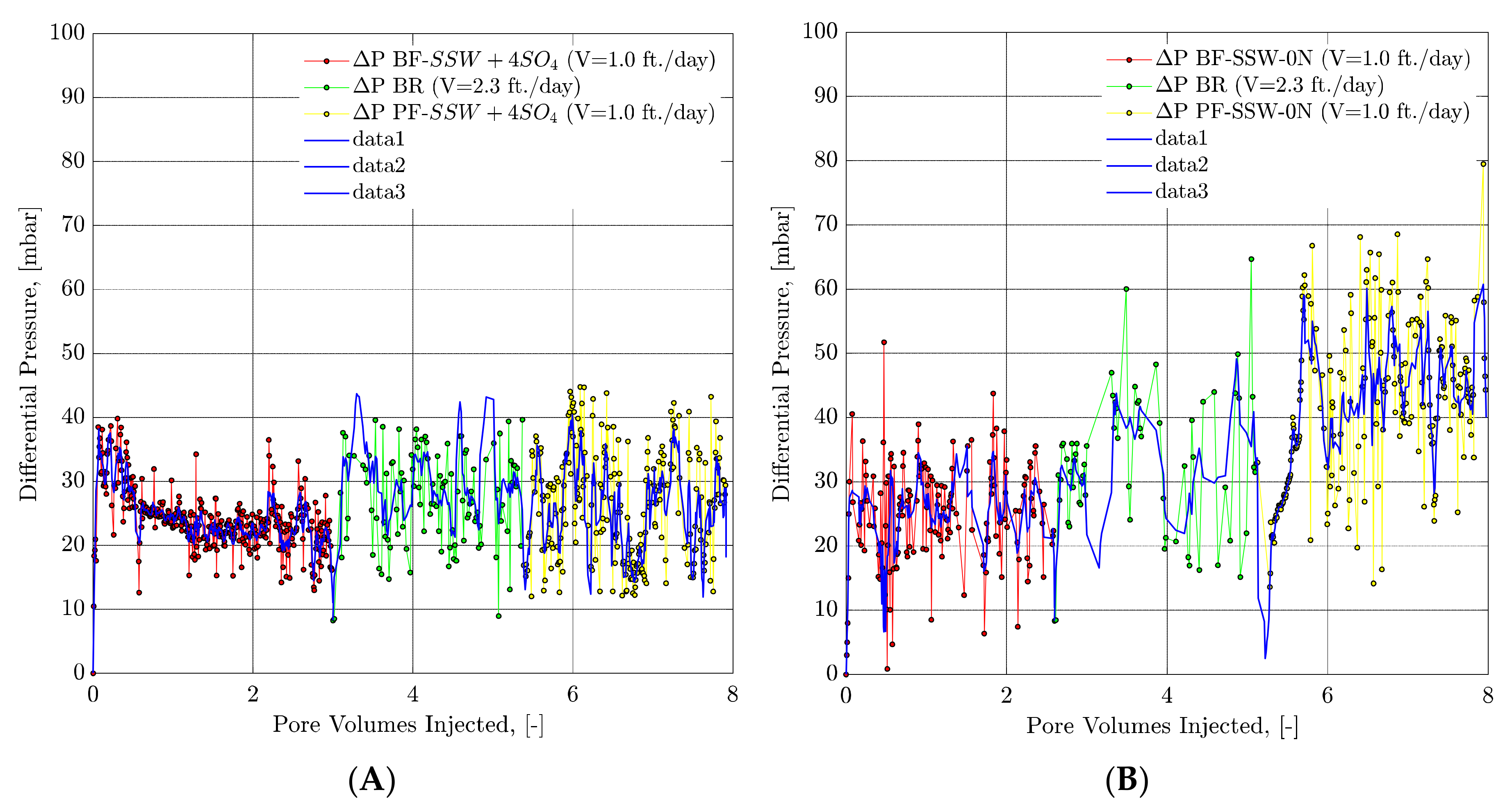
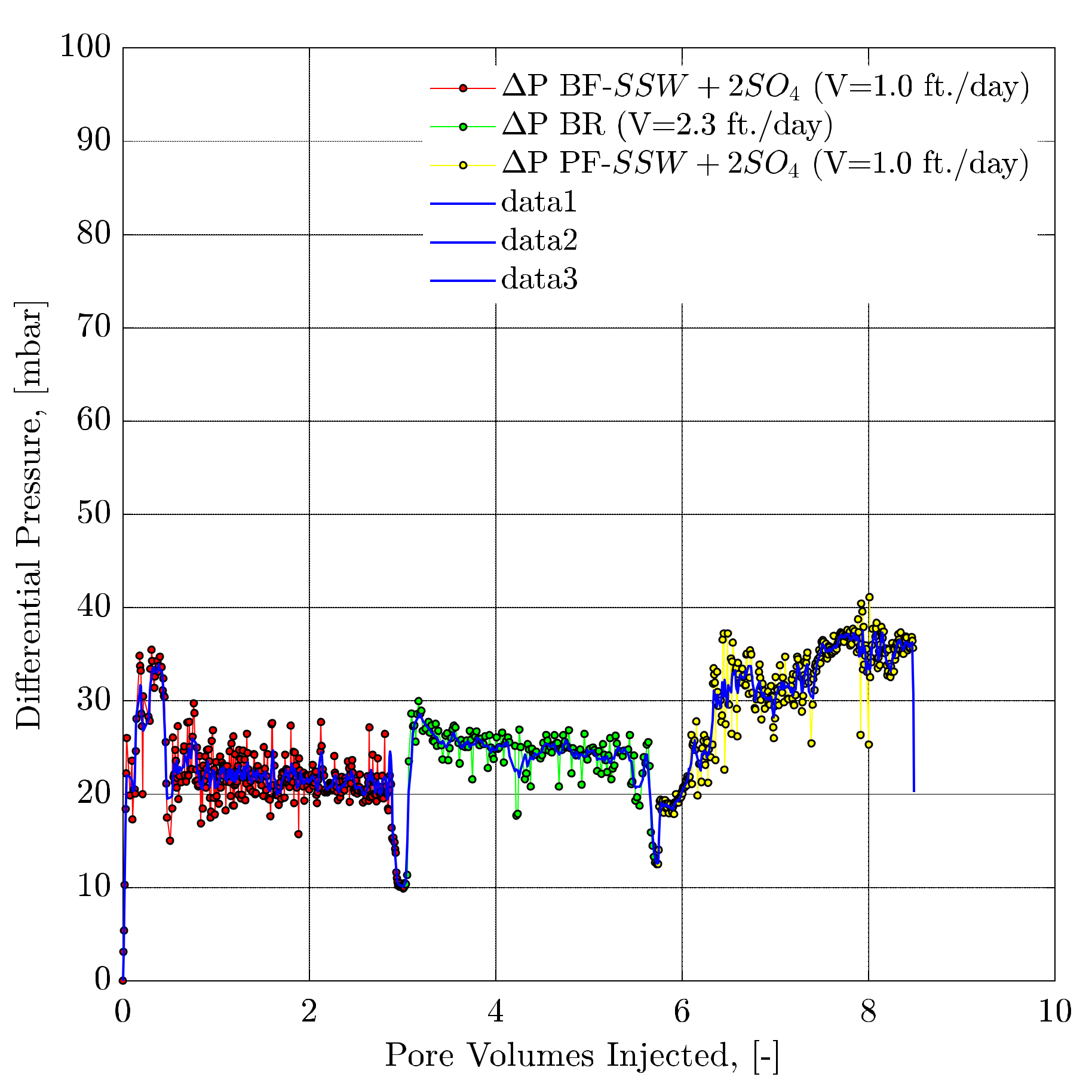
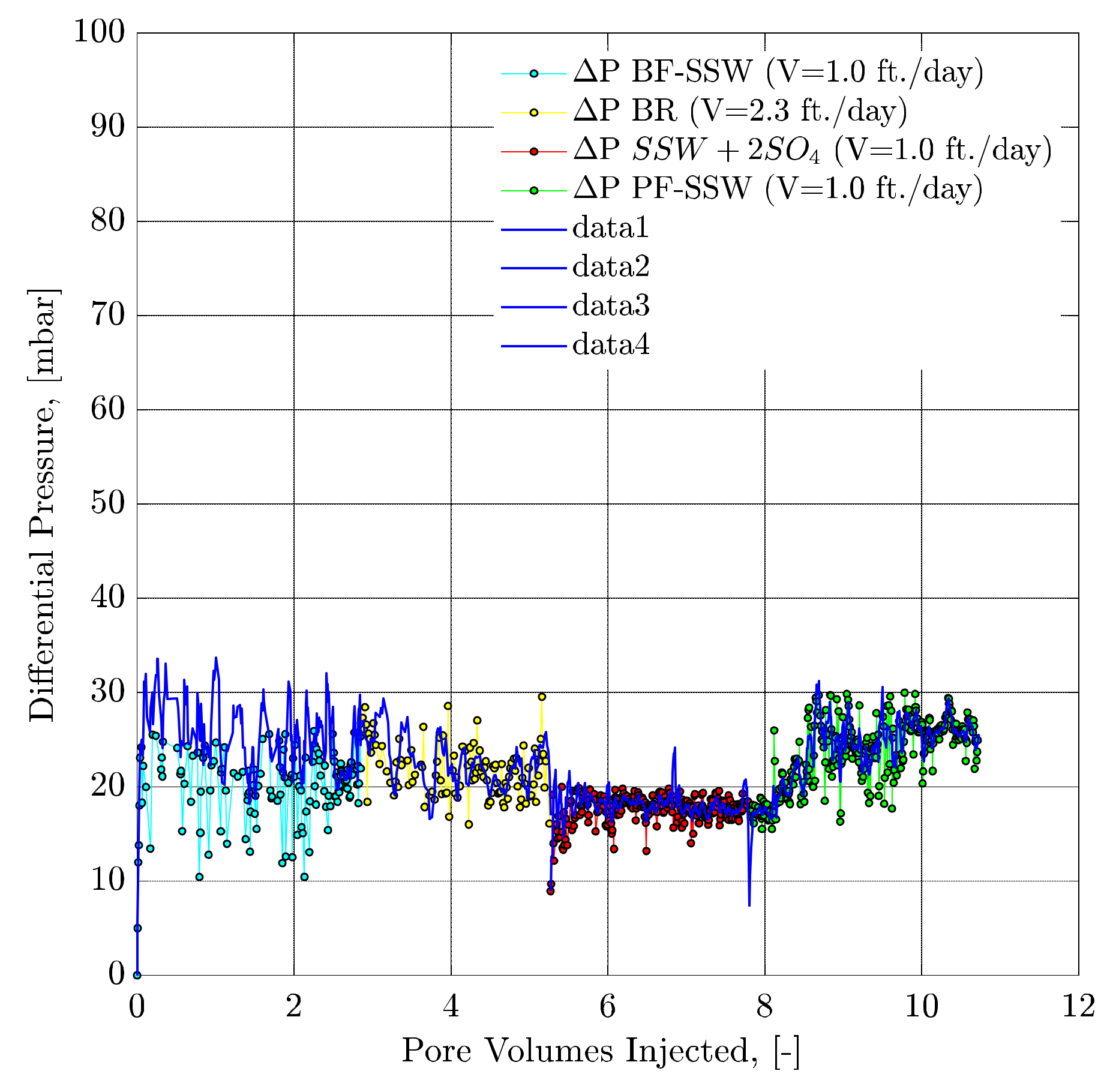
4.4.8. Pressure Response Observations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Polymer viscosity after injection through core flood pipe-system till core plug |
| B | Polymer viscosity before injection core plug |
| BF | Brine flood |
| BR | Bump rate |
| DR | Degradation rate of polymer solutions (%) |
| IFT | Interfacial tension |
| IFV | Interfacial viscoelasticity |
| PDI | Potential determining ions |
| PF | Polymer flood |
| Q | Injection rate |
| ROB | Rock–oil–brine |
| ROS | Remaining oil saturation |
| SSW | Synthetic sea water |
| SSW+2S | Synthetic sea water doubled with sulfates |
| SSW+2S | Synthetic sea water quadrupled with sulfates |
| SW | Modified Water |
| TDS | Total dissolved solids |
| FB | Formation brine |
| kg | Permeability to gas |
| Kb | Permeability to brine |
References
- Patton, C.C. Water Quality Control and Its Importance in Waterflooding Operations. J. Pet. Technol. 1988, 40, 1123–1126. [Google Scholar] [CrossRef]
- Martin, J.C. The Effects of Clay on the Displacement of Heavy Oil by Water. Venez. Annu. Meet. 1959. [Google Scholar] [CrossRef]
- Yousef, A.A.; Al-Saleh, S.; Al-Jawfi, M.S. Smart WaterFlooding for Carbonate Reservoirs: Salinity and Role of Ions. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar] [CrossRef]
- Nasralla, R.A.; Nasr-El-Din, H.A. Nasr-El-Din, Coreflood Study of Low Salinity Water Injection in Sandstone Reservoirs. In Proceedings of the SPE/DGS Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 15–18 May 2011. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Wettability and Oil Recovery by Low Salinity Injection. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Foedisch, H.; Strobel, G.-J.; Ganzer, L. Potential Benefits of Fluid Optimization for Combined Smart-Water and Polymer Flooding: Impact on Remaining Oil Saturation. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 22–24 October 2019. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Tahir, M. Influence of Sulfate Ions on the Combined Application of Modified Water and Polymer Flooding—Rheology and Oil Recovery. Energies 2020, 13, 2356. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Gaol, C.L.; Säfken, S.; Ganzer, L. Flow Dynamics of Sulfate-Modified Water/Polymer Flooding in Micromodels with Modified Wettability. Appl. Sci. 2020, 10, 3239. [Google Scholar] [CrossRef]
- Suman, Y.K.; Shirif, E.; Ibrahim, H.; Ala-Ktiwi, A. Evaluation of Low Saline “Smart Water” Enhanced Oil Recovery in Light Oil Reservoirs. World J. Eng. Technol. 2014, 2, 13–22. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Foedisch, H.; Abdullah, H.; Ganzer, L. Impact of Sulphates Presence During Application of Smart Water Flooding Combined with Polymer Flooding. In Proceedings of the SPE Europec featured at 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Morrow, N.R.; Valat, M.; Yidliz, H. Effect of Brine Composition on Recovery of an Alaskan Crude Oil by Waterflooding. In Proceedings of the Annual Technical Meeting, Calgary, AB, Canada, 10–12 June1996. [Google Scholar] [CrossRef]
- Tang, G.; Morrow, N. Salinity, Temperature, Oil Composition, and Oil Recovery by Waterflooding. SPE Reserv. Eng. 1997, 12, 269–276. [Google Scholar] [CrossRef]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 199–208. [Google Scholar] [CrossRef]
- Lager, A.; Webb, K.J.; Black, C.J.J.; Singleton, M.; Sorbie, K.S. Low salinity oil recovery—An experimental investigation. Petrophysics 2008, 49, 28–35. [Google Scholar]
- Morrow, N.R.; Tang, G.-Q.; Valat, M.; Xie, X. Prospects of improved oil recovery related to wettability and brine composition. J. Pet. Sci. Eng. 1998, 20, 267–276. [Google Scholar] [CrossRef]
- Rivet, S.; Lake, L.W.; Pope, G.A. A Coreflood Investigation of Low-Salinity Enhanced Oil Recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19–22 September 2010. [Google Scholar] [CrossRef]
- Xie, Q.; Ma, D.; Wu, J.; Liu, Q.; Jia, N.; Luo, M. Low Salinity Waterflooding in Low Permeability Sandstone: Coreflood Experiments and Interpretation by Thermodynamics and Simulation. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, 11–13 August 2015. [Google Scholar] [CrossRef]
- Winoto, W.; Loahardjo, N.; Xie, S.X.; Yin, P.; Morrow, N.R. Secondary and Tertiary Recovery of Crude Oil from Outcrop and Reservoir Rocks by Low Salinity Waterflooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar] [CrossRef]
- Aghaeifar, Z.; Strand, S.; Puntervold, T.; Austad, T.; Sajjad, F.M. Smart Water injection strategies for optimized EOR in a high temperature offshore oil reservoir. J. Pet. Sci. Eng. 2018, 165, 743–751. [Google Scholar] [CrossRef]
- AlGeer, M.A.; Gmira, A.; Al-Enezi, S.M.; Yousef, A.A. A New Insight on the Impact of Individual Ions on Fluid/Fluid Interactions and SmarWater Recovery. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 21–23 March 2016. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Morrow, N.R. Waterflood Performance by Injection of Brine with Different Salinity for Reservoir Cores. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar] [CrossRef]
- Mohamed, M.I.; Alvarado, V. Smart Water Flooding in Berea Sandstone at Low Temperature: Is Wettability Alteration the Sole Mechanism at Play? In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar] [CrossRef]
- Lager, A.; Webb, K.J.; Black, C.J.J. Impact of Brine Chemistry on Oil Recovery. In Proceedings of the IOR 2007 14th European Symposium on Improved Oil Recovery, Cairo, Egypt, 22–24 April 2007; Available online: http://www.earthdoc.org/publication/publicationdetails/?publication=6195 (accessed on 13 November 2007).
- Al-Nofli, K.; Pourafshary, P.; Mosavat, N.; Shafiei, A. Effect of Initial Wettability on Performance of Smart Water Flooding in Carbonate Reservoirs—An Experimental Investigation with IOR Implications. Energies 2018, 11, 1394. [Google Scholar] [CrossRef]
- Mamonov, A.; Puntervold, T.; Strand, S. EOR by Smart Water Flooding in Sandstone Reservoirs—Effect of Sandstone Mineralogy on Initial Wetting and Oil Recovery. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 16–18 October 2017. [Google Scholar] [CrossRef]
- Bagci, S.; Kok, M.V.; Türksoy, U.; Baǧci, S. Effect of brine composition on oil recovery by waterflooding. Pet. Sci. Technol. 2001, 19, 359–372. [Google Scholar] [CrossRef]
- Ligthelm, D.J.; Gronsveld, J.; Hofman, J.; Brussee, N.; Marcelis, F.; van der Linde, H. Novel Waterflooding Strategy By Manipulation Of Injection Brine Composition. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar] [CrossRef]
- Morrow, N.; Buckley, J. Improved Oil Recovery by Low-Salinity Waterflooding. J. Pet. Technol. 2011, 63, 106–112. [Google Scholar] [CrossRef]
- Webb, K.J.; Black, C.J.J.; Tjetland, G. A Laboratory Study Investigating Methods for Improving Oil Recovery in Carbonates. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 21–23 November 2005. [Google Scholar] [CrossRef]
- Moradi, M.; Alvarado, V. Influence of Aqueous-Phase Ionic Strength and Composition on the Dynamics of Water–Crude Oil Interfacial Film Formation. Energy Fuels 2016, 30, 9170–9180. [Google Scholar] [CrossRef]
- Horváth-Szabó, G.; Czarnecki, J.; Masliyah, J. Liquid Crystals in Aqueous Solutions of Sodium Naphthenates. J. Colloid Interface Sci. 2001, 236, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Havre, T.E.; Sjöblom, J. Emulsion stabilization by means of combined surfactant multilayer (D-phase) and asphaltene particles. Colloids Surf. A Physicochem. Eng. Asp. 2003, 228, 131–142. [Google Scholar] [CrossRef]
- Garcia-Olvera, G.; Alvarado, V.; Alvarado, V. Interfacial rheological insights of sulfate-enriched smart-water at low and high-salinity in carbonates. Fuel 2017, 207, 402–412. [Google Scholar] [CrossRef]
- Alotaibi, M.B.; Azmy, R.M.; Nasr-El-Din, H.A. Wettability Studies Using Low-Salinity Water in Sandstone Reservoirs. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 3–6 May 2010. [Google Scholar] [CrossRef]
- Czarnecki, J.; Moran, K. On the Stabilization Mechanism of Water-in-Oil Emulsions in Petroleum Systems. Energy Fuels 2005, 19, 2074–2079. [Google Scholar] [CrossRef]
- Verruto, V.J.; Kilpatrick, P.K. Water-in-Model Oil Emulsions Studied by Small-Angle Neutron Scattering: Interfacial Film Thickness and Composition. Langmuir 2008, 24, 12807–12822. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Zaki, N.N.; Sjöblom, J.; Kilpatrick, P.K. The Stability of Water-in-Crude and Model Oil Emulsions. Can. J. Chem. Eng. 2008, 85, 793–807. [Google Scholar] [CrossRef]
- Acevedo, S.; Borges, B.; Quintero, F.; Piscitelly, V.; Gutiérrez, L.B. Asphaltenes and Other Natural Surfactants from Cerro Negro Crude Oil. Stepwise Adsorption at the Water/Toluene Interface: Film Formation and Hydrophobic Effects. Energy Fuels 2005, 19, 1948–1953. [Google Scholar] [CrossRef]
- Auflem, I.H.; Westvik, A.; Sjöblom, J. Destabilization of Water-in-Crude Oil Emulsions Based on Recombined Oil Samples at Various Pressures. J. Dispers. Sci. Technol. 2003, 24, 103–112. [Google Scholar] [CrossRef]
- Kiran, S.K.; Acosta, E.; Moran, K. Evaluating the hydrophilic–lipophilic nature of asphaltenic oils and naphthenic amphiphiles using microemulsion models. J. Colloid Interface Sci. 2009, 336, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.G. Wettability Literature Survey- Part 1: Rock/Oil/Brine Interactions and the Effects of Core Handling on Wettability. J. Pet. Technol. 1986, 38, 1125–1144. [Google Scholar] [CrossRef]
- Al-Saedi, H.N.; Flori, R.E.; Al-Jaberi, S.K. Eliminate the role of clay in sandstone: EOR low salinity water flooding. J. Pet. Explor. Prod. Technol. 2018, 9, 1475–1483. [Google Scholar] [CrossRef]
- Alvarado, V.; Wang, X.; Moradi, M. Role of Acid Components and Asphaltenes in Wyoming Water-in-Crude Oil Emulsions. Energy Fuels 2011, 25, 4606–4613. [Google Scholar] [CrossRef]
- Alvarado, V.; Garcia-Olvera, G.; Manrique, E.J. Considerations of Adjusted Brine Chemistry for Waterflooding in Offshore Environments. OTC Bras. 2015. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Langanke, N.; Ganzer, L.; Jaeger, P. Coupling Microfluidics Data with Core Flooding Experiments to Understand Sulfonated/Polymer Water Injection. Polymer 2020, 12, 1227. [Google Scholar] [CrossRef]
- Moustafa, E.-A.A.; Shedid, S.A. Effects of magnesium and potassium sulfates on oil recovery by water flooding. Egypt. J. Pet. 2018, 27, 649–656. [Google Scholar] [CrossRef]
- Morin, B.; Oakey, J.; Liu, Y.; Alvarado, V. A microfluidic flow focusing platform to screen the evolution of crude oil–brine interfacial elasticity. Lab. Chip. 2016, 16, 3074–3081. [Google Scholar] [CrossRef]
- Bidhendi, M.M.; Garcia-Olvera, G.; Morin, B.; Oakey, J.; Alvarado, V. Interfacial Viscoelasticity of Crude Oil/Brine: An Alternative Enhanced-Oil-Recovery Mechanism in Smart Waterflooding. SPE J. 2018, 23, 0803–0818. [Google Scholar] [CrossRef]
- Garcia-Olvera, G.; Alvarado, V. The Potential of Sulfate as Optimizer of Crude Oil-Water Interfacial Rheology to Increase Oil Recovery during Smart Water Injection in Carbonates. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Ayirala, S.C.; AlYousef, A.A.; Li, Z.; Xu, Z. Water Ion Interactions at Crude Oil-Water Interface: A New Fundamental Understanding on SmartWater Flood. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 6–9 March 2017. [Google Scholar] [CrossRef]
- Reza, R. The Effect of Seawater on the Interfacial Tension: Smart Water Flooding. Available online: https://www.academia.edu/25203560/The_Effect_of_Seawater_on_the_Interfacial_Tension_Smart_Water_Flooding (accessed on 15 April 2020).
- Fathi, S.J.; Austad, T.; Strand, S. Water-Based Enhanced Oil Recovery (EOR) by “Smart Water”: Optimal Ionic Composition for EOR in Carbonates. Energy Fuels 2011, 25, 5173–5179. [Google Scholar] [CrossRef]
- Zhang, Y.; Morrow, N.R. Comparison of Secondary and Tertiary Recovery with Change in Injection Brine Composition for Crude-Oil/Sandstone Combinations. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar] [CrossRef]
- Yuan, M. Effect of Temperature on Barium Sulfate Scale Inhibition of Diethylene Triamine Penta (Methylene Phosphonic Acid). In Advances in Crystal Growth Inhibition Technologies; Springer: Boston, MA, USA, 2002; pp. 151–163. [Google Scholar]
- Bin Merdhah, A.B.; Yassin, A.A.M. Laboratory Study and Prediction of Calcium Sulphate at High-Salinity Formation Water. Open Pet. Eng. J. 2008, 1, 62–73. Available online: https://benthamopen.com/ABSTRACT/TOPEJ-1-62 (accessed on 14 November 2017). [CrossRef][Green Version]
- Andersen, K.I.; Halvorsen, E.; Sælensminde, T.; Østbye, N.O. Water Management in a Closed Loop - Problems and Solutions at Brage Field. In Proceedings of the SPE European Petroleum Conference, Paris, France, 24–25 October 2000. [Google Scholar] [CrossRef]
- Ghosh, B.; Sun, L.; Osisanya, S. Smart-Water EOR Made Smarter A Laboratory Development. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016. [Google Scholar] [CrossRef]
- Graham, G.M.; Boak, L.S.; Hobden, C.M. Examination of the Effect of Generically Different Scale Inhibitor Species (PPCA and DETPMP) on the Adherence and Growth of Barium Sulphate Scale on Metal Surfaces. In Proceedings of the International Symposium on Oilfield Scale, Aberdeen, UK, 30–31 January 2001. [Google Scholar] [CrossRef]
- Al-Qattan, A.; Sanaseeri, A.; Al-Saleh, Z.; Singh, B.; Al-Kaaoud, H.; Delshad, M.; Hernandez, R.; Winoto, W.; Badham, S.; Bouma, C.; et al. Low Salinity Waterflood and Low Salinity Polymer Injection in the Wara Reservoir of the Greater Burgan Field. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar] [CrossRef]
- Pourafshary, P.; Moradpour, N. Hybrid EOR Methods Utilizing Low-Salinity Water. Enhanc. Oil Recover. Process. New Technol. 2019. [Google Scholar] [CrossRef]
- Wang, D.; Hao, Y.; Delamaide, E.; Ye, Z.; Ha, S.; Jiang, X. Results of Two Polymer Flooding Pilots in the Central Area of Daqing Oil Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, Texas, 3–6 October 1993. [Google Scholar] [CrossRef]
- Kleinitz, W.; Littmann, W. Polymer Flooding: Appraisal of Four Different Field Projects in Germany. In Proceedings of the IOR 1995 8th European Symposium on Improved Oil Recovery, Vienna, Austria, 15–17 May 1995; p. cp-107-00054. [Google Scholar] [CrossRef]
- Koning, E.J.L.; Mentzer, E.; Heemskerk, J. Evaluation of a Pilot Polymer Flood in the Marmul Field, Oman. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar] [CrossRef]
- Algharaib, M.; Alajmi, A.; Gharbi, R. Improving polymer flood performance in high salinity reservoirs. J. Pet. Sci. Eng. 2014, 115, 17–23. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Enhanced Oil Recovery (EOR) by Combined Low Salinity Water/Polymer Flooding. Energy Fuels 2013, 27, 1223–1235. [Google Scholar] [CrossRef]
- Torrijos, I.D.P.; Puntervold, T.; Strand, S.; Austad, T.; Bleivik, T.H.; Abdullah, H.I. An experimental study of the low salinity Smart Water - Polymer hybrid EOR effect in sandstone material. J. Pet. Sci. Eng. 2018, 164, 219–229. [Google Scholar] [CrossRef]
- AlSofi, A.M.; Wang, J.; AlBoqmi, A.M.; AlOtaibi, M.B.; Ayirala, S.C.; AlYousef, A.A. SmartWater Synergy with Chemical EOR for a Slightly Viscous Arabian Heavy Reservoir. In Proceedings of the SPE Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 6–8 December 2016. [Google Scholar] [CrossRef]
- Santo, A.; Muggeridge, A. An Investigation into the Benefits of Combined Polymer-Low Salinity Waterflooding. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Brisbane, Australia, 23–25 October 2018. [Google Scholar] [CrossRef]
- Mohammadi, H.; Jerauld, G. Mechanistic Modeling of the Benefit of Combining Polymer with Low Salinity Water for Enhanced Oil Recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar] [CrossRef]
- Tabary, R.; Douarche, F.; Bazin, B.; Lemouzy, P.M.; Moreau, P.; Morvan, M. Design of a Surfactant/Polymer Process in a Hard Brine Context: A Case Study Applied to Bramberge Reservoir. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar] [CrossRef]
- Tay, A.; Oukhemanou, F.; Wartenberg, N.; Moreau, P.; Guillon, V.; Delbos, A.; Tabary, R. Adsorption Inhibitors: A New Route to Mitigate Adsorption in Chemical Enhanced Oil Recovery. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar] [CrossRef]
- Hincapie, R.E.; Duffy, J.; O’Grady, C.; Ganzer, L. An Approach to Determine Polymer Viscoelasticity under Flow through Porous Media by Combining Complementary Rheological Techniques. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar] [CrossRef]
- Hincapie, R.E. Pore-Scale Investigation of the Viscoelastic Phenomenon during Enhanced Oil Recovery (EOR) Polymer Flooding through Porous Media; Papierflieger: Clausthal-Zellerfeld, Germany, 2016. [Google Scholar]
- Tahir, M.; Hincapie, R.E.; Be, M.; Ganzer, L. Experimental Evaluation of Polymer Viscoelasticity during Flow in Porous Media: Elongational and Shear Analysis. In Proceedings of the SPE Europec featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E. Coexistence of Shear and Elongational Components of Flow Paths through Porous Media during Polymer-Flooding Applications. In Proceedings of the 77th EAGE Conference and Exhibition, Madrid, Spain, 1–4 June 2015. [Google Scholar]
- Be, M.; Hincapie, R.E.; Rock, A.; Gaol, C.L.; Tahir, M.; Ganzer, L. Comprehensive Evaluation of the EOR Polymer Viscoelastic Phenomenon at Low Reynolds Number. In Proceedings of the SPE Europec featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Tahir, M.; Hincapie, R.E.; Be, M.; Ganzer, L. A Comprehensive Combination of Apparent and Shear Viscoelastic Data during Polymer Flooding for EOR Evaluations. World J. Eng. Technol. 2017, 5, 585–600. [Google Scholar] [CrossRef][Green Version]
- Al-Saedi, H.; Flori, R.E.; Mortadha, A. Investigation of Smart Water Flooding in Sandstone Reservoirs: Experimental and Simulation Study Part 2. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 12–15 November 2018. [Google Scholar] [CrossRef]
- Skauge, T.; Djurhuus, K.; Reichenbach-Klinke, R. Visualization of Heavy Oil Mobilization by Associative Polymer. In Proceedings of the SPE Europec featured at 80th EAGE Conference and Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar] [CrossRef]
- Bowen, R.L. Chemical Engineering, 68th ed.; Elsevier: Amsterdam, The Netherlands, 1961; pp. 119–122. [Google Scholar]
- Vermolen, E.; Van Haasterecht, M.J.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M.A. Pushing the envelope for polymer flooding towards high-temperature and high-salinity reservoirs with polyacrylamide based ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. [Google Scholar] [CrossRef]
- Elhajjaji, R.R.; Hincapie, R.E.; Tahir, M.; Rock, A.; Wegner, J.; Ganzer, L. Systematic Study of Viscoelastic Properties During Polymer-Surfactant Flooding in Porous Media (Russian). In Proceedings of the SPE Russian Petroleum Technology Conference and Exhibition, Moscow, Russia, 24–26 October 2016. [Google Scholar] [CrossRef]
- Tetteh, J.T.; Barati, R. Crude-Oil/Brine Interaction as a Recovery Mechanism for Low-Salinity Waterflooding of Carbonate Reservoirs. SPE Reserv. Eval. Eng. 2019, 22, 877–896. [Google Scholar] [CrossRef]






| No. | Mechanisms | Approach/Evaluation | Formation | Active Salts | Ref. |
|---|---|---|---|---|---|
| 1 | Wettability alteration | Core flooding, IFT | Sandstone and Carbonates | Low-salt brine | [33] |
| 2 | Fine migration, Wettability alteration | IFT, Core flooding | Sandstone and Carbonates | Low-salt brine | [34] |
| 3 | Transition state | Sand adhesion tests for 3-pH ranges | Quartz Sands | NaCl, CaCl2 | [35] |
| 4 | Wettability alteration | Sand-pack flooding | Sand-pack | Decreasing NaCl | [9] |
| 5 | Wettability alteration | Spontaneous imbibition, Core flooding | Sandstone | Low-salt brine | [5] |
| 6 | IFT, Wettability alteration, MIE | Core flooding, Simulation | Sandstone | Low-salt brine | [36] |
| 7 | Double layer expansion | Contact angle, Core flooding | Sandstone | Low-salt brine | [37] |
| 8 | COBR | Core flooding | Sandstone | Low-salt brine | [38] |
| 9 | Wettability alteration, Pore plugging | Core flooding | Sandstone | Low-salt brine | [39] |
| 10 | Wettability alteration, PH-value | Core flooding, Sand-pack, Crushed sand | Sandstone | Low-salt brine | [25] |
| 11 | Wettability alteration | Core flooding | Sandstone | Softened Formation brine (Ca. Mg) | [40] |
| 12 | IFT | IFT | Various | Ca, SO4 | [41] |
| 17 | Various | Review publication | Various | N/A | [42] |
| 18 | Various | Review publication | Various | N/A | [43] |
| 19 | Various | Review publication | Various | N/A | [44] |
| No. | Mechanism | Approach | Formation | Active Salts | Ref. |
|---|---|---|---|---|---|
| 1 | Wettability alteration, DLE, Oil–brine int. | Spontaneous imbibition, Interfacial rheology | Sandstone | SO4, Low salt brine (36,600–5460 ppm) | [22] |
| 2 | Oil–brine interfacial interactions | Interfacial viscoelasticity, Core flooding | Sandstone | SO4, Low salt brine (1%–10% of 0.674 M) | [48] |
| 3 | Oil–brine interfacial interactions | IFT | N/A | Ca, Mg, Na, SO4 (5760 ppm) | [20] |
| 4 | Oil–brine interfacial interactions | Core flooding | Carbonate | SO4, Low salt brine (3535–37,102 ppm) | [49] |
| 5 | Oil–brine interfacial interactions | Langmuir Trough, Oil drop Volume, G’,G’’ Moduli | N/A | SO4, Low salt brine (5761–16,407 ppm) | [50] |
| 6 | IFT | IFT | N/A | Ca, SO4 | [51] |
| No. | Mechanisms | Approach | Formation | Flooding Plan | Ref. |
|---|---|---|---|---|---|
| 1 | Hybrid EOR | Pilot test | Sandstone | Diluted produced brine + Low salinity polymer | [59] |
| 2 | Hybrid EOR | Review | Sandstone | Low salt brine + Low concentration polymer | [60] |
| 3 | Hybrid EOR | Pilot test | Sandstone | Low salt brine pre-flush (800–1300 ppm) + Polymer flood (1000 ppm) | [61] |
| 4 | Hybrid EOR | Pilot test | Sandstone | Low salt brine pre-flush + Polymer flood (800 ppm) | [62] |
| 5 | Hybrid EOR | Pilot test | Sandstone | Low salt brine pre-flush (500 ppm) + Polymer flood (800 ppm) | [63] |
| 6 | Hybrid EOR | Core flooding | Sandstone | Low salt brine pre-flush (5000–30000 ppm) + Polymer flood (2000–4000 ppm) | [64] |
| 7 | Hybrid EOR | Core flooding | Sandstone | Low salt brine pre-flush (3600 ppm) + Polymer flood (300–1000 ppm) | [65] |
| 8 | Hybrid EOR | Core flooding | Sandstone | Low salt brine pre-flush (6900 ppm) + Polymer flood (1000 ppm) | [66] |
| 9 | Hybrid EOR | Core flooding | Carbonate | Low salt brine pre-flush (6900 ppm) + Polymer flood (2000–3000 ppm) | [67] |
| 10 | Hybrid EOR | Numerical simulation | Sandstone | Low salt brine (700–5000 ppm) + Polymer flood (1000–1500 ppm) | [68] |
| 11 | Hybrid EOR | Numerical simulation | Sandstone | Low salt brine (10 times seawater) + Polymer flood (13.5cP) | [69] |
| Chemical Formula | Total Dissolved Solids (g/L) | |||||
|---|---|---|---|---|---|---|
| Group 1 Formation Brines | Group 2 Injection Brines | |||||
| Brine 1 | Brine 2 | Brine 1 | Brine 3 | Brine 4 | Brine 5 | |
| SSW | 2*SSW | SSW | SSW+2SO4−2 | SSW+4SO4−2 | SSW-Na | |
| NaCl | 23.97 | 47.94 | 23.97 | 23.97 | 23.97 | 0.00 |
| KCl | 0.80 | 1.60 | 0.80 | 0.80 | 0.80 | 0.80 |
| CaCl2·2H2O | 1.11 | 2.21 | 1.11 | 1.11 | 1.11 | 1.11 |
| MgCl2·6H2O | 11.04 | 22.08 | 11.04 | 11.04 | 11.04 | 11.04 |
| SrCl2·6H2O | 0.03 | 0.05 | 0.02 | 0.03 | 0.02 | 0.02 |
| Na2SO4 | 3.93 | 7.86 | 3.93 | 7.86 | 15.73 | 3.93 |
| NaHCO3 | 0.27 | 0.55 | 0.27 | 0.27 | 0.27 | 0.27 |
| TDS | 41.15 | 82.31 | 41.15 | 45.09 | 52.95 | 17.18 |
| Hardness | 0.13 | 0.13 | 0.13 | 0.11 | 0.09 | 0.48 |
| Density (g/cm3) @22 °C | 1.03 | 1.04 | 1.03 | 1.02 | 1.04 | 1.02 |
| η (mPa.s) @45 °C | 0.63 | 0.7 | 0.63 | 0.68 | 0.68 | 0.65 |
| Core | L | D | phi, Φ | PV | kg | kb | Swc | Soi | FB* |
|---|---|---|---|---|---|---|---|---|---|
| mm | mm | % | mL | mD | mD | % | % | ||
| T1 | 59.99 | 29.52 | 27.18 | 8.95 | 3272 | 2148 | 20.61 | 79.39 | SSW |
| T2 | 60.11 | 29.36 | 26.53 | 9.18 | 3231 | 2067 | 15.66 | 84.34 | SSW |
| T3 | 60.4 | 29.08 | 26.22 | 8.19 | 3775 | 2051 | 21.39 | 78.61 | 2*SSW |
| T4 | 60.08 | 29.34 | 26.2 | 8.58 | 3464 | 2113 | 20.52 | 79.48 | 2*SSW |
| T5 | 60.37 | 29.31 | 28.56 | 8.05 | 3434 | 1946 | 20.97 | 79.03 | 2*SSW |
| T6 | 60.22 | 29.3 | 26.41 | 9.2 | 3438 | 1944 | 17.80 | 82.20 | 2*SSW |
| T7 | 60.09 | 29.44 | 26.76 | 9.2 | 3244 | 1952 | 17.89 | 82.11 | SSW |
| T8 | 59.93 | 29.33 | 26.06 | 8.8 | 3112 | 1970 | 18.67 | 81.33 | SSW |
| T10 | 59.87 | 29.33 | 26.43 | 8.4 | 3370 | 2108 | 26.36 | 73.64 | SSW |
| AVE. | 60.12 ± 0.17 | 29.33 ± 0.11 | 26.73 ± 0.73 | 8.72 ± 0.45 | 3371.11 ± 180.41 | 2033.22 ± 76.56 | 19.99 ± 2.86 | 80.01 ± 2.80 | - |
| Formation Brine | Secondary Mode Injection | Tertiary Mode Injection | ||
|---|---|---|---|---|
| 2*SSW | SSW | Bump Rate | Polymer prepared in SSW | |
| SSW+2SO4−2 | Bump Rate | Polymer prepared in SSW +2SO4−2 | ||
| SSW+4SO4−2 | Bump Rate | Polymer prepared in SSW +4SO4−2 | ||
| SSW-NaCl | Bump Rate | Polymer prepared in SSW +2SO4−2 | ||
| SSW | SSW | Bump Rate | Polymer prepared in SSW | |
| SSW+2SO4−2 | Bump Rate | Polymer prepared in SSW+2SO4−2 | ||
| SSW+4SO4−2 | Bump Rate | Polymer prepared in SSW+4SO4−2 | ||
| SSW-NaCl | Bump Rate | Polymer prepared in SSW+2SO4−2 | ||
| SSW | Bump Rate | SSW +2SO4−2 | Polymer prepared in SSW | |
| V (ft/day) | 1 | 2.3 | 1 | |
| Brine | IFT 1 | IFT 2 | IFT (Average) |
|---|---|---|---|
| mN/m | mN/m | mN/m | |
| SSW | 2.03 | 2.37 | 2.20 |
| SSW+2 SO4−2 | 4.66 | 4.28 | 4.47 |
| SSW+4SO4−2 | 2.11 | 2.01 | 2.06 |
| SSW-0NaCl | 2.85 | 3.02 | 2.94 |
| 2*SSW | 3.15 | 3.39 | 3.27 |
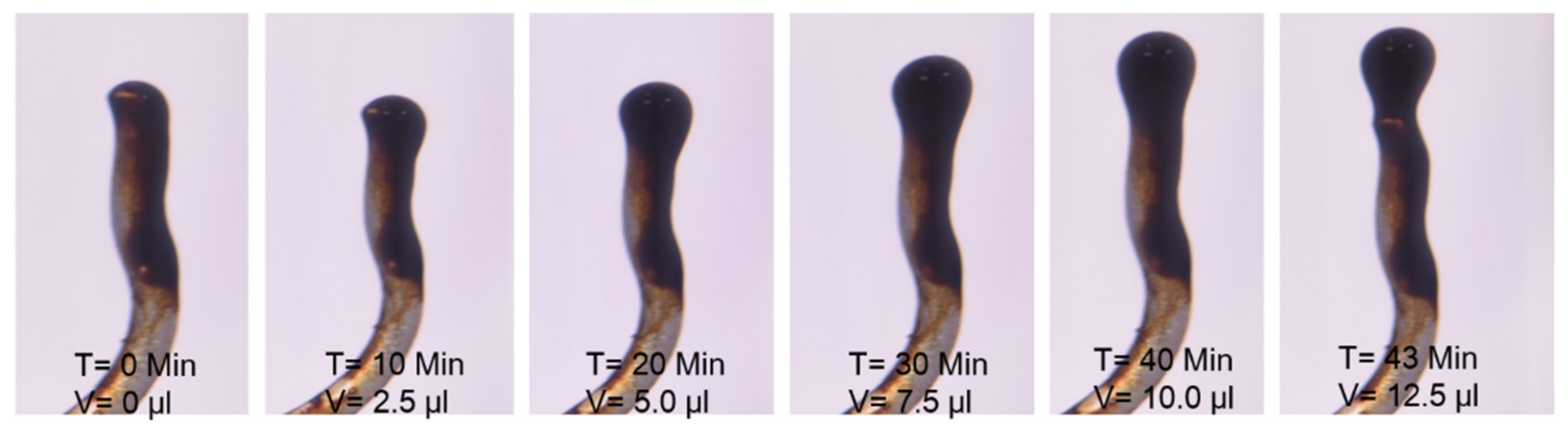
| Brine | Time Till Snap-Off | Oil Drop Size |
|---|---|---|
| Minutes | µL | |
| SSW | 21 | 7.5 |
| SSW+4SO4−2 | 11 | 5.0 |
| SSW+2SO4−2 | 43 | 12.5 |
| Nr. | Form. Brine | Inj. Brine | Core | Φ | k, brine | Soi | RF Sec. Mode | Inje. PV | RF Bump Rate | Inje. PV | RF Tert. Mode | Inje. PV | Total RF (%) | ROS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SSW | SSW | T2 | 26.53 | 2067 | 84.34 | 34.27 | ≈3 | 7.55 | ≈3 | 13.94 | ≈3 | 55.76 | 44.24 |
| 2 | SSW+2SO4−2, | T7 | 26.76 | 1952 | 82.11 | 45.69 | ≈3 | 7.07 | ≈3 | 8.84 | ≈3 | 61.60 | 38.40 | |
| 3 | SSW+4SO4−2, | T8 | 26.06 | 1970 | 81.33 | 38.98 | ≈3 | 9.28 | ≈3 | 9.90 | ≈3 | 58.16 | 41.84 | |
| 4 | SSW-0NaCl, | T1 | 27.18 | 2148 | 79.39 | 32.01 | ≈3 | 14.95 | ≈3 | 12.56 | ≈3 | 59.52 | 40.48 | |
| 5 | 2*SSW | SSW | T6 | 26.41 | 1944 | 82.2 | 34.56 | ≈3 | 10.36 | ≈3 | 12.67 | ≈3 | 57.59 | 42.41 |
| 6 | SSW+2SO4−2, | T4 | 26.20 | 2113 | 79.48 | 41.37 | ≈3 | 13.59 | ≈3 | 10.05 | ≈3 | 65.01 | 34.99 | |
| 7 | SSW+4SO4−2 | T5 | 28.56 | 1946 | 79.03 | 51.78 | ≈3 | 11.50 | ≈3 | 8.83 | ≈3 | 72.11 | 27.89 | |
| 8 | SSW-0NaCl, | T3 | 26.22 | 2051 | 78.61 | 45.35 | ≈3 | 9.67 | ≈3 | 16.32 | ≈3 | 71.34 | 28.66 |
| Nr. | Form. Brine | Inj. Brine | Core Name | Φ | k, brine | Soi | RF Sec. Mode (BF) | Inje. PV | RF Bump Rate | Inje. PV | RF Tert. Mode | RF Post-Tert. | Total RF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SSW | SSW | T10 | 26.43 | 2108 | 73.64 | 34.04 | ≈3 | 10.21 | ≈3 | 9.64 | 3.97 | 57.857 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.; Hincapie, R.E.; Ganzer, L. Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding. Energies 2020, 13, 3049. https://doi.org/10.3390/en13123049
Tahir M, Hincapie RE, Ganzer L. Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding. Energies. 2020; 13(12):3049. https://doi.org/10.3390/en13123049
Chicago/Turabian StyleTahir, Muhammad, Rafael E. Hincapie, and Leonhard Ganzer. 2020. "Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding" Energies 13, no. 12: 3049. https://doi.org/10.3390/en13123049
APA StyleTahir, M., Hincapie, R. E., & Ganzer, L. (2020). Unlocking the Effects of Fluid Optimization on Remaining Oil Saturation for the Combined Sulfate-Modified Water and Polymer Flooding. Energies, 13(12), 3049. https://doi.org/10.3390/en13123049







