Screening of the Effective Additive to Inhibit Surfactin from Forming Precipitation with Divalent Cations for Surfactin Enhanced Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used in This Study
2.2. Methodology
2.2.1. Turbidity Measurements
2.2.2. IFT Measurements
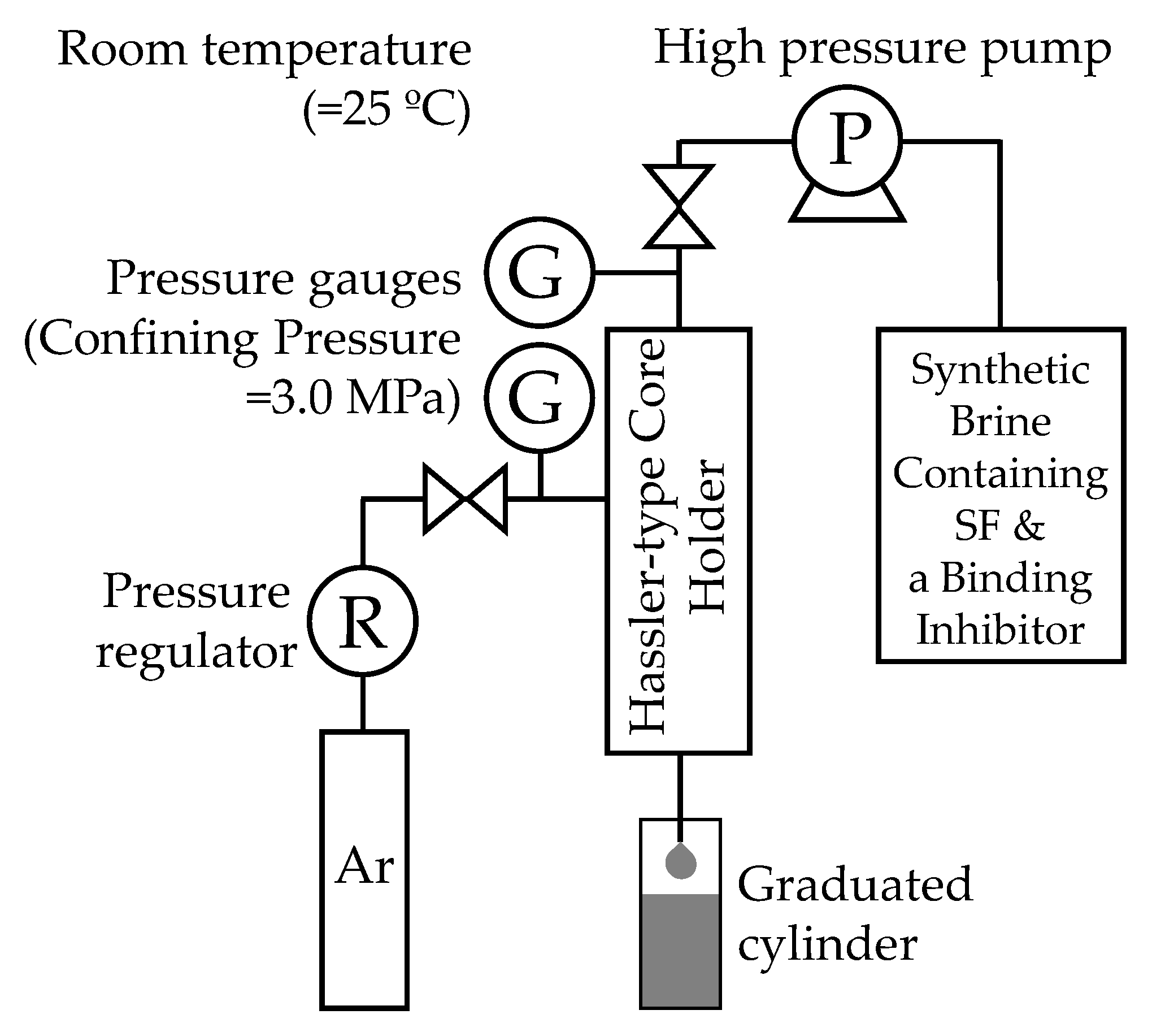
2.2.3. Surfactin Injectivity Tests
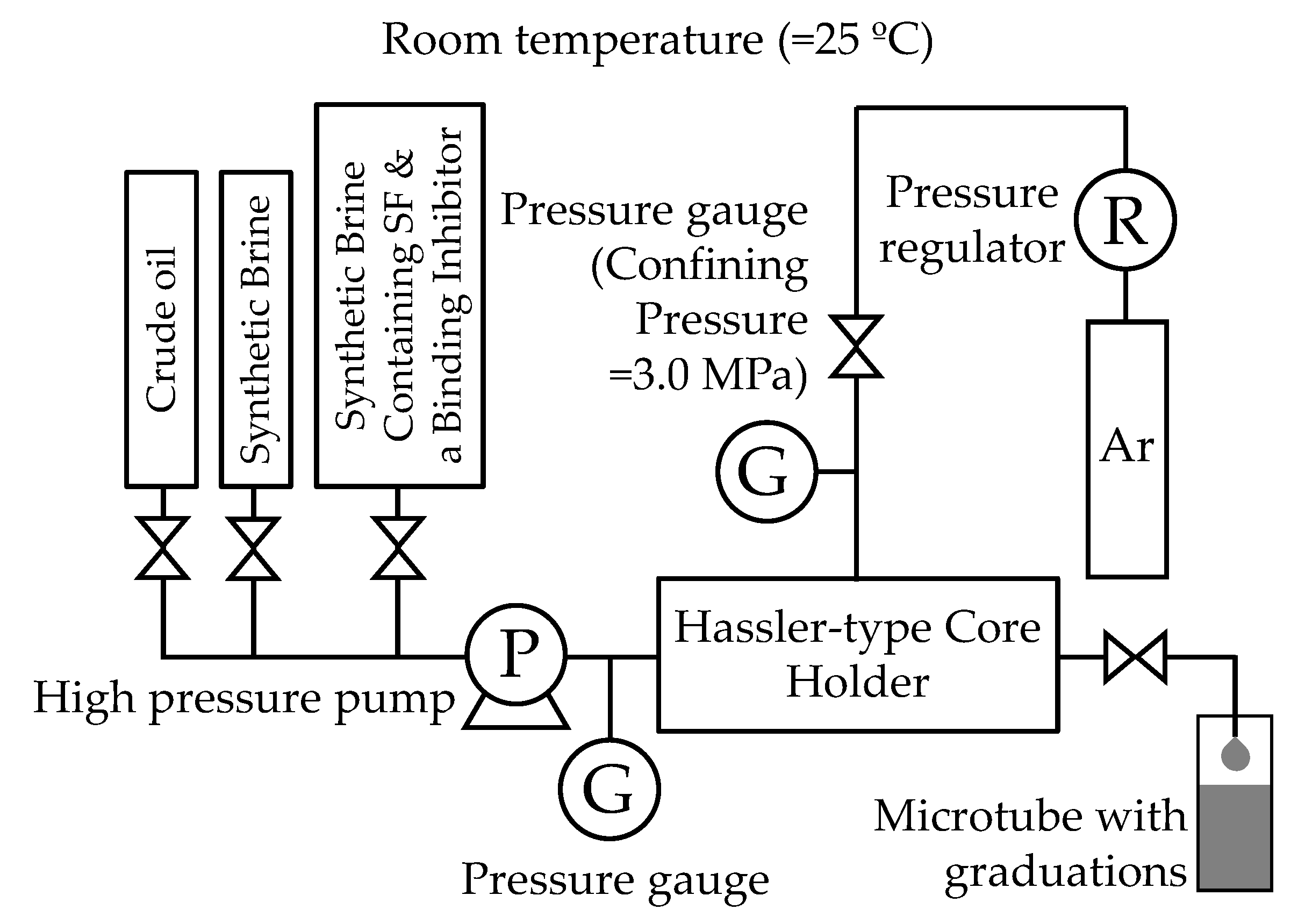
2.2.4. Core Flooding Experiments
3. Results and Discussion
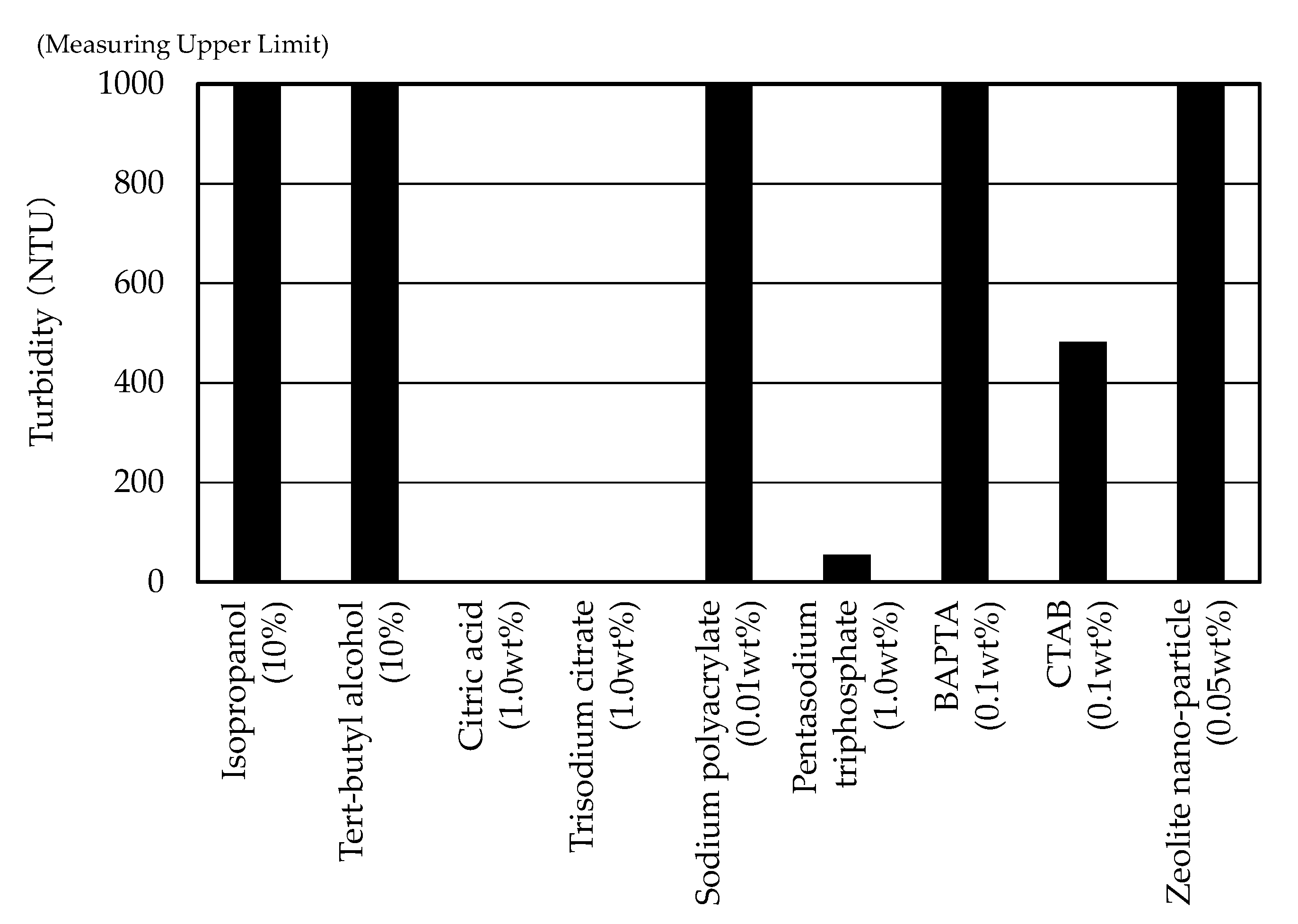
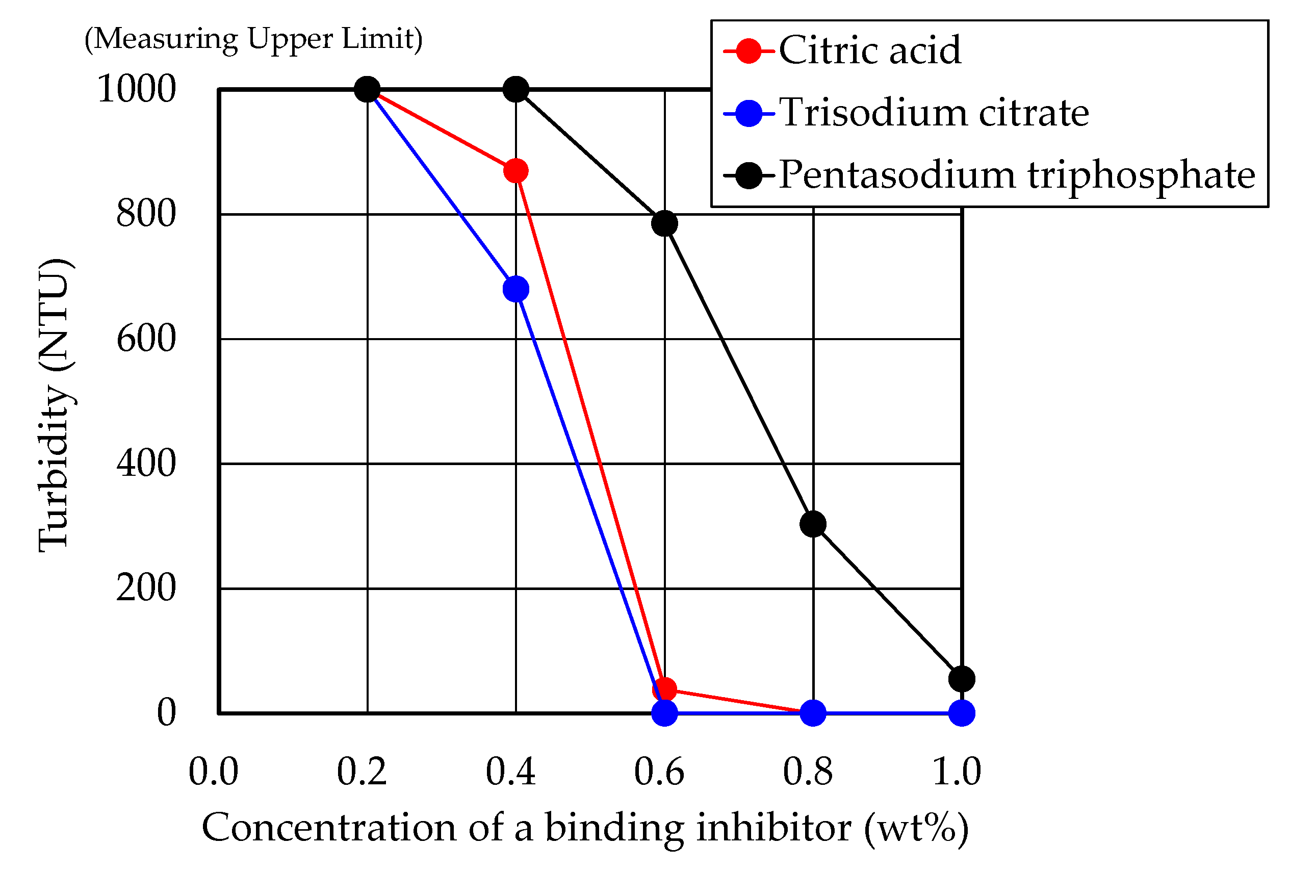
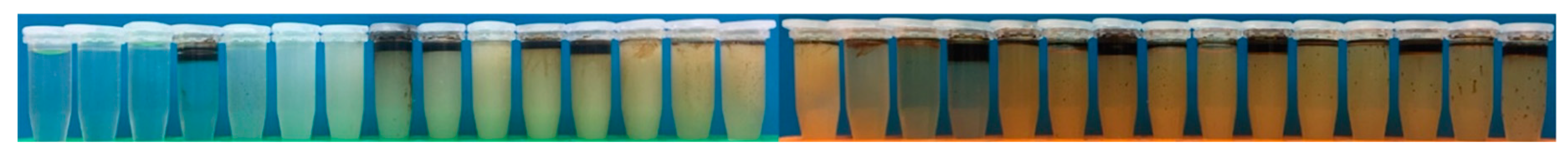
3.1. Turbidity Measurements
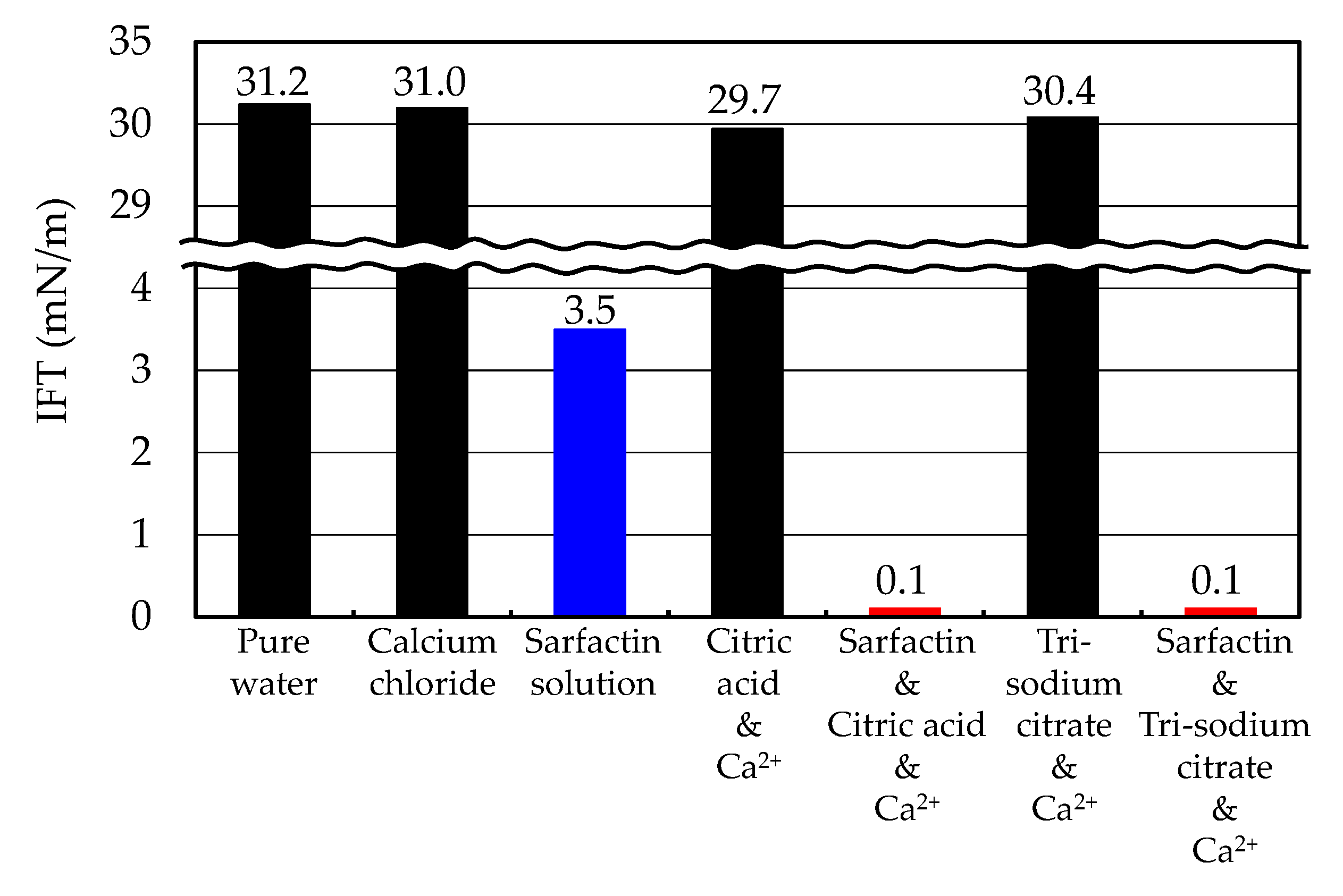
3.2. IFT Measurements
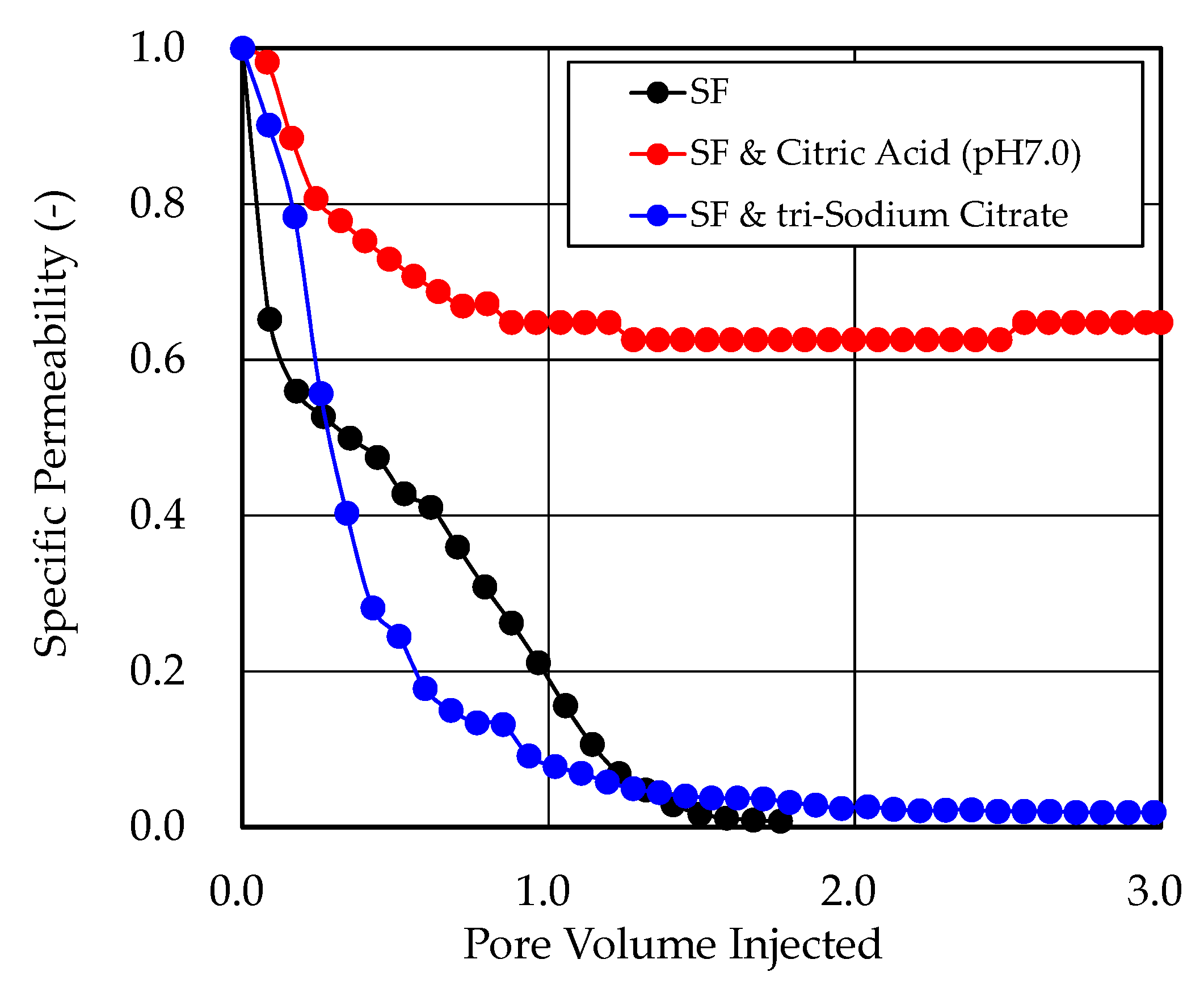
3.3. Surfactin Injectivity Tests
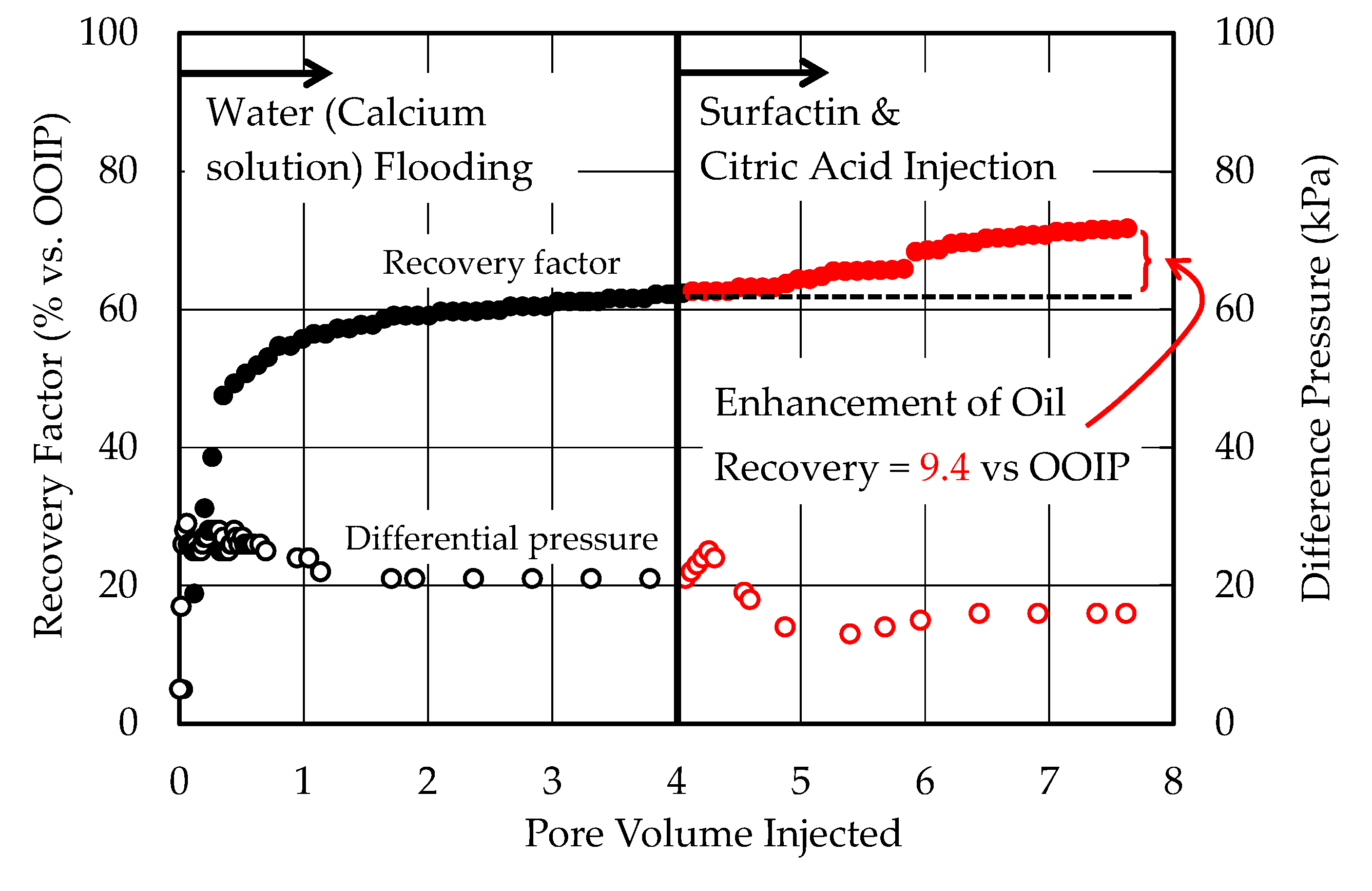
3.4. Core Flooding Experiments
4. Conclusions
- Citric acid, trisodium citrate and pentasodium triphosphate successfully prevented the surfactin from combining with calcium ion and forming the precipitation of the surfactin calcium. In particular, citric acid and trisodium citrate inhibited the binding of surfactin with calcium ions with lower concentrations, such as 0.6 wt%.
- Both citric acid and trisodium citrate had strong potential as co-surfactants of the surfactin because the IFT between the surfactin solution containing those inhibitors and oil was greatly decreased to less than 0.1 mN/m, which was less than one-thirtieth as compared with IFT between the pure surfactin solution and oil.
- Trisodium citrate, however, caused significant permeability reduction in the injectivity tests, whereas citric acid could be injected into the Berea sandstone core without significant permeability reduction. The high pH value of trisodium citrate solution might cause the dissolution of ferrum and aluminum in the core and the colloids of ferrous hydroxide and aluminum hydroxide were formed in the core, which caused the significant reduction in the permeability. Citric acid was therefore selected as the best inhibitor.
- Citric acid was subjected to the core flooding experiments as the inhibitor. While the synthetic brine containing 0.3 wt% of surfactin, 0.6 wt% of citric acid and 900 ppm of calcium ion was injected into the oil reservoir, rise in the differential pressure was not observed in the experiments using the high permeability (175 md) reservoir while a small rise in the differential pressure was observed in the experiment using the low permeability (5.9 md) reservoir. The influence of the decrease in the permeability may be reduced in more permeable oil reservoir.
- Overall, 13.1% and 24.9% of oil remaining in the oil reservoir after the primary oil recovery was recovered from the low permeability reservoir and high permeability reservoir, respectively, by injecting the synthetic brine containing 0.3 wt% of surfactin, 0.6 wt% of citric acid and 900 ppm of calcium ion. In particular, the 24.9% recovery factor was 0.9% higher than the recovery factor obtained by injecting pure surfactin solution reported by previous work. Citric acid is also effective for enhancing the surfactin capacity for increasing the recovery factor.
- The above results support the notion that citric acid has a dual role as the binding inhibitor and co-surfactant for the surfactin. As citric acid is an environmentally friendly and cheap chemical, it can be a promising additive which can increase the applicable reservoir and potential of surfactant EOR.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Cross-sectional area of the core samples |
| K | Absolute permeability |
| ks | Specific permeability |
| ka | Apparent permeability |
| L | Length of the core |
| Q | Flow rate of brine |
| ΔP | Pressure different between both ends of the core |
| µ | Viscosity of the brine injected |
References
- Mohan, K. Alkaline Surfactant Flooding for Tight Carbonate Reservoirs. Presented at the SPE Annual Technical Conference and Exhibition 2009, New Orleans, LA, USA, 4–7 October 2009. Paper SPE-129516-STU. [Google Scholar]
- Wang, D.; Zhang, Z.; Cheng, J.; Demin, W.; Zhenhua, Z.; Jiecheng, C.; Jingchun, Y.; Shutang, G.; Li, L. Pilot Test of Alkaline/Surfactant/Polymer Flooding in Daqing Oil Field. SPE Reserv. Eng. 1997, 12, 229–233. [Google Scholar]
- Clara, H.; Larry, J.C.; Lorenzo, A.; Baldonedo, A.; Jie, Q.; Dowling, P.C.; Pitts, M.J. ASP System Design for an Offshore Application in the La Salina Field, Lake Maracaibo. SPE Reserv. Eval. Eng. 2001, 6, 147–156. [Google Scholar]
- Pratap, M.; Gauma, M.S. Field Implementation of Alkaline–Surfactant–Polymer (ASP) Flooding: A Maiden Effort in India. Presented at the Asia Pacific Oil and Gas. Conference and Exhibition, Perth, Australia, 18–20 October 2004. Paper SPE-88455-MS. [Google Scholar]
- Chang, H.L.; Zhang, Z.Q.; Wang, Q.M.; Xu, Z.S.; Guo, Z.D.; Sun, H.Q.; Qiao, Q. Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People’s Republic of China. J. Pet. Technol. 2004, 58, 84–89. [Google Scholar] [CrossRef]
- Pitts, M.J.; Dowling, P.; Wyatt, K.; Surkalo, H.; Adams, K.C. Alkaline–Surfactant–Polymer Flood of the Tanner Field. Presented at the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. Paper SPE-100004-MS. [Google Scholar]
- Wang, D.; Cheng, J.; Wu, J.; Demin, W.; Jiecheng, C.; Junzheng, W.; Zhenyu, Y.; Yuming, Y. Summary of ASP Pilots in Daqing Oil Field. Presented at the SPE Asia Pacific Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 25–26 October 1999. Paper SPE-57288-MS. [Google Scholar]
- Li, H.; Liao, G.; Han, P.; Hongfu, L.; Guangzhi, L.; Peihui, H.; Zhenyu, Y.; Xiaolin, W.; Chen Guangyu, C.; Peiqiang, J.; et al. Alkaline/Surfactant/Polymer (ASP) Commercial Flooding Test in Central Xing2 Area of Daqing Oilfield. Presented at the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 20–21 October 2003. Paper SPE-84896-MS. [Google Scholar]
- Cheng, J.; Xu, D.; Bai, W. Commercial Test of ASP Flooding in Daqing Oil Field. Presented at the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 3–6 November 2008. Paper SPE-117824-MS. [Google Scholar]
- Hong, F.L.; Dian, P.X.; Jiang, J.; Li, H.; Xu, D.; Jiang, J.; Du, X.; Hong, J.; Jiang, Y.; Xu, Y. Performance Analysis of ASP Commercial Flooding in Central Xing2 Area of Daqing Oilfield. Presented at the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. Paper SPE-114348-MS. [Google Scholar]
- Wang, F.; Yang, Z.; Wu, J.; Li, Y.; Wang, F.; Wu, J.; Yang, Z.; Chen, G.; Zong, L.; Peng, S. Current Status and Prospects of ASP Flooding in Daqing Oil Fields. Presented at the SPE/DOE Symposium on Improved Oil Recovery 2008, Tulsa, OK, USA, 20–23 April 2008. Paper SPE-114343-MS. [Google Scholar]
- Shahri, M.P.; Shadizadeh, S.R.; Jamialahmadi, M. Applicability Test of New Surfactant Produced from Zizyphus Spina-Christi Leaves for Enhanced Oil Recovery in Carbonate Reservoirs. J. Jpn. Pet. Inst. 2011, 55, 27–32. [Google Scholar] [CrossRef]
- Austad, T.; Matre, B.; Milter, J.; Saevareid, A.; Øyno, L. Chemical Flooding of Oil Reservoirs 8. Spontaneous Oil Expulsion from Oil- and Water-Wet Low Permeable Chalk Material by Imbibition of Aqueous Surfactant Solutions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 137, 117–129. [Google Scholar] [CrossRef]
- Sheng, J.J. Status of Surfactant EOR Technology. Petroleum 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Nazina, T.N.; Sokolova, D.; Shestakova, N.M.; Grigor’ian, A.A.; Mikhaĭlova, E.M.; Babich, T.L.; Lysenko, A.M.; Turova, T.P.; Poltaraus, A.B.; Feng, T.; et al. The Phylogenetic Diversity of Aerobic Organotrophic Bacteria from the Dagan High-Temperature Oil Field. Microbiology 2005, 74, 343–351. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sivapathasekaran, C.; Sen, R. Antimicrobial Biosurfactants from Marine Bacillus Circulans: Extracellular Synthesis and Purification. Lett. Appl. Microbiol. 2009, 48, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.E. The Effect of a New in Situ Precipitation Inhibitor on Chemical EOR. J. Pet. Explor. Prod. Technol. 2013, 3, 133–137. [Google Scholar]
- Zhang, J.; Nguyen, Q.P.; Flaaten, A.; Pope, G.A. Mechanisms of Enhanced Natural Imbibition with Novel Chemicals. SPE Reserv. Eval. Eng. 2009, 12, 912–920. [Google Scholar] [CrossRef]
- Flaaten, A.; Nguyen, Q.P.; Pope, G.A.; Zhang, G. A systematic laboratory approach to low-cost, high-performance chemical flooding. SPE Reserv. Eval. Eng. 2009, 12, 713–723. [Google Scholar] [CrossRef]
- Saito, M.; Sugai, Y.; Sasaki, K.; Okamoto, Y.; Ouyang, C. Experimental and Numerical Studies on EOR Using a Biosurfactant. Presented at the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 7–10 November 2016. Paper SPE-183496-MS. [Google Scholar]
- Mohanty, K.K.; Chandrasekhar, S. Wettability Alteration with Brine Composition in High Temperature Carbonate Reservoirs. Presented at the SPE Annual Technical Conference and Exhibition 2013, New Orleans, LA, USA, 30 September–2 October 2013. Paper SPE-166280-MS. [Google Scholar]
- Fathi, S.J.; Austad, T.; Strand, S. Smart Water as a Wettability Modifier in Chalk: The Effect of Salinity and Ionic Composition. Energy Fuels 2010, 24, 2514–2519. [Google Scholar] [CrossRef]
- Gupta, R.; Smith, P.G.J.; Hu, L.; Willingham, T.W.; Cascio, M.L.; Shyeh, J.J.; Harris, C.R. Enhanced Waterflood for Middle East Carbonate Cores–Impact of Injection Water Composition. Presented at the SPE Middle East. Oil and Gas. Show and Conference, Manama, Bahrain, 25–28 September 2011. Paper SPE-142668-MS. [Google Scholar]

| Class | Chemicals | Final Concentration in the Testing Solution | Expected Effects |
|---|---|---|---|
| Alcohols | Isopropanol | 10.0% | Enhancing solubility of surfactin calcium |
| Tertiary butyl alcohol | 10.0% | ||
| Chelating Agents | Citric acid (pH7.0) | 1.00 wt% | Capturing calcium ion |
| Tri-sodium citrate | 1.00 wt% | ||
| Sodium poly-acrylate | 0.01 wt% | ||
| Penta-sodium triphosphate | 1.00 wt% | ||
| BAPTA | 0.10 wt% | ||
| Cationic Surfactant | CTAB | 0.10 wt% | Preventing SF from binding with calcium ion by binding with SF |
| Ion Exchanger | Zeolite nano particle | 0.05 wt% | Capturing calcium ion |
| Testing Solution | Initial Permeability, md | Apparent Permeability, md | Specific Permeability |
|---|---|---|---|
| 0.3%-Surfactin | 32.2 | 0.258 | 0.008 |
| 0.3%-Surfactin 0.6%-Citric Acid (pH7.0) 900 ppm-Calcium | 26.1 | 16.9 | 0.648 |
| 0.3%-Surfactin 0.6%-Tri-Sodium citrate 900 ppm-Calcium | 27.0 | 0.511 | 0.019 |
| Type of Oil Reservoir | OOIP, mL | Oil Recovered by Primary Recovery, mL | Primary Oil Recovery, % | Residual Oil, mL | Oil Recovered by Surfactin and Citric Acid Injection, mL | EOR vs. OOIP, % | EOR vs. Residual Oil, % |
|---|---|---|---|---|---|---|---|
| Low Permeability | 11.9 | 6.5 | 54.8 | 5.4 | 0.7 | 5.9 | 13.1 |
| High Permeability | 10.1 | 6.3 | 62.3 | 3.8 | 1.0 | 9.4 | 24.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, N.; Sugai, Y.; Sasaki, K.; Okamoto, Y.; Yanagisawa, S. Screening of the Effective Additive to Inhibit Surfactin from Forming Precipitation with Divalent Cations for Surfactin Enhanced Oil Recovery. Energies 2020, 13, 2430. https://doi.org/10.3390/en13102430
Miyazaki N, Sugai Y, Sasaki K, Okamoto Y, Yanagisawa S. Screening of the Effective Additive to Inhibit Surfactin from Forming Precipitation with Divalent Cations for Surfactin Enhanced Oil Recovery. Energies. 2020; 13(10):2430. https://doi.org/10.3390/en13102430
Chicago/Turabian StyleMiyazaki, Nao, Yuichi Sugai, Kyuro Sasaki, Yoshifumi Okamoto, and Satohiro Yanagisawa. 2020. "Screening of the Effective Additive to Inhibit Surfactin from Forming Precipitation with Divalent Cations for Surfactin Enhanced Oil Recovery" Energies 13, no. 10: 2430. https://doi.org/10.3390/en13102430
APA StyleMiyazaki, N., Sugai, Y., Sasaki, K., Okamoto, Y., & Yanagisawa, S. (2020). Screening of the Effective Additive to Inhibit Surfactin from Forming Precipitation with Divalent Cations for Surfactin Enhanced Oil Recovery. Energies, 13(10), 2430. https://doi.org/10.3390/en13102430





