Effect of Environment-Friendly Non-Ionic Surfactant on Interfacial Tension Reduction and Wettability Alteration; Implications for Enhanced Oil Recovery
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Dodecanoyl-Glucosamine Surfactant
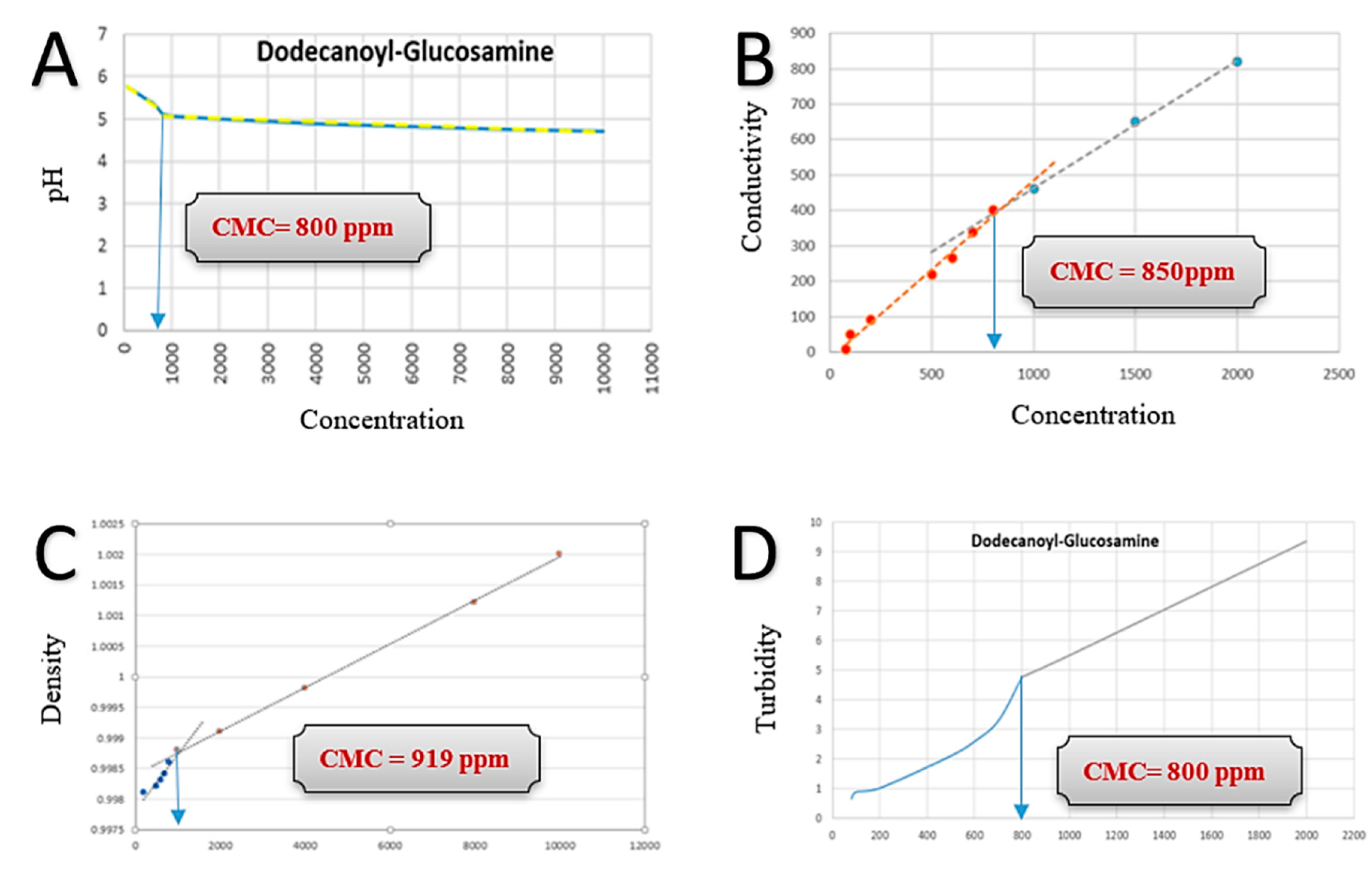
2.3. Mechanistic Quantification of Critical Micelle Concentration (CMC)
2.4. Core Cleaning and Core Ageing Procedure
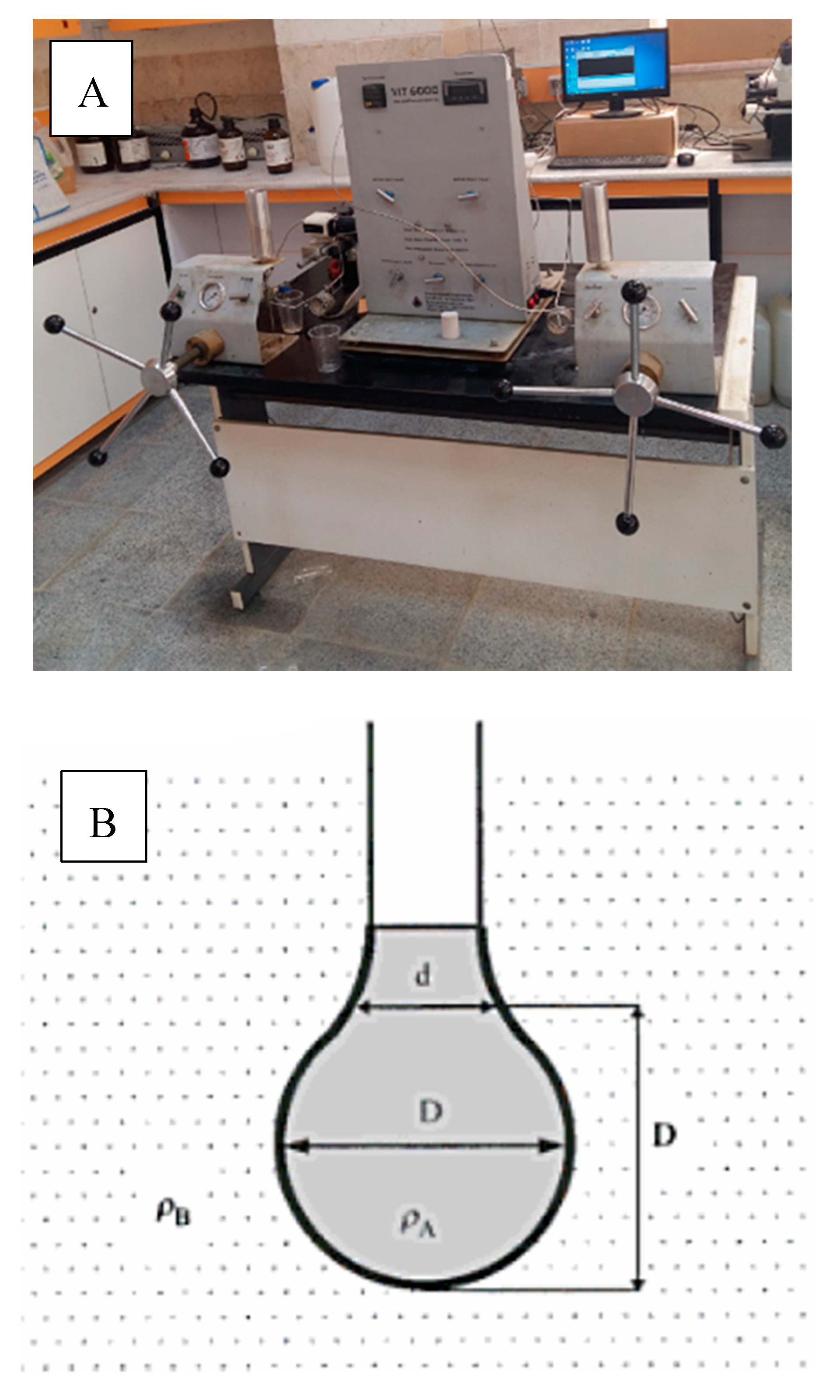
2.5. IFT Measurement Systems
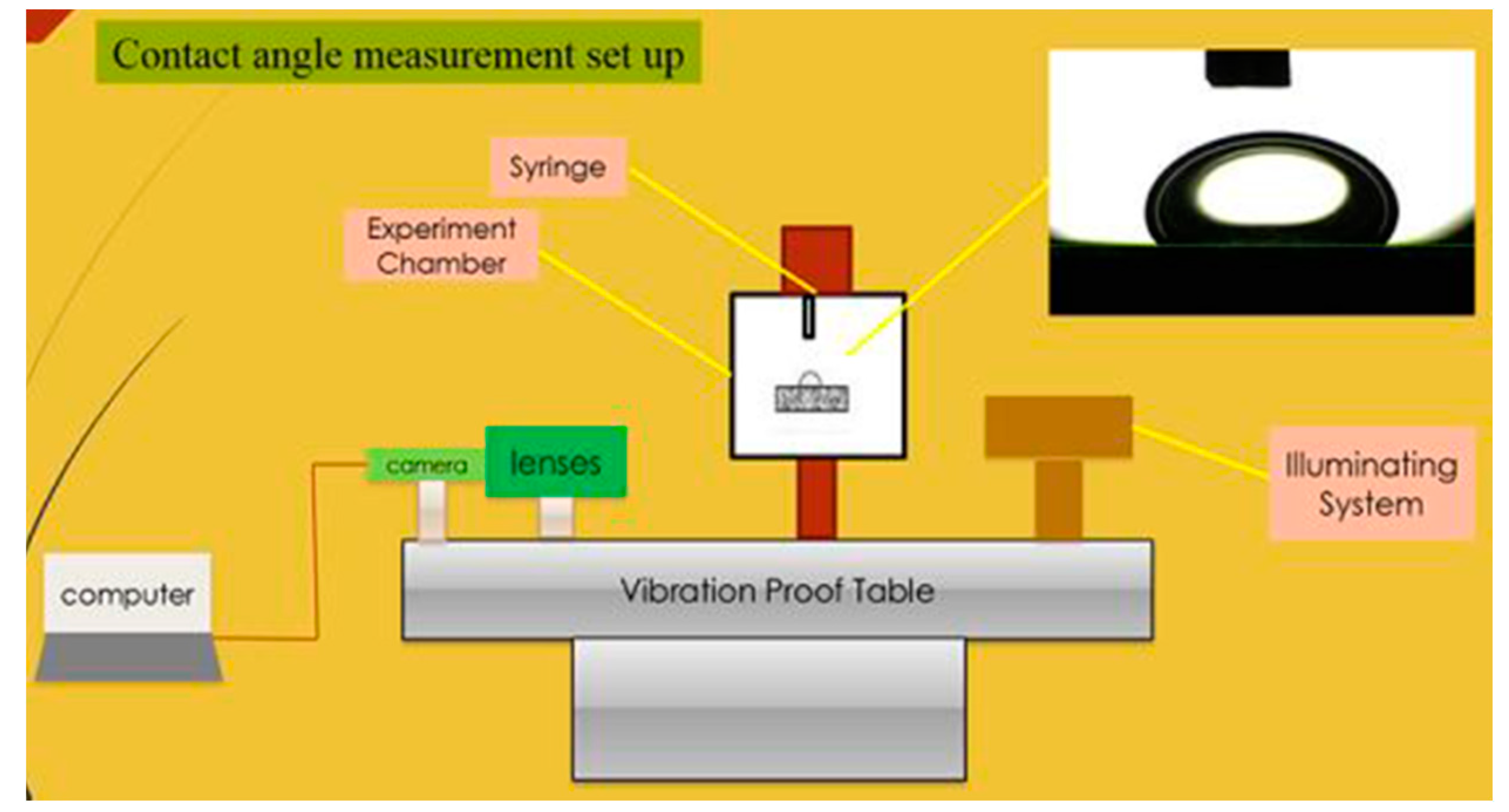
2.6. Contact Angle Measurement System
2.7. Core Flooding Measurements
3. Results and Discussion
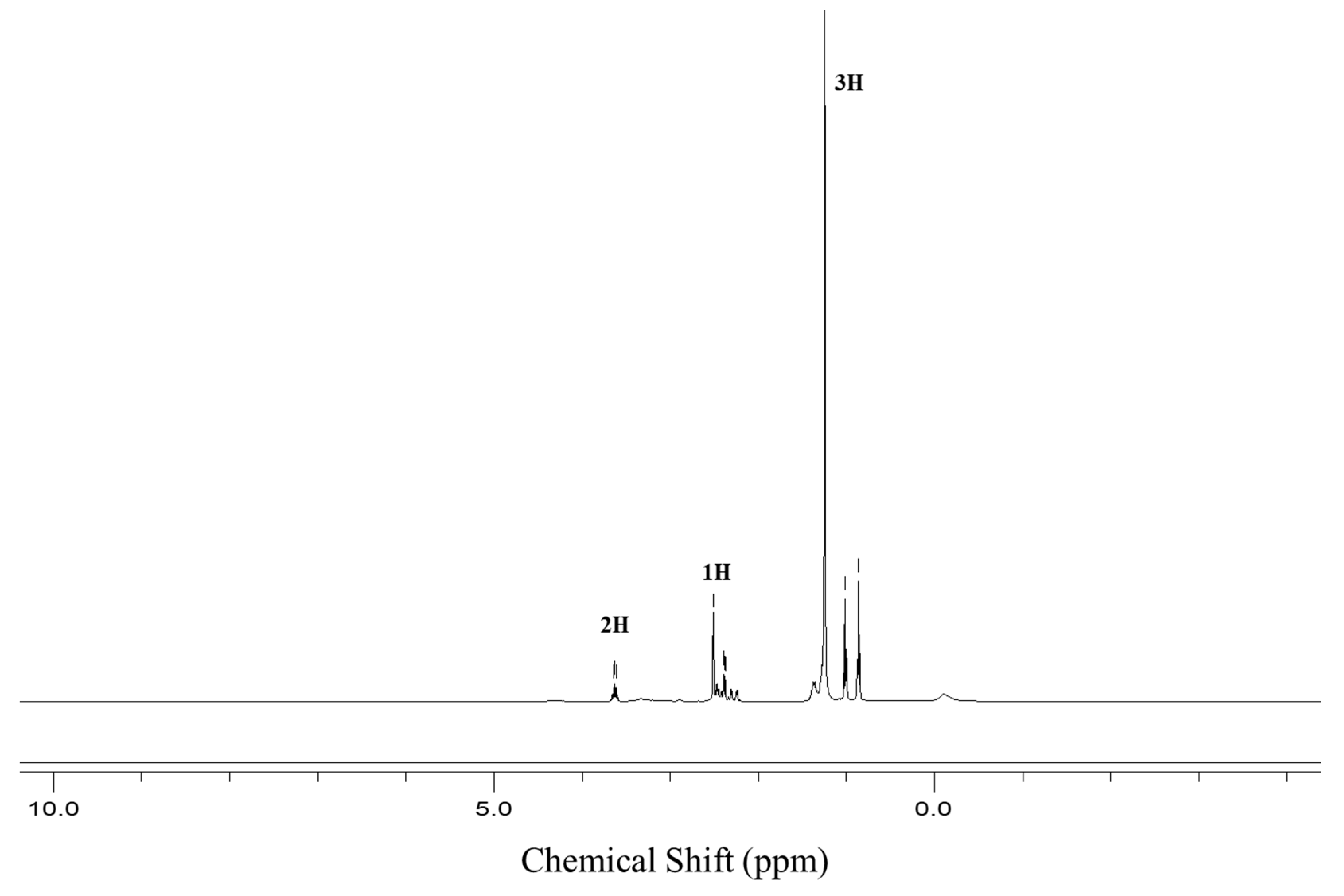
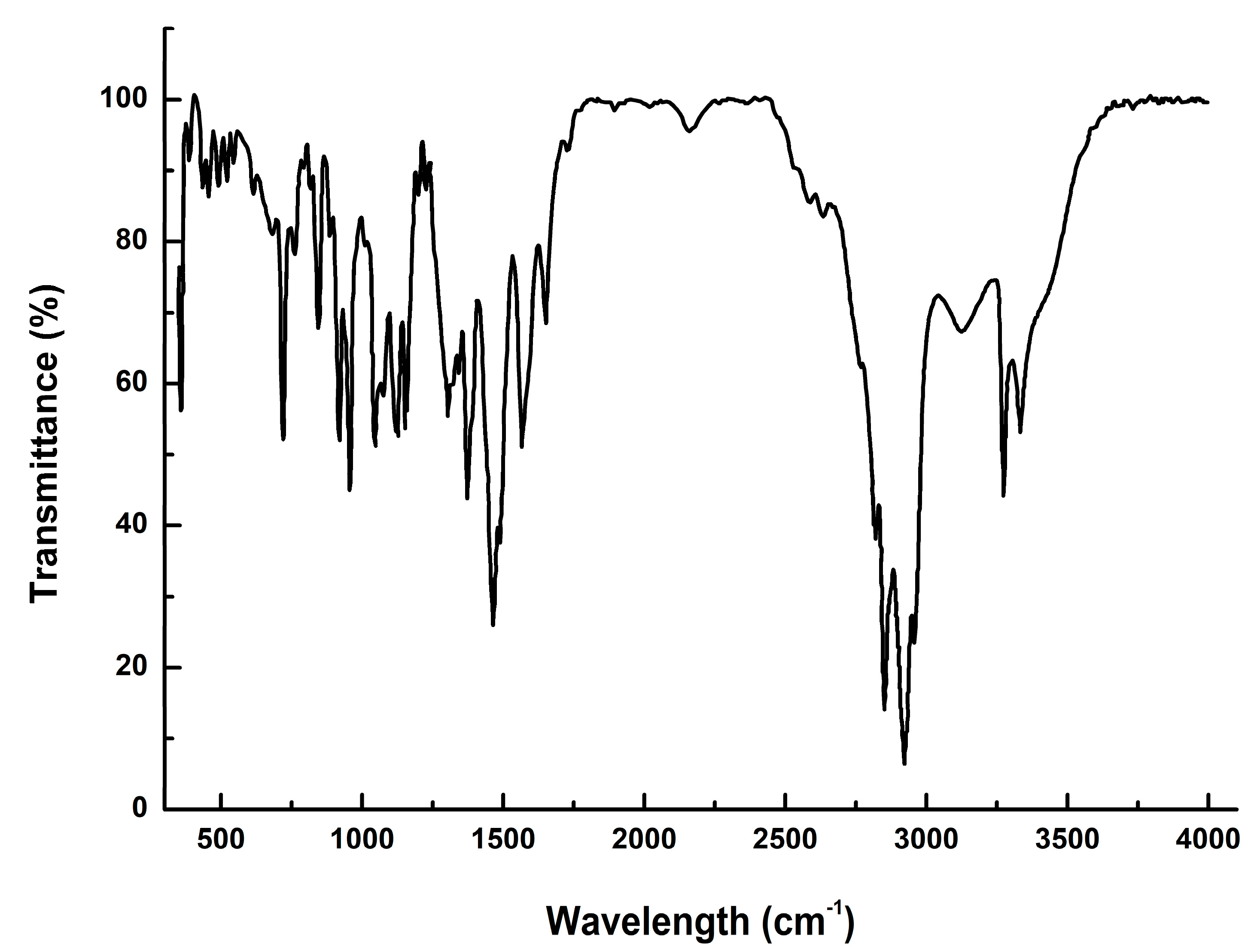
3.1. Characterization of Dodecanoyl-Glucosamine Surfactant
3.2. Critical Micelle Concentration Measurements
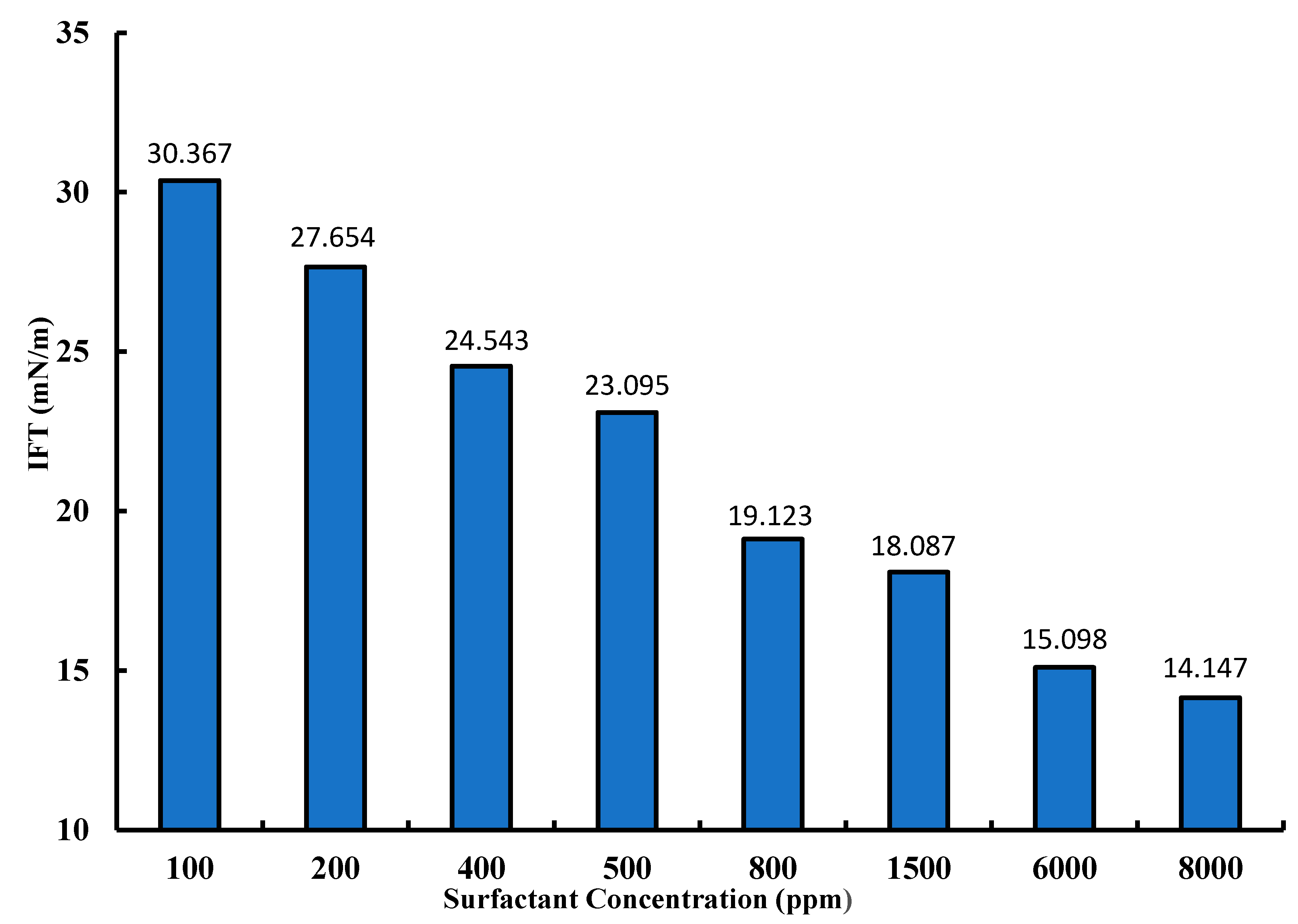
3.3. Interfacial Tension Measurements
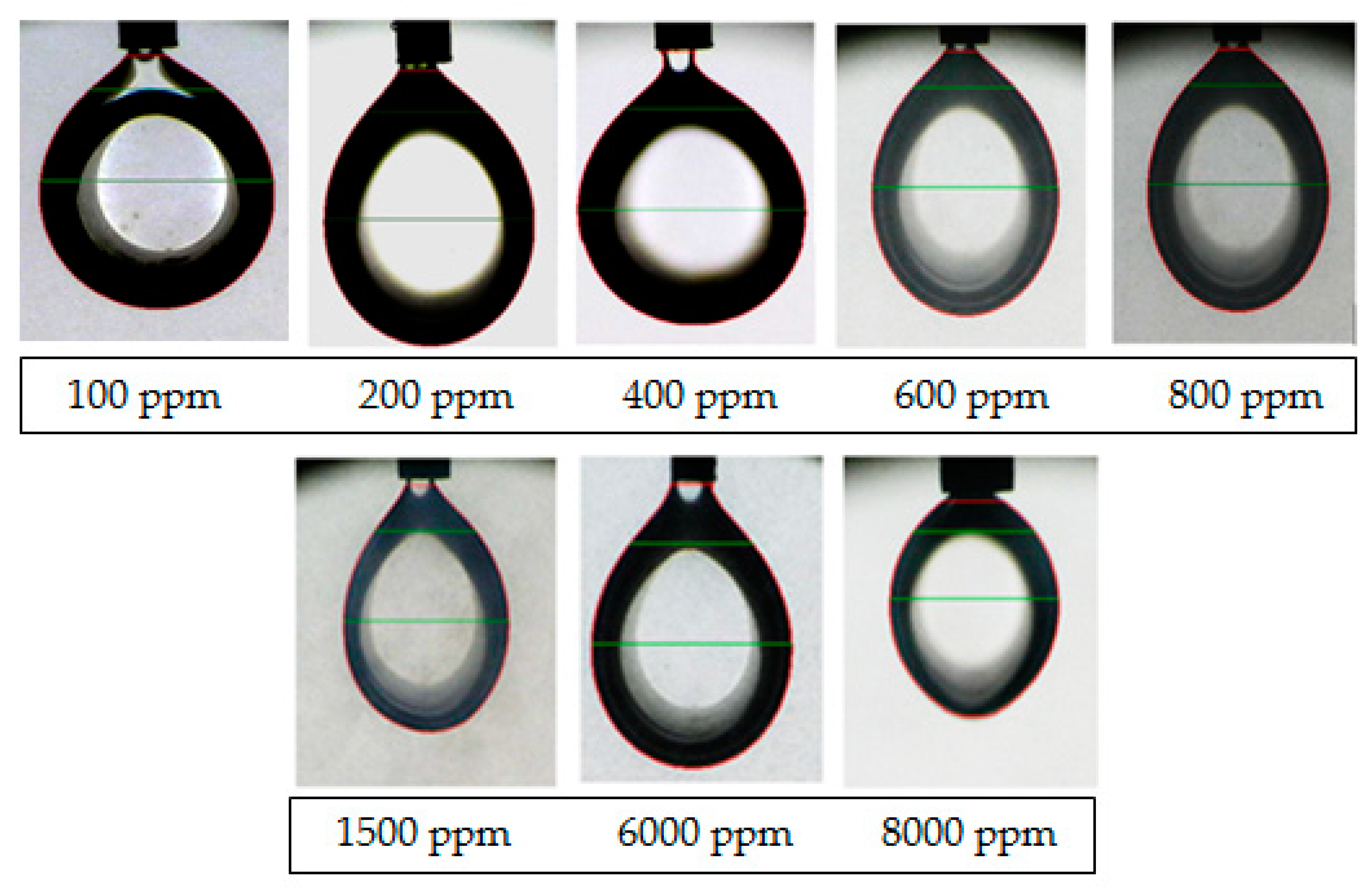
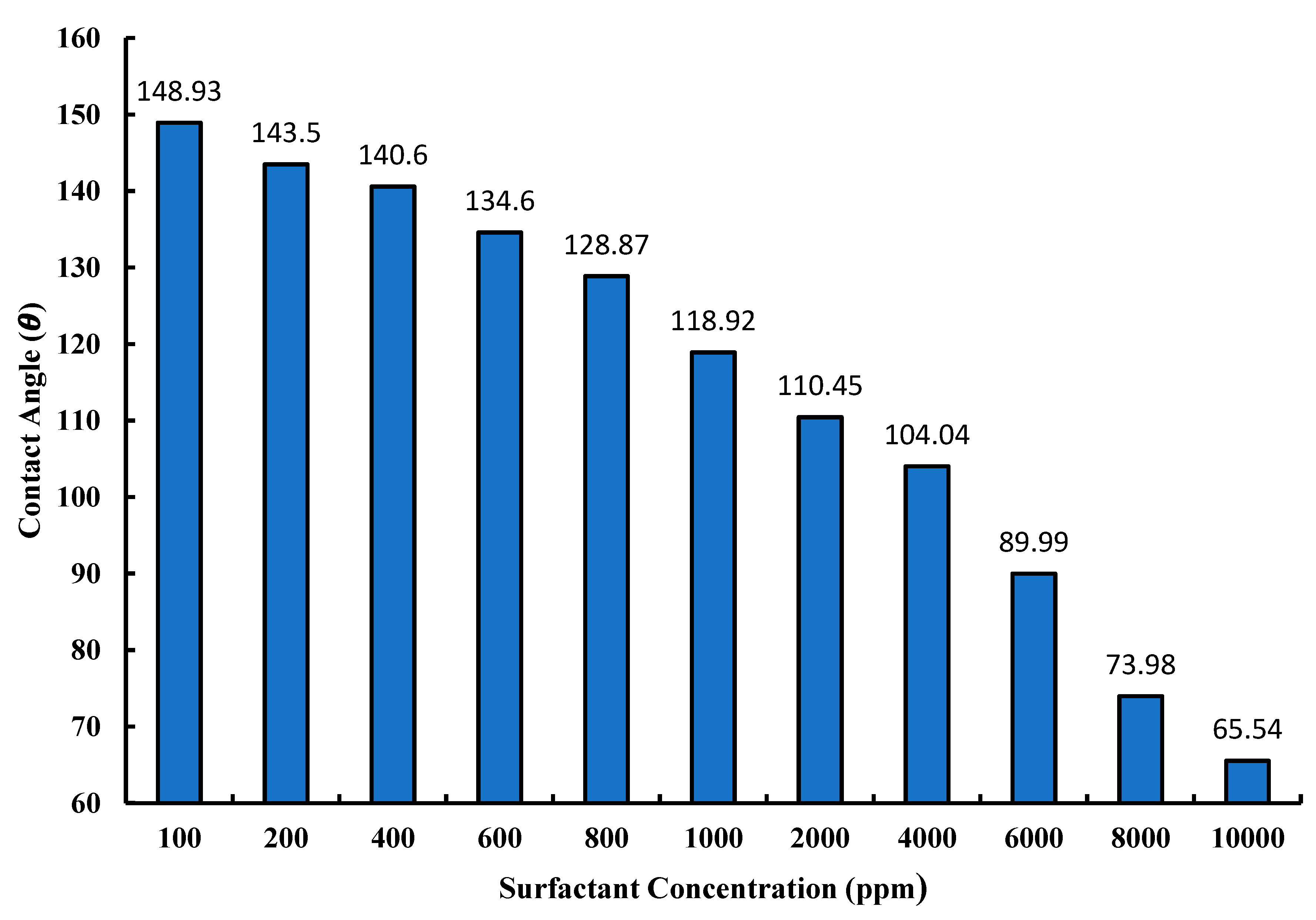
3.4. Contact Angle Measurements
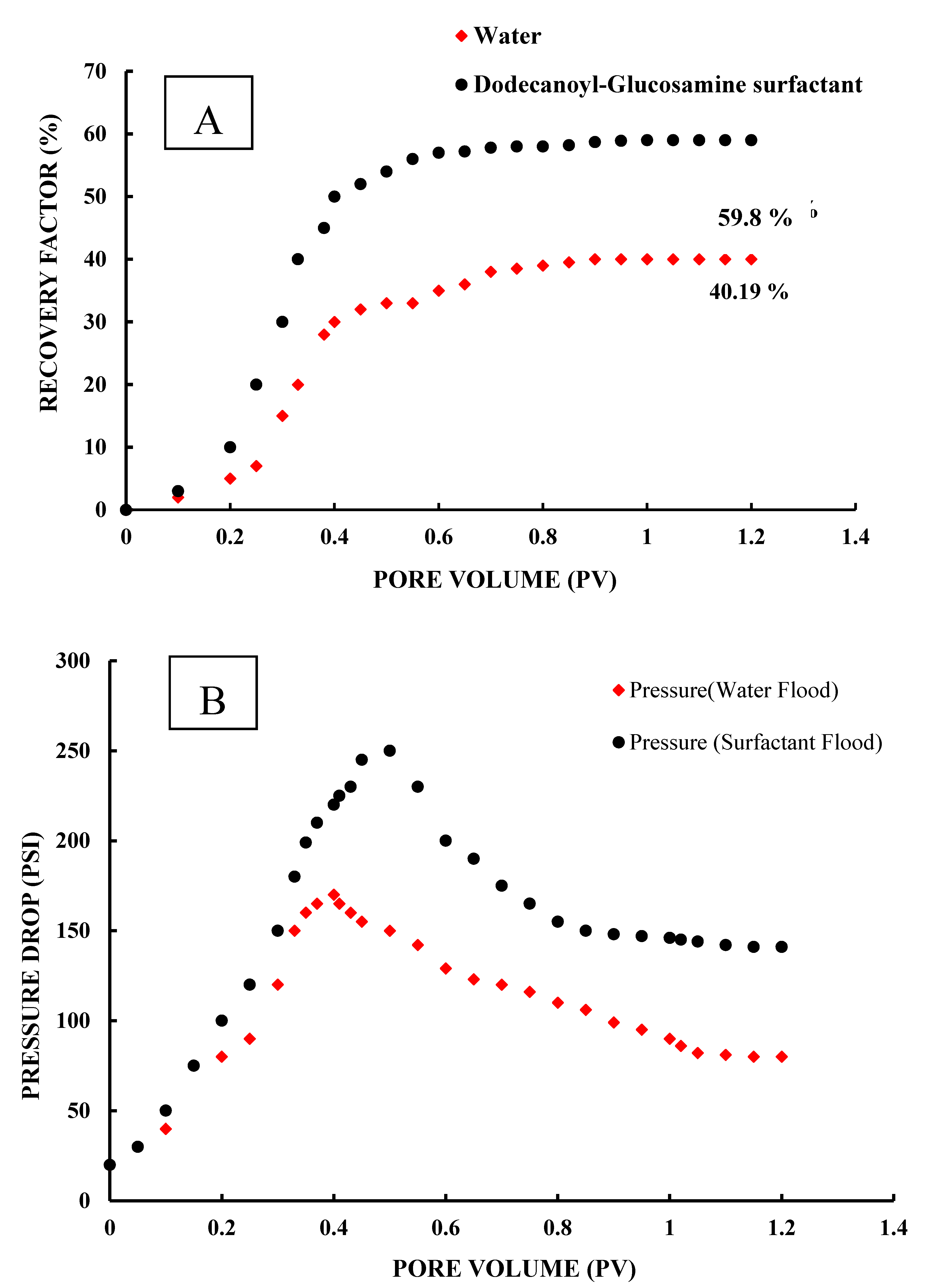
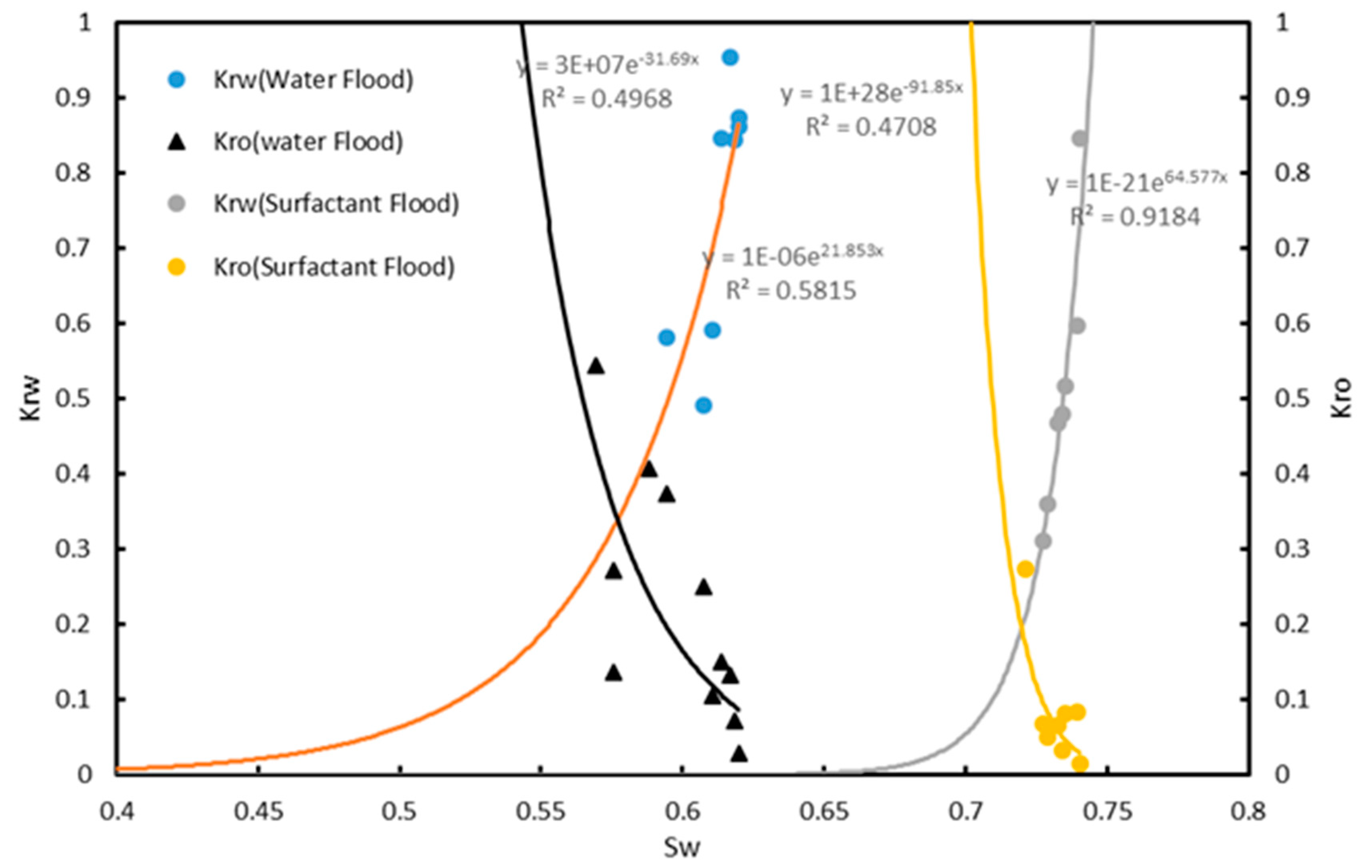
3.5. Core Flooding
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Memon, K.R.; Mahesar, A.A.; Ali, M.; Tunio, A.H.; Mohanty, U.S.; Akhondzadeh, H.; Awan, F.U.R.; Iglauer, S.; Keshavarz, A. Influence of cryogenic liquid nitrogen on petro-physical characteristics of mancos shale: An experimental investigation. Energy Fuels 2020, 34, 2160–2168. [Google Scholar] [CrossRef]
- Mahesar, A.A.; Shar, A.M.; Ali, M.; Tunio, A.H.; Uqailli, M.A.; Mohanty, U.S.; Akhondzadeh, H.; Iglauer, S.; Keshavarz, A. Morphological and petro physical estimation of eocene tight carbonate formation cracking by cryogenic liquid nitrogen; a case study of Lower Indus basin, Pakistan. J. Pet. Sci. Eng. 2020, 192, 107318. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Blunt, M.; Fayers, F.J.; Orr, F.M., Jr. Carbon dioxide in enhanced oil recovery. Energy Convers. Manag. 1993, 34, 1197–1204. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Emadi, S.; Shadizadeh, S.R.; Manshad, A.K.; Rahimi, A.M.; Mohammadi, A.H. Effect of nano silica particles on Interfacial Tension (IFT) and mobility control of natural surfactant (Cedr Extraction) solution in enhanced oil recovery process by nano-surfactant flooding. J. Mol. Liq. 2017, 248, 163–167. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent Advances in Surfactant EOR, Proceedings of the IPTC 2008: International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 3–5 December 2008; Society of Petroleum Engineers: Denver, CO, USA, 2008. [Google Scholar]
- Chukwudeme, E.A.; Hamouda, A.A. Enhanced oil recovery (EOR) by miscible CO2 and water flooding of asphaltenic and non-asphaltenic oils. Energies 2009, 2, 714–737. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Ali, M.; Al-Anssari, S.; Shakeel, M.; Arif, M.; Dahraj, N.U.; Iglauer, S. Influence of Miscible CO2 Flooding on Wettability and Asphaltene Precipitation in Indiana Lime Stone, Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017; Society of Petroleum Engineers: Jakarta, Indonesia, 2017. [Google Scholar]
- Ali, M.; Dahraj, N.U.; Haider, S.A. Study of Asphaltene Precipitation during CO2 Injection in Light Oil Reservoirs, Proceedings of the SPE/PAPG Pakistan Section Annual Technical Conference, Islamabad, Pakistan, 24–25 November 2015; Society of Petroleum Engineers: Islamabad, Pakistan, 2015. [Google Scholar]
- Nowrouzi, I.; Manshad, A.K.; Mohammadi, A.H. Effects of ions and dissolved carbon dioxide in brine on wettability alteration, contact angle and oil production in smart water and carbonated smart water injection processes in carbonate oil reservoirs. Fuel 2019, 235, 1039–1051. [Google Scholar] [CrossRef]
- Le Van, S.; Chon, B.H. Chemical flooding in heavy-oil reservoirs: From technical investigation to optimization using response surface methodology. Energies 2016, 9, 711. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Bagalkot, N. Effect of salts on interfacial tension and CO2 mass transfer in carbonated water injection. Energies 2019, 12, 748. [Google Scholar] [CrossRef]
- Najimi, S.; Nowrouzi, I.; Manshad, A.K.; Farsangi, M.H.; Hezave, A.Z.; Ali, J.A.; Keshavarz, A.; Mohammadi, A.H. Investigating the effect of [C 8 Py][Cl] and [C 18 Py][Cl] ionic liquids on the water/oil interfacial tension by considering Taguchi method. J. Pet. Explor. Prod. Technol. 2019, 9, 2933–2941. [Google Scholar] [CrossRef]
- Jha, N.; Ali, M.; Sarmadivaleh, M.; Iglauer, S.; Barifcani, A.; Lebedev, M.; Sangwai, J. Low Salinity Surfactant Nanofluids for Enhanced CO2 Storage Application at High Pressure and Temperature, Proceedings of the Fifth CO2 Geological Storage Workshop, Utrecht, The Netherlands, 21–23 November 2018; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2018; pp. 1–4. [Google Scholar]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J. Mol. Liq. 2019, 284, 735–747. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Bahraminejad, H.; Khaksar Manshad, A.; Riazi, M.; Ali, J.A.; Sajadi, S.M.; Keshavarz, A. CuO/TiO2/PAM as a novel introduced hybrid agent for water—oil interfacial tension and wettability optimization in chemical enhanced oil recovery. Energy Fuels 2019, 33, 10547–10560. [Google Scholar] [CrossRef]
- Howe, A.M.; Clarke, A.; Mitchell, J.; Staniland, J.; Hawkes, L.; Whalan, C. Visualising surfactant enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 449–461. [Google Scholar] [CrossRef]
- Jha, N.K.; Ali, M.; Iglauer, S.; Lebedev, M.; Roshan, H.; Barifcani, A.; Sangwai, J.S.; Sarmadivaleh, M. Wettability alteration of quartz surface by low-salinity surfactant nanofluids at high-pressure and high-temperature conditions. Energy Fuels 2019, 33, 7062–7068. [Google Scholar] [CrossRef]
- Jha, N.K.; Lebedev, M.; Iglauer, S.; Ali, M.; Roshan, H.; Barifcani, A.; Sangwai, J.S.; Sarmadivaleh, M. Pore scale investigation of low salinity surfactant nanofluid injection into oil saturated sandstone via X-ray micro-tomography. J. Colloid Interface Sci. 2020, 562, 370–380. [Google Scholar] [CrossRef]
- Ali, M.; Al-Anssari, S.; Arif, M.; Barifcani, A.; Sarmadivaleh, M.; Stalker, L.; Lebedev, M.; Iglauer, S. Organic acid concentration thresholds for ageing of carbonate minerals: Implications for CO2 trapping/storage. J. Colloid Interface Sci. 2019, 534, 88–94. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.A.; Zhang, Y.; Iglauer, S. Experimental investigation of carbonate wettability as a function of mineralogical and thermo-physical conditions. Fuel 2020, 264, 116846. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Arain, Z.-U.-A.; Barifcani, A.; Keshavarz, A.; Ali, M.; Iglauer, S. Influence of Pressure and Temperature on CO2-Nanofluid Interfacial Tension: Implication for Enhanced Oil Recovery and Carbon Geosequestration, Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018; Society of Petroleum Engineers: At Abu Dhabi, UAE, 2018. [Google Scholar]
- Ali, M. Effect of Organic Surface Concentration on CO2-Wettability of Reservoir Rock. Master’s Thesis, Curtin University, Bentley, WA, Australia, 2018. [Google Scholar]
- Cayias, J.; Schechter, R.S.; Wade, W. The utilization of petroleum sulfonates for producing low interfacial tensions between hydrocarbons and water. J. Colloid Interface Sci. 1977, 59, 31–38. [Google Scholar] [CrossRef]
- Al-Sabagh, A. Surface activity and thermodynamic properties of water-soluble polyester surfactants based on 1, 3-dicarboxymethoxybenzene used for enhanced oil recovery. Polym. Adv. Technol. 2000, 11, 48–56. [Google Scholar] [CrossRef]
- Shuler, P.J.; Tang, H.; Lu, Z.; Tang, Y. Chemical Process for Improved Oil Recovery from Bakken Shale, Proceedings of the Canadian Unconventional Resources Conference, Calgary, AB, Canada, 15–17 November 2011; Society of Petroleum Engineers: Calgary, AB, Canada, 2011. [Google Scholar]
- Cheng, A.; Kwan, J.T. Optimal Injection Design Utilizing Tracer and Simulation in a Surfactant Pilot for a Fractured Carbonate Yates Field, Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; Society of Petroleum Engineers: Tulsa, OK, USA, 2012. [Google Scholar]
- Asl, H.F.; Zargar, G.; Manshad, A.K.; Takassi, M.A.; Ali, J.A.; Keshavarz, A. Experimental investigation into l-Arg and l-Cys eco-friendly surfactants in enhanced oil recovery by considering IFT reduction and wettability alteration. Pet. Sci. 2020, 17, 105–117. [Google Scholar] [CrossRef]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Zhou, X. A review on wettability alteration in carbonate rocks: Wettability modifiers. Energy Fuels 2019, 34, 31–54. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Galedarzadeh, M.; Shadizadeh, S.R. Wettability alteration in carbonate rocks by implementing new derived natural surfactant: Enhanced oil recovery applications. Transp. Porous Media 2015, 106, 645–667. [Google Scholar] [CrossRef]
- Karimi, M.; Al-Maamari, R.S.; Ayatollahi, S.; Mehranbod, N. Wettability alteration and oil recovery by spontaneous imbibition of low salinity brine into carbonates: Impact of Mg2+, SO42− and cationic surfactant. J. Pet. Sci. Eng. 2016, 147, 560–569. [Google Scholar] [CrossRef]
- Ghosh, B.; Li, X. Effect of surfactant composition on reservoir wettability and scale inhibitor squeeze lifetime in oil wet carbonate reservoir. J. Pet. Sci. Eng. 2013, 108, 250–258. [Google Scholar] [CrossRef]
- Alveskog, P.; Holt, T.; Torsaeter, O. The effect of surfactant concentration on the Amott wettability index and residual oil saturation. J. Pet. Sci. Eng. 1998, 20, 247–252. [Google Scholar] [CrossRef]
- Akhondzadeh, H.; Keshavarz, A.; Al-Yaseri, A.Z.; Ali, M.; Awan, F.U.R.; Wang, X.; Yang, Y.; Iglauer, S.; Lebedev, M. Pore-scale analysis of coal cleat network evolution through liquid nitrogen treatment: A micro-computed tomography investigation. Int. J. Coal Geol. 2020, 219, 103370. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M. Surfactants: Toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-based drilling fluids: A review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Epelle, E.I.; Gerogiorgis, D.I. A multiparametric CFD analysis of multiphase annular flows for oil and gas drilling applications. Comput. Chem. Eng. 2017, 106, 645–661. [Google Scholar] [CrossRef]
- Giger, W.; Brunner, P.H.; Schaffner, C. 4-Nonylphenol in sewage sludge: Accumulation of toxic metabolites from nonionic surfactants. Science 1984, 225, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Kvestak, R.; Ahel, M. Occurrence of toxic metabolites from nonionic surfactants in the Krka river estuary. Ecotoxicol. Environ. Saf. 1994, 28, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Ríos, F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Ali, M.; Jarni, H.H.; Aftab, A.; Ismail, A.R.; Saady, N.M.C.; Sahito, M.F.; Keshavarz, A.; Iglauer, S.; Sarmadivaleh, M. Nanomaterial-based drilling fluids for exploitation of unconventional reservoirs: A review. Energies 2020, 13, 3417. [Google Scholar] [CrossRef]
- Aftab, A.; Ali, M.; Sahito, M.F.; Mohanty, U.S.; Jha, N.K.; Akhondzadeh, H.; Azhar, M.R.; Ismail, A.R.; Keshavarz, A.; Iglauer, S. Environmental friendliness and high performance of multifunctional tween 80/ZnO-Nanoparticles-Added water-based drilling fluid: An experimental approach. Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Manshad, A.K.; Rezaei, M.; Moradi, S.; Nowrouzi, I.; Mohammadi, A.H. Wettability alteration and interfacial tension (IFT) reduction in enhanced oil recovery (EOR) process by ionic liquid flooding. J. Mol. Liq. 2017, 248, 153–162. [Google Scholar] [CrossRef]
- Hou, B.-F.; Wang, Y.-F.; Huang, Y. Mechanistic study of wettability alteration of oil-wet sandstone surface using different surfactants. Appl. Surf. Sci. 2015, 330, 56–64. [Google Scholar] [CrossRef]
- Nabipour, M.; Ayatollahi, S.; Keshavarz, P. Application of different novel and newly designed commercial ionic liquids and surfactants for more oil recovery from an Iranian oil field. J. Mol. Liq. 2017, 230, 579–588. [Google Scholar] [CrossRef]
- Tavakoli, P.; Shadizadeh, S.R.; Hayati, F.; Fattahi, M. Effect of synthesized nanoparticles and Henna-Tragacanth solutions on oil/water interfacial tension: Nanofluids stability considerations. Petroleum 2020. [Google Scholar] [CrossRef]
- Ravi, S.; Shadizadeh, S.; Moghaddasi, J. Core flooding tests to investigate the effects of IFT reduction and wettability alteration on oil recovery: Using mulberry leaf extract. Pet. Sci. Technol. 2015, 33, 257–264. [Google Scholar] [CrossRef]
- Iglauer, S. CO2–water–rock wettability: Variability, influencing factors, and implications for CO2 geostorage. Acc. Chem. Res. 2017, 50, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Al-Anssari, S.; Nwidee, L.; Ali, M.; Sangwai, J.S.; Wang, S.; Barifcani, A.; Iglauer, S. Retention of Silica Nanoparticles in Limestone Porous Media, Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017; Society of Petroleum Engineers: Jakarta, Indonesia, 2017. [Google Scholar]
- Ali, M.; Arif, M.; Sahito, M.F.; Al-Anssari, S.; Keshavarz, A.; Barifcani, A.; Stalker, L.; Sarmadivaleh, M.; Iglauer, S. CO2-wettability of sandstones exposed to traces of organic acids: Implications for CO2 geo-storage. Int. J. Greenh. Gas. Control. 2019, 83, 61–68. [Google Scholar] [CrossRef]
- Takassi, M.A.; Zargar, G.; Madani, M.; Zadehnazari, A. The preparation of an amino acid-based surfactant and its potential application as an EOR agent. Pet. Sci. Technol. 2017, 35, 385–391. [Google Scholar] [CrossRef]
- Ramshini, A. Synthesis and Study of a Novel Lauroyl Cysteine Surfactant as Water–Oil Demulsifier and Modeling with Fuzzy Logic Design. (Unpublished doctoral dissertation). Ph.D. Thesis, PUT, Abadan, Iran, 2017. [Google Scholar]
- Madani, M.; Zargar, G.; Takassi, M.A.; Daryasafar, A.; Wood, D.A.; Zhang, Z. Fundamental investigation of an environmentally-friendly surfactant agent for chemical enhanced oil recovery. Fuel 2019, 238, 186–197. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Laxmi, K.D.; Mandal, A. Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: Implications for enhanced oil recovery. Chem. Eng. Sci. 2018, 187, 200–212. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Ali, M.; Memon, S.; Bhatti, M.A.; Lagat, C.; Sarmadivaleh, M. Reversible and irreversible adsorption of bare and hybrid silica nanoparticles onto carbonate surface at reservoir condition. Petroleum 2019. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.; Iglauer, S. Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems: Critical parametric analysis and future outlook. Adv. Colloid Interface Sci. 2019. [Google Scholar] [CrossRef]
- Ali, M.; Sahito, M.F.; Jha, N.K.; Memon, S.; Keshavarz, A.; Iglauer, S.; Saeedi, A.; Sarmadivaleh, M. Effect of Nanofluid on CO2-wettability reversal of sandstone formation; implications for CO2 geo-storage. J. Colloid Interface Sci. 2019. [Google Scholar] [CrossRef]
- Alizadeh, A.H.; Keshavarz, A.; Haghighi, M. Flow Rate Effect on Two-Phase Relative Permeability in Iranian Carbonate Rocks, Proceedings of the SPE Middle East oil and gas show and conference, Manama, Bahrain, 11–14 March 2007; Society of Petroleum Engineers: Manama, Bahrain, 2007. [Google Scholar]
- Ali, M.; Aftab, A.; Arain, Z.-U.-A.; Al-Yaseri, A.; Roshan, H.; Saeedi, A.; Iglauer, S.; Sarmadivaleh, M. Influence of Organic Acids Concentration on Wettability Alteration of Cap-rock: Implications for CO2 Trapping/Storage. Appl. Mater. Interfaces. 2020. [Google Scholar] [CrossRef]



| Surfactant | IFT, mN/m | Contact Angle, Degree | Recovery Factor (RF) % | References | ||
|---|---|---|---|---|---|---|
| Without Surfactant | With Surfactant | Without Surfactant | With Surfactant | |||
| (C12mim)(Cl) | 27.55 | 6.33 | - | - | - | Manshad et al. (2017) [47] |
| (C18mim)(Cl) | 27.55 | 1.20 | 149 | 124 | 48 | - |
| (C8Py)(Cl) | 27.55 | 19.05 | - | - | - | - |
| (C18Py)(Cl) | 27.55 | 1.81 | 142 | 120 | 43 | - |
| CTAB | - | 0.050 | 140 | 56 | - | Hou et al. (2015) [48] |
| TX-100 | - | 0.322 | 140 | 98 | - | - |
| POE | - | 2.329 | 140 | 110 | - | - |
| lauroyl arginine (L-Arg) | 34.5 | 18 | 144 | 78 | 55.6 | Asl. et al. (2020) [31] |
| lauroyl cysteine (L-Cys) | 34.5 | 15.4 | 144 | 75 | 52.6 | - |
| (C12mim)(Br) | 43.97 | 9.10 | - | - | Nabipour et al. (2017) [49] | |
| (C14mim)(Br) | 43.97 | 7.20 | - | - | - | |
| (C16mim)(Br) | 43.97 | 4.70 | - | - | 75.6 | - |
| CTAB | 43.97 | 7.70 | - | - | 71 | - |
| Henna extract | 37 | 15.24 | - | - | - | Tavakoli et al. (2020) [50] |
| TiO2 + Henna extract | 37 | 14.57 | - | - | - | - |
| Mulberry Leaf Extract | 42 | 20 | 150 | 30 | 63 | Ravi et al. (2015) [51] |
| Organic Acid | Molar Mass (g/mol) | Formula | Density (g/cm3) | Chemical Structure |
|---|---|---|---|---|
| Glucosamine | 179.17 | C6H13NO5 | 1.563 |  |
| Lauryl Chloride | 218.765 | C12H23ClO | 0.946 |  |
| Methanol | 32.04 | CH3OH | 0.792 | 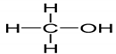 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, O.M.; Zargar, G.; Khaksar Manshad, A.; Ali, M.; Takassi, M.A.; Ali, J.A.; Keshavarz, A. Effect of Environment-Friendly Non-Ionic Surfactant on Interfacial Tension Reduction and Wettability Alteration; Implications for Enhanced Oil Recovery. Energies 2020, 13, 3988. https://doi.org/10.3390/en13153988
Haghighi OM, Zargar G, Khaksar Manshad A, Ali M, Takassi MA, Ali JA, Keshavarz A. Effect of Environment-Friendly Non-Ionic Surfactant on Interfacial Tension Reduction and Wettability Alteration; Implications for Enhanced Oil Recovery. Energies. 2020; 13(15):3988. https://doi.org/10.3390/en13153988
Chicago/Turabian StyleHaghighi, Omid Mosalman, Ghasem Zargar, Abbas Khaksar Manshad, Muhammad Ali, Mohammad Ali Takassi, Jagar A. Ali, and Alireza Keshavarz. 2020. "Effect of Environment-Friendly Non-Ionic Surfactant on Interfacial Tension Reduction and Wettability Alteration; Implications for Enhanced Oil Recovery" Energies 13, no. 15: 3988. https://doi.org/10.3390/en13153988
APA StyleHaghighi, O. M., Zargar, G., Khaksar Manshad, A., Ali, M., Takassi, M. A., Ali, J. A., & Keshavarz, A. (2020). Effect of Environment-Friendly Non-Ionic Surfactant on Interfacial Tension Reduction and Wettability Alteration; Implications for Enhanced Oil Recovery. Energies, 13(15), 3988. https://doi.org/10.3390/en13153988







