Effects of Pyrolysis Temperature and Retention Time on Fuel Characteristics of Food Waste Feedstuff and Compost for Co-Firing in Coal Power Plants
Abstract
:1. Introduction
2. Experimental
2.1. Raw Material
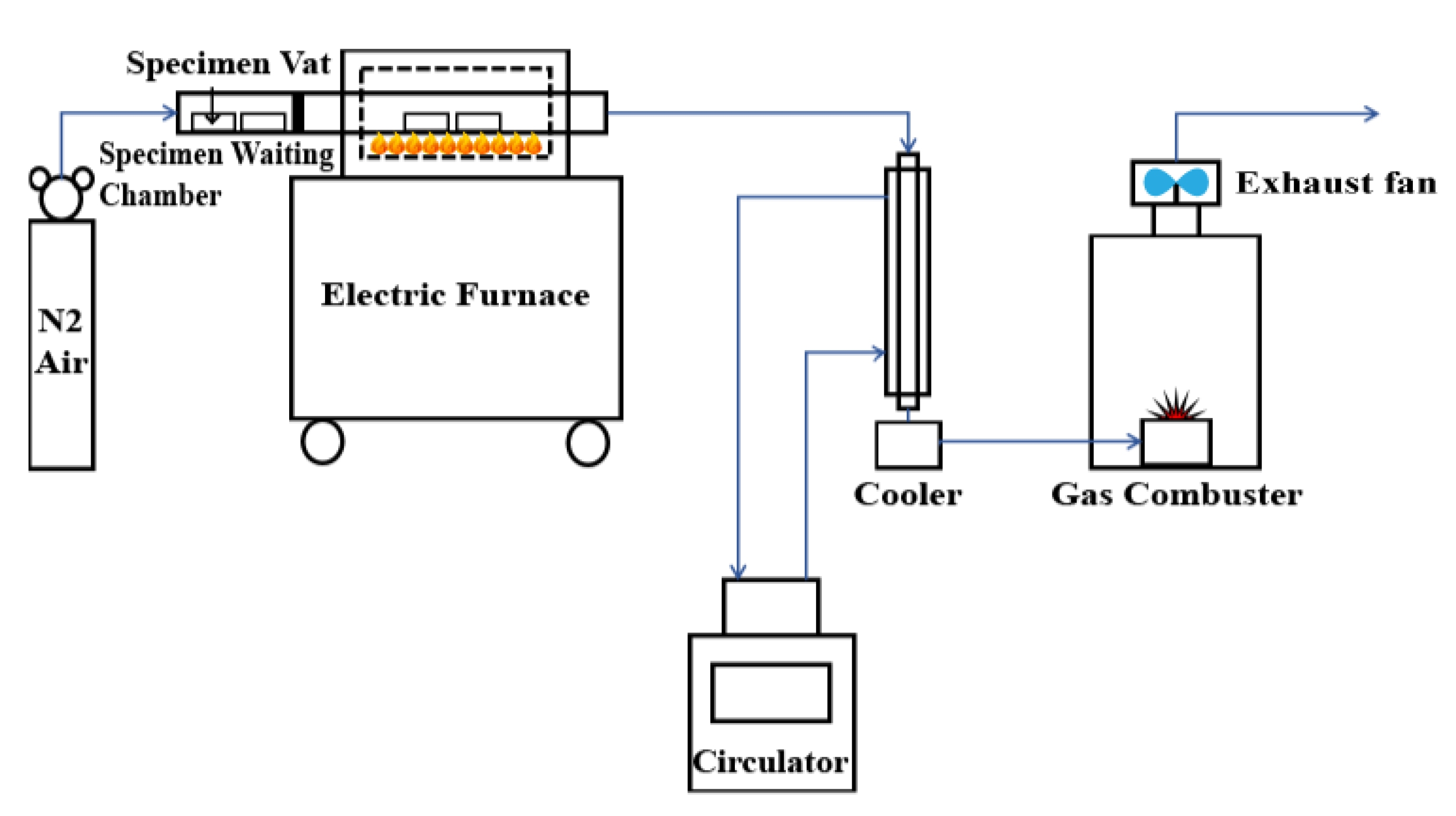
2.2. Experimental Apparatus and Procedures
2.2.1. Pyrolysis
2.2.2. Analytical Methods
3. Results and Discussion
3.1. Elemental Fuel Characteristics
3.1.1. Basic Properties
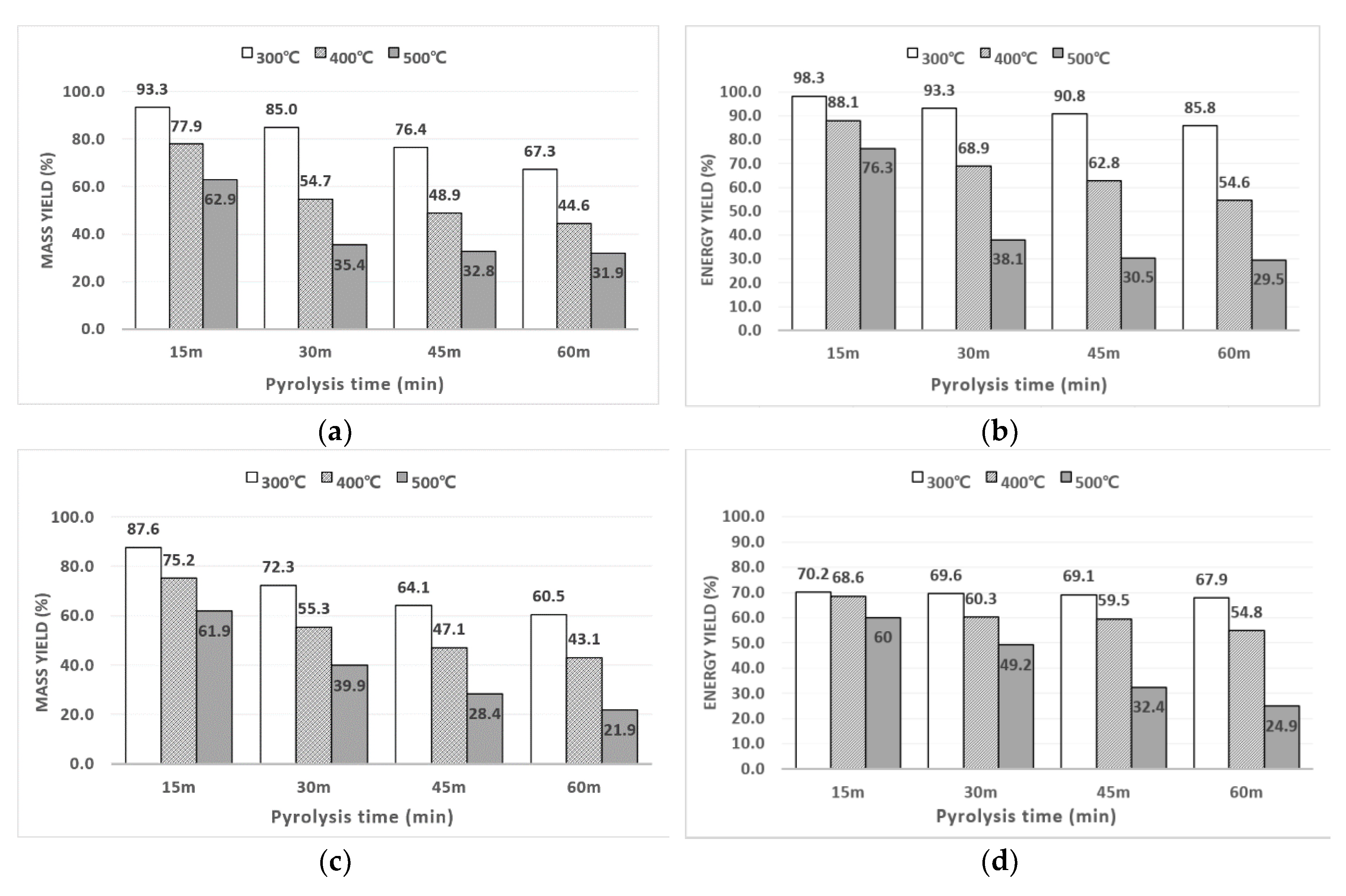
3.1.2. Energy and Mass Yield
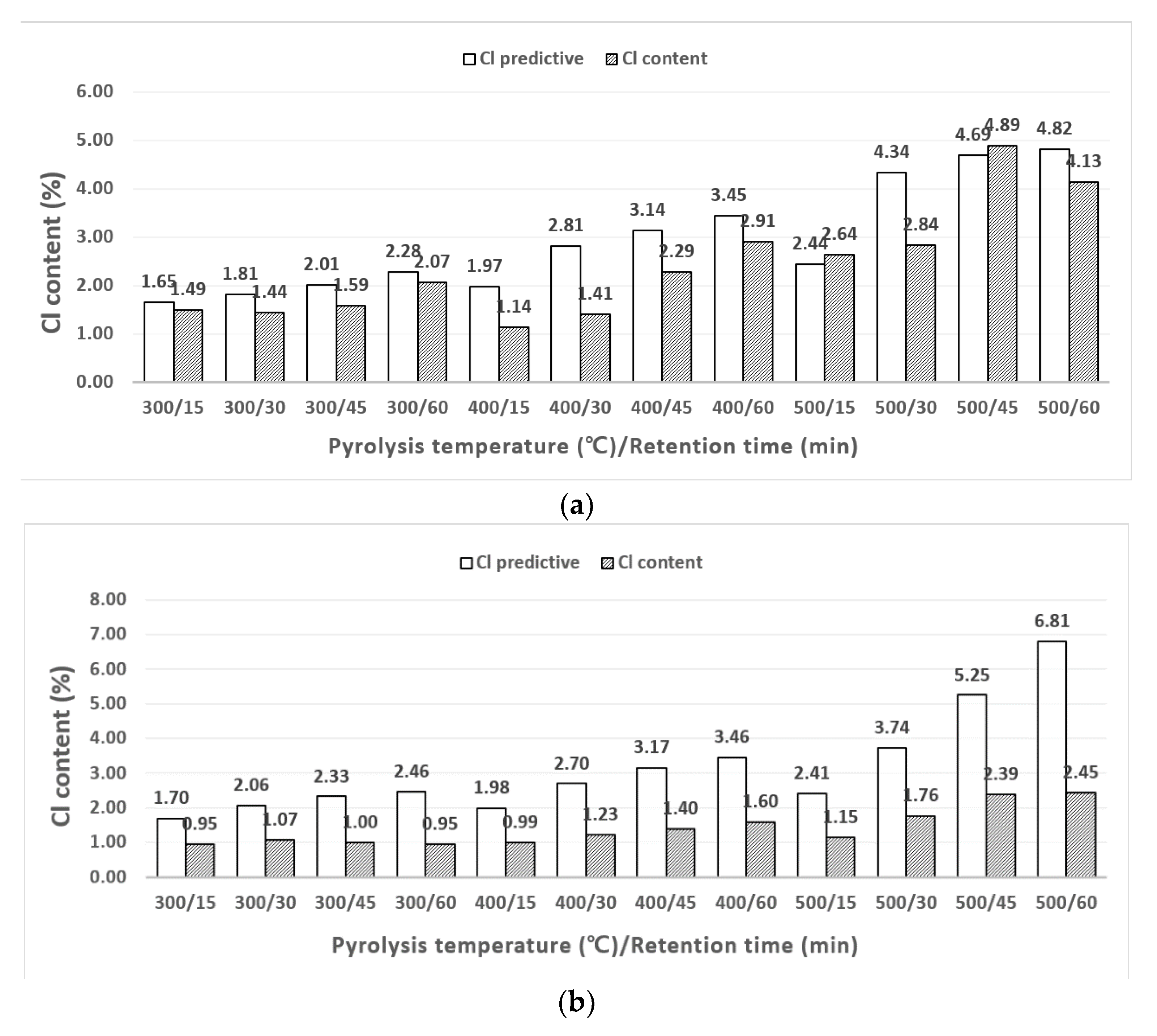
3.2. Chlorine Content Change with Different Pyrolysis Conditions
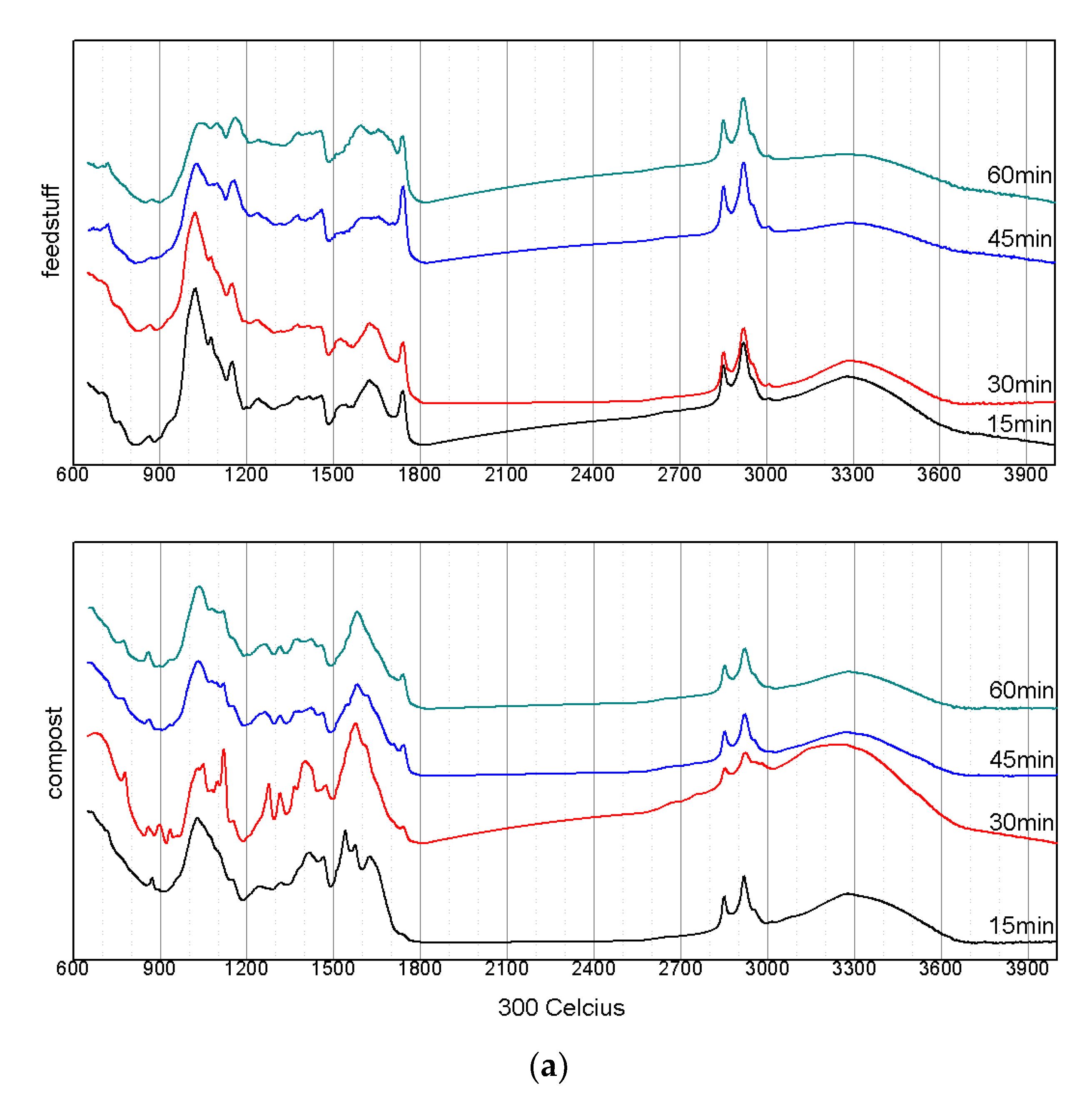
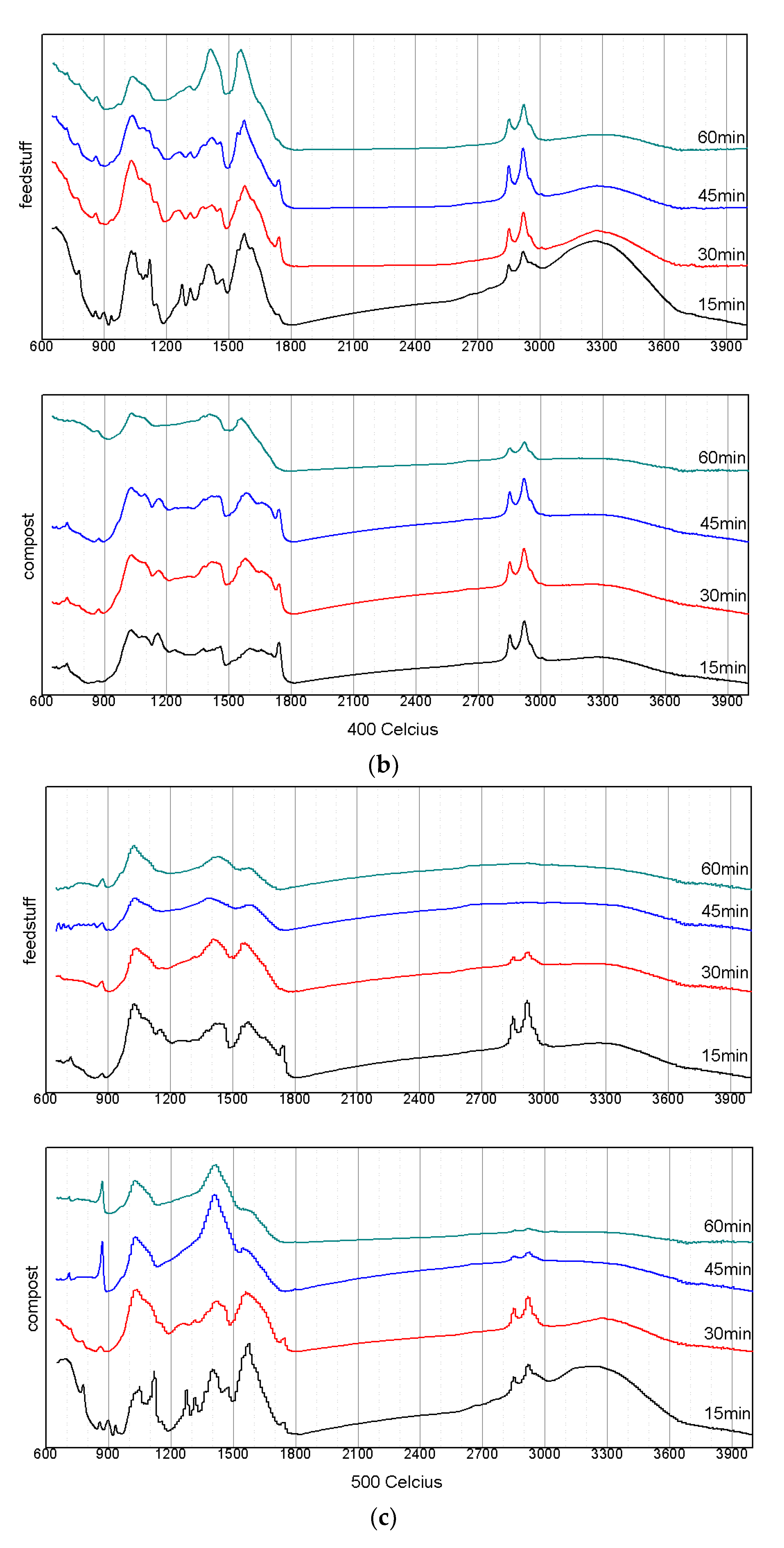
3.3. Changes in the Structuralized Characteristics According to the Pyrolysis Conditions
3.4. Slagging and Fouling Indices Analysis
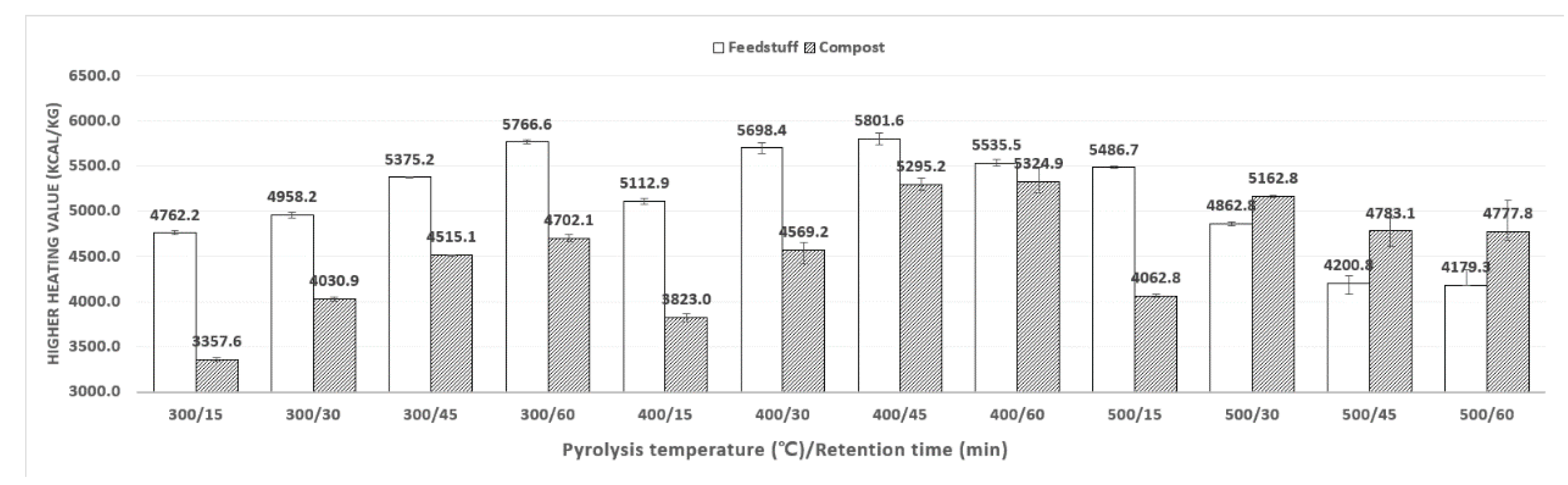
3.5. Higher Heating Value (HHV) Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Wang, Y.; Li, S.; Wei, X. Release of alkali metals during co-firing biomass and coal. Renew. Energy 2016, 96, 91–97. [Google Scholar] [CrossRef]
- Daood, S.S.; Javed, M.T.; Gibbs, B.M.; Nimmo, W. NOx control in coal combustion by combining biomass co-firing, oxygen enrichment and SNCR. Fuel 2013, 105, 283–292. [Google Scholar] [CrossRef]
- Munir, S.; Nimmo, W.; Gibbs, B.M. The effect of air staged, co-combustion of pulverised coal and biomass blends on NOx emissions and combustion efficiency. Fuel 2011, 90, 126–135. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Yang, W.; Blasiak, W. Effects of flue gas internal recirculation on NOx and SOx emissions in a co-firing boiler. Int. J. Clean Coal Energy 2013, 2, 13–21. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Baxter, L.L.; Miles, T.R.; Miles, T.R., Jr.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1998, 54, 47–78. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.I.; Oh, S.C. A study on torrefaction of food waste. Fuel 2015, 140, 275–281. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Lee, Y.E.; Jo, J.H.; Kim, S.M.; Yoo, Y.S. Recycling possibility of the salty food waste by pyrolysis and water scrubbing. Energies 2017, 10, 210. [Google Scholar] [CrossRef]
- Keipi, T.; Tolvanen, H.; Kokko, L.; Raiko, R. The effect of torrefaction on the chlorine content and heating value of eight woody biomass samples. Biomass Bioenergy 2014, 66, 232–239. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qiao, Z.; Liu, H. Release and transformation characteristics of K and Cl during straw torrefaction and mild pyrolysis. Fuel 2016, 167, 31–39. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Sárossy, Z.; Dong, C.; Glarborg, P. Release and transformation of chlorine and potassium during pyrolysis of KCl doped biomass. Fuel 2017, 197, 422–432. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qiao, Z.; Liu, H. Release and transformation behavior of Cl during pyrolysis of torrefied rice straw. Fuel 2016, 183, 145–154. [Google Scholar] [CrossRef]
- Saleh, S.B.; Flensborg, J.P.; Shoulaifar, T.K.; Sárossy, Z.; Hansen, B.B.; Egsgaard, H.; DeMartini, N.; Jensen, P.A.; Glarborg, P.; Dam-Johansen, K. Release of chlorine and sulfur during biomass torrefaction and pyrolysis. Energy Fuels 2014, 28, 3738–3746. [Google Scholar] [CrossRef]
- Lawrence, A.; Kumar, R.; Nandakumar, K.; Narayanan, K. A novel tool for assessing slagging propensity of coals in PF boilers. Fuel 2008, 87, 946–950. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of slagging and fouling tendency during biomass co-firing with coal in a fluidized bed. Biomass Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- López, C.; Unterberger, S.; Maier, J.; Hein, K.R.G. Overview of actual methods for characterization of ash deposition. In Proceedings of the Heat Exchanger Fouling and Cleaning: Fundamentals and Applications, ECI Symposium Series, Santa Fe, NM, USA, 18–22 May 2003. [Google Scholar]
- Lee, Y.E.; Jo, J.H.; Kim, I.T.; Yoo, Y.S. Value-added performance and thermal decomposition characteristics of dumped food waste compost by pyrolysis. Energies 2018, 11, 1061. [Google Scholar] [CrossRef]
- Park, S.-W.; Jang, C.-H. Effects of pyrolysis temperature on changes in fuel characteristics of biomass char. Energy 2012, 39, 187–195. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Bekiaris, G.; Bruun, S.; Peltre, C.; Houot, S.; Jensen, L.S. FTIR-PAS: A powerful tool for characterising the chemical composition and predicting the labile C fraction of various organic waste products. Waste Manag. 2015, 39, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Singh, B.R.; DeOliveira, D.B.; Fu, F.N.; Fuller, M.P. Fourier transform infrared analysis of amide III bands of proteins for the secondary structure estimation. In Biomolecular Spectroscopy III, Proceedings of SPIE 1890, Los Angeles, CA, USA, 17–22 January 1993; Nafie, L.A., Mantsch, H.H., Eds.; International Society for Optics and Photonics: Bellingham, WA, USA, 1993; pp. 47–55. [Google Scholar] [CrossRef]
- Hadjoudja, S.; Deluchat, V.; Baudu, M. Cell surface characterisation of Microcystis aeruginosa and Chlorella vulgaris. J. Colloid Interface Sci. 2010, 342, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jebsen, C.; Norici, A.; Wagner, H.; Palmucci, M.; Giordano, M.; Wilhelm, C. FTIR spectra of algal species can be used as physiological fingerprints to assess their actual growth potential. Physiol. Plant. 2012, 146, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta Part A 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Shi, W.J.; Kong, L.X.; Bai, J.; Xu, J.; Li, W.C.; Bai, Z.Q.; Li, W. Effect of CaO/Fe2O3 on fusion behaviors of coal ash at high temperatures. Fuel Process. Technol. 2018, 181, 18–24. [Google Scholar] [CrossRef]
- Holubcik, M.; Jandacka, J.; Malcho, M. Ash melting temperature prediction from chemical composition of biomass ash. Holist. Approach Environ. 2015, 5, 119–125. [Google Scholar]
- Gao, Y.; Ding, L.; Li, X.; Wang, W.; Xue, Y.; Zhu, X.; Hu, H.; Luo, G.; Naruse, I.; Bai, Z.; et al. Na&Ca removal from Zhundong coal by a novel CO2-water leaching method and the ashing behavior of the leached coal. Fuel 2017, 210, 8–14. [Google Scholar] [CrossRef]

| Index | Formula | Slagging and Fouling Potential | Ref. | |||
|---|---|---|---|---|---|---|
| Low | Med | High | Severe | |||
| Silica ratio | 72–80 | 65–72 | 50–65 | - | [16] | |
| B/A ratio 1 | <0.11 | 0.11–0.14 | 0.14 | - | ||
| Sulfur content | <0.6 | 0.6–2.0 | 2.0–2.6 | >2.6 | ||
| Fu 2 | ≤0.6 | 0.6–1.6 | 1.6–40 | >40 | [17] | |
| AFI 3 (°C) | >1343 | 1232–1343 | 1149–1232 | <1149 | [18,19] | |
| Parameter | Feedstuff (wt.%) | Compost (wt%) |
|---|---|---|
| Carbon | 45.26 | 41.57 |
| Hydrogen | 6.39 | 5.94 |
| Nitrogen | 3.5 | 3.03 |
| Sulfur 1 | - | - |
| Oxygen | 32.35 | 35.54 |
| Moisture | 6.17 | 10.77 |
| Volatile | 72.32 | 68.96 |
| Ash | 10.45 | 10.4 |
| Fixed carbon | 11.07 | 9.88 |
| HHV 2 (kcal/kg) | 4520.19 | 4187.37 |
| Sample | Proximate Analysis (wt. %) | Ultimate Analysis (wt. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Ash | Fixed Carbon | C | H | S | N | O | ||
| Food waste fodder | ||||||||||
| 300 °C | 15 m | 2.05 | 75.6 | 10.51 | 11.84 | 45.05 | 6.28 | - | 4.16 | 31.73 |
| 30 m | 2.2 | 72.67 | 11.55 | 13.58 | 49.53 | 6.43 | - | 4.54 | 29.09 | |
| 45 m | 0.41 | 70.41 | 13.22 | 15.96 | 55.5 | 6.65 | - | 4.88 | 23.13 | |
| 60 m | 0.6 | 64.58 | 15.61 | 19.21 | 57.86 | 6.35 | - | 5.33 | 17.77 | |
| 400 °C | 15 m | 0.61 | 72.7 | 12.59 | 14.1 | 51.42 | 6.78 | - | 4.2 | 25 |
| 30 m | 0.37 | 62.86 | 16.73 | 20.04 | 54.08 | 5.75 | - | 4.98 | 15.15 | |
| 45 m | 0.79 | 53.63 | 20.18 | 25.4 | 61.16 | 5.88 | - | 5.11 | 11.14 | |
| 60 m | 2.76 | 50.79 | 20.83 | 25.62 | 61.35 | 5.87 | - | 5.49 | 12.05 | |
| 500 °C | 15 m | 0.69 | 57.92 | 18.05 | 23.34 | 58.19 | 5.73 | - | 5.3 | 16.33 |
| 30 m | 1.78 | 52.11 | 21.78 | 24.32 | 52.56 | 5.34 | - | 3.98 | 24.45 | |
| 45 m | 0.64 | 24.98 | 33.31 | 41.06 | 52.67 | 2.37 | - | 4.83 | 20.73 | |
| 60 m | 0.69 | 24.6 | 33.57 | 41.14 | 37.21 | 1.37 | - | 2.89 | 11.45 | |
| Food waste compost | ||||||||||
| 300 °C | 15 m | 28.58 | 57.06 | 7.68 | 6.68 | 35.79 | 5.48 | - | 2.51 | 36.87 |
| 30 m | 16.69 | 65.62 | 9 | 8.69 | 40.83 | 5.82 | - | 2.82 | 32.74 | |
| 45 m | 7.38 | 72.05 | 10.36 | 10.21 | 46.06 | 6.42 | - | 3.22 | 34.26 | |
| 60 m | 3.26 | 74.41 | 11.33 | 11 | 48.26 | 6.35 | - | 3.73 | 31.8 | |
| 400 °C | 15 m | 19.81 | 63.38 | 8.76 | 8.04 | 40.63 | 5.97 | - | 3.11 | 34.6 |
| 30 m | 6.59 | 71 | 11.96 | 10.46 | 46.39 | 6.19 | - | 3.46 | 29.87 | |
| 45 m | 1.26 | 66.66 | 16.2 | 15.88 | 53.63 | 5.89 | - | 4.09 | 23.87 | |
| 60 m | 3.27 | 58.89 | 18.95 | 18.89 | 54.12 | 5.69 | - | 3.82 | 17.25 | |
| 500 °C | 15 m | 17.4 | 63.16 | 10.25 | 9.19 | 40.92 | 5.82 | - | 3.31 | 33.4 |
| 30 m | 1.17 | 64.56 | 17.47 | 16.79 | 50.66 | 5.4 | - | 3.52 | 21.14 | |
| 45 m | 0.64 | 45.55 | 27.34 | 26.47 | 52.14 | 4.36 | - | 3.39 | 14.55 | |
| 60 m | 0.9 | 40.44 | 28.22 | 30.44 | 51.74 | 3.91 | - | 3.32 | 12.99 | |
| Undetected (<0.05 wt %) | ||||||||||
| Sample | Silica Ratio | B/A Ratio | Sulphur Content | Fu | Ash Fusibility Index (AFI) | |
|---|---|---|---|---|---|---|
| Food waste compost | 300 °C | 6.90 (High) | 11.61 (High) | 0.58 (Low) | 92.76 (Severe) | >1500 °C (Low) |
| 400 °C | 6.82 (High) | 11.54 (High) | 0.58 (Low) | 46.39 (Severe) | >1500 °C (Low) | |
| 500 °C | 7.13 (High) | 11.87 (High) | 0.59 (Low) | 106.00 (Severe) | >1500 °C (Low) | |
| Food waste feedstuff | 300 °C | 5.38 (High) | 16.85 (High) | 0.84 (Medium) | 185.35 (Severe) | >1500 °C (Low) |
| 400 °C | 6.33 (High) | 14.89 (High) | 0.74 (Medium) | 170.49 (Severe) | >1500 °C (Low) | |
| 500 °C | 6.96 (High) | 13.02 (High) | 0.65 (Medium) | 140.10 (Severe) | >1500 °C (Low) | |
| Food Waste Compost | Food Waste Feedstuff | |||||
|---|---|---|---|---|---|---|
| 300 °C | 400 °C | 500 °C | 300 °C | 400 °C | 500 °C | |
| CaO | 59.67 | 61.72 | 59.1 | 60.21 | 56.92 | 55.32 |
| P2O5 | 13.07 | 14.15 | 11.41 | 12.62 | 14.4 | 16.69 |
| Na2O | 5.42 | 1.91 | 4.63 | 6.59 | 6.91 | 6.08 |
| Cl | 4.03 | 5.1 | 5.73 | 6.22 | 7.06 | 6.79 |
| SiO2 | 4.91 | 5.01 | 5.01 | 3.59 | 4.06 | 4.37 |
| Fe2O3 | 4.56 | 4.63 | 4.83 | 0.79 | 0.74 | 0.74 |
| K2O | 2.57 | 2.11 | 4.3 | 4.41 | 4.54 | 4.68 |
| MgO | 1.98 | 2.09 | 1.35 | 2.16 | 2.37 | 2.34 |
| SO3 | 1.89 | 1.75 | 2.08 | 2.23 | 1.89 | 1.65 |
| Al2O3 | 1.39 | 1.22 | 1.16 | 0.73 | 0.67 | 0.85 |
| SrO | 0.11 | 0.1 | 0.11 | 0.11 | 0.1 | 0.1 |
| BaO | - | - | - | 0.13 | 0.14 | 0.17 |
| ZnO | 0.1 | - | - | - | - | - |
| MnO | - | 0.09 | 0.11 | - | - | - |
| TiO2 | 0.09 | 0.06 | 0.08 | 0.08 | 0.07 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-E.; Shin, D.-C.; Jeong, Y.; Kim, I.-T.; Yoo, Y.-S. Effects of Pyrolysis Temperature and Retention Time on Fuel Characteristics of Food Waste Feedstuff and Compost for Co-Firing in Coal Power Plants. Energies 2019, 12, 4538. https://doi.org/10.3390/en12234538
Lee Y-E, Shin D-C, Jeong Y, Kim I-T, Yoo Y-S. Effects of Pyrolysis Temperature and Retention Time on Fuel Characteristics of Food Waste Feedstuff and Compost for Co-Firing in Coal Power Plants. Energies. 2019; 12(23):4538. https://doi.org/10.3390/en12234538
Chicago/Turabian StyleLee, Ye-Eun, Dong-Chul Shin, Yoonah Jeong, I-Tae Kim, and Yeong-Seok Yoo. 2019. "Effects of Pyrolysis Temperature and Retention Time on Fuel Characteristics of Food Waste Feedstuff and Compost for Co-Firing in Coal Power Plants" Energies 12, no. 23: 4538. https://doi.org/10.3390/en12234538
APA StyleLee, Y.-E., Shin, D.-C., Jeong, Y., Kim, I.-T., & Yoo, Y.-S. (2019). Effects of Pyrolysis Temperature and Retention Time on Fuel Characteristics of Food Waste Feedstuff and Compost for Co-Firing in Coal Power Plants. Energies, 12(23), 4538. https://doi.org/10.3390/en12234538





