Ignition of Slurry Fuel Droplets with Different Heating Conditions
Abstract
1. Introduction
2. Typical Nomenclature of Industrial Waste
3. Experimental Setup and Methods
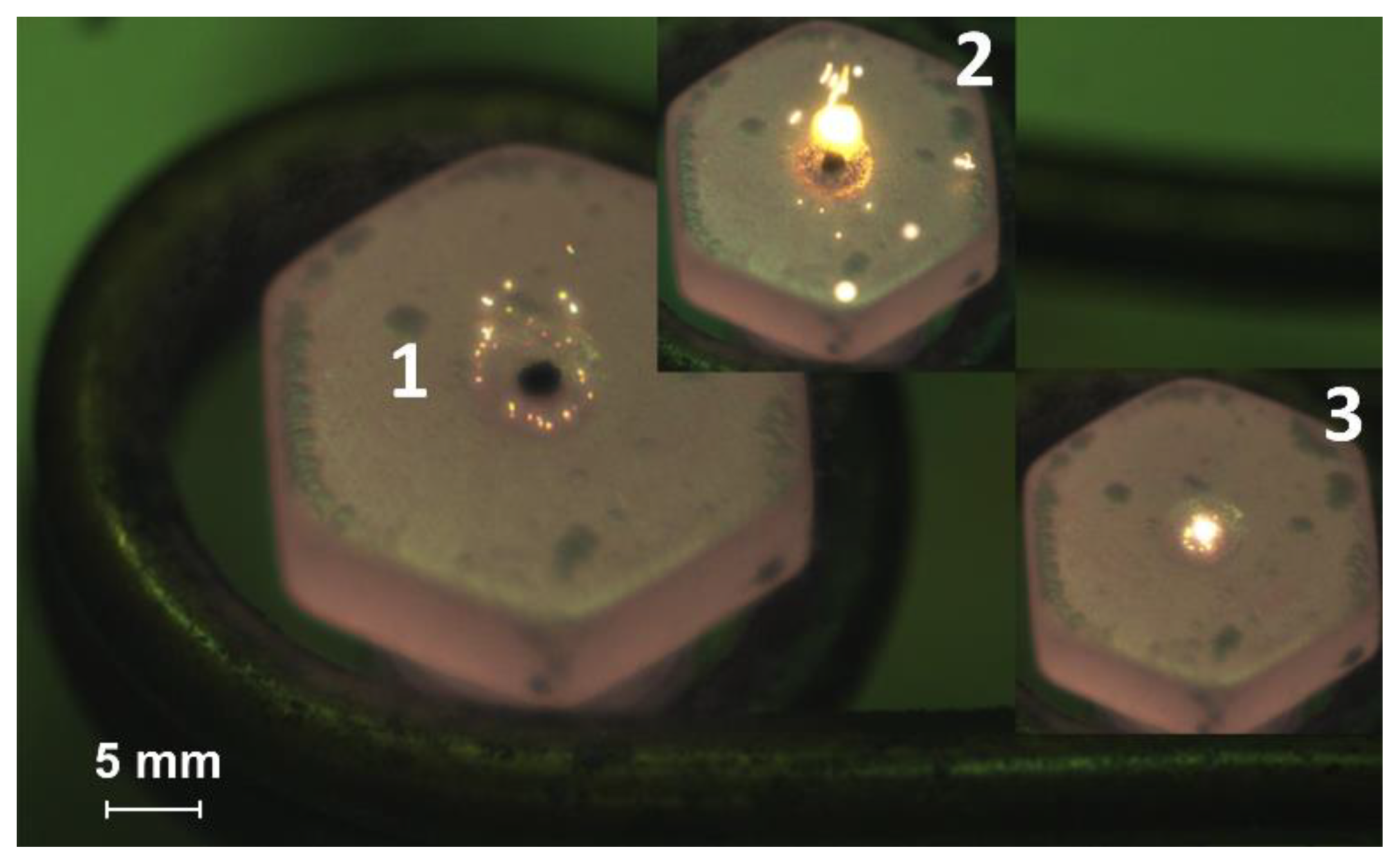
3.1. Heating of the Fuel Particles on the Hot Surface
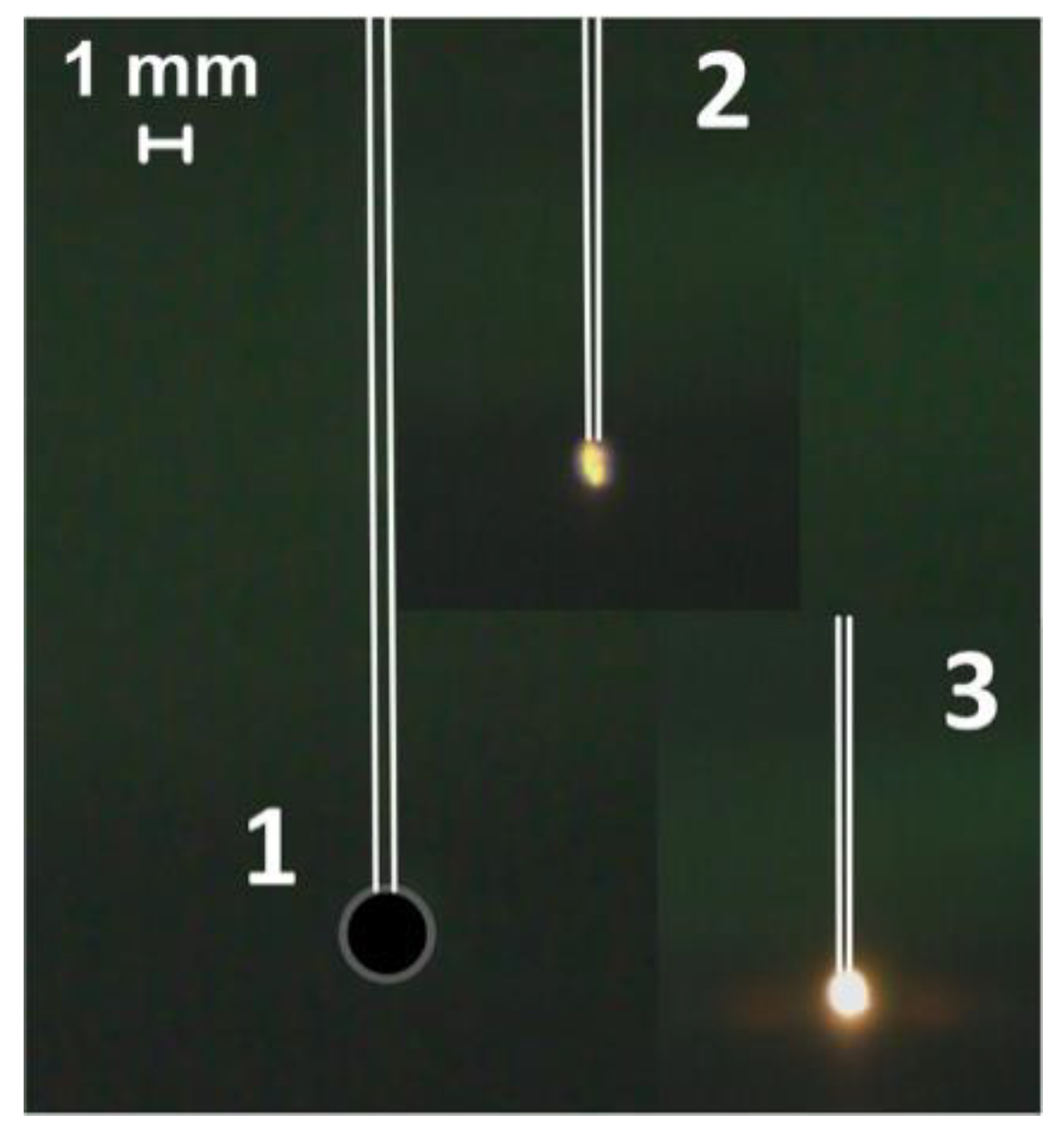
3.2. Heating of a Fuel Particle on the Holder in the Flow of Heated Air
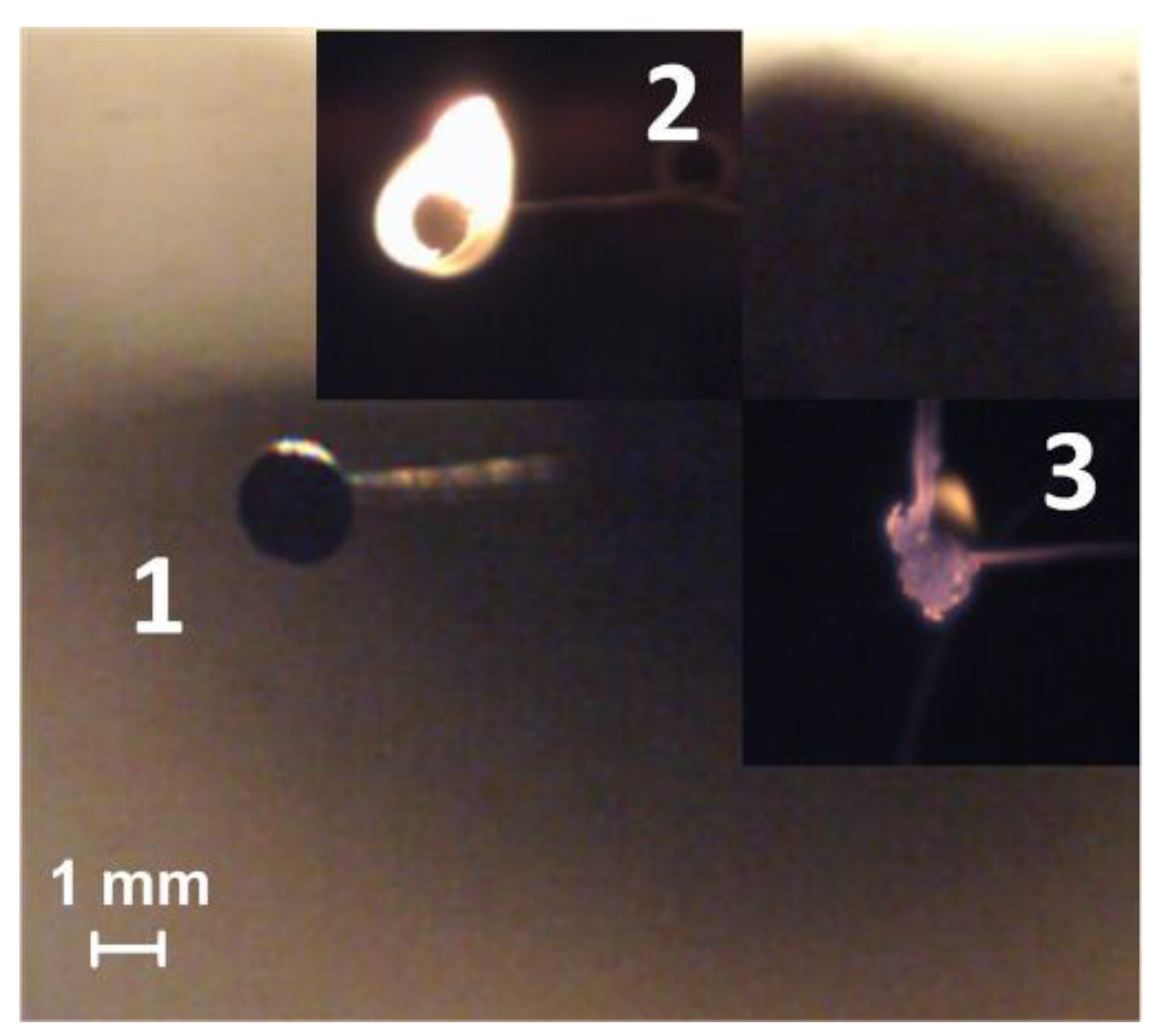
3.3. Heating of the Fuel Particle in the Conditions of Intense Radiation Heating on the Holder
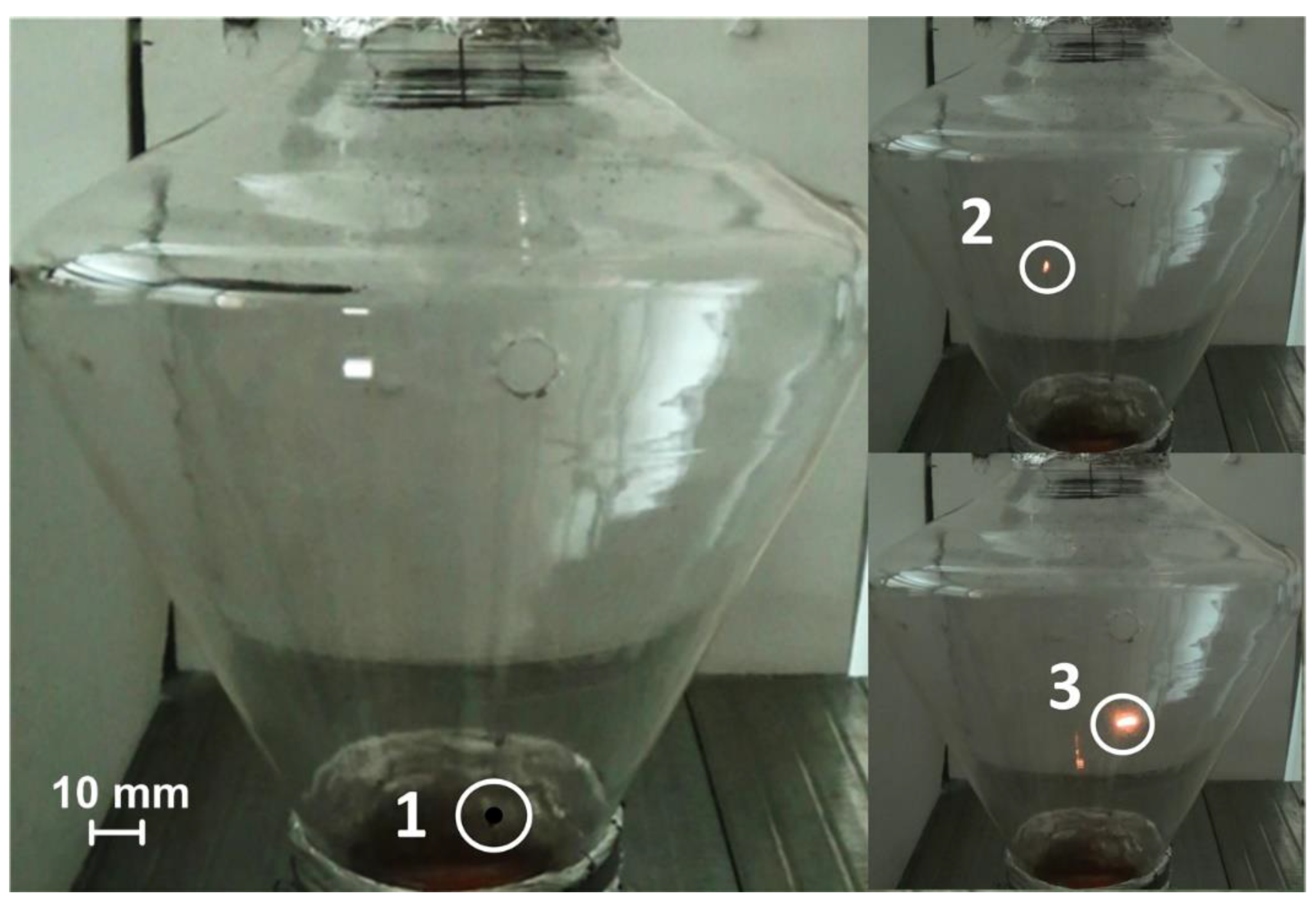
3.4. Use of a Quartz Combustion Chamber
4. Results and Discussion
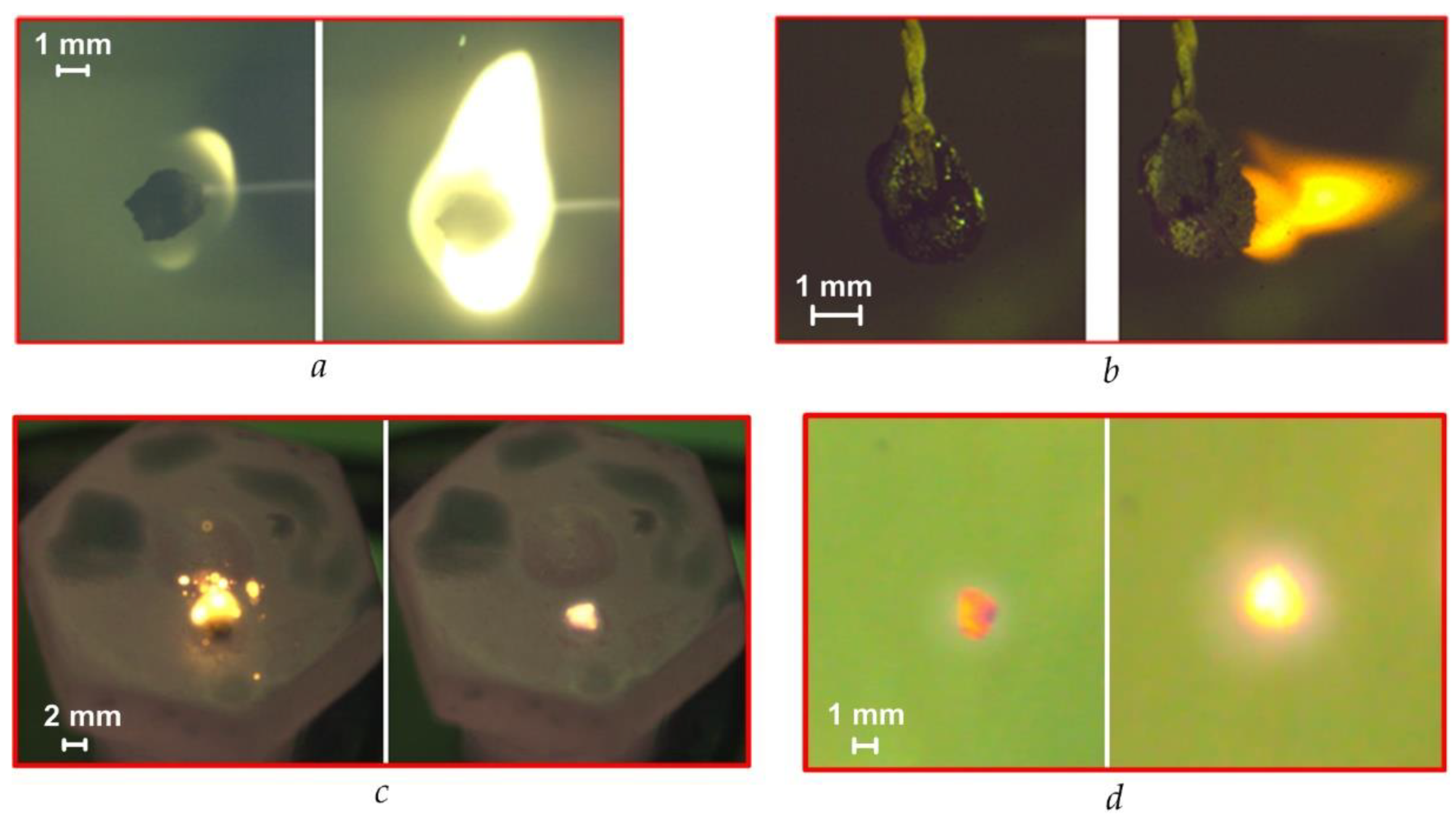
4.1. Stages of Fuel Ignition and Combustion with Different Heating Schemes
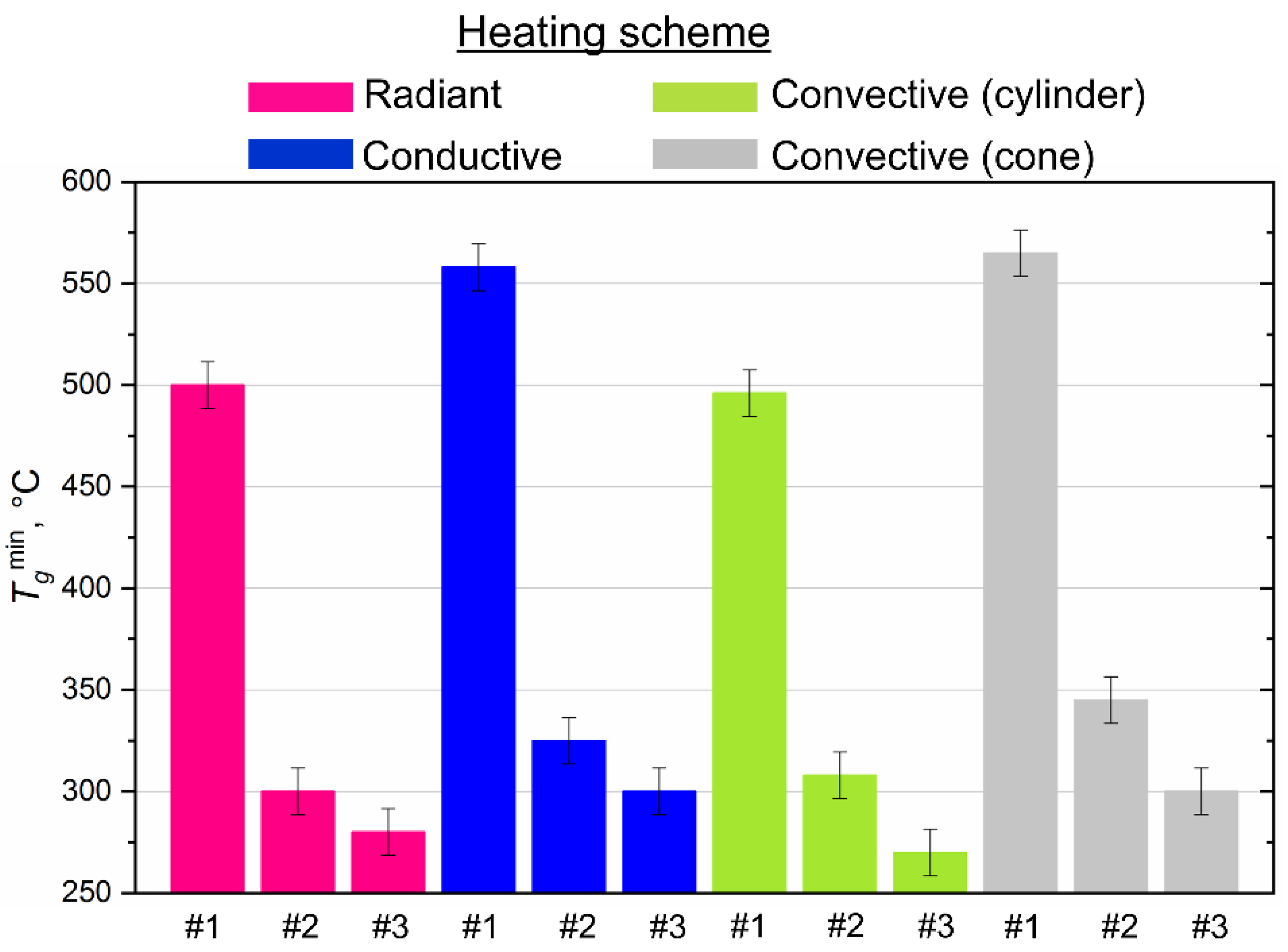
4.2. Minimum Temperatures of Combustion Initiation
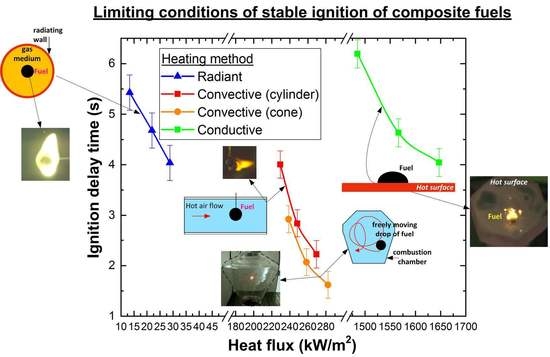
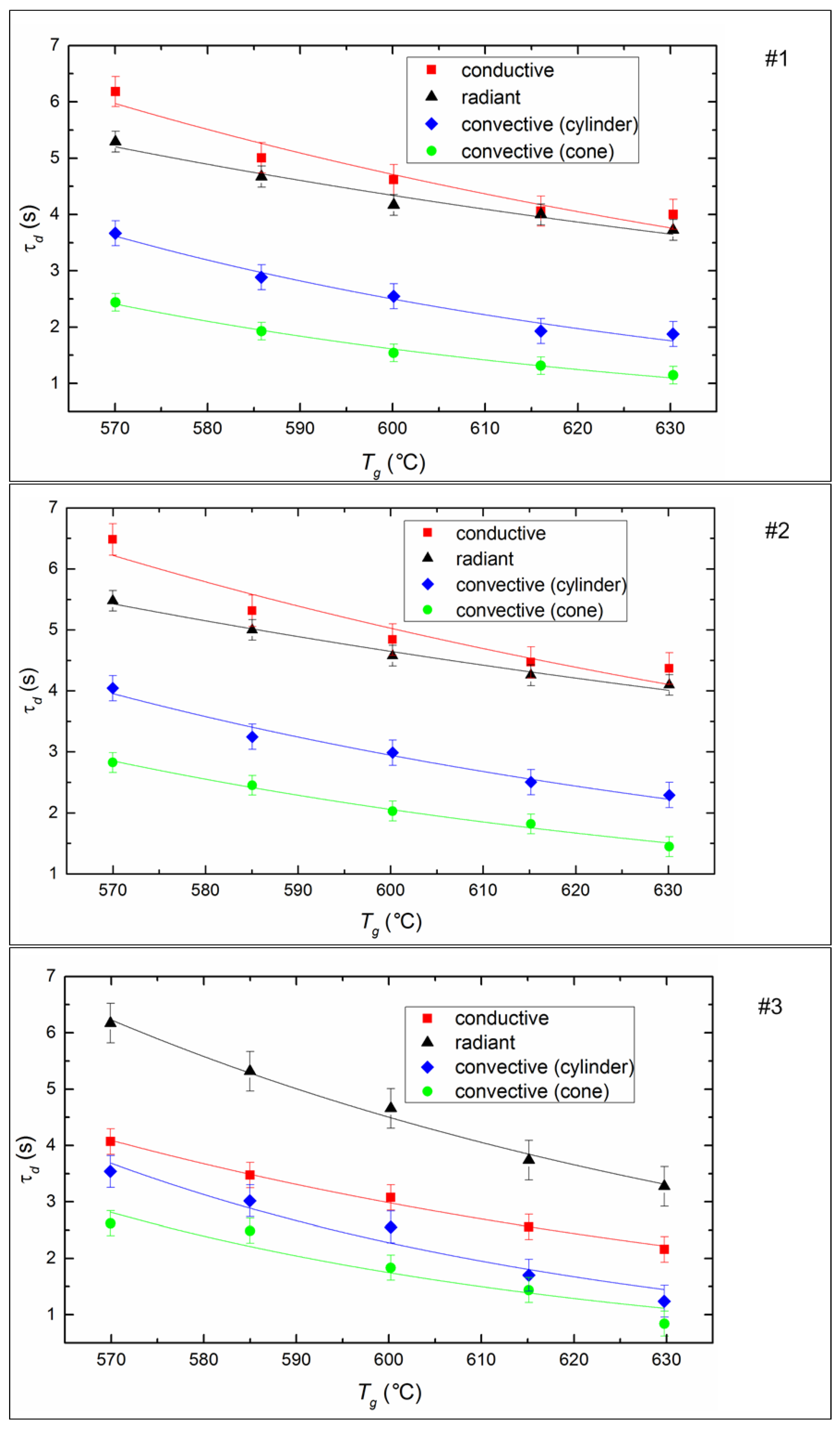
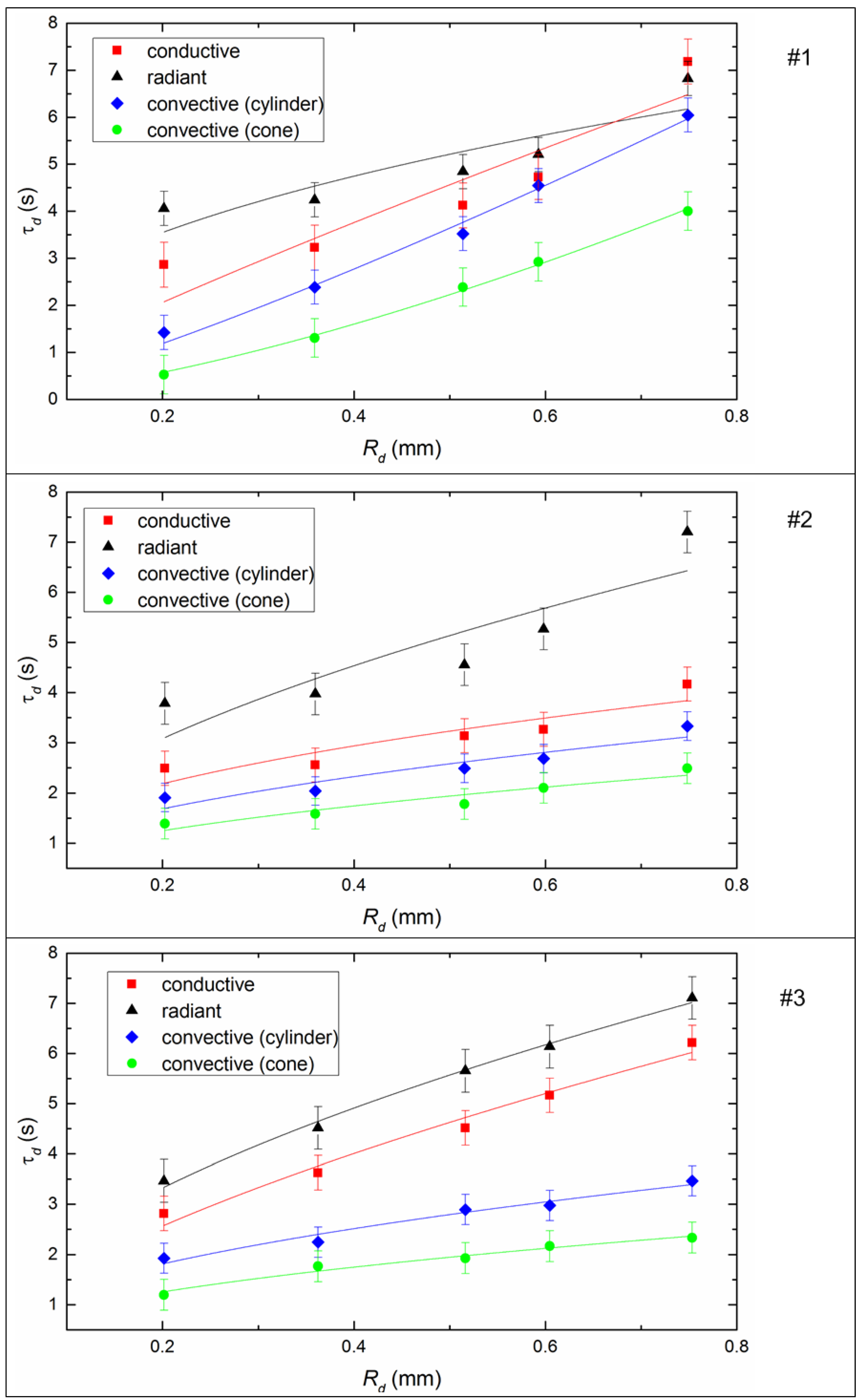
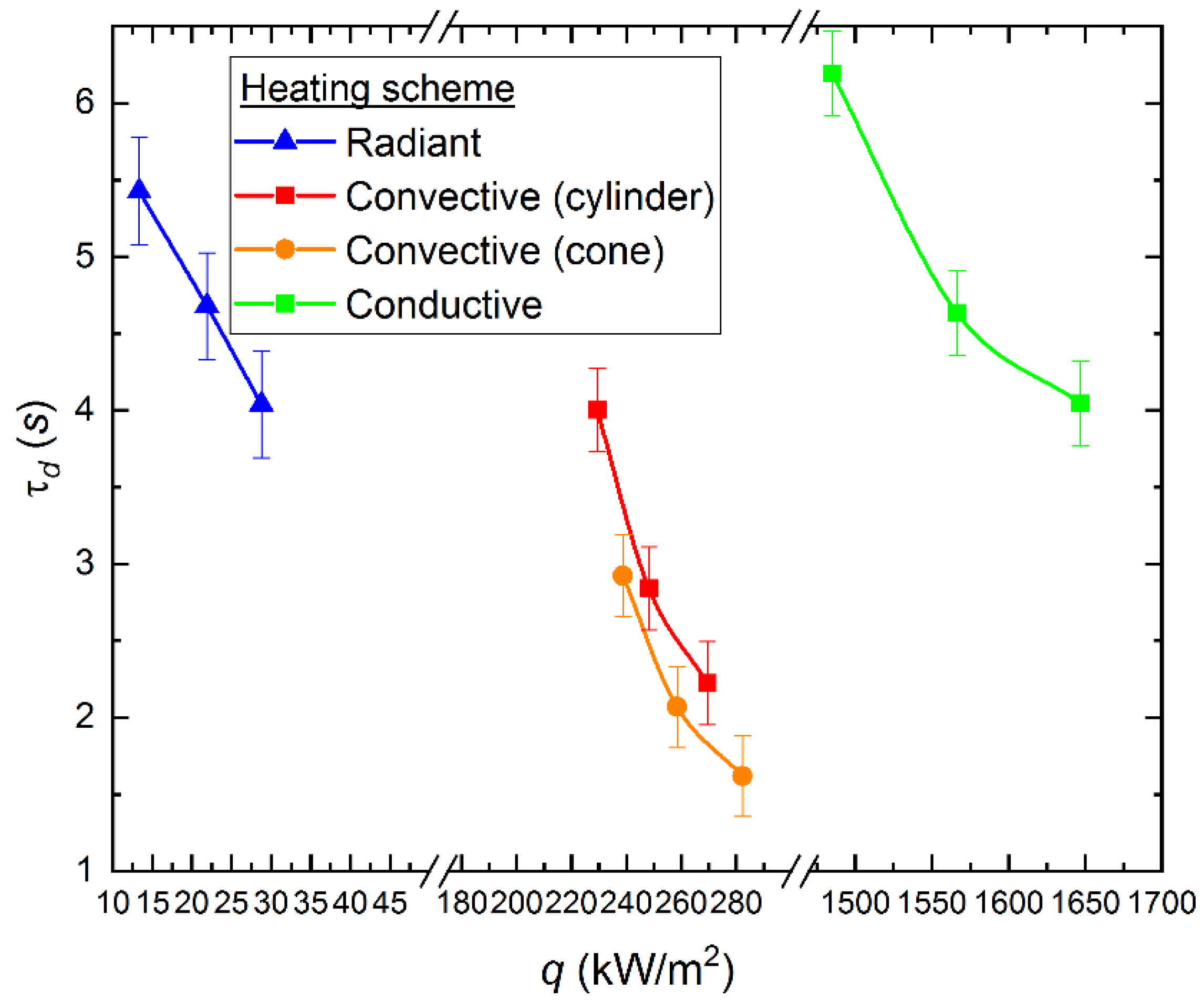
4.3. Ignition Delay Times
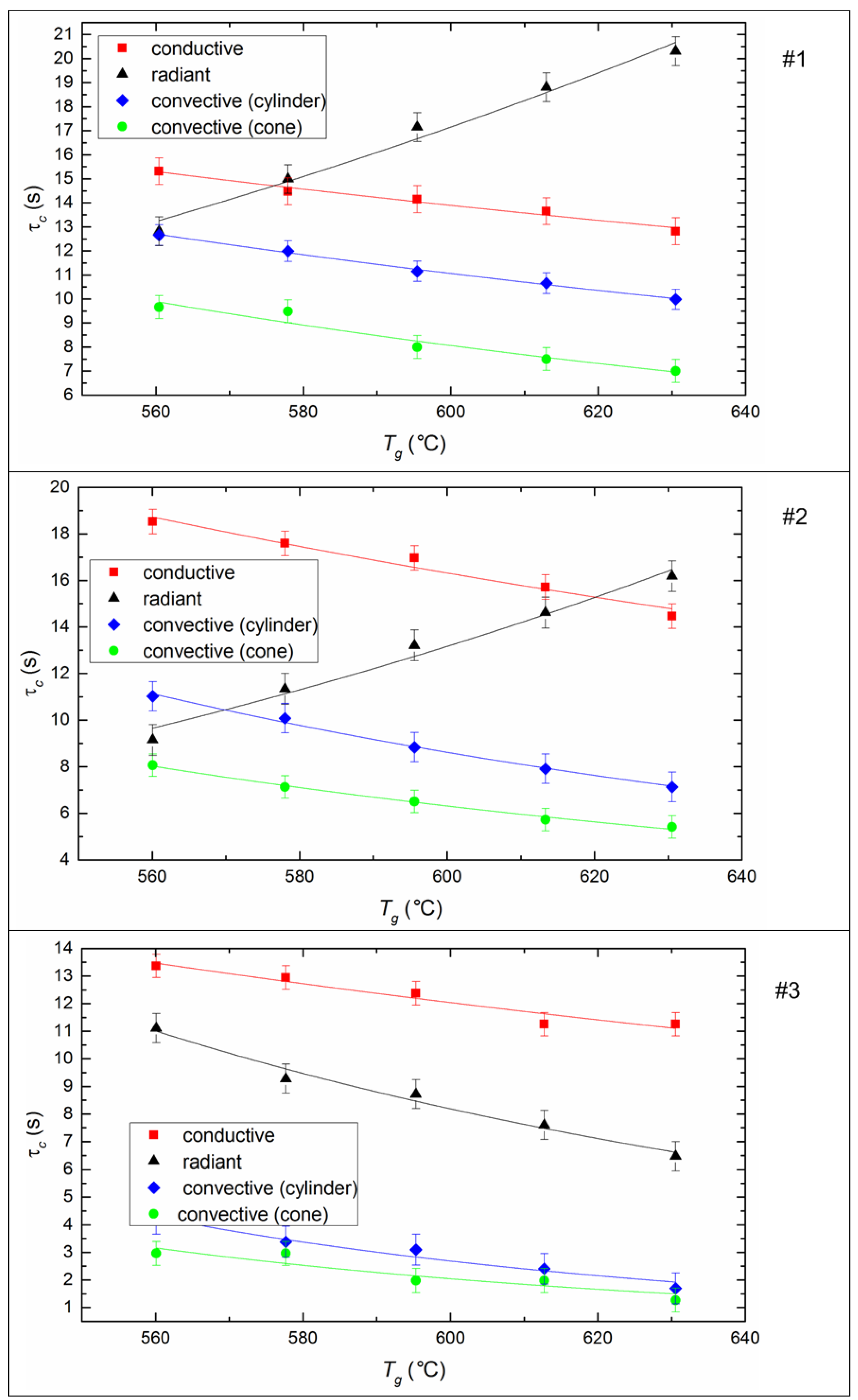
4.4. Maximum Combustion Temperatures of Fuel
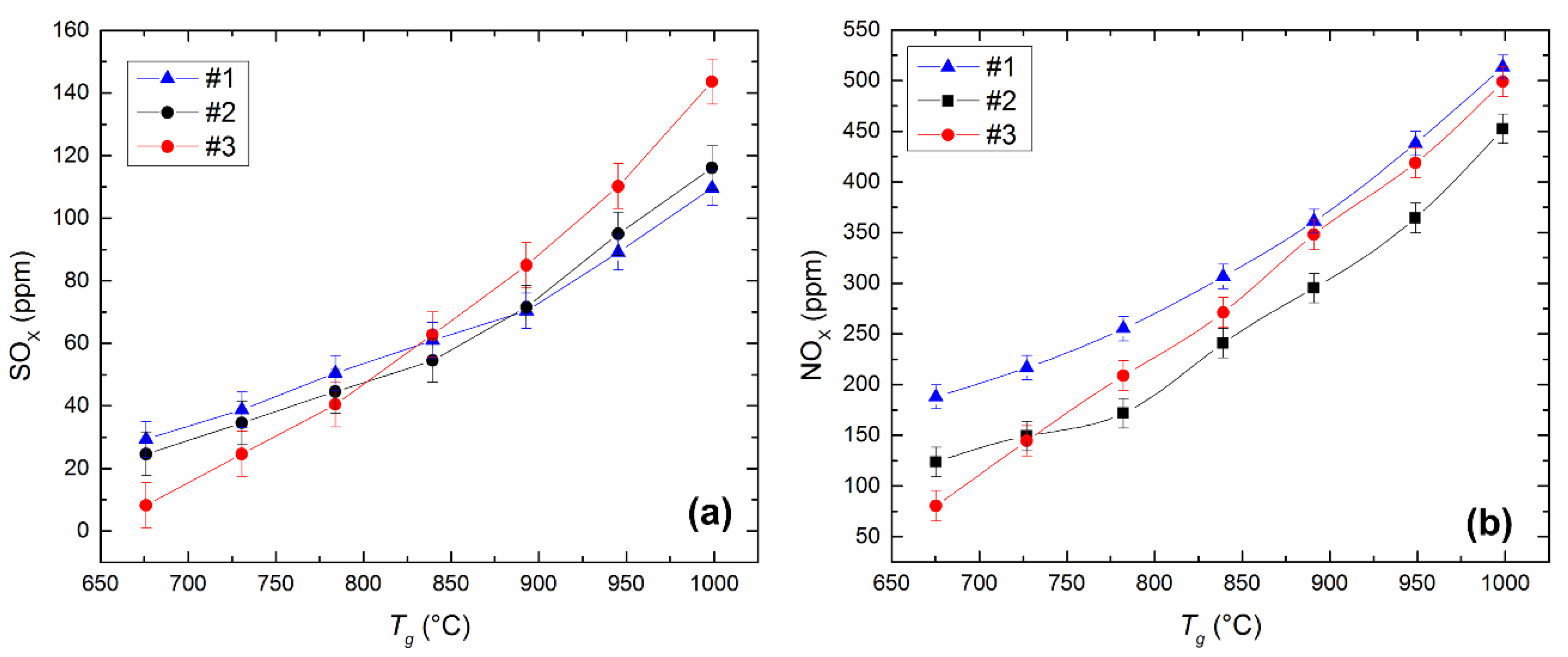
4.5. Anthropogenic Emissions
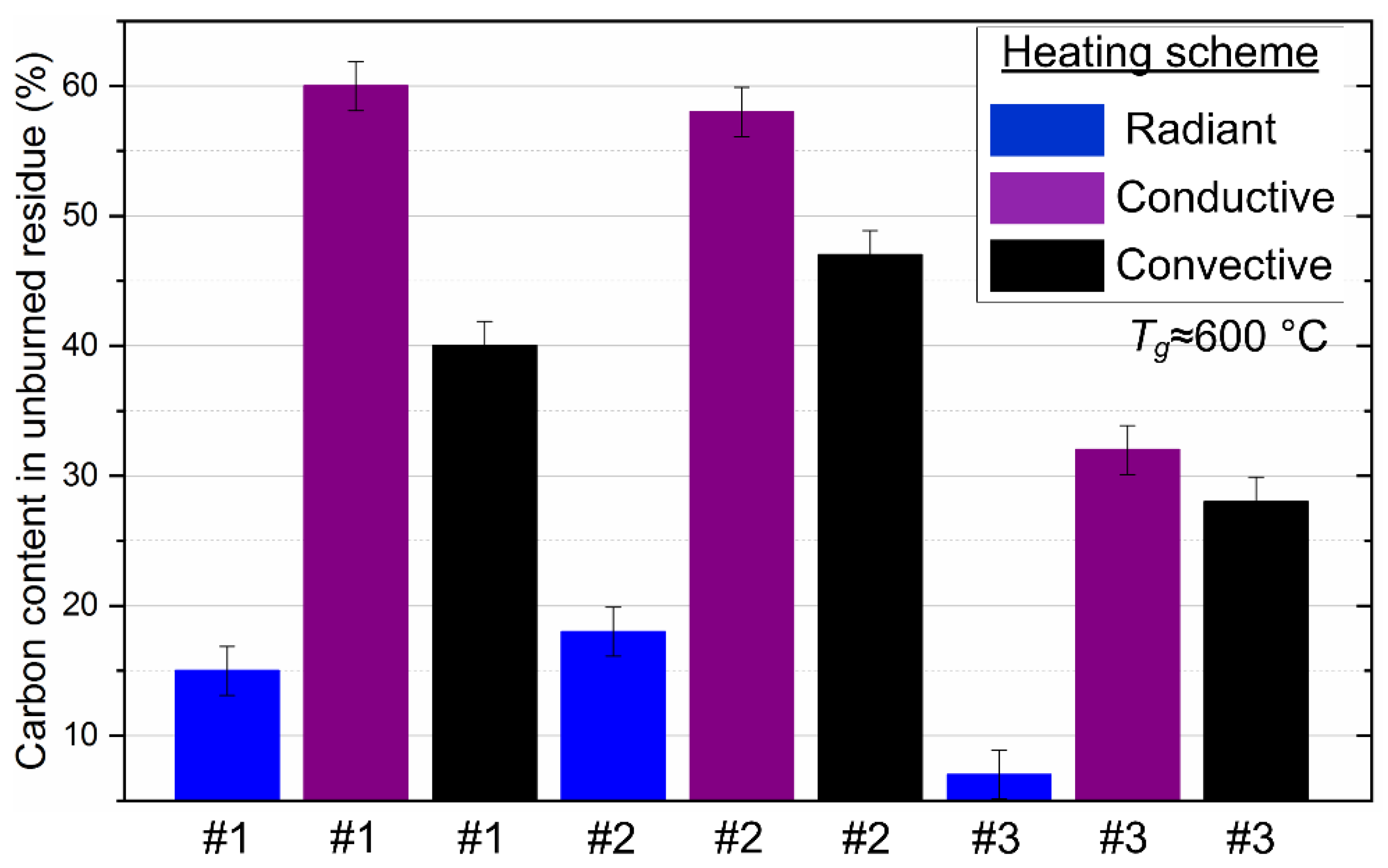
4.6. Analysis of the Ash Residue during Fuel Combustion
5. Conclusions
- (i)
- All of the considered heating schemes can be used to study the ignition and combustion of slurry fuels (with the dominance of the conductive, convective and radiation mechanisms). The differences in the basic characteristics of the heating, ignition and combustion established during the experiments make it possible to predict similar differences for real combustion processes. The mechanism of the slurry fuel droplet ignition is common and based on intensive dehydration of the surface layers of fuel fragments, the formation and ignition of the gas-vapor mixture (volatile and vapors) and the subsequent heterogeneous ignition of the carbon base.
- (ii)
- Based on a comparative analysis, it can be concluded that the most effective in terms of minimizing the ignition delay times and the extreme temperatures of combustion initiation is convective heating of the fuel. The maximum combustion temperatures and the minimum ash residue, consequently, correspond to the experiments with radiation heating. We can ensure such conditions by organizing vortex combustion processes in which fuel droplets spin in the combustion chamber and burn out for a long time. The completeness of fuel underburning is reduced, and anthropogenic emissions are minimized.
- (iii)
- According to the properties and concentration of the slurry fuel components, as well as the method for their heating, there are three possible modes of ignition and combustion of slurry fuel particles: flame, smoldering and micro-explosion. As a result, the ignition delay times, the extreme temperatures of the ignition and especially the temperature and duration of the slurry combustion may be different. The obtained experimental data on the characteristics and conditions for the implementation of these processes allow us to explain the differences of the parameters considered in the present work, which are recorded by various scientific groups throughout the world.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLF | composite liquid fuels |
| Nomenclature | |
| Ad | ash level of dry sample (%) |
| Ca | heat capacity of air (J/(kg K)) |
| Cdaf, Hdaf, Ndaf, Odaf | fraction of carbon, hydrogen, nitrogen, oxygen in the sample converted to a dry ash-free state (%) |
| NOx | concentration of nitrogen oxides (ppm) |
| Q | heat flux (kW/m2) |
| qcond | conductive heat flux (kW/m2) |
| qconv | convective heat flux (kW/m2) |
| qrad | radiative heat flux (kW/m2) |
| Qas | heat of combustion (MJ/kg) |
| Rd | initial droplet radius (mm) |
| Std | fraction of sulfur in the sample converted to a dry state (%) |
| SOx | concentration of sulfur oxides (ppm) |
| Td | temperature in the center of a droplet (°C) |
| Tdmax | maximum temperature in the center of a droplet (°C) |
| Tg | temperature in combustion chamber (°C) |
| Tgmin | minimum heating temperature sufficient for sustainable ignition (°C) |
| Ts | droplet surface temperature (°C) |
| Tsub | heated substrate temperature (°C) |
| Vdaf | yield of volatiles of filter cake converted to a dry ash-free state, (s) |
| Vg | gas flow velocity (m/s) |
| Wa | initial sample moisture (%) |
| Greek symbols | |
| A | heat transfer coefficient (W/(m2·K)) |
| εd | emissivity factor of water droplet |
| εa | emissivity factor of air |
| Λ | thermal conductivity (W/(m·K)) |
| λd | thermal conductivity coefficient of water droplet (W/(m·K)) |
| λa | thermal conductivity coefficient of air (W/(m·K)) |
| νa | kinematic viscosity of air (m2/s) |
| ρa | air density (kg/m3) |
| σ | Stefan‒Boltzmann constant (W/(m2·K4)) |
| τd | ignition delay time (s) |
| τc | time of complete burnout (s) |
| Dimensionless numbers | |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Re | Reynolds number |
References
- Gorlov, E.G. Composite water-containing fuels from coals and petroleum products. Solid Fuel Chem. 2004, 38, 40–50. [Google Scholar]
- Khodakov, G.S. Coal-water suspensions in power engineering. Therm. Eng. 2007, 54, 36–47. [Google Scholar] [CrossRef]
- Lishtvan, I.I.; Falyushin, P.L.; Smolyachkova, E.A.; Kovrik, S.I. Fuel suspensions based on fuel oil, peat, waste wood, and charcoal. Solid Fuel Chem. 2009, 43, 1–4. [Google Scholar] [CrossRef]
- Vedruchenko, V.R. The dynamics of droplet transformations in the flame of a water-fuel oil emulsion used as the fuel for boiler installations. Therm. Eng. 2000, 47, 156–160. [Google Scholar]
- Glushkov, D.O.; Strizhak, P.A.; Chernetskii, M.Y. Organic Coal-Water Fuel: Problems and Advances (Review). Therm. Eng. 2016, 63, 707–717. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Coal-water slurries containing petrochemicals to solve problems of air pollution by coal thermal power stations and boiler plants: An introductory review. Sci. Total Environ. 2018, 613–614, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, W.; Kijo-Kleczkowska, A.; Leszczynski, J. Analysis of cyclic combustion of solid fuels. Fuel 2009, 88, 221–234. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A. Combustion of coal-water suspensions. Fuel 2011, 90, 865–877. [Google Scholar] [CrossRef]
- Singh, G.; Esmaeilpour, M.; Ratner, A. Investigation of combustion properties and soot deposits of various US crude oils. Energies 2019, 12, 2368. [Google Scholar] [CrossRef]
- Wu, D.; Song, Z.; Schmidt, M.; Zhang, Q.; Qian, X. Theoretical and numerical study on ignition behavior of coal dust layers on a hot surface with corrected kinetic parameters. J. Hazard. Mater. 2019, 368, 156–162. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, X.; Shi, Y.; Qin, X.; Song, C. Ignition and Combustion Behaviors of Coal Slime in Air. Energy Fuels 2017, 31, 11439–11447. [Google Scholar] [CrossRef]
- Chen, G.B.; Chatelier, S.; Lin, H.T.; Wu, F.H.; Lin, T.H. A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies 2018, 11, 3436. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Egorov, R.I.; Strizhak, P.A. The ignition parameters of the coal-water slurry droplets at the different methods of injection into the hot oxidant flow. Appl. Therm. Eng. 2016, 107, 10–20. [Google Scholar] [CrossRef]
- Atal, A.; Levendis, Y.A. Observations on the combustion behavior of coal water fuels and coal water fuels impregnated with calcium magnesium acetate. Combust. Flame 1993, 93, 61–89. [Google Scholar] [CrossRef]
- Atal, A.; Levendis, Y.A. Combustion of CWF agglomerates from pulverized or micronized bituminous coal, carbon black, and diesel soot. Combust. Flame 1994, 93, 326–342. [Google Scholar] [CrossRef]
- Bogomolov, A.; Valiullin, T.; Vershinina, K.; Shevyrev, S.; Shlegel, N. Igniting Soaring Droplets of Promising Fuel Slurries. Energies 2019, 12, 208. [Google Scholar] [CrossRef]
- Valiullin, T.R.; Strizhak, P.A. Influence of the shape of soaring particle based on coal-water slurry containing petrochemicals on ignition characteristics. Therm. Sci. 2017, 21, 1399–1408. [Google Scholar] [CrossRef]
- ISO. Solid Mineral Fuels—Hard Coal—Determination of Moisture in the General Analysis Test Sample by Drying in Nitrogen; ISO 11722:1999; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 1999. [Google Scholar]
- ISO. Solid Mineral Fuel—Determination of Ash; ISO 1171:2010; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2010. [Google Scholar]
- ISO. Hard Coal and Coke—Determination of Volatile Matter; BS ISO 562:2010; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2010. [Google Scholar]
- ISO. Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value; ISO 1928:2009; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2009. [Google Scholar]
- ASTM. Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter; D240-92 (1997) e2; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- ISO. Petroleum Products and Bituminous Materials: Determination of Water—Distillation Method; ISO 3733:1999; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 1999. [Google Scholar]
- ISO. Petroleum Products: Determination of Ash; ISO 6245:2001; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2001. [Google Scholar]
- ISO. Petroleum Products: Determination of Flash and Fire Points—Cleveland Open Cup Method; ISO 2592:2000; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2000. [Google Scholar]
- ASTM. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke; D5373-14e1; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants; D5291-10(2015); ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Lei, K.; Ye, B.; Cao, J.; Zhang, R.; Liu, D. Combustion Characteristics of Single Particles from Bituminous Coal and Pine Sawdust in O2/N2, O2/CO2, and O2/H2O Atmospheres. Energies 2017, 10, 1695. [Google Scholar] [CrossRef]
- Dieck, R.H. Measurement Uncertainty, Fourth Edition: Methods and Applications; ISA Publishing: Research Triangle Park, NC, USA, 2006; p. 277. [Google Scholar]
- Yao, S.C.; Manwani, P. Burning of suspended coal-water slurry droplet with oil as combustion additive. Combust. Flame 1986, 66, 87–89. [Google Scholar] [CrossRef]
- Saito, M.; Sadakata, M.; Sakai, T. Single droplet combustion of coal-oil/methanol/water mixtures. Fuel 1983, 62, 1481–1486. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Z.; Zhang, Y.; Liu, P.; Zhang, D. An experimental investigation into the ignition and combustion characteristics of single droplets of biochar water slurry fuels in air. Appl. Energy 2017, 185, 2160–2167. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Su, H.; Chen, J.; Liu, B.; Zhang, Y. Explosion Characteristics and Flame Propagation Behavior of Mixed Dust Cloud of Coal Dust and Oil Shale Dust. Energies 2019, 12, 3807. [Google Scholar] [CrossRef]

| Sample | Proximate Analysis | Ultimate Analysis, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ad, % | Vdaf, % | Qas, MJ/kg | Wa, % | Cdaf | Hdaf | Ndaf | Std | Odaf | |
| Lignite | 4.12 | 47.63 | 22.91 | 14.11 | 73.25 | 6.516 | 0.79 | 0.435 | 18.99 |
| Low-caking coal | 21.68 | 27.40 | 26.23 | 2.76 | 77.30 | 4.783 | 1.93 | 0.326 | 15.32 |
| Filter cake of low-caking coal | 50.89 | 30.16 | 15.23 | 37.9 | 87.47 | 5.039 | 2.15 | 0.444 | 4.77 |
| Density at 20 °C, kg/m3 | Humidity, % | Flash Point, °C | Temperature of Ignition, °C | Heat of Combustion, MJ/kg |
|---|---|---|---|---|
| 868 | 0.07 | 175 | 193 | 45.1 |
| CLF (Composite Liquid Fuel) (Relative Mass Concentration of Components, %) | ||
|---|---|---|
| #1 | #2 | #3 |
| 90% of filter cake of low-caking coal, 10% of waste turbine oil | 40% of low-caking coal, 10% of lignite, 40% of water, 10% of waste turbine oil | 50% of lignite, 40% of water, 10% of waste turbine oil |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiullin, T.; Vershinina, K.; Strizhak, P. Ignition of Slurry Fuel Droplets with Different Heating Conditions. Energies 2019, 12, 4553. https://doi.org/10.3390/en12234553
Valiullin T, Vershinina K, Strizhak P. Ignition of Slurry Fuel Droplets with Different Heating Conditions. Energies. 2019; 12(23):4553. https://doi.org/10.3390/en12234553
Chicago/Turabian StyleValiullin, Timur, Ksenia Vershinina, and Pavel Strizhak. 2019. "Ignition of Slurry Fuel Droplets with Different Heating Conditions" Energies 12, no. 23: 4553. https://doi.org/10.3390/en12234553
APA StyleValiullin, T., Vershinina, K., & Strizhak, P. (2019). Ignition of Slurry Fuel Droplets with Different Heating Conditions. Energies, 12(23), 4553. https://doi.org/10.3390/en12234553